Abstract
The fish species undergo diel and seasonal changes in coastal (littoral) ocean zones. Many factors affect these seasonal and diel patterns, thus it is difficult to determine which are the most important. Concerning the Adriatic Sea, studies on the temporal changes of fish communities are rare. Therefore, the primary aim of this study was to evaluate, in terms of abundance, diversity and species composition, the diel and seasonal changes in a one-year cycle of the fish community living in the infralittoral zone of the Eastern Central Adriatic and the main drivers affecting them. This study revealed the presence of a very rich and diverse community in the coastal zone of the Eastern Central Adriatic with a total of 63 identified fish species. The results showed that the differences in fish assemblage were more strongly influenced by seasonal variations rather than diurnal variations. The primary environmental factor that drives fish to undertake seasonal variations is water temperature. Considering that many species are important commercial species, especially for small scale fisheries, and taking into account future climate-driven changes that would affect the seasonality of the target species, it is essential that future management will be tailored accordingly and in due time.
1. Introduction
The abundance of individual fish species and the composition as well as the abundance and diversity of the total fish fauna undergo diel and seasonal changes in coastal (littoral) ocean zones. Diel biological processes of fish assemblages inhabiting temperate areas are associated with feeding activity, shelter usage and predator avoidance and are synchronized to changes in photoperiod length and tidal cycles [1,2,3]. In coastal habitats with small tides, especially shallower ones, the photoperiod light intensity, which is related to solar irradiance, is among the most important environmental factors controlling the biological rhythms of coastal fishes as environmental illumination determines the timing of activity of predators and preys, that perform their ecological tasks according to a trade-off between the maximum opportunities of visual-based feeding and minimum mortality risk [4]. The exposure of marine costal ecosystems to solar light also produces seasonal changes of photoperiod length which is impacting coastal fishes on a seasonal level. The photoperiod length is not the only factor that affects seasonal changes in fish assemblages as fishes are interacting with other environmental factors and habitat parameters and/or by factors relating to life history, including spawning and recruitment patterns [1]. While a multitude of factors affect the seasonal patterns of species composition and diversity, making it challenging to pinpoint the most influential variables, it becomes evident that, in coastal zones with minimal salinity fluctuations, temperature exerts the strongest influence on fish populations [5,6].
Seasonality occurs in all marine ecosystems, but its duration and intensity vary depending on the geographical area [7]. The Adriatic Sea is a semi-enclosed basin within the larger semi-enclosed sea constituted by the Mediterranean and is characterized by the largest shelf area of the Mediterranean, which extends over the Northern and Central areas where the bottom depth is no more than about 75 and 100 m, respectively, with the exception of the Pomo/Jabuka Pit (200–260 m) in the Central Adriatic. On the other side, the Southern Adriatic has a relatively narrow continental shelf and a marked, steep slope and it reaches a maximum depth of 1223 m [8]. The Adriatic Sea shows large interannual as well as seasonal and shorter-term variability in the circulation and in the physical and bio-geochemical properties of the upper sea layer, which is mostly influenced by modifications in meteorological forcing and continental inputs [9,10]. The thermohaline properties of the Adriatic Sea are determined mainly by the air–sea interaction, water exchange through the Otranto Strait, river discharge, mixing, currents and topography of the basin. More than 1200 large and small islands lie along the eastern coast, while there are almost no islands along the western shore. Thus, the eastern and western Adriatic coasts have different morphological and topographic properties: the eastern coast is composed of limestone, and its steep and narrow shelf deepens fast while the western coast has a wider shelf because of sediments brought by the river. Hence, the eastern Adriatic channel areas, compared to the open waters, are also more productive given that they are impacted by freshwater inflow, coves, bays and river mouths as well as the lower depths [9]. In the Eastern Adriatic, the coastal area is traditionally the most important fishing area for commercial, small-scale and recreational fisheries. The coastal area is characterized by a higher productivity rate compared to the open sea due to the relatively lower depth, proximity of the land and fresh-water inflow. The marine biodiversity in the Adriatic is attributed to its geological history, limited by its present bathymetric, hydrographic and climatic characteristics, and influenced by present geographical connectivity and anthropogenic processes. These factors have been crucial in shaping the peculiarities of Adriatic ichthyofauna which still depends on it [9,11]. A recently updated checklist of Adriatic Sea fishes with a critical assessment of each species using an evidence approach included a total of 444 species [12]. Within these species, the vast majority of species are attributed to the marine benthic environment, nearly 72%. Most benthic fishes are benthic littoral species, representing 56% of all benthic fish diversity and 40% of total Adriatic fish diversity, followed by species occurring both in the littoral and in the bathyal area representing 37% of all benthic fish diversity and 27% of total Adriatic fish diversity [11]. Within the benthic species that inhabit continental shelf, infralittoral species and species of widespread littoral occurrence are common in both the infralittoral and circalittoral zones while only a few species are exclusively circalittoral [11].
