Assessing the Contribution of Posidonia oceanica to Mediterranean Secondary Production Through Stable Isotope Analysis
Abstract
1. Introduction
2. Materials and Methods
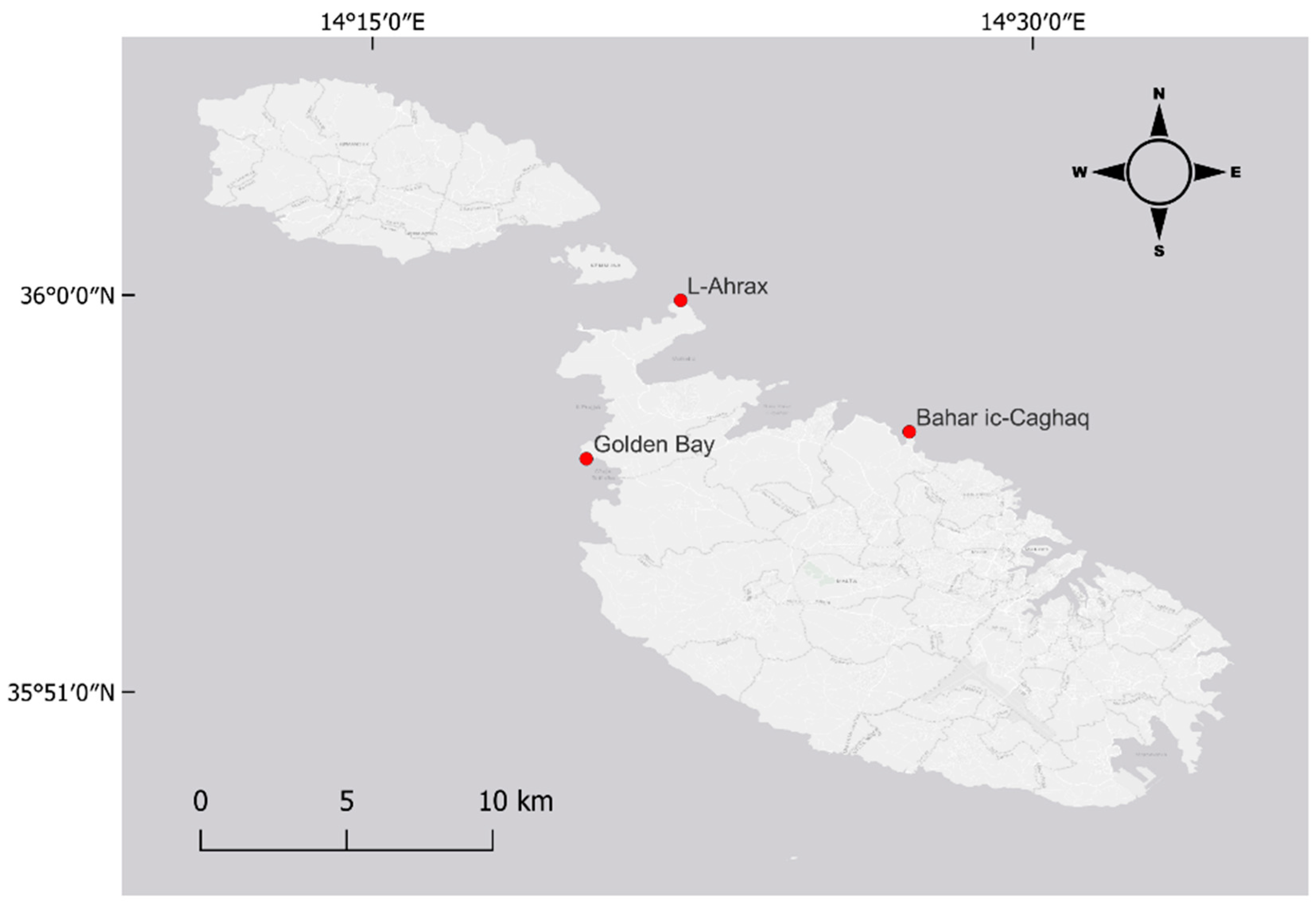
2.1. Study Area and Sample Collection
2.2. Stable Isotope Analysis (IRMS)
2.3. Statistical Analyses
3. Results
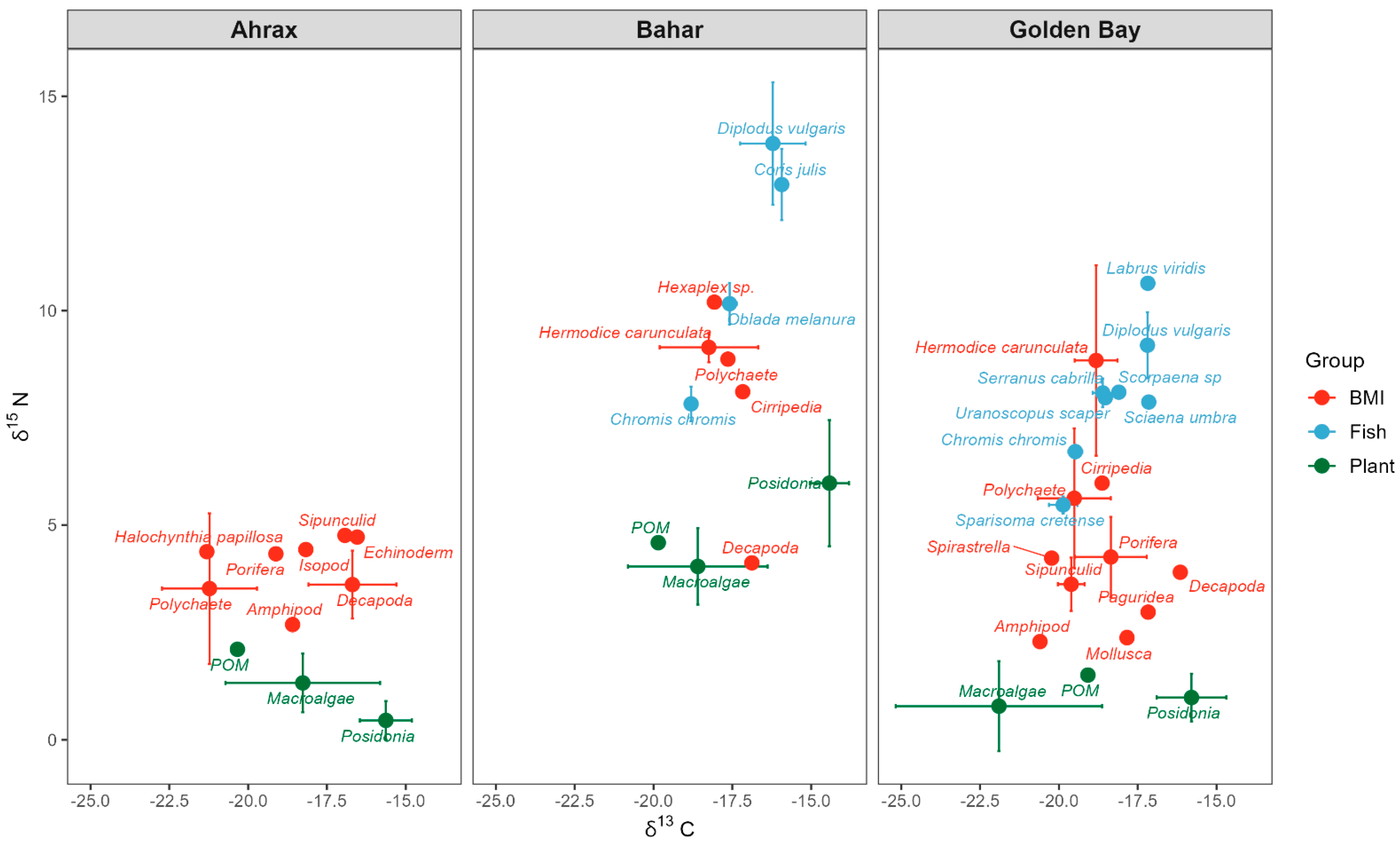
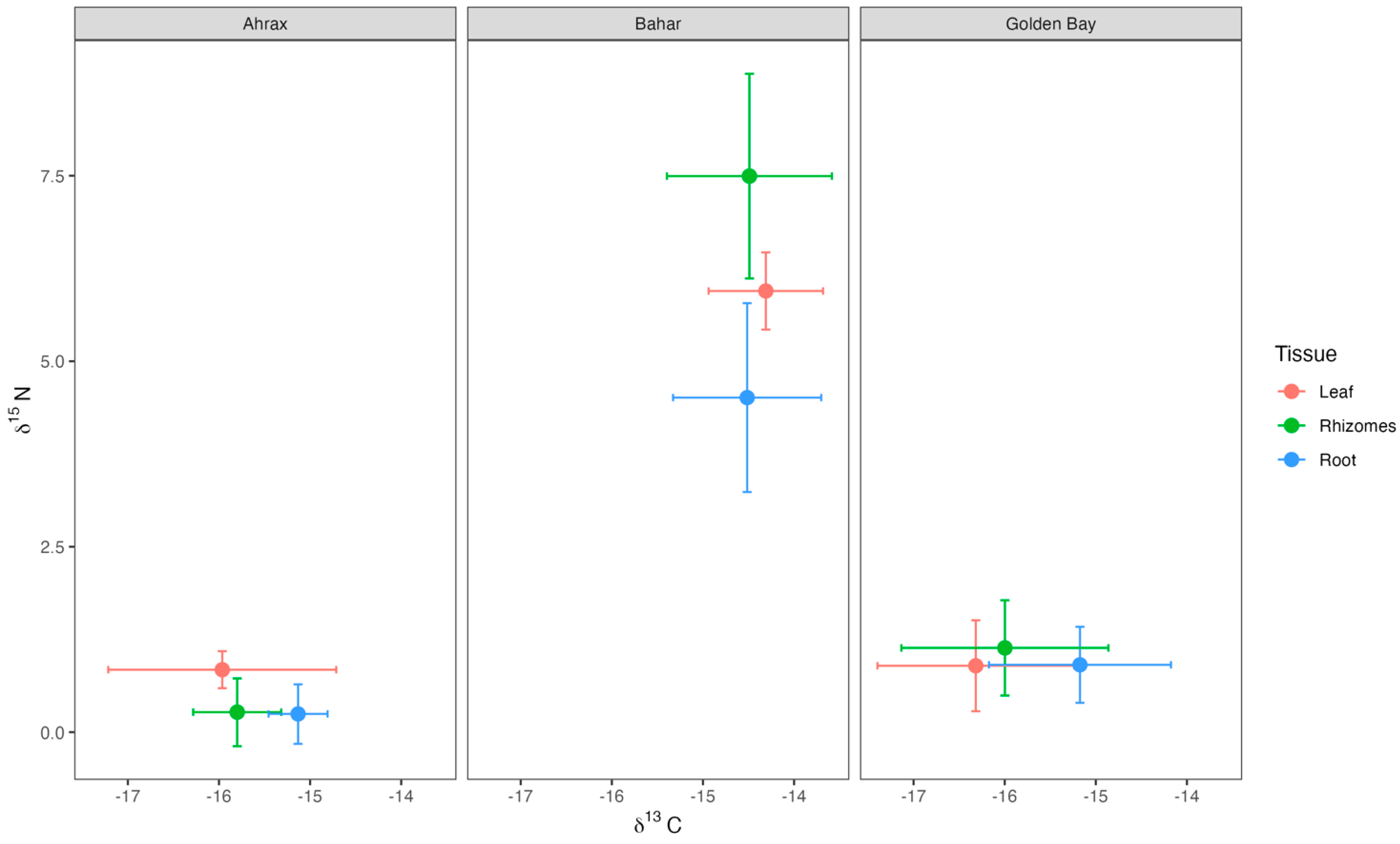
3.1. Stable Isotope Values
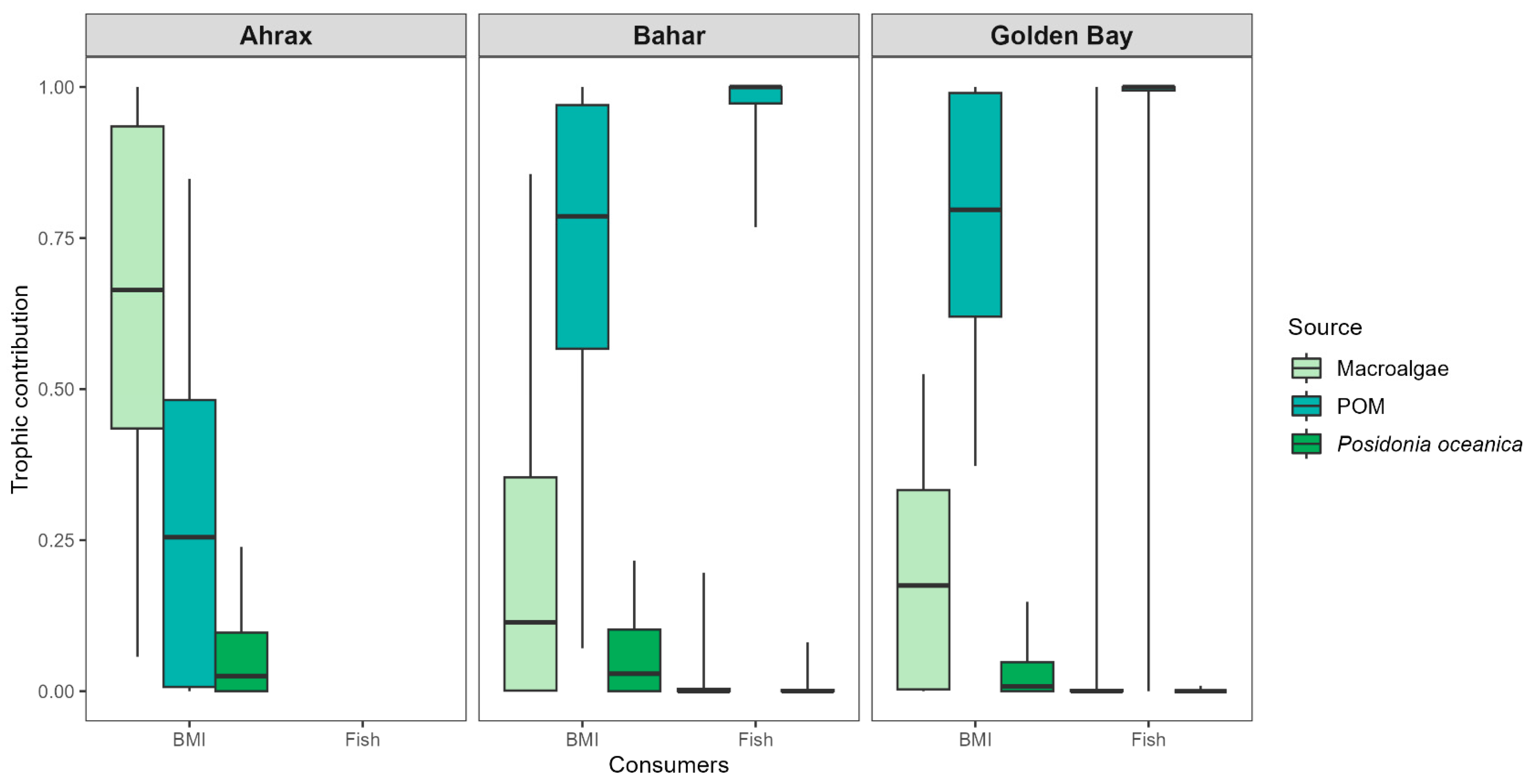
