Study of the Combustion Characteristics of a Compression Ignition Engine Fueled with a Biogas–Hydrogen Mixture and Biodiesel
Abstract
1. Introduction
2. Materials and Methods
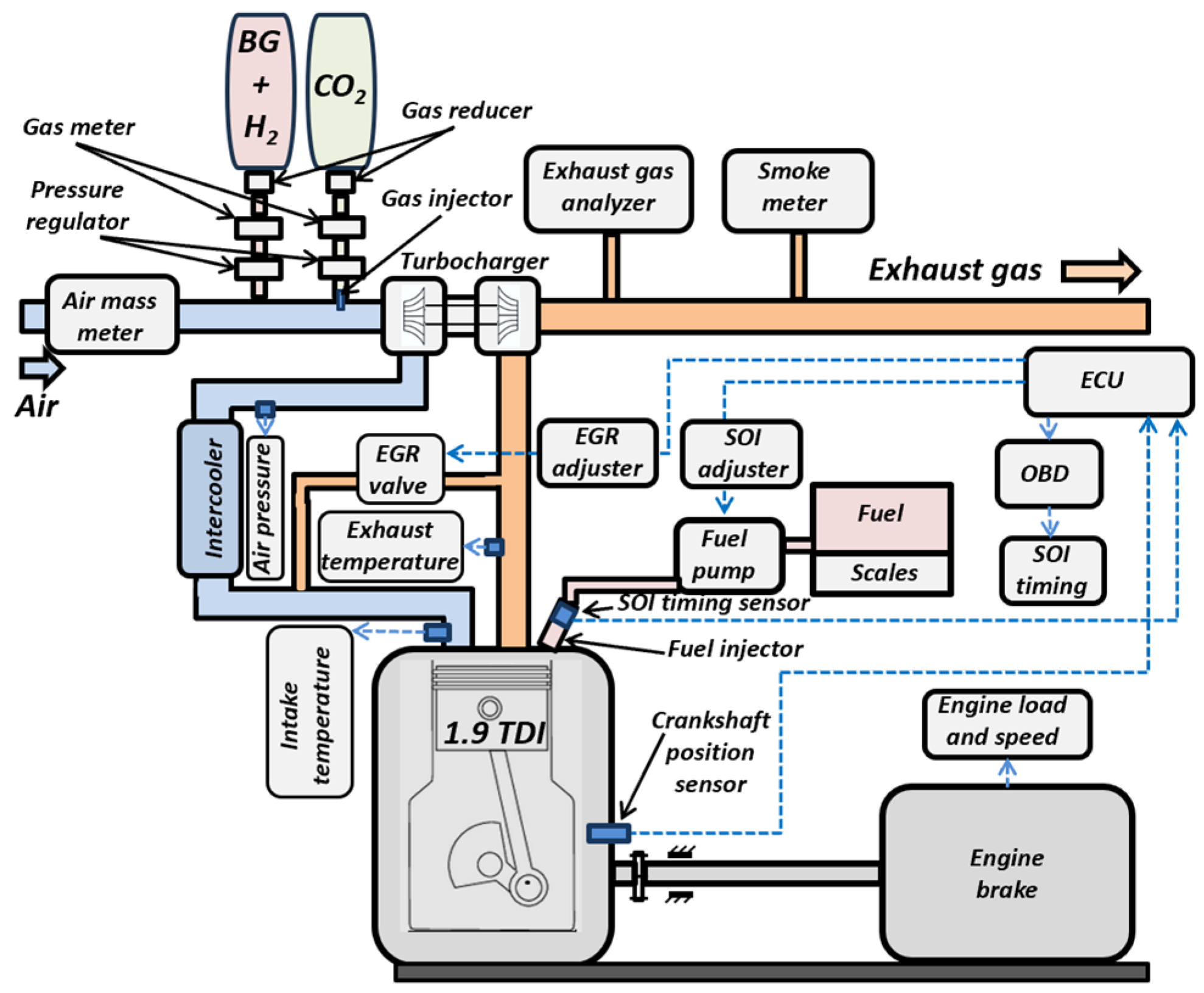
2.1. Experimental Equipment
2.2. Fuels and Blends
2.3. Numerical Analysis Methodology for Combustion
| — | change in the internal energy in the cylinder; | |
| — | piston work; | |
| — | fuel heat input; | |
| — | wall heat losses; | |
| — | enthalpy flow due to blow-by; | |
| — | mass in the cylinder; | |
| — | specific internal energy; | |
| — | cylinder pressure; | |
| — | cylinder volume; | |
| — | fuel energy; | |
| — | wall heat loss; | |
| — | crank angle; | |
| — | enthalpy of blow-by; | |
| — | blow-by mass flow; | |
| — | mass element flowing into the cylinder; | |
| — | mass element flowing out of the cylinder; | |
| — | enthalpy of the in-flowing mass; | |
| — | enthalpy of the mass leaving the cylinder; | |
| — | evaporation heat of the fuel; | |
| — | fraction of evaporation heat from the cylinder charge; | |
| — | mass of evaporating fuel. |
| — | total fuel heat input; | |
| — | crank angle; | |
| — | start of combustion; | |
| — | combustion duration; | |
| — | shape parameter; | |
| — | Vibe parameter, typically a = 6.9 for complete combustion. |
| — | mass fraction burned. |
3. Results and Discussion
4. Conclusions
- (1)
- Increasing the GES to 80% (in the case of 20HVO_80BG) decreased the excess air ratio from 2.49 (HVO100) to λ = 1.95 and brought it closer to the flammability limit of biogas (λ = 1.90). Increasing the H2 concentration to 30% of the volume of methane in the biogas (20HVO_80(BG+H3)) increased the excess air ratio to λ = 2.03, but the better flammability properties of H2 extended the flammable limit of the mixture.
- (2)
- In the case of the D-F mode, by compressing the air with NG or BG additive and increasing the GES to 40%, 60%, and 80%, the pressure at the end of the compression stroke decreased by ~2.5%, ~5.1%, and ~7.5%, respectively, because the air composed of two-atom molecules (N2, O2) was partially replaced by four-atom molecules (CH4) and three-atom molecules (CO2), which have a lower compressibility polytropic index. The increased energy demand for the compression of NG and biogas also reduced the temperature at the end of the compression and caused a deterioration in the ignition conditions of the pilot fuels.
- (3)