Although there are studies in the Mediterranean, as well as in the Adriatic, that investigated temporal changes of the infralittoral fish community, most of them were focusing on very shallow areas (up to 5 m of depth) or on a particular group of fishes, e.g., cryptobenthic or juveniles or on only one temporal period (diel or seasonal) [13,14,15,16,17,18].
Comprehensive studies on temporal changes of fish communities in the Adriatic are, thus, rare. Taking that into account it is necessary to fill this knowledge gap that might hide important aspects of the dynamics and relationships of coastal infralittoral fish communities. We hypothesized that the infralittoral fish community in the Eastern Central Adriatic undertakes both diel and seasonal variations which are influenced by a combination of different factors. Consequently, the main objectives of this study were as follows: (1) to determine the abundance, diversity and fish species composition; (2) to analyse the diel and seasonal changes of the fish community along a one-year cycle; and (3) to determine the main factors affecting the distribution of fish occurring in the infralittoral zone of the Eastern Central Adriatic.
2. Materials and Methods
2.1. Study Area and Sampling
The study area was located in island of Hvar channel in the Eastern Central Adriatic (Figure 1). The channel is surrounded by the mainland along the north to east, by the island of Brač at the west and by the island of Hvar at the south. This area represents the typical Eastern Adriatic area where different fisheries activities are carried out. Since that trawl is considered the best method for collecting fish at such depth (16) a small experimental trawl was used with a mesh size of 12 mm, total length of 19.4 m and lead line length of 8.4 m, towed at a speed of 2 knots by a small commercial fishing boat of 7.6 m length and powered by 13.40 kW engine. Considering that in the sampling area the presence of Posidonia oceanica seagrass meadows was known, a special lead line was used to slide a trawl over the Posidonia leaves to prevent any cutting and damaging.
Figure 1.
The study area indicated by the dotted rectangle.
Fish were collected every month during for a one-year period from January to December 2018. Each month the sampling was conducted during the day and night, with 12 h difference in between and each haul lasted 30 min.
Taking into account the coast configuration and its suitability for trawling, the sampling was carried out at depths from 21 to 45 m and at the distance of 100 to 600 m from the coastline. The sea surface temperature ranged from a minimum of 11.9 °C in January to a maximum of 27.1 °C in August.
Each fish was identified at a species level and its total length was measured to the nearest mm and weight to the nearest 0.1 g. Species names, orders and families were arranged according to Eschmeyer’s Catalog of Fishes [19,20].
2.2. Data Analysis
The analysis involved a dataset of 24 trawling samples. To investigate the relationship between fish assemblages, quantified in terms of species abundance, and the variables of day period, season and the interaction between these temporal factors, we used the univariate Permanova procedure developed by Anderson [21]. This methodology was chosen due to the dataset’s non-compliance with the normality assumptions necessary for parametric analysis of variance (ANOVA). When the Permanova analysis yielded statistically significant outcomes, we performed pairwise comparisons.
For the visualization of temporal patterns revealed by the Permanova procedure, a nonparametric multidimensional scaling (nMDS) approach was applied. for each month, we calculated the Shannon diversity index (H) and the Simpson diversity index using the ‘diversity’ function in the ‘vegan’ R-package [22]. To evaluate dissimilarities in fish composition across different times of the day and seasons, we implemented a one-way Analysis of Similarity (ANOSIM) [23]. Subsequently, SIMPER analysis [24] was utilized to identify the species contributing the most to dissimilarity within the samples, particularly concerning time of day and season. Prior to this analysis, the dataset underwent a logarithmic (ln(x + 1)) transformation, with Bray–Curtis distances serving as the measure of dissimilarity.
A univariate Permanova analysis based on Euclidean distances was used to test for differences in total biomass and total abundance across different day periods and throughout the seasons. All Permanova tests were performed with 9999 permutations, utilizing the ‘adonis’ function in the R package ‘vegan’ [22].
