The Impact of Flow-Thermal Characteristics in Ship-Board Solid Oxide Fuel Cells
Abstract
1. Introduction
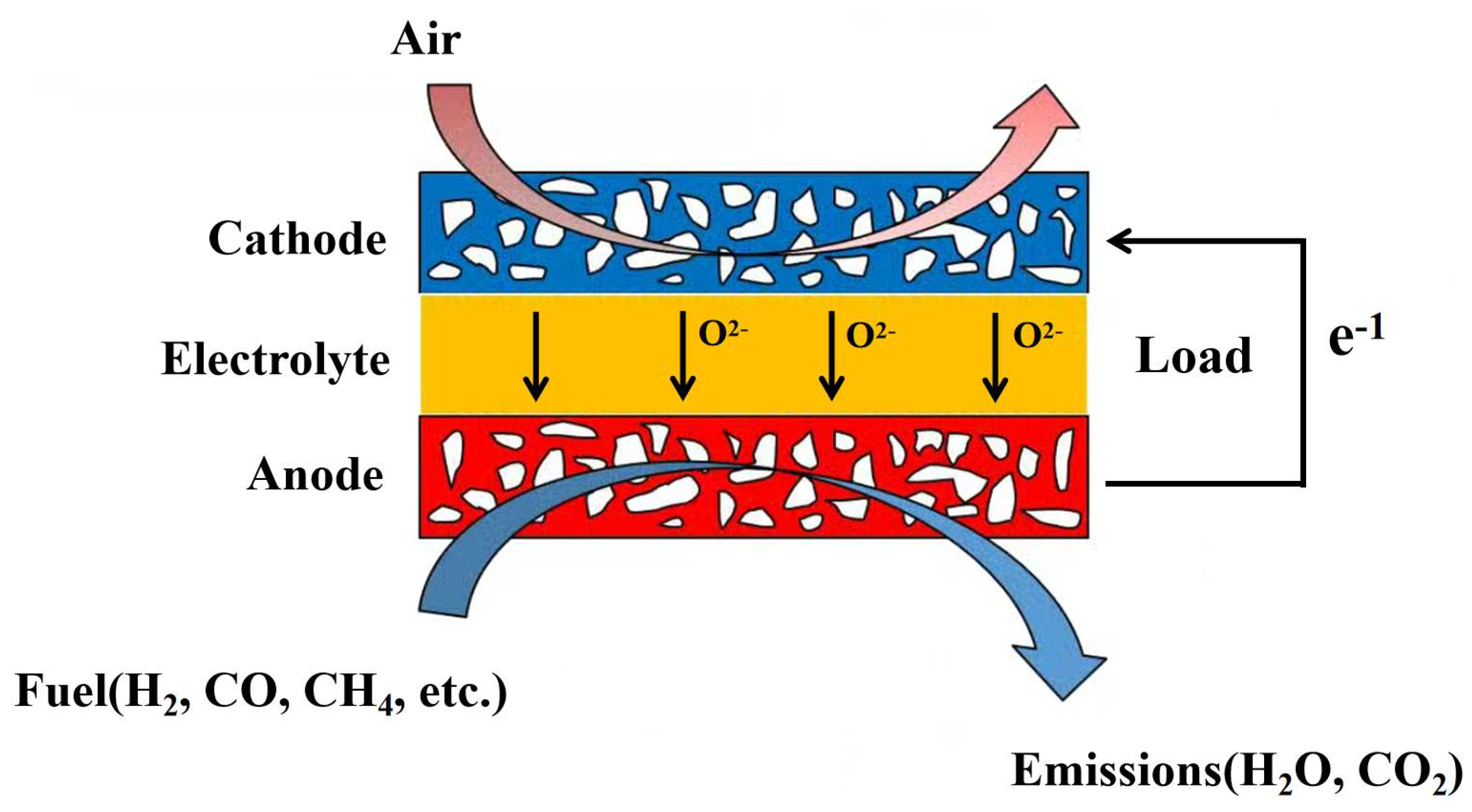
2. Mathematical Model and Three-Dimensional Numerical Model of SOFC
2.1. Model Assumption
- The SOFC operates normally under steady and adiabatic conditions.