- HVO100 fuels have a higher cetane number compared to D100 due to their altered chemical–physical properties (higher H/C ratio, better atomization and vaporization, etc.), which shortens the ID phase. HVO100 is therefore better suited as a pilot fuel in the D-F mode, especially when using BG, as it partially compensates for the slower combustion.
- (4)
- As the GES was increased, the ROHR of combustion during the premixed combustion phase increased slightly as the combustion of the liquid fuel was simultaneously accompanied by the combustion of the gas. Increasing the H2 addition to the biogas further did not increase the ROHR in this combustion phase, because the mixture was extremely lean at the start of combustion and the gas burned at a low velocity. In the second phase, diffusion combustion, the ROHR decreased as the GES increased to 80% because the HVO injection and combustion ended earlier due to the reduction in the pilot fuel, and the combustion of gases, in particular BG, was slower. However, in this phase, the addition of H2 to the BG already had a positive combustion velocity enhancement effect because the excess air from the combustion was reduced and the pressure and temperature were increased. The tendency for more intense combustion was repeated in both the pressure rise and temperature rise characteristics.
- (5)
- The MBF analysis showed that increasing the GES from 40% to 80%, with HVO_NG, increased the duration of the MBF10 and MBF50, indicating slower combustion. However, the duration of the MBF90 did not increase with an increase in the GES from 60% to 80%, and considering that the MBF50 ended later, we can say that in this case, the diffusion combustion phase was shortened due to the faster NG combustion after the flammability limit was reached. When the engine was operated on the HVO_BG dual biofuels, only increasing the H2 addition (up to 30% methane by volume) led to higher combustion speeds and a shortening of the diffusion combustion and later combustion phases. In this case, the addition of hydrogen resulted in the combustion characteristics of HVO_BG being closer to those of HVO_NG. This was confirmed by the decreasing value of Tex, indicating an increase in the thermal efficiency of the combustion process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Union. Directive 2018/2001 on the Promotion of the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 12 August 2024).
- European Commission. White Paper 2011: Roadmap to a Single European Transport Area. Available online: https://transport.ec.europa.eu/white-paper-2011_en (accessed on 12 August 2024).