3.2. Stable Isotope Mixing Model
4. Discussion
4.1. δ13C and δ15N Isotopes of Sources and Consumers
4.2. Trophic Contribution of Sources to Consumers
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orth, R.J.; Carruthers, T.J.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hays, G.C.; Marsh, H.; Olyarnik, S.; Kendrick, G.A.; et al. A global crisis for seagrass ecosystems. BioScience 2010, 60, 222–233. [Google Scholar] [CrossRef]
- Gullström, M.; Bodin, M.; Nilsson, P.G.; Öhman, M.C. Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Mar. Ecol. Prog. Ser. 2008, 363, 241–255. [Google Scholar] [CrossRef]
- Vizzini, S. Analysis of the trophic role of Mediterranean seagrasses in marine coastal ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 52, 383–393. [Google Scholar] [CrossRef]
- Gacia, E.; Duarte, C.M.; Middelburg, J.J. Carbon and nutrient deposition in a Mediterranean seagrass (Posidonia oceanica) meadow. Limnol. Oceanogr. 2002, 47, 23–32. [Google Scholar] [CrossRef]
- Terrados, J.; Borum, J. Why are seagrasses important?—Goods and services provided by seagrass meadows. In European seagrasses: An Introduction to Monitoring and Management; M&MS Project, 2004; pp. 8–10. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.vliz.be/imisdocs/publications/67177.pdf&ved=2ahUKEwjBufvJvYWKAxW1s1YBHZZZGloQFnoECBYQAw&usg=AOvVaw1G2Dyf9GlRwR_ztHJ_Qv7Z (accessed on 24 November 2024).