- The gas mixture is an incompressible ideal gas due to the high operating temperature and ambient pressure, and it obeys the ideal gas state equation.
- The flow is laminar in the channel as the flow rate is low (Re < 2400).
- In the SOFC, all electrochemical and heterogeneous chemical reactions occur at the surface of the electrolyte.
- The effects of gravity and the heat transfer in the stack are neglected.
- The electrode is a homogeneous isotropic medium with a porous microstructure.
2.2. Model Governing Equation
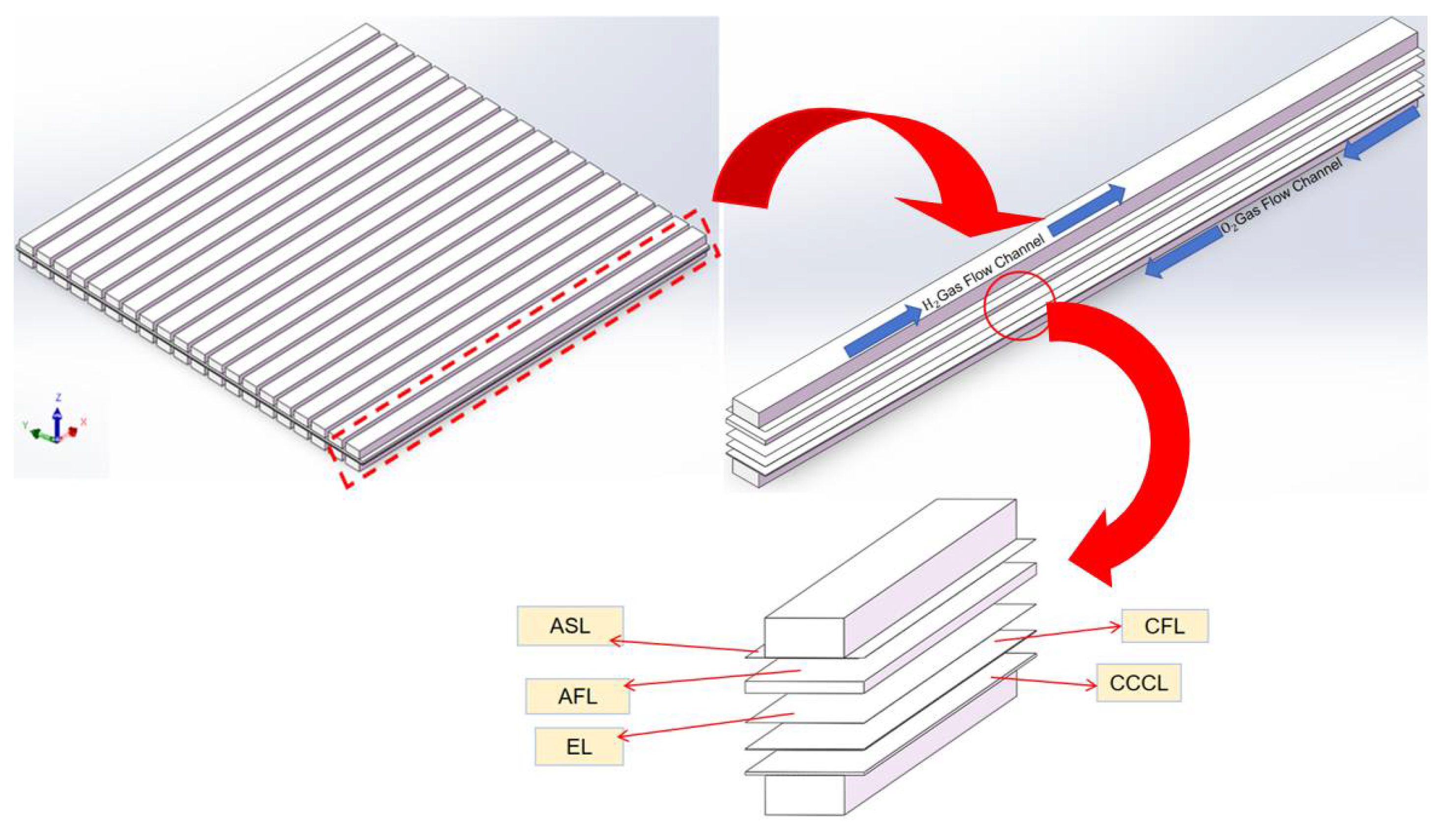
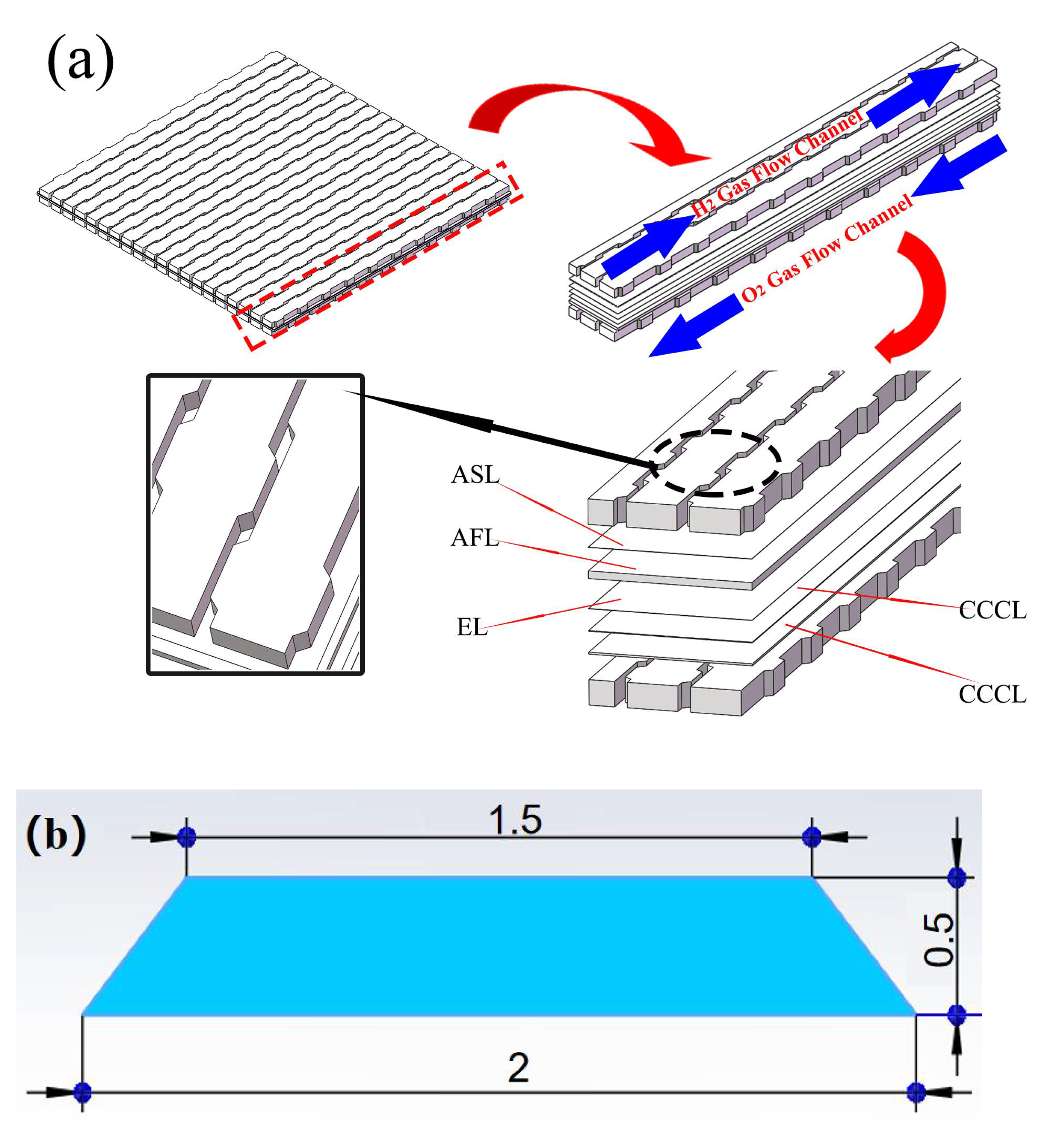
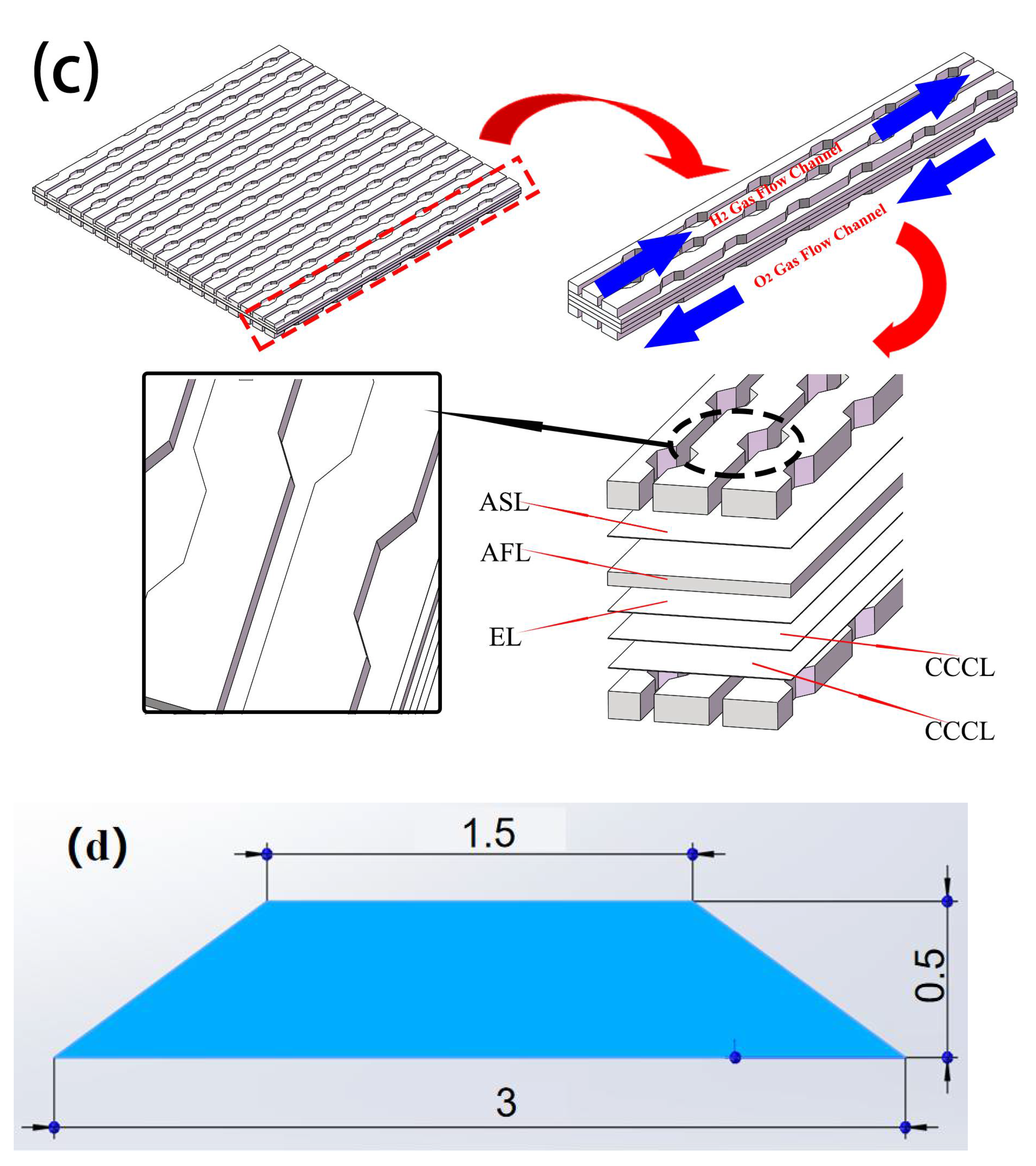
2.3. Classic and Curved SOFC Model
2.4. Boundary Conditions
3. Analysis of Classical Channel Structure