- Srivastava, R.; Kumar, R. Advances in the Field of Bioenergy. In Handbook of Bioenergy; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Diano, N.; Riccardi, C. Greenhouse Gas Reduction Technologies in Industrial Processes. Chem. Eng. Trans. 2015, 43, 409–414. Available online: https://www.aidic.it/cet/15/43/069.pdf (accessed on 15 August 2024).
- Ao, T.-J.; Liu, C.-G.; Sun, Z.-Y.; Zhao, X.-Q.; Tang, Y.-Q.; Bai, F.-W. Anaerobic Digestion Integrated with Microbial Electrolysis Cell to Enhance Biogas Production and Upgrading In Situ. Biotechnol. Adv. 2024, 73, 108372. [Google Scholar] [CrossRef] [PubMed]
- Barbusiński, K.; Kalemba, K. Removal of H₂S in Biogas Systems. Arch. Civ. Eng. Environ. 2016, 9, 53–58. [Google Scholar] [CrossRef]
- Alrbai, M.; Ahmad, A.D.; Al-Dahidi, S.; Abubaker, A.M.; Al-Ghussain, L.; Hayajneh, H.S.; Akafuah, N.K. Hydrogen Sulfide Impacts in Biogas Combustion. Case Stud. Therm. Eng. 2022, 35, 102509. [Google Scholar] [CrossRef]
- Ghimire, A.; Gyawali, R.; Lens, P.N.L.; Lohani, S.P. Technologies for H₂S Removal in Biogas. In Renewable Energy Technologies. Emerg. Technol. Biol. Syst. Biogas Upgrad. 2021, 295–320. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Composition Analysis of Agricultural Biogas. Renew. Energy 2022, 189, 1249–1260. [Google Scholar] [CrossRef]
- Shin, J.; Kim, W.; Lee, J.M.; Lee, M.J.; Song, H.W.; Kim, S.D. Simultaneous H₂S and Siloxane Removal in Biogas. Detritus 2023, 23, 18276. [Google Scholar] [CrossRef]
- Sidi Habib, S.; Torii, S.; Kavitha Mol, S.; Achuthan Nair, A.C. Optimization of the Factors Affecting Biogas Production Using the Taguchi Design of Experiment Method. Biomass 2024, 4, 687–703. [Google Scholar] [CrossRef]
- European Commission. Methane Emissions Management in Energy Sector. Available online: https://energy.ec.europa.eu/topics/carbon-management-and-fossil-fuels/methane-emissions_en (accessed on 30 August 2024).
- U.S. Environmental Protection Agency (EPA). Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials#:~:text=Methane%20(CH4)%20is%20estimated,is%20reflected%20in%20the%20GWP (accessed on 30 August 2024).
- Melnyk, O.; Onyshchenko, S.; Onishchenko, O.; Shumylo, O.; Voloshyn, A.; Ocheretna, V.; Fedorenko, O. Implementation Research of Alternative Fuels and Technologies in Maritime Transport. In Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2023; Available online: https://link.springer.com/chapter/10.1007/978-3-031-44351-0_2 (accessed on 5 September 2024).