3. Results
The study area is characterized by several different habitats, namely muddy, muddy-sandy, sandy and Posidonia oceanica seagrass meadows. On these grounds, a total of 9541 fish weighing 166,397.3 g were caught during a 12-month period. A total of 63 fish species were identified, 61 belonging to the teleost fishes and 2 to Chondrichthyes (Table 1). Within 25 represented families the most dominant family was Sparidae with 13 species, followed by 7 species from Labridae and 6 from Gobiidae. Among all species, Picarel Spicara smaris (Linnaeus, 1758) was the most dominant and composed 28.38% of the total number of fish. Picarel was followed by Painted comber Serranus scriba (Linnaeus, 1758) (13.65%), Damselfish Chromis chromis (Linnaeus, 1758) (12.79%), Spicara flexuosum Rafinesque, 1810 (8.88%) and Brown comber Serranus hepatus (Linnaeus, 1758) (5.35%). These five species accounted for 69% of the total number of fish. In terms of weight, the order of the three most dominant species is the same as the list starts with S. smaris (23.84%), S. scriba (16.04%) and C. chromis (10.79%), but the fourth and fifth are Black scorpionfish Scorpaena porcus Linnaeus, 1758 (5.75%) and East Atlantic peacock wrasse Symphodus tinca (Linnaeus, 1758) (5.67%). These species contribute 62.09% to total biomass.

Table 1.
The total number of individuals and the biomass of fish species collected during a one-year period.
The fish assemblage structure in the samples exhibited significant variations in total biomass and total abundance, both seasonally and diurnally (Figure 2). The results of the Permanova analysis (Table 2) revealed highly significant differences in fish assemblage between seasons (p = 0.001) and between day periods (p = 0.0015). However, the interaction between seasons and day periods was statistically insignificant (p = 0.1164).
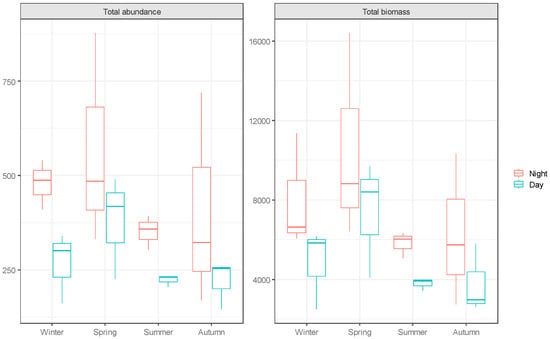
Figure 2.
Boxplot of seasonal and diurnal changes in total abundance and total biomass.

Table 2.
Results of the Permanova and pairwise tests, examining the differences in fish assemblage and environmental variables among seasons (Winter/Spring/Summer/Autumn) and between day periods (day/night) and differences in total biomass and total abundance.
Pairwise comparisons were conducted to examine the specific differences between seasons. The results revealed significant differences in fish assemblage between winter and spring (p = 0.0092), winter and summer (p = 0.0027) and summer and autumn (p = 0.0279). Furthermore, a marginally significant difference was observed between spring and autumn (p = 0.0541).
However, no significant differences were found between winter and autumn (p = 0.6368) or between spring and summer (p = 0.101).
Table 2 presents the statistical results of the Permanova and pairwise tests, examining the differences in fish assemblage and environmental variables between seasons (Winter/Spring/Summer/Autumn) and between day periods (Day/Night). For pairwise comparison only statistically significant results are included. Additionally, it includes the results of the pairwise Permanova analysis for the differences in total biomass and total abundance.
The nMDS plots (stress = 0.136) indicate a partial separation of fish assemblage between day and night, and autumn and summer and a larger separation between winter and spring and winter and summer (Figure 3).
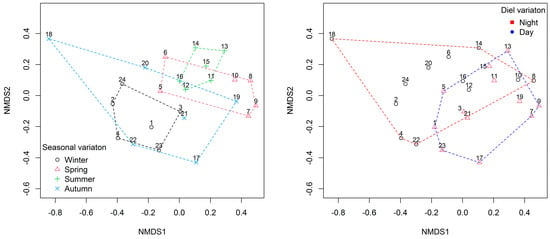
Figure 3.
Non-metric multi-dimensional scaling (nMDS) ordination plot of fish assemblage comparing diurnal and seasonal variations in composition.