- James, R.K.; Silva, R.; Van Tussenbroek, B.I.; Escudero-Castillo, M.; Mariño-Tapia, I.; Dijkstra, H.A.; Bouma, T.J. Maintaining tropical beaches with seagrass and algae: A promising alternative to engineering solutions. BioScience 2019, 69, 136–142. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Van Belzen, J.; Herman, P.M.J.; Van Katwijk, M.M.; Lamers, L.P.M.; Van Leent, P.J.M.; Bouma, T.J. Low-canopy seagrass beds still provide important coastal protection services. PLoS ONE 2013, 8, e62413. [Google Scholar] [CrossRef]
- Ascioti, F.A.; Mangano, M.C.; Marcianò, C.; Sarà, G. The sanitation service of seagrasses—Dependencies and implications for the estimation of avoided costs. Ecosyst. Serv. 2022, 54, 101418. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Glob. Change Biol. 2018, 24, 4919–4928. [Google Scholar] [CrossRef]
- Diaz-Almela, E.; Marbà, N.; Duarte, C.M. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob. Chang. Biol. 2007, 13, 224–235. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Chang. Biol. 2010, 16, 2366–2375. [Google Scholar] [CrossRef]
- Litsi-Mizan, V.; Efthymiadis, P.T.; Gerakaris, V.; Serrano, O.; Tsapakis, M.; Apostolaki, E.T. Decline of seagrass (Posidonia oceanica) production over two decades in the face of warming of the Eastern Mediterranean Sea. New Phytol. 2023, 239, 2126–2137. [Google Scholar] [CrossRef]
- Rinaldi, A.; Martinez, M.; Badalamenti, F.; D’Anna, G.; Mirto, S.; Marín-Guirao, L.; Montalto, V. The ontogeny-specific thermal sensitivity of the seagrass Posidonia oceanica. Front. Mar. Sci. 2023, 10, 1183728. [Google Scholar] [CrossRef]
- Hoffman, R. Alien benthic algae and seagrasses in the Mediterranean Sea and their connection to global warming. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, Netherlands, 2014. [Google Scholar]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef]
- Richir, J.; Salivas-Decaux, M.; Lafabrie, C.; Lopez Y Royo, C.; Gobert, S.; Pergent, G.; Pergent-Martini, C. Bioassessment of trace element contamination of Mediterranean coastal waters using the seagrass Posidonia oceanica. J. Environ. Manag. 2015, 151, 486–499. [Google Scholar] [CrossRef]
- Richir, J. Trace Elements in Marine Environments: Occurrence, Threats and Monitoring with Special Focus on the Coastal Mediterranean. J. Environ. Anal. Toxicol. 2016, 6. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Buckley, L.B.; Moore, P.; Poloczanska, E.S.; Brander, K.M.; Brown, C.; Bruno, J.F.; Duarte, C.M.; Halpern, B.S.; et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 2011, 334, 652–655. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Buñuel, X.; Alcoverro, T.; Romero, J.; Arthur, R.; Ruiz, J.M.; Pérez, M.; Pages, J.F. Warming intensifies the interaction between the temperate seagrass Posidonia oceanica and its dominant fish herbivore Sarpa salpa. Mar. Environ. Res. 2021, 165, 105237. [Google Scholar] [CrossRef]
- Vacchi, M.; De Falco, G.; Simeone, S.; Montefalcone, M.; Morri, C.; Ferrari, M.; Bianchi, C.N. Biogeomorphology of the Mediterranean Posidonia oceanica seagrass meadows. Earth Surf. Process. Landf. 2017, 42, 42–54. [Google Scholar] [CrossRef]
- Vizzini, S.; Sarà, G.; Michener, R.H.; Mazzola, A. The role and contribution of the seagrass Posidonia oceanica (L.) Delile organic matter for secondary consumers as revealed by carbon and nitrogen stable isotope analysis. Acta Oecol. 2002, 23, 277–285. [Google Scholar] [CrossRef]
