Abstract
Suitable colonization materials are a pursued target in marine restoration programs. Known for making nutrients available while reducing pollutants and the risk of pathogens in terrestrial ecosystems, Biochar and Bioferment materials of organic origin were tested during a two-year experiment. We tested the efficacy of these materials for restoration purposes through experimental concrete tiles treated with Biochar (B) and Bioferment (F) and tiles made of concrete, which were used as controls (Ct) for the colonization of marine organisms in the marine protected area of Porto Cesareo, Southern Italy (20 m depth). Tiles were monitored for photographs from October 2019 to September 2021. Initially, Biochar treatment presented a higher percentage of total benthic cover (81.23 ± 2.76, median ± SE), differing from Bioferment treatment and control tiles (45.65 ± 5.43 and 47.95 ± 3.69, respectively). Significant interaction between treatments and times suggests changes in community structure related to Polychaeta cover increase in Bioferment and control materials from the second monitoring time. Furthermore, the underwater instability of Bioferment on the tiles could explain the similarity with control tiles in marine organisms’ covers. Hence, Biochar is shown to be a material with optimal stability in seawater, demonstrating greater capacity for marine organisms’ colonization in less time compared to the other two materials.
1. Introduction
With around 40% of the world’s population living in coastal zones, marine ecosystems are responsible for providing environmental, cultural, and economic goods and services that are essential for human survival [1]. Nevertheless, the indiscriminate and increasing use of natural resources, together with the increase in human population density, affects coastal and marine ecosystems with the loss of habitats and species richness [2,3]. Cumulative impacts require urgent and clear responses based on a solid understanding of the mechanisms affecting the functioning of key marine ecosystems and reliable restoration programs. Ongoing transformations and shifts are so complex and synergistic that they may not be perceived as dramatic changes but as part of our natural adaptation to current social and economic needs [4].
The Mediterranean Sea is one of the most affected by different stressors, endangered by pollution and overfishing, and also heated faster than other areas of the planet [5,6]. Porto Cesareo is a multiple-use marine protected area (MPA) of the Mediterranean Sea, which includes areas of relevant conservation value, but the enhancement of human activities has led to the degradation of the quality of the environment [7]. Thus, the improvement of tools that allow the restoration of marine and coastal ecosystems in these areas needs to be addressed to recover the functionality of the systems.
New concepts of restoration, going beyond the already well-structured know-how that we have gathered during the last decades [8,9], have to be applied to effective scaling-up with wider integration and participation. Artificial structures or artificial reefs can be employed as a tool for the restoration of marine ecosystems, seeking to mimic natural hard substrates and creating underwater gardens covered with typical species found within confined environments, such as filter feeder Polychaetas, sponges, and ascidians [4,10]. Still, the interest in artificial reefs also leads to the possible negative impacts of using unsuitable materials [10]. Increasing the biodiversity and complexity of ecosystem functioning through active hard corals, macroalgae, sponges, and gorgonian gardening may be a good solution, but we still lack clear information about the optimal conditions in terms of materials to make a reasonable and sustainable upscaling [4].
In general, it is assumed that the type of material will determine the development of the first steps of the benthic community [11]. In this sense, the selection of materials for artificial structures for marine ecosystem restoration must involve environmental and ethical considerations [12], as well as ecological requirements of the biological community, alongside economic, logistic, and engineering factors [13]. Although guidelines have been developed to support the placement of artificial reefs in European seas [10], further studies are needed to evaluate the specificity of material needs for each locality to be restored.
Some concrete processing techniques aimed at mitigating impacts have been tested using different mixtures, whether for cost reduction [14], for mitigation of heavy metals in the reuse of waste materials [15], mixed with biogenic material [16], in adapting concrete to promote the growth of marine organisms [17,18], or in limiting the life cycle of the structures [19].
Biochar is a material that valorizes biomass residues (i.e., a solid product of biomass pyrolysis) while having environmental benefits, such as climate change mitigation for its potential for carbon sequestration and the reduction of nutrient leaching [20]. Biochar is commonly used as a bioremediatory component in agricultural soils and recently as an admixture to cement, used in construction with excellent mechanical, electrical, thermal, and chemical stability [21,22]. Furthermore, Biochar is a carbon-rich and porous solid material that can be produced through the thermochemical conversion of biomass (pyrolysis, hydrothermal carbonization, gasification, and torrefaction) in the presence of little or no oxygen. Its unique qualities, such as large surface area, calorific value, hydrophobicity, high porosity, high cation exchange capacity, and stability, help in the mitigation of various environmental impacts such as soil amendment, remediation of environmental contaminants, and wastewater, as well as contributing to the sequestration or immobilization of carbon, among others. For this reason, Biochar has received more attention in environmental matters [23,24].
This material has been applied mainly in terrestrial ecosystem remediation, immobilizing solidified radioactive waste when submerged [21], immobilizing heavy metals for soil remediation [25], and in construction for absorbing electromagnetic waves [26]. Few studies have evaluated the performance of Biochar material on marine sediment remediation [27,28,29,30]. However, Biochar has not been tested on the colonization of marine organisms, particularly with restoration purposes during the early stages of succession, which can reflect the ecosystem dynamics and anticipated changes over time [31], and due to the interest in the velocity of the restoration action on the degraded ecosystem.