The Anosim analysis (for all pairwise comparisons) confirmed that there were higher dissimilarities in fish assemblage attributed to seasonal variations (Global R = 0.353, p = 0.0003) compared to diurnal variations (Global R = 0.1268, p = 0.0345). These results indicate that the differences in fish assemblage were more strongly influenced by seasonal variations rather than diurnal variations.
The Simper identified Spicara smaris as a major contributor to dissimilarities between day periods and among seasons. Regarding diurnal dissimilarities Spicara smaris (25.65%) together with Spicara flexuosum (13.24%), Chromis chromis (12.12%), Serranus scriba (9.54%), Serranus hepatus (5.22%) and Bogue Boops boops (Linnaeus, 1758) (4.25%) contributed to 70% of the total dissimilarity between groups. Hence, when comparing winter and spring seasons S. smaris is again the species who is a major contributor to the dissimilarity (17.22%) and together with S. flexuosum (15.53%), S. scriba (13.99%), C. chromis (11.17%), S. hepatus (4.64%), B. boops (4.22%) and Annular seabream Diplodus annularis (Linnaeus, 1758) (4.05%) contributed nearly 71% to the total dissimilarity between groups.
Comparing winter and spring seasons S. smaris again contributed the most with 24.59% to the dissimilarity and together with S. flexuosum (18.58%), C. chromis (10.55%), S. scriba (7.77%), B. boops (5.43%) and S. hepatus these species contributed more than 72% to the total dissimilarity between groups. S. smaris is also a major contributor (31.75%) to the total dissimilarity between groups when comparing the winter season and autumn and together with S. flexuosum (19.00%), C. chromis (7.74%), B. boops (7.54%) and S. scriba (5.09%) makes more than 71% of the total dissimilarity. S. smaris (22.34%), C. chromis (15.15%), S. scriba (13.16%), S. hepatus (7.35%), C. julis (5.41%), D. annularis (5.34%) and S. porcus (3.70%) contributed to the total dissimilarity between groups with more than 72% when comparing spring and summer seasons. When comparing spring and autumn season Simper analysis revealed that seven species contributed to the total dissimilarity by more than 71%, namely S. smaris (22.20%), S. scriba (15.03%), C. chromis (11.88%), S. flexuosum (10.01%), S. hepatus (4.31%), D. annularis (4.06) and B. boops (3.76%). S. smaris is also a major contributor to the dissimilarity between summer and autumn with 22.46%, followed by S. flexuosum (13.15%), C. chromis (12.19%), S. scriba (10.82%), S. hepatus (6.71%) and B. boops (4.13%) which together contribute with nearly 70% to the total dissimilarity.
With regard to the difference in total biomass and total abundance between seasons, no significant differences were found (p = 0.1346 and p = 0.2122, respectively). In contrast, when comparing day and night, the total biomass and total abundance values were significantly higher during the night (p = 0.0297 and p = 0.0072, respectively).
Shannon’s H and the Simpson diversity index both indicate greater diversity in the daytime trawl samples compared to the nighttime samples across most months, with the exception of January (Figure 4).
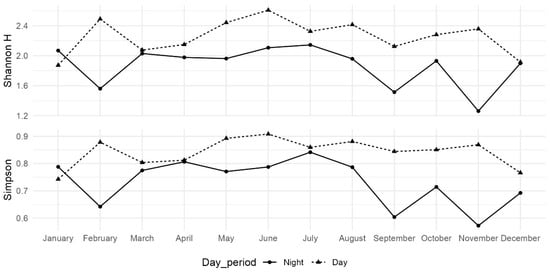
Figure 4.
Diurnal monthly trends of Shannon diversity (H) and Simpson diversity index during Solid line represents night period and dotted line daylight period.
4. Discussion
The high number of species constituting the fish community of the infralittoral zone of the Eastern Adriatic is likely in relation to the high variety of habitats in the studied area, from muddy, sandy or muddy-sandy bottoms to seagrass beds. The results from this study showed high variations in the fish community in the infralittoral zone, both seasonally and diurnally. Picarel was the species that contributed the most to the differences, followed by close relative S. flexuosum, and C. chromis, S. hepatus, B. boops, D. annularis and S. scriba. Throughout the study period picarel was also the most abundant species on a month and day/night basis. However, during the warmer months from April to August and October, the species with the highest abundance during the day period was S. scriba. In February S. flexuosum had the highest abundance as well as during the night period in August, C. chromis abundance was the highest among the species during the night period in April, August and October, while during the night in July the most abundant species was S. hepatus.