- Ramsay, W.; Fridell, E.; Michan, M. Maritime Energy Transition: Future Fuels and Future Emissions. J. Mar. Sci. Appl. 2023, 22, 369–381. Available online: https://link.springer.com/article/10.1007/s11804-023-00369-z (accessed on 5 September 2024). [CrossRef]
- Schönsteiner, K.; Massier, T.; Hamacher, T. Sustainable Transport by Use of Alternative Marine and Aviation Fuels: A Well-to-Tank Analysis to Assess Interactions with Singapore’s Energy System. Renew. Sustain. Energy Rev. 2016, 66, 190–202. [Google Scholar] [CrossRef]
- Al-Abboodi, N.K.F.; Ridha, H. Design and Thermodynamics Analysis of Marine Dual Fuel Low-Speed Engine with Methane Reforming Integrated High-Pressure Exhaust Gas Recirculation System. Fuel 2022, 324, 123747. [Google Scholar] [CrossRef]
- Curran, S.; Onorati, A.; Payri, R.; Agarwal, A.K.; Arcoumanis, C.; Bae, C.; Boulouchos, K.; Chuahy, F.D.F.; Gavaises, M.; Hampson, G.J.; et al. The Future of Ship Engines: Renewable Fuels and Enabling Technologies for Decarbonization. Int. J. Eng. Res. 2023. [Google Scholar] [CrossRef]
- Rueda-Vázquez, J.M.; Serrano, J.; Pinzi, S.; Jiménez-Espadafor, F.J.; Dorado, M.P. A Review of the Use of Hydrogen in Compression Ignition Engines with Dual-Fuel Technology and Techniques for Reducing NOx Emissions. Sustainability 2024, 16, 3462. [Google Scholar] [CrossRef]
- Feroskhan, M.F.; Ismail, S.; Natarajan, G.; Manavalla, S.; Yunus Khan, T.M.; Abdul Khadar, S.D.; Azam Ali, M. A Comprehensive Study of the Effects of Various Operating Parameters on a Biogas-Diesel Dual Fuel Engine. Sustainability 2023, 15, 21232. [Google Scholar] [CrossRef]
- Rimkus, A.; Stravinskas, S.; Matijošius, J. Comparative Study on the Energetic and Ecologic Parameters of Dual Fuels (Diesel–NG and HVO–Biogas) and Conventional Diesel Fuel in a CI Engine. Appl. Sci. 2020, 10, 359. [Google Scholar] [CrossRef]
- Harish, H.; Kempaiah, U.N. Experimental Analysis of a CI Engine Running on Dual-Fuel Mode with Gaseous and Liquid Fuels. Int. J. Eng. Res. Technol. 2016, 4, 31013. [Google Scholar]
- Zhang, Z.; Lv, J.; Li, W.; Long, J.-X.; Wang, S.; Tan, D.M.; Yin, Z. Performance and Emission Evaluation of a Marine Diesel Engine Fueled with Natural Gas Ignited by Biodiesel-Diesel Blended Fuel. Energy 2022, 256, 124662. [Google Scholar] [CrossRef]
- Costa, R.B.R.; Valle, R.M.; Hernández, J.J.; Malaquias, A.C.T.; Coronado, C.J.R.; Pujatti, F.J.P. Experimental Investigation on the Potential of Biogas/Ethanol Dual-Fuel Spark-Ignition Engine for Power Generation: Combustion, Performance and Pollutant Emission Analysis. Appl. Energy 2020, 261, 114438. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Nayak, S.K.; Le, H.S.; Kowalski, J.; Deepanraj, B.; Duong, X.Q.; Truong, T.H.; Tran, V.D.; Cao, D.N.; Nguyen, P.Q.P. Performance and Emission Characteristics of Diesel Engines Running on Gaseous Fuels in Dual-Fuel Mode. Int. J. Hydrogen Energy 2024, 49 Pt B, 868–909. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Saleh, K.; Wandel, A.P.; Fattah, I.M.R.; Yusaf, T.; Alrazen, H.A. Influence of Natural Gas and Hydrogen Properties on Internal Combustion Engine Performance, Combustion, and Emissions: A Review. Fuel 2023, 344, 130844. [Google Scholar] [CrossRef]
- Ng, W.C.; Yaw, C.S.; Shaffee, S.N.A.; Samad, N.A.A.; Koi, Z.K.; Chong, M.N. Elevating the Prospects of Green Hydrogen (H₂) Production Through Solar-Powered Water Splitting Devices: A Systematic Review. Sustain. Mater. Technol. 2024, 40, e00972. [Google Scholar] [CrossRef]