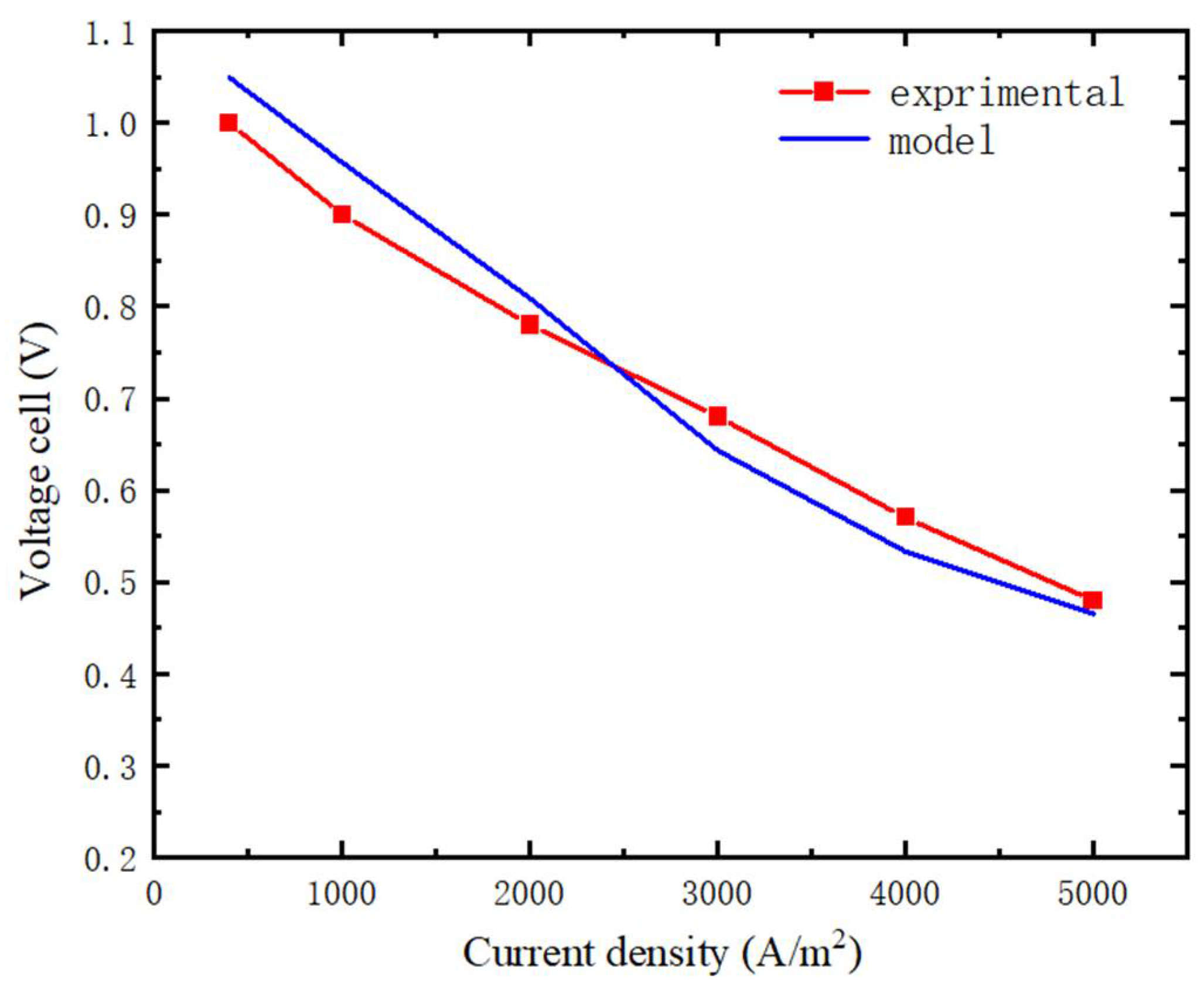
3.1. Model Verification
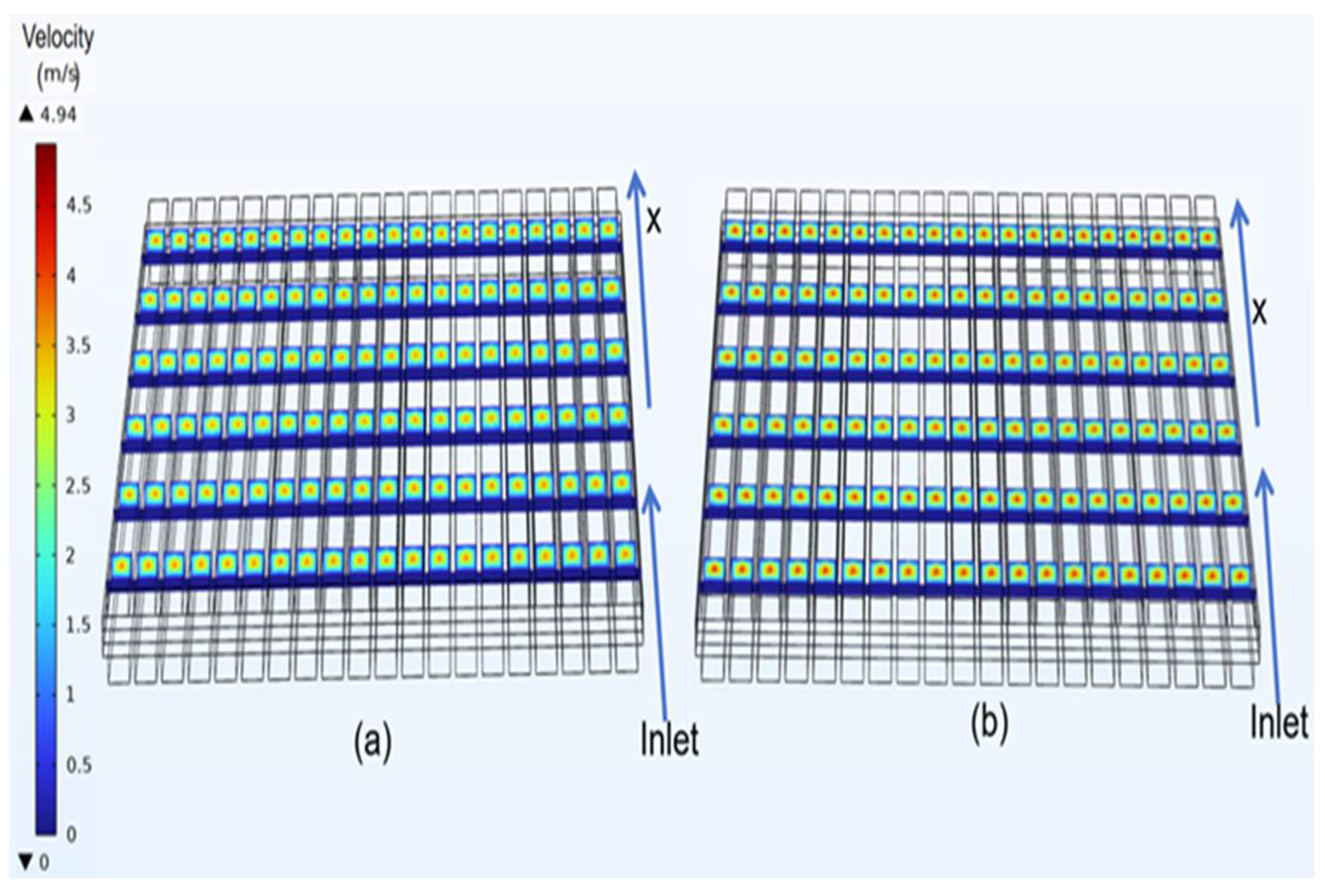
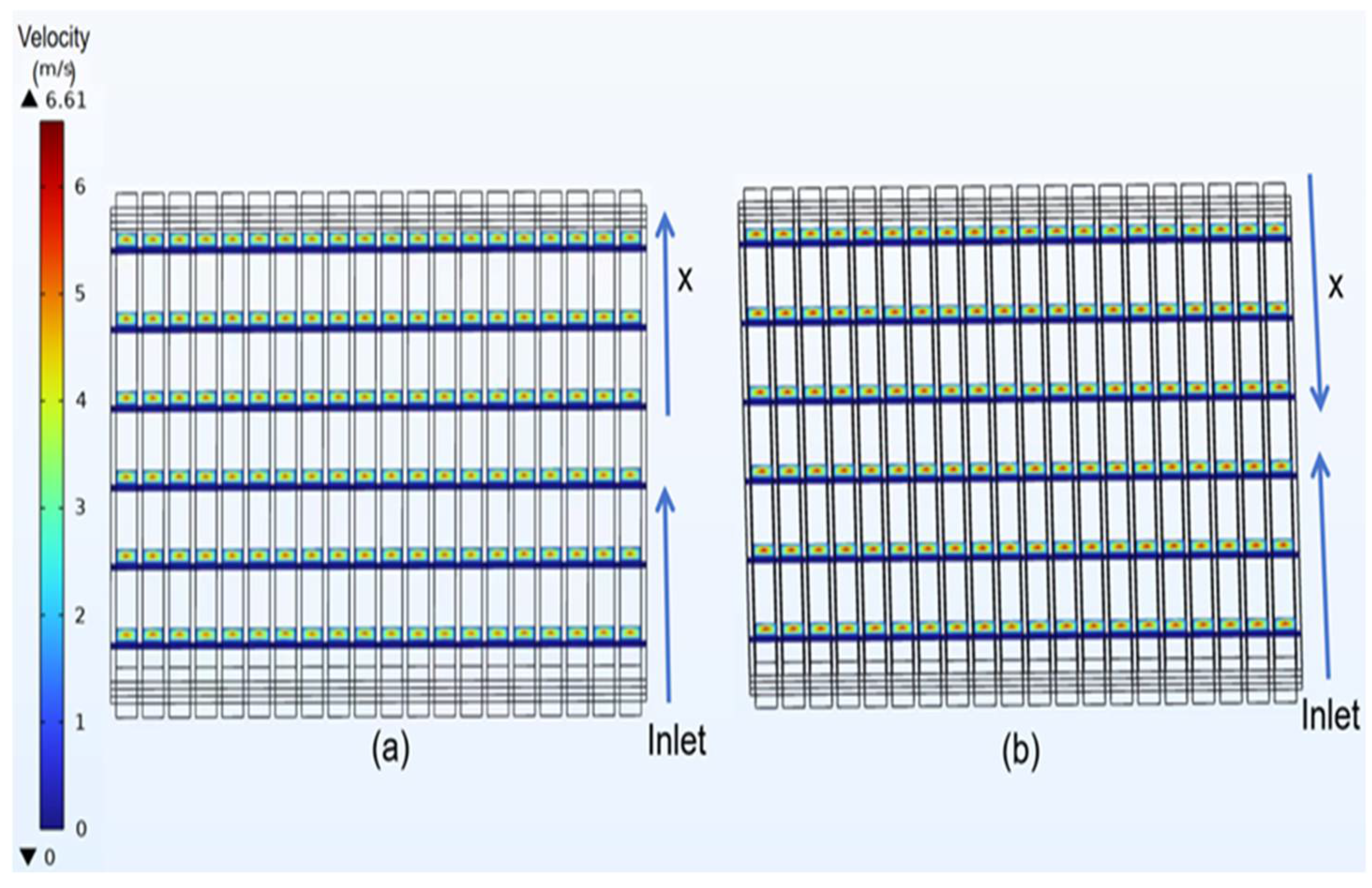
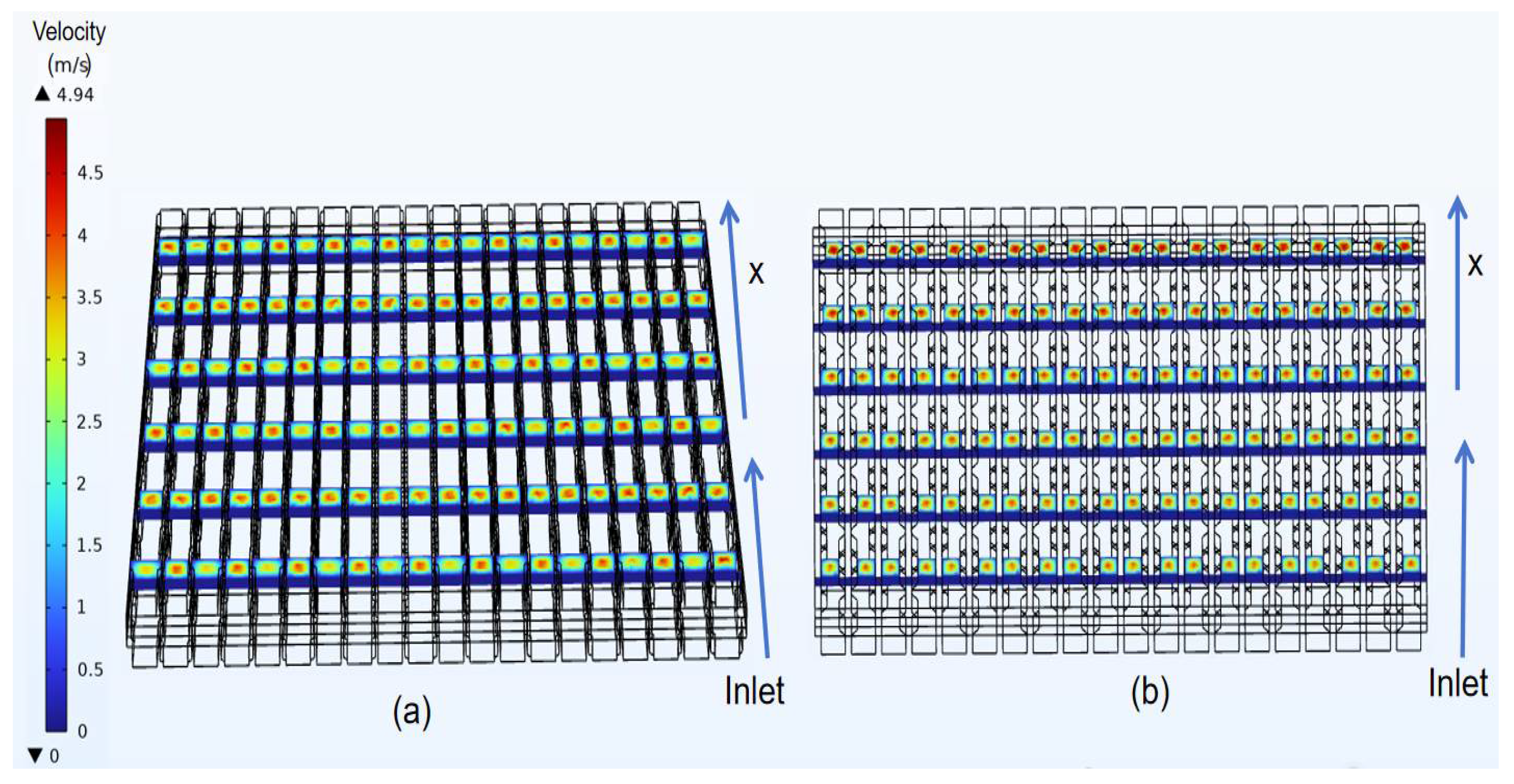
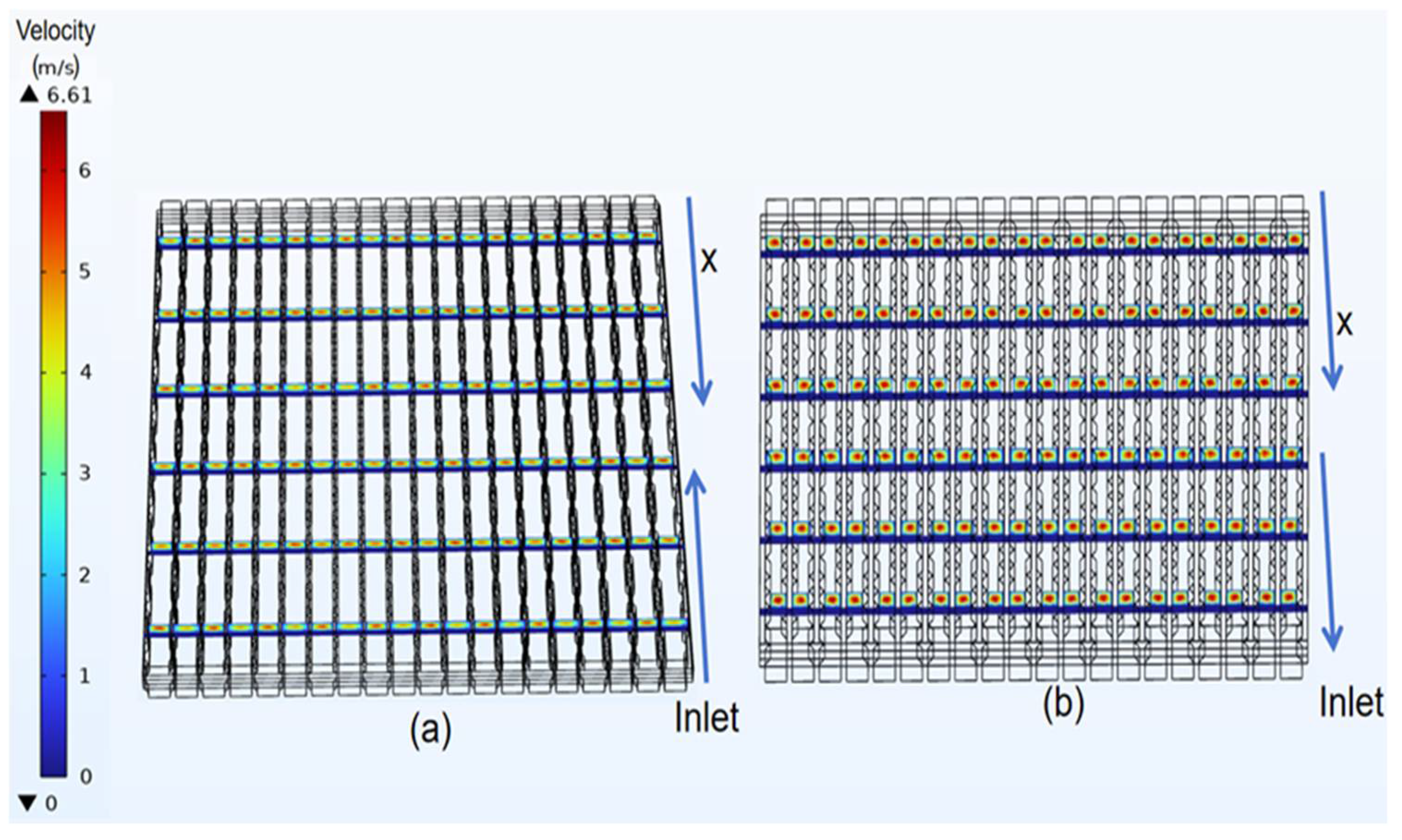
3.2. Velocity Distribution of Classical Model
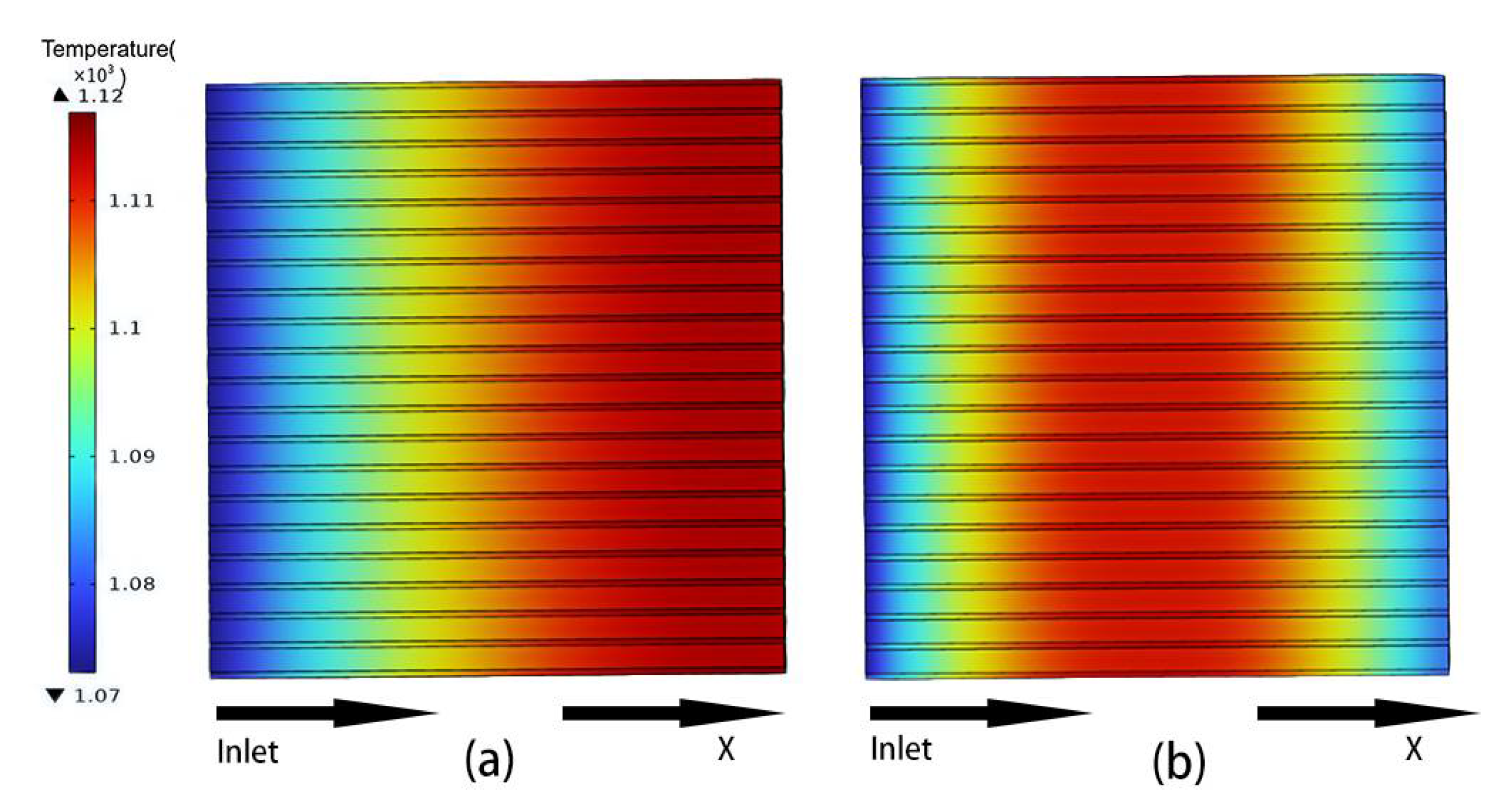
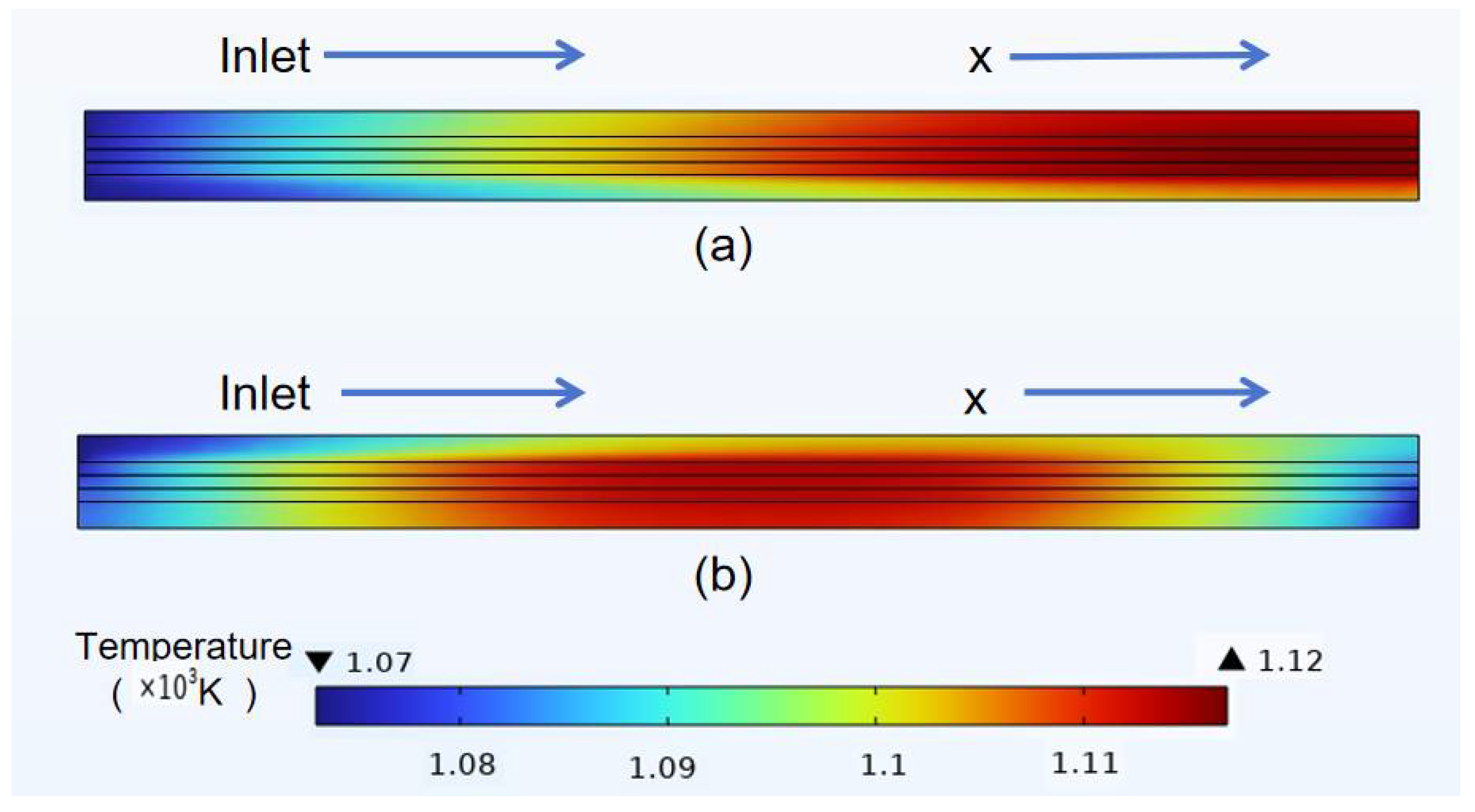