The high abundance of picarel observed during the colder months and especially during the night period coincides with knowledge related to the traditional small-scale fishing activities targeting picarel. Cetinić et al. [25] reported that, in the Eastern Adriatic, several pieces of fishing gear were designed and used for targeting picarel during winter months in coastal areas, particularly throughout the night period. Even more, the fishing gear for picarel intended to be used during the day needed to be a much larger in height to be able to fish picarel present during the daytime in the water column while moving down closer to the bottom with the decrease of the daylight [25]. The similar behaviour pattern of the picarel was also observed in other areas of the Mediterranean, e.g., Deudero et al. [16] in Balearics recorded low densities of picarel during the daytime and explained that by sampling gear characteristics (low height of beam trawl) which not allowed to fish picarel in a water column. Such diel variations of picarel and other species are probably related to the possibility of finding food and predator avoidance. Picarel is a benthopelagic species that feed mainly on zooplankton [26] but it is also preyed on by larger demersal and benthopelagic fish species (e.g., Conger conger, Merluccius merluccius, Scorpaena scrofa, Uranoscopus scaber, Zeus faber) [27] that are found in the same area. That means picarel plays a crucial role in the flux of energy from low to high trophic levels of the Eastern Adriatic benthic and pelagic food which is in agreement with the results obtained for the North Aegean Sea [26].
The list of fish species from this study is similar to the results of other studies focusing on infralittoral fish in the Mediterranean [16,28,29] and the Adriatic Sea [30,31]. Certain variations can be attributed to different sampling techniques, e.g., visual census study will record more cryptic and small species [13,15,18,31], while on the other hand using trawl allows recording of large and fast-swimming fish and species buried in mud and sand. Hence, depth also plays an important role [28] and the restriction of this study is related to not recording species inhabiting the first 20 m of depth, such as some small shallow water species from families Gobiidae and Bleniidae [13,15,18,31]. Nevertheless, in all studies, the fish community was characterized predominately by the species belonging to the families Sparidae, Labridae, Serranidae and Pomacentridae. Interestingly, the list of species is similar to the fish community of the single coralligenous habitat of the Eastern Adriatic, although more species (76), particularly small and cryptic, were recorded then due to the use of two different underwater research techniques that used divers for sampling [31]. Considering that this study was not performed on the coralligenous habitat while, on the other hand, the study on fish community of coralligenous habitat was completed at a similar depth (from 9 to 60 m) as this study, that suggests that many fish species inhabiting the infralittoral zone are generalist species that can prefer one habitat but can also use any other if in a certain period of their life (or season), such habitat can provide them with food supply and/or shelter from predation.
The results from this study demonstrate that the infralittoral fish community in the Eastern Central Adriatic undergoes both diel and seasonal variations in abundance, diversity and species composition. These distinctive diel and seasonal variations in fish assemblages are known to be directly or indirectly related to diel changes in foraging activity and predator avoidance [1,13]. However, the results of this study also showed that the differences in fish assemblage were more strongly influenced by seasonal variations than by diel variations. Seasonal fluctuations in species abundance are closely associated with life history characteristics, mostly spawning and recruitment, and also with seasonal food availability. Hence, seasonal variations are also affected by physical factors. Salinity is not considered an important factor as it varies a little in the investigated area. Thus, temperature is considered to be the most important factor causing seasonal variations. Obtained results revealed significant differences in fish assemblage between seasons with the highest difference in temperature, e.g., winter and spring, winter and summer and summer and autumn, while between seasons that were characterized by a low difference in temperature, the variations were marginal or not observed. The significance of water temperature as the environmental driver has been described in many species of fish, particularly those from coastal zones [32]. Water temperature is considered as a main determinant of the distribution and behaviour of organisms because it has a direct impact on the physiological performance of fish [33]. Consequently, water temperature intensely impacts the presence or absence of fish species in certain areas, because, for example, fish with warmer water affinity used shallower waters when water temperature increased in summer, and shifted to deeper, warmer areas when shallow waters cooled during winter, and vice versa.