- Campagne, C.S.; Salles, J.-M.; Boissery, P.; Deter, J. The seagrass Posidonia oceanica: Ecosystem services identification and economic evaluation of goods and benefits. Mar. Pollut. Bull. 2015, 97, 391–400. [Google Scholar] [CrossRef]
- Borg, J.A.; Attrill, M.J.; Rowden, A.A.; Schembri, P.J.; Jones, M.B. Architectural characteristics of two bed types of the seagrass Posidonia oceanica over different spatial scales. Estuar. Coast. Shelf Sci. 2005, 62, 667–678. [Google Scholar] [CrossRef]
- Borg, J.A.; Rowden, A.A.; Attrill, M.J.; Schembri, P.J.; Jones, M.B. Occurrence and distribution of different bed types of seagrass Posidonia oceanica around the Maltese Islands. Medit. Mar. Sci. 2009, 10, 45. [Google Scholar] [CrossRef]
- Monnier, B.; Pergent, G.; Mateo, Á.M.; Clabaut, P.; Pergent-Martini, C. Quantification of blue carbon stocks associated with Posidonia oceanica seagrass meadows in Corsica (NW Mediterranean). Sci. Total Environ. 2022, 838, 155864. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Gérard, P.; Christine, P.-M.; Sandrine, R.; Thierry, T.; Marc, V. The necromass of the Posidonia oceanica seagrass meadow: Fate, role, ecosystem services and vulnerability. Hydrobiologia 2016, 781, 25–42. [Google Scholar] [CrossRef]
- Smit, A.J.; Brearley, A.; Hyndes, G.A.; Lavery, P.S.; Walker, D.I. Carbon and nitrogen stable isotope analysis of an Amphibolis griffithii seagrass bed. Estuar. Coast. Shelf Sci. 2005, 65, 545–556. [Google Scholar] [CrossRef]
- Smit, A.J.; Brearley, A.; Hyndes, G.A.; Lavery, P.S.; Walker, D.I. δ15N and δ13C analysis of a Posidonia sinuosa seagrass bed. Aquat. Bot. 2006, 84, 277–282. [Google Scholar] [CrossRef]
- Lanyon, J.M.; Limpus, C.J.; Marsh, H. Dugongs and turtles: Grazers in the seagrass system. In Biology of Seagrasses: A Treatise on the Biology of Seagrasses with Special Reference to the Australian Region; Elsevier: Amsterdam, Netherlands, 1989. [Google Scholar]
- Guidetti, P. Invertebrate borers in the Mediterranean sea grass Posidonia oceanica: Biological impact and ecological implications. J. Mar. Biol. Assoc. United Kingd. 2000, 80, 725–730. [Google Scholar] [CrossRef]
- Champenois, W.; Lepoint, G.; Borges, A.V. Community Gross Primary Production and Respiration in Epilithic Macroalgae and Posidonia oceanica Macrophytodetritus accumulation in the Bay of Revellata (Corsica). Estuar. Coast. Shelf Sci. 2024, 309, 108971. [Google Scholar] [CrossRef]
- Stock, B.C.; Jackson, A.L.; Ward, E.J.; Parnell, A.C.; Phillips, D.L.; Semmens, B.X. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 2018, 6, e5096. [Google Scholar] [CrossRef]
- Marley, G.; Lawrence, A.J.; Phillip, D.A.; Hayden, B. Mangrove and mudflat food webs are segregated across four trophic levels, yet connected by highly mobile top predators. Mar. Ecol. Prog. Ser. 2019, 632, 13–25. [Google Scholar] [CrossRef]
- Docmac, F.; Araya, M.; Hinojosa, I.A.; Dorador, C.; Harrod, C. Habitat coupling writ large: Pelagic-derived materials fuel benthivorous macroalgal reef fishes in an upwelling zone. Ecology 2017, 98, 2267–2272. [Google Scholar] [CrossRef]
- Dubois, S.; Jean-Louis, B.; Bertrand, B.; Lefebvre, S. Isotope trophic-step fractionation of suspension-feeding species: Implications for food partitioning in coastal ecosystems. J. Exp. Mar. Biol. Ecol. 2007, 351, 121–128. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, Version 2.5-7. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 1 July 2024).