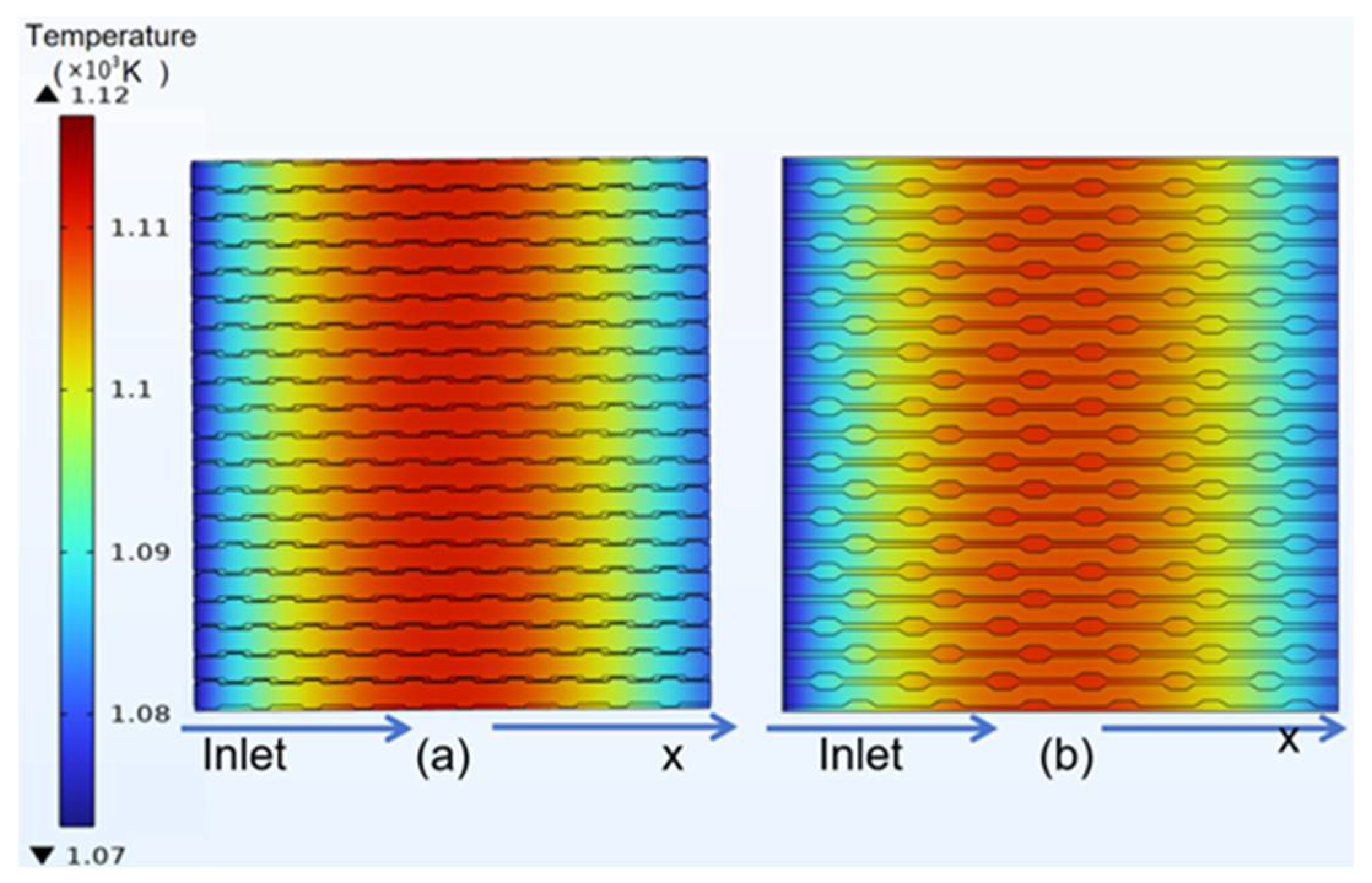
3.3. Temperature Distribution of Classical Model
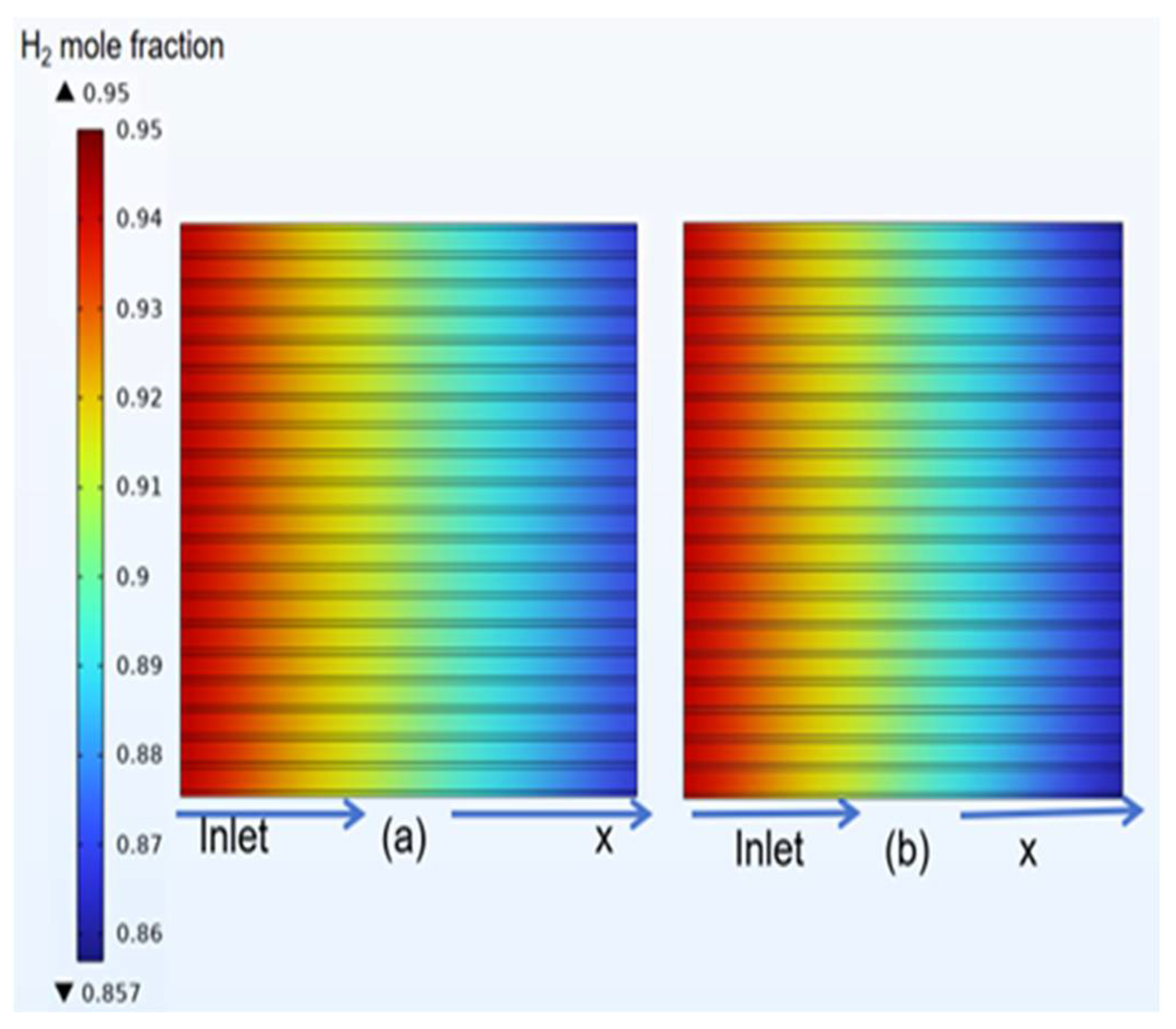
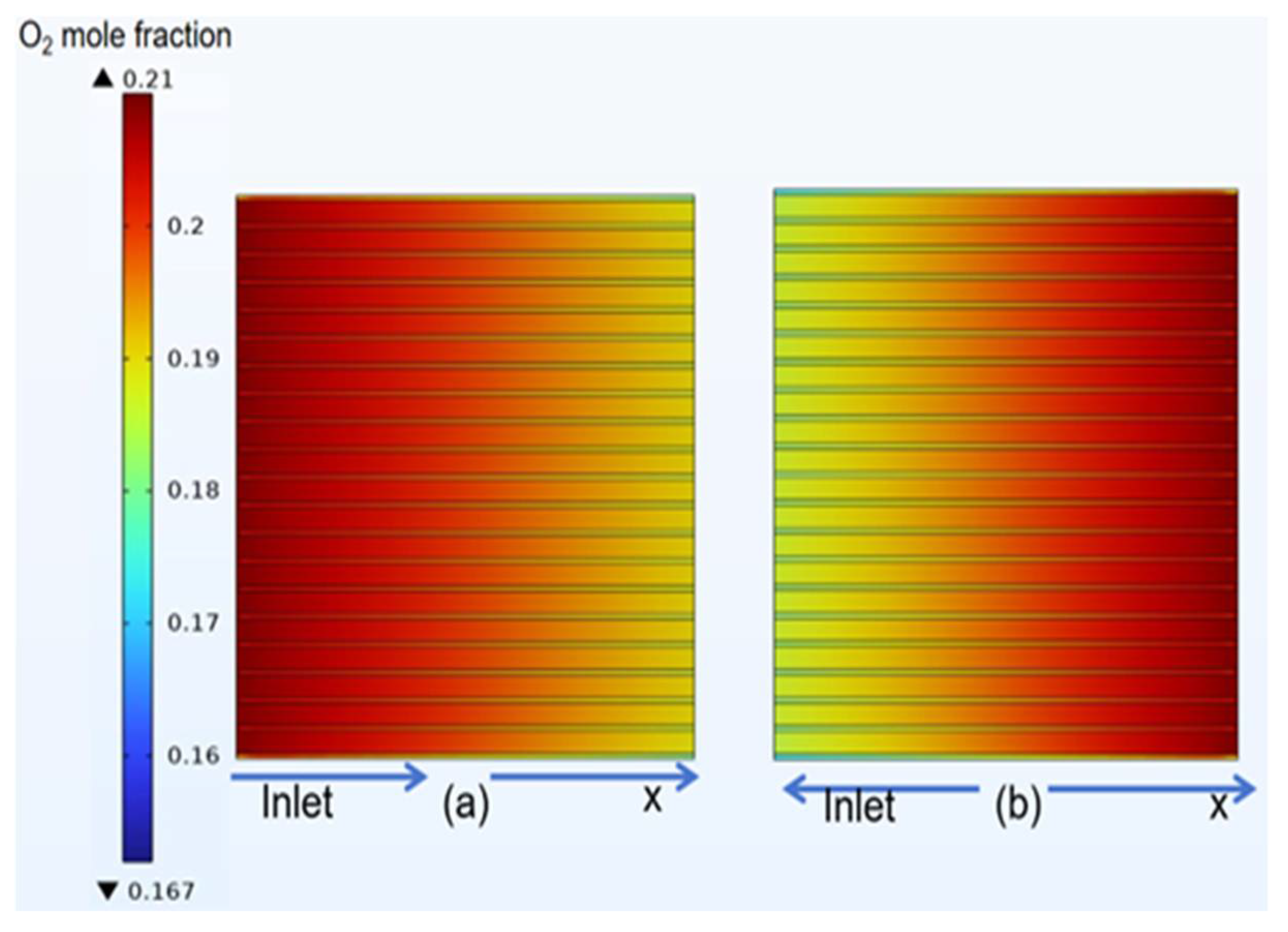
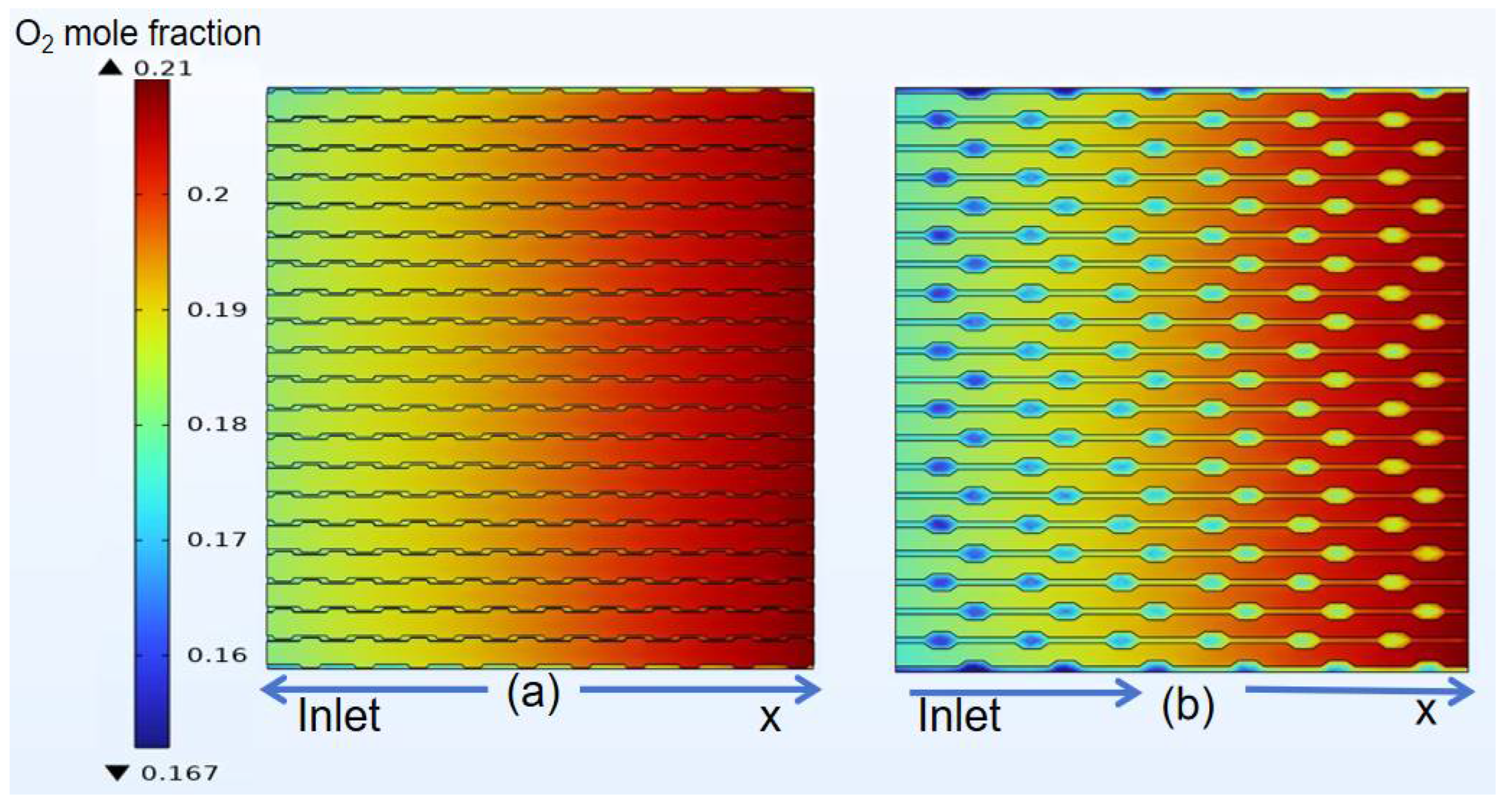
3.4. Species Distribution of Classical Model
4. Analysis of Curved Channel Structure
4.1. Velocity Distribution of Curved Model