On the other hand, diel variations are affected by diurnal-nocturnal activity patterns. Many infralittoral species of fish exhibit activity rhythms that cause changes in abundance between day and night in coastal areas, as detected by different sampling systems and methodologies [34]. Diurnal and nocturnal activity is often described as a product of fish behavioural response to solar irradiance variations. Hence, diel dynamics of marine fish also reflect habitat use and activity patterns, which are species-specific as well as size-specific, and mostly attributed to the variation in feeding behaviour and to predator avoidance [13,35]. However, what must be taken into account during the analysis of diel variations is a certain restriction that can be attributed to daytime fishing gear evasion. Considering the sampling depth investigated in this study it is obvious that no fishing gear can sample from the surface to the bottom. Thus, fishing gear evasion should be considered also as one of the factors producing observed diel variations.
Our study revealed the presence of a very rich and diverse infralittoral fish community in the coastal zone of the Eastern Central Adriatic that undertakes seasonal variations in abundance, diversity and species composition with the water temperature as the primary environmental factor that drives fish community structure. Considering that in recent decades we have been witness to climate change that affects the physical and chemical properties of the ocean, particularly the water temperature that has increased on a global scale, as well as in the Adriatic, we can presume that climate change will lead to shifts in species communities across the Adriatic, particularly in its coastal zone [36]. Many of the species observed in this study are important commercial species in the Adriatic, especially for small-scale fisheries which is a highly dominant fishery category in the Eastern Adriatic where small-scale coastal fishing boats, less than 12 m in length, make up 95.7% of the fleet. [37,38]. Therefore, it can be presumed that future climate-driven changes in the biophysical characteristics of the marine environment and frequent occurrence of extreme events will lead to extensive fluctuations in opportunities for commercial fisheries, particularly small-scale. Seasonality in catches, which is one of the main characteristics of small-scale fisheries [39], now appears to be a significant problem, as climate change would affect seasonality of the target species which, consequently, can have serious implications for the sustainability of small-scale fisheries and its management. To preserve the economic value of a fishery and to manage fisheries resources effectively under these new circumstances, fishery management should strengthen the links between the government and all stakeholders, including local coastal communities that are highly dependent on fisheries, small-scale in particular. Despite increasing awareness of the importance of healthy oceans for climate change mitigation most fisheries management legislation, approaches and tools are still very general and do not consider the effects of climate change. Furthermore, it can be expected that climate changes will have different effects in different areas, with some areas benefiting while others lose out. Thus, a greater participatory role of all stakeholders is essential in determining whether or not the potential negative impacts of climate change on fisheries can be mitigated and how. Only such adaptive and active fisheries management that integrates ecological, social and economic factors will ensure that the fisheries sector will survive and continue to provide economic benefits to coastal communities.
In conclusion, future studies should continue to monitor changes in fish communities and evaluate the effects of water temperature, particularly on commercially important fish stocks, thus providing climate-informed reference points. Although this may be challenging in terms of funding and capacity given that many potential factors affecting species in combination with water temperature changes are mostly unknown (persistence of species with limited migratory capacity, level of evolutionary adaptability to changing conditions, inflow and impact of a new warm-affinity species, etc.), it is essential for effective and climate-adaptive management that needs to be tailored accordingly and in due time.
Author Contributions