- Rameez, P.V.; Ibrahim, M.M. A Comprehensive Review on the Utilization of Hydrogen in Low Temperature Combustion Strategies: Combustion, Performance and Emission Attributes. J. Ocean Eng. Sci. 2023, 113, 101511. [Google Scholar] [CrossRef]
- Sterlepper, S.; Fischer, M.; Claßen, J.; Huth, V.; Pischinger, S. Concepts for Hydrogen Internal Combustion Engines and Their Implications on the Exhaust Gas Aftertreatment System. Energies 2021, 14, 8166. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green Hydrogen: A Pathway to a Sustainable Energy Future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Roque, L.F.A.; da Costa, R.B.R.; de Souza, T.A.Z.; Coronado, C.J.R.; Pinto, G.M.; Cintra, A.J.A.; Raats, O.O.; Oliveira, B.M.; Frez, G.V.; Alves, L.F.R. Experimental Analysis and Life Cycle Assessment of Green Diesel (HVO) in Dual-Fuel Operation with Bioethanol. J. Clean. Prod. 2023, 389, 135989. [Google Scholar] [CrossRef]
- Dobrzyńska, E.; Szewczyńska, M.; Pośniak, M.; Szczotka, A.; Puchałka, B.; Woodburn, J. Exhaust Emissions from Diesel Engines Fueled by Different Blends with the Addition of Nanomodifiers and Hydrotreated Vegetable Oil HVO. Environ. Pollut. 2020, 259, 113772. [Google Scholar] [CrossRef]
- No, S.-Y. Parffinic Biofuels: HVO, BTL Diesel, and Farnesane. In Advanced Biofuels and Bioproducts; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Ferguson, C.R.; Kirkpatrick, A.T. Internal Combustion Engines: Applied Thermosciences, 3rd ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Smigins, R.; Sondors, K.; Pirs, V.; Dukulis, I.; Birzietis, G. Studies of Engine Performance and Emissions at Full-Load Mode Using HVO, Diesel Fuel, and HVO5. Energies 2023, 16, 4785. [Google Scholar] [CrossRef]
- Bullermann, J.; Meyer, N.-C.; Krafft, A.; Wirz, F. Comparison of Fuel Properties of Alternative Drop-In Fuels with Standard Marine Diesel and the Effects of Their Blends. Fuel 2024, 357, 129937. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Sahoo, N.; Saha, U.K. Effect of Engine Parameters and Type of Gaseous Fuel on the Performance of Dual-Fuel Gas Diesel Engines—A Critical Review. Renew. Sustain. Energy Rev. 2009, 13, 1151–1184. [Google Scholar] [CrossRef]
- Yang, B.; Xi, C.; Wei, X.; Zeng, K.; Lai, M.-C. Parametric Investigation of Natural Gas Port Injection and Diesel Pilot Injection on the Combustion and Emissions of a Turbocharged Common Rail Dual-Fuel Engine at Low Load. Appl. Energy 2015, 143, 130–137. [Google Scholar] [CrossRef]
- Khatri, N.; Khatri, K.K. Hydrogen Enrichment on Diesel Engine with Biogas in Dual Fuel Mode. Int. J. Hydrogen Energy 2020, 45, 7128–7140. [Google Scholar] [CrossRef]
- Pizzuti, L.; Martins, C.A.; Lacava, P.T. Laminar burning velocity and flammability limits in biogas: A literature review. Renew. Sustain. Energy Rev. 2016, 62, 856–865. [Google Scholar] [CrossRef]
- Verma, S.; Das, L.M.; Bhatti, S.S.; Kaushik, S.C. A comparative exergetic performance and emission analysis of pilot diesel dual-fuel engine with biogas, CNG and hydrogen as main fuels. Energy Convers. Manag. 2017, 151, 764–777. [Google Scholar] [CrossRef]
- Pinto, G.M.; da Costa, R.B.R.; de Souza, T.A.Z.; Rosa, A.J.A.C.; Raats, O.O.; Roque, L.F.A.; Frez, G.V.; Coronado, C.J.R. Experimental investigation of performance and emissions of a CI engine operating with HVO and farnesane in dual-fuel mode with natural gas and biogas. Energy 2023, 277, 127648. [Google Scholar] [CrossRef]