4.2. Temperature Distribution of Curved Model
4.3. Species Distribution of Curved Model
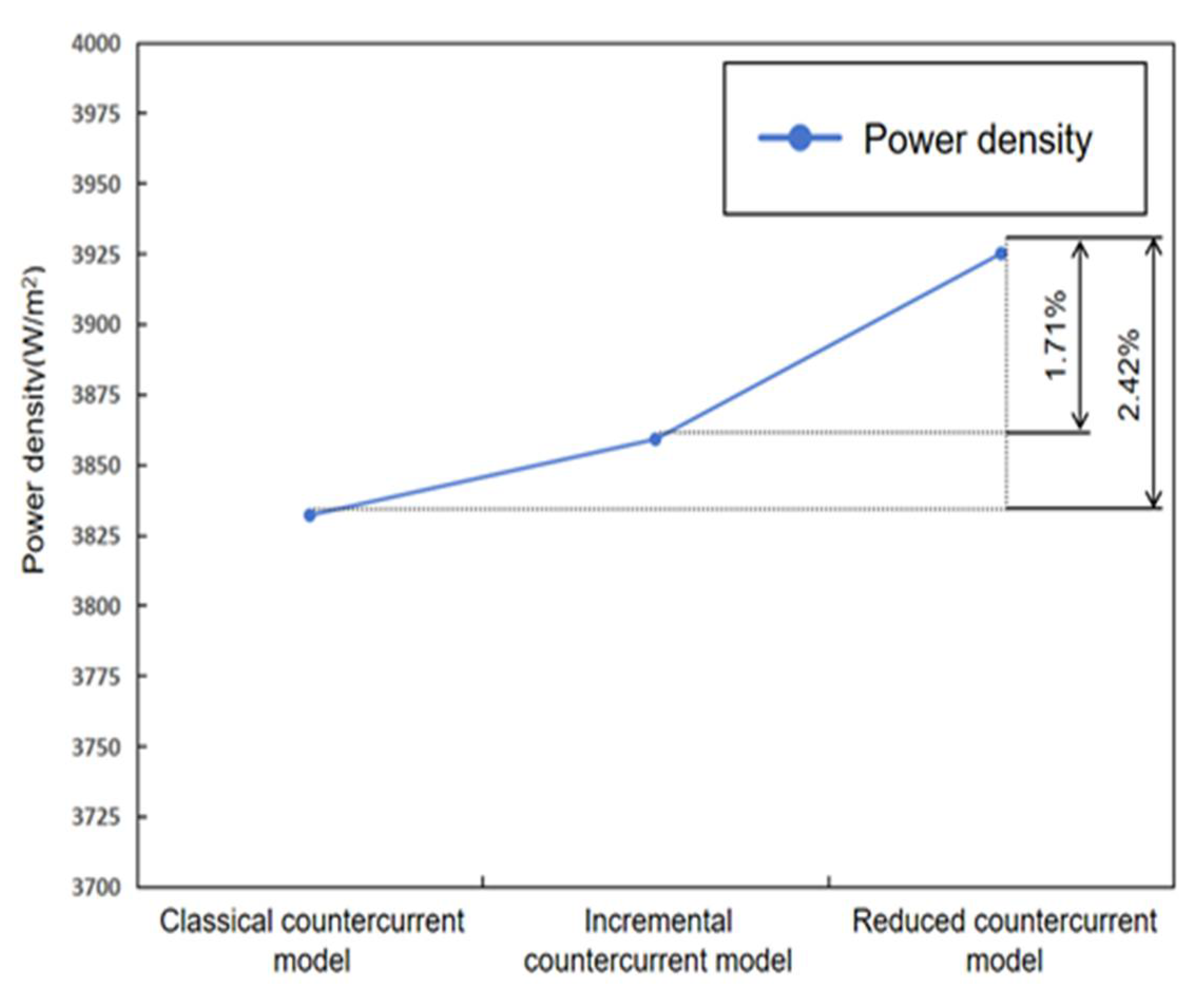
4.4. Performance Comparison
5. Conclusions
- (1)
- Analysis of the power density curve indicated that the power density of the optimized model was 2.42% higher than that of the classical model and 1.71% higher than that of the asymptotic model.