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 2014, 92, 823–835. [Google Scholar] [CrossRef]
- ERA. The 3rd River Basin Management Plan for the Malta Water Catchment District 2021–2027. March 2024, 192 pp. Available online: https://sustainabledevelopment.gov.mt/wp-content/uploads/2024/10/3rd-River-Basin-Management-Plan-Malta-1.pdf (accessed on 28 November 2024).
- Mazzola, A.; Sarà, G.; Venezia, F.; Caruso, M.; Catalano, D.; Hauser, S. Origin and distribution of suspended organic matter as inferred from carbon isotope composition in a Mediterranean semi-enclosed marine system. Chem. Ecol. 1999, 16, 215–238. [Google Scholar] [CrossRef]
- Prado, P.; Tomas, F.; Alcoverro, T.; Romero, J. Extensive direct measurements of Posidonia oceanica defoliation confirm the importance of herbivory in temperate seagrass meadows. Mar. Ecol. Prog. Ser. 2007, 340, 63–71. [Google Scholar] [CrossRef]
- Mazzella, L.; Spinoccia, L. Epiphytic diatoms of leaf blades of the Mediterranean seagrass Posidonia oceanica (L.) Delile. Plant Biosyst. 1992, 126, 752–754. [Google Scholar]
- Cebrián, J.; Duarte, C.M.; Marbà, N. Herbivory on the seagrass Cymodocea nodosa (Ucria) Ascherson in contrasting Spanish Mediterranean habitats. J. Exp. Mar. Biol. Ecol. 1996, 204, 103–111. [Google Scholar] [CrossRef]
- Cocheret De La Morinière, E.; Pollux, B.; Nagelkerken, I.; Hemminga, M.; Huiskes, A.; Van Der Velde, G. Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: Stable isotopes and gut-content analysis. Mar. Ecol. Prog. Ser. 2003, 246, 279–289. [Google Scholar] [CrossRef]
- Lesser, M.P.; Slattery, M.; Macartney, K.J. Using stable isotope analyses to assess the trophic ecology of scleractinian corals. Oceans 2022, 3, 527–546. [Google Scholar] [CrossRef]
| Taxon | L’Aħrax | Baħar | Golden Bay | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | δ13C | δ15N | n | δ13C | δ15N | n | δ13C | δ15N | |
| Carbon sources | |||||||||
| Macroalgae | 12 | −18.3 (0.3) | 1.3 (0.7) | 7 | −18.6 (2.2) | 4 (0.9) | 26 | −21.9 (3.3) | 0.8 (1) |
| Marine POM * | 1 | −20.3 | 2.1 | 1 | −19.9 | 4.6 | 1 | −19.1 | 1.5 |
| Posidonia oceanica | 15 | −15.6 (0.8) | 0.5 (0.5) | 7 | −14.4 (0.6) | 6 (1.5) | 14 | −15.8 (1.1) | 1.0 (0.6) |
| Invertebrates | |||||||||
| Amphipod | 1 | −18.6 | 2.7 | 1 | −20.6 | 2.3 | |||
| Decapod | 4 | −16.7 (1.4) | 3.6 (0.8) | 1 | −16.9 | 4.1 | 1 | −16.2 | 3.9 |
| Echinoderm | 1 | −16.7 | 4.7 | ||||||
| Halochynthis papillosa | 1 | −21.3 | 2.7 | ||||||
| Isopod | 1 | −18.2 | 4.4 | ||||||
| Polychaete | 2 | −21.2 (1.5) | 3.5 (1.8) | 1 | −17.6 | 8.9 | 5 | −19.5 (1.2) | 5.6 (1.6) |
| Porifera | 1 | −19.1 | 4.3 | 5 | −18.4 (1.1) | 4.3 (0.9) | |||