- Verma, S.; Das, L.M.; Kaushik, S.C. Effects of varying composition of biogas on performance and emission characteristics of compression ignition engine using exergy analysis. Energy Convers. Manag. 2017, 138, 346–359. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Bąkowski, A.; Radziszewski, L.; Milan, Ž. Determining the Polytrophic Exponent of the Process Occurring During the Working Cycle of a Diesel. Procedia Eng. 2016, 136, 220–226. [Google Scholar] [CrossRef]
- Lee, Y.; Min, K. Estimation of the polytropic index for in-cylinder pressure prediction in engines. Appl. Therm. Eng. 2019, 158, 113703. [Google Scholar] [CrossRef]
- Kubica, G.; Flekiewicz, M.; Marzec, P. Selected aspects of the use of gaseous fuels blends to improve efficiency and emission of SI engine. Transp. Probl. 2019, 14, 95–103. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Somers, L.M.T.; van de Beld, B. Ignition and combustion characteristics of hydrotreated pyrolysis oil in a combustion research unit. Fuel 2022, 316, 123419. [Google Scholar] [CrossRef]
- Marasri, S.; Ewphun, P.-P.; Srichai, P.; Charoenphonphanich, C.; Karin, P.; Tongroon, M.; Kosaka, H. Combustion Characteristics of Hydrotreated Vegetable Oil-Diesel Blends under EGR and Low Temperature Combustion Conditions. Int. J. Automot. Technol. 2019, 20, 569–578. [Google Scholar] [CrossRef]
- Parravicini, M.; Barro, C.; Boulouchos, K. Experimental characterization of GTL, HVO, and OME based alternative fuels for diesel engines. Fuel 2021, 292, 120177. [Google Scholar] [CrossRef]
- Rahman, K.A.; Ramesh, A. Studies on the effects of methane fraction and injection strategies in a biogas diesel common rail dual fuel engine. Fuel 2019, 236, 147–165. [Google Scholar] [CrossRef]
- Swami Nathan, S.; Mallikarjuna, J.M.; Ramesh, A. An experimental study of the biogas–diesel HCCI mode of engine operation. Energy Convers. Manag. 2010, 51, 1347–1353. [Google Scholar] [CrossRef]
- Bora, B.J.; Saha, U.K. Optimisation of injection timing and compression ratio of a raw biogas powered dual fuel diesel engine. Appl. Therm. Eng. 2016, 92, 111–121. [Google Scholar] [CrossRef]
- Bora, B.J.; Saha, U.K. Experimental evaluation of a rice bran biodiesel—Biogas run dual fuel diesel engine at varying compression ratios. Renew. Energy 2016, 87, 782–790. [Google Scholar] [CrossRef]
- Bora, B.J.; Saha, U.K. Theoretical Performance Limits of a Biogas–Diesel Powered Dual Fuel Diesel Engine for Different Combinations of Compression Ratio and Injection Timing. J. Energy Eng. 2016, 142. [Google Scholar] [CrossRef]
- Bora, B.J.; Saha, U.K. Comparative assessment of a biogas run dual fuel diesel engine with rice bran oil methyl ester, pongamia oil methyl ester and palm oil methyl ester as pilot fuels. Renew. Energy 2015, 81, 490–498. [Google Scholar] [CrossRef]
- Talibi, M.; Hellier, P.; Ladommatos, N. Combustion and exhaust emission characteristics, and in-cylinder gas composition, of hydrogen enriched biogas mixtures in a diesel engine. Energy 2017, 124, 397–412. [Google Scholar] [CrossRef]
- Park, O.; Veloo, P.S.; Liu, N.; Egolfopoulos, F.N. Combustion characteristics of alternative gaseous fuels. Proc. Combust. Inst. 2011, 33, 887–894. [Google Scholar] [CrossRef]
- Pham, Q.; Park, S.; Agarwal, A.K.; Park, S. Review of dual-fuel combustion in the compression-ignition engine: Spray, combustion, and emission. Energy 2022, 250, 123778. [Google Scholar] [CrossRef]









| Parameter | Value |
|---|---|
| Number of cylinders | 4 |
| Displacement (cm3) | 1896 |
| Bore/stroke, mm | 79.5/95.5 |
| Compression ratio | 19.5 |
| Power | 66 kW (at 4000 rpm) |
| Torque | 180 Nm (at 2000–2500 rpm) |
| Aspiration | Turbocharger |