Conceptualization, A.S.; methodology, A.S. and D.P.; software, D.P.; validation, A.S. and D.P.; formal analysis, A.S. and D.P.; investigation, A.S.; resources, A.S.; data curation, A.S.; writing—original draft preparation, A.S.; writing—review and editing, A.S. and D.P.; visualization, A.S. and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as the authors were only analysing the existing catch.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pessanha, A.L.M.; Araújo, F.G.; De Azevedo, M.C.C.; Gomes, I.D. Diel and seasonal changes in the distribution of fish on a southeast Brazil sandy beach. Mar. Biol. 2003, 143, 1047–1055. [Google Scholar] [CrossRef]
- Helm, B.; Ben-Shlomo, R.; Sheriff, M.J.; Hut, R.A.; Foster, R.; Barnes, B.M.; Dominoni, D. Annual rhythms that underlie phenology: Biological time-keeping meets environmental change. Proc. R. Soc. B 2013, 280, 20130016. [Google Scholar] [CrossRef] [PubMed]
- Kronfeld-Schor, N.; Bloch, G.; Schwartz, W.J. Animal clocks: When science meets nature. Proc. R. Soc. B 2013, 280, 20131354. [Google Scholar] [CrossRef] [PubMed]
- Mittelbach, G.G.; Ballew, N.G.; Kjelvik, M.K. Fish behavioral types and their ecological consequences. Can. J. Fish. Aquat. Sci. 2014, 71, 927–944. [Google Scholar] [CrossRef]
- Castillo-Rivera, M.; Zárate-Hernández, R.; Ortiz-Burgos, S.; Zavala-Hurtado, J. Diel and seasonal variability in the fish community structure of a mud-bottom estuarine habitat in the Gulf of Mexico. Mar. Ecol. 2010, 31, 633–642. [Google Scholar] [CrossRef]
- Francescangeli, M.; Sbragaglia, V.; Trullols, E.; Antonijuan, J.; Massana, I.; Prat, J.; Nogueras Cervera, M.; Mihai Toma, D.; Aguzzi, J. Long-Term Monitoring of Diel and Seasonal Rhythm of Dentex dentex at an Artificial Reef. Front. Mar. Sci. 2022, 9, 837216. [Google Scholar] [CrossRef]
- Lloret-Lloret, E.; Navarro, J.; Giménez, J.; López, N.; Pennino, M.G.; Coll, M. The Seasonal Distribution of a Highly Commercial Fish Is Related to Ontogenetic Changes in Its Feeding Strategy. Front. Mar. Sci. 2020, 7, 566686. [Google Scholar] [CrossRef]
- Farrugio, H.; Soldo, A. Adriatic Sea: Status and Conservation of Fisheries; UNEP-MAP-RAC/SPA: Tunis, Tunisia, 2012; p. 58. [Google Scholar]
- Dulčić, J.; Soldo, A.; Jardas, I. Adriatic Fish Biodiversity and Review Of Bibliography Related to Croatian Small-Scale Coastal Fisheries; AdriaMed Technical Documents No. 15; AdriaMed: Termoli, Italy, 2005; pp. 103–125. [Google Scholar]
- Lipizer, M.; Partescano, E.; Rabitti, A.; Giorgetti, A.; Crise, A. Qualified temperature, salinity and dissolved oxygen climatologies in a changing Adriatic Sea. Ocean Sci. 2014, 10, 771–797. [Google Scholar] [CrossRef]
- Lipej, L.; Kovačić, M.; Dulčić, J. An Analysis of Adriatic Ichthyofauna—Ecology, Zoogeography, and Conservation Status. Fishes 2022, 7, 58. [Google Scholar] [CrossRef]
- Kovačić, M.; Lipej, L.; Dulčić, J. Evidence approach to checklists: Critical revision of the checklist of the Adriatic Sea fishes. Zootaxa 2020, 4767, 1–55. [Google Scholar] [CrossRef]
- Azzurro, E.; Pais, A.; Consoli, P.; Andaloro, F. Evaluating day-night changes in shallow Mediterranean rocky reef fish assemblages by visual census. Mar. Biol. 2007, 151, 2245–2253. [Google Scholar] [CrossRef]
- Dulčić, J.; Kraljević, M.; Grbec, B.; Pallaoro, A. Composition and temporal fluctuations of inshore juvenile fish populations in the Kornati Archipelago, eastern middle Adriatic. Mar. Biol. 1997, 129, 267–277. [Google Scholar] [CrossRef]
- Azzurro, E.; Aguzzi, J.; Maynou, F.; Chiesa, J.J.; Savini, D. Diel rhythms in shallow Mediterranean rocky-reef fishes: A chronobiological approach with the help of trained volunteers. J. Mar. Biol. Ass. UK 2013, 932, 461–470. [Google Scholar] [CrossRef]
- Deudero, S.; Morey, G.; Frau, A.; Moranta, J.; Moreno, I. Temporal trends of littoral fishes at deep Posidonia oceanica seagrass meadows in a temperate coastal zone. J. Mar. Syst. 2008, 70, 82–195. [Google Scholar] [CrossRef]
- Condal, F.; Aguzzi, J.; Sardà, F.; Nogueras, M.; Cadena, J.; Costa, C.; Del Río, J.; Mànuel, A. Seasonal rhythm in a Mediterranean coastal fish community as monitored by a cabled observatory. Mar. Biol. 2012, 159, 2809–2817. [Google Scholar] [CrossRef]
- Glavičić, I.; Kovačić, M.; Soldo, A.; Schliewen, U. A quantitative assessment of the diel influence on the cryptobenthic fish assemblage of the shallow Mediterranean infralittoral zone. Sci. Mar. 2020, 84, 49–57. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalogue of Fishes: Genera, Species, References. 2022. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 1 October 2023).