- (2)
- In the temperature field, it was observed that the temperature distribution of the counter-flow field was more uniform, with lower maximum temperatures, compared to different flow directions. This shows that the counter-current flow field is more conducive to enhancing SOFC performance than the forward flow field. Additionally, this study identified a gradual temperature increase from the fuel inlet and outlet sides toward the middle of the structure, with overall temperatures at the electrolyte–anode interface greater than those at the electrolyte–cathode interface.
- (3)
- In the velocity field, the gas velocity is highest at the center of the gas channel. As the gas diffuses toward the diffusion electrode, its velocity gradually decreases, reaching near-zero velocity upon reaching the diffusion-layer wall.
- (4)
- The gas concentration at the electrode decreases rapidly in the first half of the air inlet, followed by a slower rate of concentration decrease. However, hydrogen exhibits a more uniform distribution on the same horizontal interface compared to oxygen.
- (1)
- The length of the flow channels and the arrangement of baffle blocks affect the thermal characteristics of the fuel cell.
- (2)
- This study focused on the internal flow field, and it did not study the influence of the gas flow mode of the external flow field on the SOFC performance. The gas flow mode in the external flow field involves the uniformity of the temperature distribution of the SOFC, which in turn affects the cell life.
- (3)
- The model established in this study is a steady-state model, and in actual operation, the temperature, flow rate, pressure and electrochemical reaction rate all change with time. It is necessary to establish a transient model for further studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Symbols’ Meanings
| Symbol | Representative Significance | Unit | Symbol | Representative Significance | Unit |
| AFL | Anode functional layer | —— | ASL | Anode-supported layer | |
| CCCL | Cathode current collector layer | —— | CFL | Cathode functional layer | —— |
| Reference concentrations of hydrogen and water | mol/m3 | Specific constant pressure heat capacity of the gas j | J/(kg·K) | ||
| Molar concentration ratios in the SOFC activation region | —— | Effective binary diffusion coefficient (i and j are different substances) | m2/s | ||
| Molecular diffusion coefficient | m2/s | Knudsen diffusion | m2/s | ||
| Standard electromotive force | V | Activation energies | J/mol | ||
| EL | Electrolyte layer | —— | Faraday constant | C/mol | |
| Current density | A/m2 | Unit matrix | |||
| IN | Interconnect | —— | Pre-exponential constant | —— | |
| Effective heat conductivity | W/(m·K) | Heat conduction coefficient of gas | W/(m·K) | ||
| Heat conduction coefficient of solid | W/(m·K) | Molar amount of each gas | kg/mol | ||
| Proton exchange membrane fuel cell | —— | Total pressure of the gas | Pa | ||
| Partial pressure of substance i | Pa | Heat source term | W/m3 | ||
| Gas constant | J/(kg·K) | Effective pore diameter | m | ||
| Change in entropy before and after the electrochemical reaction | J/(mol·K) | Activated surface area | 1/m | ||
| Solid-state oxide fuel cells | —— | T | Temperature of the gas | K | |
| Speed field vector | m/s | Activation polarization | —— | ||
| Concentration polarization | —— | Volume diffusion coefficient | M3 | ||
| Ohmic polarization | Mole fraction | ||||
| Anodic and cathodic transfer coefficients of the electrodes | —— | Thickness of the anode layer | mm | ||
| Porosity | —— | Overpotential of activation polarization | V | ||
| Volume fraction of conductive material | —— | Permeability | m2 | ||
| Viscosity | Pa·s | Density | kg/m3 | ||
| Conductivity of the anode | S/m | Effective electronic conductivity | —— | ||
| Effective ionic conductivity | —— | Tortuosity of gas transfer in the porous electrode | —— | ||
| Potential | V | —— | —— | —— |
References
- Yang, J.; Yu, Y.; Ma, T.; Zhang, C.; Wang, Q. Evolution of energy and metal demand driven by industrial revolutions and its trend analysis. Chin. J. Popul. Resour. Environ. 2022, 19, 256–264. [Google Scholar] [CrossRef]
- Jiang, F.F.; Wei, Z.B.; Zhang, C.Z.; He, H.W.; Song, R.; Gao, F. Cathodic Supply Optimization of PEMFC System Under Variable Altitude. IEEE Trans. Transp. Electrif. 2024, 71, 14298–14307. [Google Scholar] [CrossRef]
- Watanabe, M.D.B.; Cherubini, F.; Tisserant, A.; Cavalett, O. Drop-in and hydrogen-based biofuels for maritime transport: Country based assessment of climate change impacts in Europe up to 2050. Energy Convers. Manag. 2022, 273, 116403. [Google Scholar] [CrossRef]
- Menon, N.V.; Chan, S.H. Technoeconomic and environmental assessment of HyForce, a hydrogen-fuelled harbour tug. Int. J. Hydrogen Energy 2022, 47, 6924–6935. [Google Scholar] [CrossRef]
- Chen, Z.; Lam, J.S. Life cycle assessment of diesel and hydrogen power systems in tugboats. Transp. Res. Part D Transp. Environ. 2022, 103, 103192. [Google Scholar] [CrossRef]
- Tomos BA, D.; Stamford, L.; Welfle, A.; Larkin, A. Decarbonising international shipping—A life cycle perspective on alternative fuel options. Energy Convers. Manag. 2024, 299, 117848. [Google Scholar] [CrossRef]
- IMO Fuel Cell Power Systems for Marine and Offshore Applications. August 2023. Available online: https://ww2.eagle.org/content/dam/eagle/rules-and-guides/current/other/312_requirements-for-fuel-cell-power-systems-for-marine-and-offshore-applications/312-fuel-cell-reqts-aug23.pdf (accessed on 5 September 2024).
- YANMAR Yanmar Develops Hydrogen Fuel Cell System for Maritime Applications. 4 June 2020. Available online: https://www.yanmar.com/global/news/2020/06/04/75041.html (accessed on 5 September 2024).
- Alfa Laval. Maritime Industry Players Join Forces to Realize the Decarbonization Potential of Solid Oxide Fuel Cells. 2 January 2021. Available online: https://www.alfalaval.com/industries/marine-transportation/marine/marine-news/maritime-industry-players-join-forces-to-realize-the-decarbonization-potential-of-solid-oxide-fuel-cells/ (accessed on 5 September 2024).
- Sheikh, A.A.; Bianchi, F.R.; Bove, D.; Bosio, B. A review on MCFC matrix: State-of-the-art, degradation mechanisms and technological improvements. Heliyon 2024, 10, e25847. [Google Scholar] [CrossRef]
- Biebl, M.; Verheyen, J.; Roes, J. Investigation of a Hydrogen Admixture in a Natural Gas Powered Phosphoric Acid Fuel Cell (PAFC). In Proceedings of the 2022 International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), Maldives, Maldives, 16–18 November 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–6. [Google Scholar]
- Kwon, L.; Kang, J.G.; Baik, K.D.; Kim, K.; Ahn, C. Advancement and applications of PEMFC energy systems for large-class unmanned underwater vehicles: A review. Int. J. Hydrogen Energy 2024, 79, 277–294. [Google Scholar] [CrossRef]
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent advances on electrolyte materials for SOFC: A review. Inorg. Chem. Commun. 2023, 152, 110724. [Google Scholar] [CrossRef]
- Latapí, M.; Davíðsdóttir, B.; Jóhannsdóttir, L. Drivers and barriers for the large-scale adoption of hydrogen fuel cells by Nordic shipping companies. Int. J. Hydrogen Energy 2023, 48, 6099–6119. [Google Scholar] [CrossRef]
- IMO Hot Topic—Reducing Greenhouse Gas Emissions from Ships. 2018. Available online: https://cimrc.shmtu.edu.cn/2022/1214/c5091a195998/page.htm (accessed on 5 September 2024).