| Fuel injection | Direct injection (single) |
| Camshaft location | Overhead camshaft engine (OHC) |
| Equipment | Indicator, Units | Measurement Range | Accuracy |
|---|---|---|---|
| Engine load bench KI-5543 (GOSNITI, Russia) | MB, Nm | 0–440 | ±1.2 Nm |
| Electronic fuel scales SK-5000 (A&D, Germany) | Bf, kg | 0–5.0 | ±1.0 g |
| Air mass meter BOSCH HFM 5 (BOSCH, Germany) | Bair, kg/h | 8–370 | ±2% |
| Gas mass flow meter RHM 015 (RHEONIK, Germany) | Bgas, kg/h | 0.09–36 | ±0.1% |
| In-cylinder pressure piezoelectric sensor AVL GH13P (AVL, Austria) | pc, bar | 0–250 | Sensitivity 15.84 ± 0.09 pC/bar |
| Crankshaft encoder A58M-F (Precizika Metrology, Lithuania) | CAD | 0–360 | Repeatability 0.176 CAD |
| Pressure meter Delta OHM HD 2304.0/TP704-5BAI (Delta Ohm, Italy) | PTC, bar | 0–360 | ±0.4% |
| Temperature sensor (K-type thermocouple) (Cartridge Heaters, United Kingdom) | Tex, °C | 0–1200 | ±1.5° C |
| SOI measurement equipment VAG-Com (Ross-Tech, Germany) | SOI, CAD bTDC | 0–20 | ±1.0°CAD |
| Title | D100 | HVO100 | NG | BG | H2 |
|---|---|---|---|---|---|
| Lower heating value LHV, MJ/kg | 42.5 | 43.63 | 48.6 | ~19.2 | 120 |
| Density, 15 °C, kg/m3 | 840 | 780 | 0.722 | ~1.2 | 0.09 |
| Cetane number | 52.1 | 74.3 | - | - | - |
| Octane number | - | - | 130 | 120 | 130 |
| Elemental composition, C % | 87 | 84.7 | 75 | - | 0 |
| Elemental composition, H % | 13 | 15.3 | 25 | - | 100 |
| Stoichiometric air/fuel ratio lo, kg/kg | 14.6 | 15.1 | 17.2 | ~6.2 | 34.8 |
| Title | D (ES) | HVO (ES) | GAS (GES) | NG (GVS) | CO2 (GVS) | H2 (GVS) |
|---|---|---|---|---|---|---|
| D100 | 100% | - | - | - | - | - |
| HVO100 | - | 100% | - | - | - | - |
| 60HVO_40NG | - | 60% | 40% | 100% | - | - |
| 60HVO_40BG | - | 60% | 40% | 60% | 40% | - |
| 60HVO_40(BG+H1) | - | 60% | 40% | 54% | 40% | 6% |
| 60HVO_40(BG+H2) | - | 60% | 40% | 48% | 40% | 12% |
| 60HVO_40(BG+H3) | - | 60% | 40% | 42% | 40% | 18% |
| 40HVO_60NG | - | 40% | 60% | 100% | - | - |
| 40HVO_60BG | - | 40% | 60% | 60% | 40% | - |
| 40HVO_60(BG+H1) | - | 40% | 60% | 54% | 40% | 6% |
| 40HVO_60(BG+H2) | - | 40% | 60% | 48% | 40% | 12% |
| 40HVO_60(BG+H3) | - | 40% | 60% | 42% | 40% | 18% |
| 20HVO_80NG | - | 20% | 80% | 100% | - | - |
| 20HVO_80BG | - | 20% | 80% | 60% | 40% | - |
| 20HVO_80(BG+H1) | - | 20% | 80% | 54% | 40% | 6% |
| 20HVO_80B(G+H2) | - | 20% | 80% | 48% | 40% | 12% |
| 20HVO_80B(G+H3) | - | 20% | 80% | 42% | 40% | 18% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimkus, A.; Žaglinskis, J. Study of the Combustion Characteristics of a Compression Ignition Engine Fueled with a Biogas–Hydrogen Mixture and Biodiesel. J. Mar. Sci. Eng. 2024, 12, 2192. https://doi.org/10.3390/jmse12122192
Rimkus A, Žaglinskis J. Study of the Combustion Characteristics of a Compression Ignition Engine Fueled with a Biogas–Hydrogen Mixture and Biodiesel. Journal of Marine Science and Engineering. 2024; 12(12):2192. https://doi.org/10.3390/jmse12122192
Chicago/Turabian StyleRimkus, Alfredas, and Justas Žaglinskis. 2024. "Study of the Combustion Characteristics of a Compression Ignition Engine Fueled with a Biogas–Hydrogen Mixture and Biodiesel" Journal of Marine Science and Engineering 12, no. 12: 2192. https://doi.org/10.3390/jmse12122192
APA StyleRimkus, A., & Žaglinskis, J. (2024). Study of the Combustion Characteristics of a Compression Ignition Engine Fueled with a Biogas–Hydrogen Mixture and Biodiesel. Journal of Marine Science and Engineering, 12(12), 2192. https://doi.org/10.3390/jmse12122192