- Van der Laan, R.; Fricke, R.; Eschmeyer, W.N. (Eds.) Eschmeyer’s Catalogue of Fishes: Classification. 2022. Available online: http://www.calacademy.org/scientists/catalog-of-fishes-classification/ (accessed on 1 October 2023).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, J. Vegan: Ecological diversity. R Proj. 2013, 368, 1–11. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Similarity-based testing for community pattern: The two-way layout with no replication. Mar. Biol. 1994, 118, 167–176. [Google Scholar] [CrossRef]
- Cetinić, P.; Jardas, I.; Dulčić, J.; Pallaoro, A.; Kraljević, M.; Soldo, A. The effects of the beach seine “migavica” on coastal fish communities. Fol. Univ. Agric. Stetin. 1999, 192, 25–35. [Google Scholar]
- Karachle, P.K.; Stergiou, K.I. Diet and feeding habits of Spicara maena and S. smaris (Pisces, Osteichthyes, Centracanthidae) in the North Aegean Sea. Acta Adriat. 2014, 55, 75–84. [Google Scholar]
- Dulčić, J.; Pallaoro, A.; Cetinić, P.; Kraljević, M.; Soldo, A.; Jardas, I. Age, growth and mortality of picarel, Spicara smaris L. (Pisces: Centracanthidae), from the Eastern Adriatic (Croatian coast). J. Appl. Ichthyol. 2003, 19, 10–14. [Google Scholar] [CrossRef]
- Tunesi, L.; Molinari, A.; Salvati, E.; Mori, M. Depth and substrate type driven patterns in the infralittoral fish assemblage of the NW Mediterranean Sea. Cybium 2006, 30, 151–159. [Google Scholar]
- La Mesa, G.; Molinari, A.; Tunesi, L. Coastal fish assemblage characterisation to support the zoning of a new Marine Protected Area in north-western Mediterranean. Ital. J. Zool. 2010, 77, 197–210. [Google Scholar] [CrossRef]
- Lipej, L.; Bonaca, M.O.; Šiško, M. Coastal fish diversity in three marine protected areas and one unprotected area in the Gulf of Trieste (Northern Adriatic). Mar. Ecol. 2003, 24, 259–273. [Google Scholar] [CrossRef]
- Soldo, A.; Glavičić, I.; Kovačić, M. Combining Methods to Better Estimate Total Fish Richness on Temperate Reefs: The Case of a Mediterranean Coralligenous Cliff. J. Mar. Sci. Eng. 2021, 9, 670. [Google Scholar] [CrossRef]
- Van Der Walt, K.A.; Porri, F.; Potts, W.M.; Duncan, M.I.; James, N.C. Thermal tolerance, safety margins and vulnerability of coastal species: Projected impact of climate change induced cold water variability in a temperate African region. Mar. Environ. Res. 2021, 169, 105346. [Google Scholar] [CrossRef]
- Waldock, C.; Stuart-Smith, R.D.; Edgar, G.J.; Bird, T.J.; Bates, A.E. The shape of abundance distributions across temperature gradients in reef fishes. Ecol. Lett. 2019, 22, 685–696. [Google Scholar] [CrossRef]
- Schalm, G.; Bruns, K.; Drachenberg, N.; Geyer, N.; Foulkes, N.S.; Bertolucci, C.; Gerlach, G. Finding Nemo’s clock reveals switch from nocturnal to diurnal activity. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Arrington, D.A.; Winemiller, K.O. Diel changeover in sandbank fish assemblages in a neotropical floodplain river. J. Fish. Biol. 2003, 63, 442–459. [Google Scholar] [CrossRef]
- Rutterford, L.A.; Simpson, S.D.; Bogstad, B.; Devine, J.A.; Genner, M.J. Sea temperature is the primary driver of recent and predicted fish community structure across Northeast Atlantic shelf seas. Glob. Chang. Biol. 2023, 29, 2510–2521. [Google Scholar] [CrossRef] [PubMed]
- Cetinić, P.; Soldo, A. Some basic characteristics of Croatian Marine Fisheries and its regulation. Acta Adriat. 1999, 40, 91–97. [Google Scholar]
- Soldo, A.; Bosnić, N. Characteristics of the Croatian anchovy purse seiner fleet. Acta Adriat. 2019, 60, 79–86. [Google Scholar] [CrossRef]
- Dulčić, J.; Soldo, A.; Jardas, I. Small-scale Fisheries in Croatia. AdriaMed Tech. Doc. 2005, 15, 22–32. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).