- Li, J.; Wang, J.; Wu, T.; Li, J.; Kwon, J.-T. A Study on the Thermal Behavior of Series and Parallel Connection Methods in the Process of Hydrogenation of Ship-Borne Hydrogen Storage Cylinder. Processes 2024, 12, 366. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Cheng, C.; Li, J.; Zhou, K. A Review of the Research Progress and Application of Key Components in the Hydrogen Fuel Cell System. Processes 2024, 12, 249. [Google Scholar] [CrossRef]
- Venâncio, S.A.; de Miranda, P.E.V. Direct utilization of carbonaceous fuels in multifunctional SOFC anodes for the electrosynthesis of chemicals or the generation of electricity. Int. J. Hydrogen Energy 2017, 42, 13927–13938. [Google Scholar] [CrossRef]
- Song, Y.; Zhong, Q.; Tan, W. Synthesis and electrochemical behaviour of ceria-substitution LSCM as a possible symmetric solid oxide fuel cell electrode material exposed to H2 fuel containing H2S. Int. J. Hydrogen Energy 2014, 39, 13694–13700. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Q.T. On the role and development status of solid oxide fuel cells in China’s future energy system. Sci. Technol. Innov. Her. 2010, 56. [Google Scholar]
- Kong, W.; Zhang, W.; Huang, H.; Zhang, Y.; Wu, J.; Xu, Y. Analysis of micro-tubular SOFC stability under ambient and operating temperatures. J. Mater. Sci. Technol. 2018, 34, 1436–1440. [Google Scholar] [CrossRef]
- Liu, H. Preparation of Cu/Ni-LSCM Anode and Its Performance in Methane Dry Reforming. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2020. [Google Scholar] [CrossRef]
- Yu, F.Y.; Wang, Y.S.; Xie, Y.J.; Zhang, W.M.; Zhang, J.J.; Meng, X.X.; Xiao, J.; Yang, N.T. A Micro-tubular Direct Carbon Solid Oxide Fuel Cell Operated on the Biochar Derived from Pepper Straw. Energy Technol. 2020, 8, 1901077. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, H.; Wang, Z.; Sun, W.; Li, H.; Sun, K. Enhancing the catalytic activity of Y0.08Sr0.92TiO3−δ anodes through in situ Cu exsolution for direct carbon solid oxide fuel cells. Ind. Eng. Chem. Res. 2020, 59, 13105–13112. [Google Scholar] [CrossRef]
- Cai, W.; Cao, D.; Zhou, M.; Yan, X.; Li, Y.; Wu, Z.; Wang, H. Sulfur-tolerant Fe-doped La0.3Sr0.7TiO3 perovskite as anode of direct carbon solid oxide fuel cells. Energy 2020, 211, 118958. [Google Scholar] [CrossRef]
- Jeon, O.S.; Park, M.G.; Song, R.H.; Ryu, K.H.; Na, C.W.; Shul, Y.G.; Lee, J.G. Effects of Fe2O3 doping on structural and electrical properties of 8 mol% yttria-stabilized zirconia electrolyte for solid oxide fuel cells. J. Mater. Sci. Mater. Electron. 2022, 33, 3208–3214. [Google Scholar] [CrossRef]
- Kuterbekov, K.; Nikonov, A.; Bekmyrza, K.; Khrustov, V.; Pavzderin, N.; Kabyshev, A.; Kubenova, M. Co-sintering of gradient anode–electrolyte structure for microtubular SOFC. Ceram. Int. 2024, 50, 17242–17251. [Google Scholar] [CrossRef]
- Xu, G.P. Study on Performance Optimization and Flow Field Structure of Solid Oxide Fuel Cells. Master’s Thesis, Shandong University, Jinan, China, 2024. Available online: https://kns.cnki.net/kcms2/article/abstract?v=ad5XPF-Jk6DZ-nWuoMw-_QxccRr3uUXI6EZV45tW7O4u5Ju1La7DQr4fSvQOqsgId-Lj9hjVpYcPjMlvQaaJEXL5P13wV9eoaPdOoXjZakmXBe_E-hOXvkB9wVwTIvEtwZKI8eLznEhc3nHR3eVA16hDTYmzglhw5niFUPk-1Y9dA7Meu2qDGoi6kZ5BqMt1rrCUOB3hb0s=&uniplatform=NZKPT&language=CHS (accessed on 5 September 2024).
- Wei, S.; Ni, S.; Ma, W.; Du, Z.; Lu, P. Exergy analysis and parameters optimization for zero-carbon/low-carbon fuels on SOFC-engine hybrid system. Fuel 2024, 364, 131076. [Google Scholar] [CrossRef]
- Saberi Mehr, A.; Ilkhani, M.; Sabernia, S.; Nooshmand, S.; Ebrahimpour, A.; Heydari, B. Thermodynamic modelling and optimisation of a green hydrogen-blended syngas-fueled integrated PV-SOFC system. Appl. Therm. Eng. 2024, 236, 121506. [Google Scholar] [CrossRef]
- Hussain, J.; Ali, R.; Akhtar, M.N.; Jaffery, M.H.; Shakir, I.; Raza, R. Modeling and simulation of planar SOFC to study the electrochemical properties. Curr. Appl. Phys. 2020, 20, 660–672. [Google Scholar] [CrossRef]
- Li, G.; Wu, M.; Zeng, D.; Wu, M.; Zhang, Y.; Tao, Y.; Shao, J. Mass and current uniformity for planar solid oxide fuel cells with discrete landing structured flow fields: A three-dimensional numerical analysis. Int. J. Hydrogen Energy 2022, 47, 33039–33057. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, Y.; Han, S.; Anatoly, L.; Andrey, M.; Lu, L. Investigate the effect of a parallel-cylindrical flow field on the solid oxide fuel cell stack performance by 3D multiphysics simulating. J. Energy Storage 2023, 60, 106587. [Google Scholar] [CrossRef]
- Gong, C.; Tu, Z.; Chan, S.H. A novel flow field design with flow re-distribution for advanced thermal management in Solid oxide fuel cell. Appl. Energy 2023, 331, 120364. [Google Scholar] [CrossRef]
- Guo, M.; He, Q.; Cheng, C.; Zhao, D.; Ni, M. New interconnector designs for electrical performance enhancement of solid oxide fuel cells: A 3D modelling study. J. Power Sources 2022, 533, 231373. [Google Scholar] [CrossRef]
- Liu, X.; Sun, S.D.; Dai, Y.; Zhang, H.-Y.; Li, C.-X. Numerical study of temperature distribution in tubular segmented-in-series SOFC with co-flow and counter-flow arrangements. Int. J. Hydrogen Energy 2024, 74, 447–458. [Google Scholar] [CrossRef]
- Todd, B.; Young, J.B. Thermodynamic and transport properties of gases for use in solid oxide fuel cell modelling. J. Power Sources 2002, 110, 186–200. [Google Scholar] [CrossRef]
- Xiong, X.; Liang, K.; Ma, G.; Ba, L. Three-dimensional multi-physics modelling and structural optimization of SOFC large-scale stack and stack tower. Int. J. Hydrogen Energy 2023, 48, 2742–2761. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Mukhopadhyay, J.; Biswas, N.; Basu, R.N.; Das, P.K. Performance evaluation of different bipolar plate designs of 3D planar anode-supported SOFCs. Int. J. Heat. Mass. Transf. 2018, 123, 382–396. [Google Scholar] [CrossRef]
- Lu, P.Z.; Wei, S.L.; Du, Z.H.; Ma, W.; NI, S. Analysis and comparison of multi-physics fields for different flow field configurations in SOFC. Int. J. Heat Mass Transf. 2024, 229, 125708. [Google Scholar] [CrossRef]
- Chan, S.H.; Khor, K.A.; Xia, Z.T. A complete polarization model of a solid oxide fuel cell and its sensitivity to the change of cell component thickness. J. Power Sources 2001, 93, 130–140. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hanamura, K. Prediction of overpotential and effective thickness of Ni/YSZ anode for solid oxide fuel cell by improved species territory adsorption model. J. Power Sources 2017, 353, 115–122. [Google Scholar] [CrossRef]
- Fu, Q.; Li, Z.; Wei, W.; Liu, F.; Xu, X.; Liu, Z. Performance enhancement of a beam and slot interconnector for anode-supported SOFC stack. Energy Convers. Manag. 2021, 241, 114277. [Google Scholar] [CrossRef]
- Khazaee, I.; Rava, A. Numerical simulation of the performance of solid oxide fuel cell with different flow channel geometries. Energy 2017, 119, 235–244. [Google Scholar] [CrossRef]


| Parameter | Value (mm) | |
|---|---|---|
| Cell length | 60 | |
| Gas channel | Width | 2 |
| Height | 1 | |
| Width of the channel | 2 | |
| Height of the interconnect | 1 | |
| Anode | Thickness of the ASL | 0.01 |
| Thickness of the AFL | 0.3 | |
| Thickness of the EL | 0.01 | |
| Cathode | Thickness of the CFL | 0.03 |
| Thickness of the CCCL | 0.08 | |
| Parameter | Value (mm) | |
|---|---|---|
| Cell length | 60 | |
| Gas channel | Width | 2 |
| Height | 1 | |
| Width of the channel | 2 | |
| Height of the interconnect | 1 | |
| Anode | Thickness of the ASL | 0.01 |
| Thickness of the AFL | 0.3 | |
| Thickness of the EL | 0.01 | |
| Cathode | Thickness of the CFL | 0.03 |
| Thickness of the CCCL | 0.08 | |
| Parameter | Value | Parameter | Value | ||
|---|---|---|---|---|---|
| Thermal conductivity | Anode | 15 | Density | Anode | 6870 |
| EL | 2.7 | EI | 5900 | ||
| Cathode | 20 | Cathode | 6570 | ||
| Porosity | Anode | 0.452 | Exchange coefficient | Anode | 1 |
| Cathode | 0.452 | Cathode | 1 | ||
| Pre-exponential constant | Anode | R [J/(kg·K)] | 8.314 | ||
| Cathode | F [C/mol] | 96,500 | |||
| Activation energy | Anode | —— | —— | —— | |
| Cathode | |||||
| Parameter | Temperature (K) | Species | Velocity (m/s) | |
|---|---|---|---|---|
| Fuel | Inlet | 1073 | H2∶H2O = 0.95∶0.05 | 2.5 |
| Outlet | 1073 | - | - | |
| Air | Inlet | 1073 | O2∶N2 = 0.21∶0.79 | 1.5 |
| Outlet | 1073 | - | - | |
| Top wall of the anode interconnect | Adiabatic | Convection | Wall | |
| Bottom wall of the cathode interconnect | Adiabatic | Convection | Wall | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ding, Y.; Wu, T.; Gong, Z.; Fan, Y.; Ma, H.; Kwon, J.-T.; Ni, W.; Li, J. The Impact of Flow-Thermal Characteristics in Ship-Board Solid Oxide Fuel Cells. J. Mar. Sci. Eng. 2024, 12, 1779. https://doi.org/10.3390/jmse12101779
Li J, Ding Y, Wu T, Gong Z, Fan Y, Ma H, Kwon J-T, Ni W, Li J. The Impact of Flow-Thermal Characteristics in Ship-Board Solid Oxide Fuel Cells. Journal of Marine Science and Engineering. 2024; 12(10):1779. https://doi.org/10.3390/jmse12101779
Chicago/Turabian StyleLi, Jiqiang, Yexun Ding, Tong Wu, Zhenyu Gong, Yong Fan, Haoran Ma, Jeong-Tae Kwon, Weixin Ni, and Jichao Li. 2024. "The Impact of Flow-Thermal Characteristics in Ship-Board Solid Oxide Fuel Cells" Journal of Marine Science and Engineering 12, no. 10: 1779. https://doi.org/10.3390/jmse12101779
APA StyleLi, J., Ding, Y., Wu, T., Gong, Z., Fan, Y., Ma, H., Kwon, J.-T., Ni, W., & Li, J. (2024). The Impact of Flow-Thermal Characteristics in Ship-Board Solid Oxide Fuel Cells. Journal of Marine Science and Engineering, 12(10), 1779. https://doi.org/10.3390/jmse12101779