| Sipunculid | 1 | −16.9 | 4.8 | 2 | −19.6 (0.4) | 3.6 (0.6) | |||
| Cirriped | 1 | −17.2 | 8.1 | 1 | −18.6 | 6 | |||
| Hermodice carunculata | 2 | −18.2 (1.6) | 9.1 (0.3) | 2 | −18.8 (0.7) | 8.8 (2.2) | |||
| Hexaplex sp. | 1 | −18.1 | 10.2 | ||||||
| Mollusc | 1 | −17.8 | 2.4 | ||||||
| Pagurid | 1 | −17.2 | 3 | ||||||
| Spirastrella sp. | 1 | −20.2 | 4.2 | ||||||
| Fishes | |||||||||
| Chromis chromis | 4 | −18.8 (0.2) | 7.8 (0.4) | 5 | −19.5 (0.2) | 6.7 (0.2) | |||
| Coris julis | 4 | −15.9 (0.2) | 12.9 (0.8) | ||||||
| Diplodus vulgaris | 2 | −16.2 ± (1) | 13.9 (1.4) | 2 | −17.2 (0.1) | 9.2 (0.8) | |||
| Oblada melanura | 3 | −17.6 ± (0.2) | 10.2 (0.5) | ||||||
| Labrus viridis | 1 | −17.2 | 10.6 | ||||||
| Sciana umbra | 1 | −17.2 | 7.9 | ||||||
| Scorpaena sp. | 1 | −18.1 | 8.1 | ||||||
| Serranus cabrilla | 3 | −18.6 (0.3) | 8.1 (0.3) | ||||||
| Sparisoma cretense | 4 | −19.9 (0.5) | 5.5 (0.2) | ||||||
| Uranoscopus scaber | 1 | −18.5 | 8 | ||||||
| Model | Location | Macroalgae | POM | P. oceanica |
|---|---|---|---|---|
| BMI | All | 34 (5–74) | 45 (8–82) | 12 (1–60) |
| L’Aħrax | 66 (6–100) | 25 (0–85) | 3 (0–24) | |
| Baħar iċ-Ċagħaq | 11 (0–86) | 79 (7–100) | 3 (0–21) | |
| Golden Bay | 18 (0–52) | 80 (37–100) | 1 (0–15) | |
| Fish | All | 26 (2–75) | 37 (5–84) | 25 (2–74) |
| Baħar iċ-Ċagħaq | 0 (0–20) | 1 (77–100) | 0 (0–8) | |
| Golden Bay | 0 (0–100) | 100 (0–100) | 0 (0–1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deidun, A.; Azzopardi, F.; Marrone, A.; Massa-Gallucci, A.; Cutajar, K.; Hayden, B. Assessing the Contribution of Posidonia oceanica to Mediterranean Secondary Production Through Stable Isotope Analysis. J. Mar. Sci. Eng. 2024, 12, 2197. https://doi.org/10.3390/jmse12122197
Deidun A, Azzopardi F, Marrone A, Massa-Gallucci A, Cutajar K, Hayden B. Assessing the Contribution of Posidonia oceanica to Mediterranean Secondary Production Through Stable Isotope Analysis. Journal of Marine Science and Engineering. 2024; 12(12):2197. https://doi.org/10.3390/jmse12122197
Chicago/Turabian StyleDeidun, Alan, Freja Azzopardi, Alessio Marrone, Alexia Massa-Gallucci, Karl Cutajar, and Brian Hayden. 2024. "Assessing the Contribution of Posidonia oceanica to Mediterranean Secondary Production Through Stable Isotope Analysis" Journal of Marine Science and Engineering 12, no. 12: 2197. https://doi.org/10.3390/jmse12122197
APA StyleDeidun, A., Azzopardi, F., Marrone, A., Massa-Gallucci, A., Cutajar, K., & Hayden, B. (2024). Assessing the Contribution of Posidonia oceanica to Mediterranean Secondary Production Through Stable Isotope Analysis. Journal of Marine Science and Engineering, 12(12), 2197. https://doi.org/10.3390/jmse12122197