On the other hand, it is known that microorganisms in ferments can help with the degradation process of solid organic waste [32], as well as the degradation of pollutants in terrestrial and marine environments [33,34]. The process implies three principal benefits: (i) the element’s decomposition of the organic matter used for fermentation, turning them into available nutrients; (ii) the remediation of the substrate; and (iii) the reduction of pathogenic microbiota and phytotoxicity by the action of some bacteria in ferments [32]. In addition, animal manure, among which cow manure, makes available large amounts of solid organic waste due to a great demand for livestock production that provides food for the human population, working as raw material to produce Bioferments [32]. Thus, Bioferment production from cow manure could assist for several purposes, from recycling organic solid waste [35] to bioremediation through the disintegration of hydrocarbon and plastic pollutants [32,33] to the production of bio-hydrogen [36]. Such material may be interesting in terms of testing for the first stages of colonization, boosting the surface microbiota, and facilitating its settlement.
In this study, we hypothesize that the qualities of Biochar and Bioferment materials may assist marine organisms in the first steps of colonization in terms of diversity and abundance, as they should make available nutrients through the decomposition process by bacteria while degrading the possible toxicities of materials used for artificial structures such as concrete and also reducing the risk of pathogens for marine organisms. Different materials might influence the structure of marine organisms colonizing, and to test it, we assessed benthic community development on Biochar and Bioferment materials, compared with concrete material as a control, with restoration purposes in Porto Cesareo.
2. Materials and Methods
2.1. Study Area
The Porto Cesareo is an MPA established in 1997 (40°15′ N–18°53′ E, Figure 1). It is located along the Ionian Sea coast (Apulia, Southern Italy), between Torre Colimena and Torre dell’Inserraglio, facing the village of Porto Cesareo. It extends for about 18,000 m along the Porto Cesareo coastline and 6000 m along the Nardò coastline, occupying 17,156 ha at sea [37]. The area has a moderate human impact due to a relevant increase in the human population since 1991 and to the high tourist pressure [7].
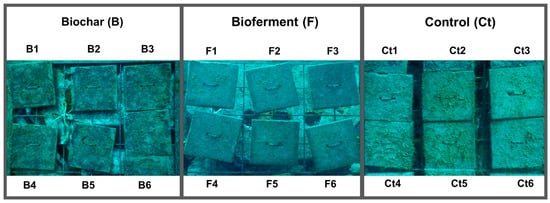
Figure 1.
Experimental design with treatments of different materials at the first monitoring time. Codes correspond to replicates for each material type.
The substrate of the study area is mainly composed of coarse sand, although it has coralligenous formations with algal dominance [7,38]. The temperature ranges from 15 to 25 °C (maximum peaks of 30 on the surface in the summer), with a peak of high temperatures between May and July and lower temperatures between December and March [39]. Although a marine protected area, the study area is dominated by colonizer organisms with low meiobenthic taxa richness (Copepods and Annelids), which indicates an immature stage of the community, commonly due to intervened or impacted ecosystems [7,40].
2.2. Experimental Design and Construction
The construction of the tiles for the Biochar treatment involved mixing biochar with commercial cement (Portland cement) and sand at a ratio of 1:1:4. The composition was mixed, blended, and controlled at 25° for 3 days, then molded and dried. The Bioferment treatment consisted of a liquid mixture, resulting in a process of decomposition and fermentation in the absence of oxygen (anaerobic) from organic vegetable and animal residues (straw and crop residues). Contains nutrients of high nutritional value (ammonia, nitrogen, hormones, vitamins, and amino acids). Its production is a relatively simple and low-cost process, as the preparation inputs are local, although its elaboration takes a period of two to three months. It was applied in layers after the activation of the Bioferment mixture, which consists of mixing the Bioferment liquid for 30 min and applying it to concrete tiles before their submersion.
We tested the efficacy of different material types for the colonization of marine organisms. Experimental tiles of 20 cm × 20 cm, consisting of Biochar treatment (identified as B), Bioferment treatment (identified as F), and control tiles (identified as Ct), were submerged for macrofauna colonization. Six replicates (six tiles) of each material, from now on mentioned as treatments and control tiles, were deployed attached to a frame in an artificial reef (Figure 1) located in Zone A of the integral reserve (zone A corresponds to the maritime space parallel to the coast between the geographic points 40°14′39.59″ N, 17°53′26.41″ E, and 40°14′8.99″ N, 17°54′13.21″ E). On vertical surfaces, abiotic factors may be more important for structuring community settlement [41]. Hence, all tiles were deployed in similar conditions: at 20 m depth, in vertical orientation, and at the same angle to the currents. The tiles of each treatment were deployed close to each other to facilitate identification during sampling.
2.3. Data Collection and Processing
Tiles were monitored through underwater photographs from October 2019 in a 4–5 month period (February 2020, July 2020), except for the last sampling (September 2021), which was 14 months after due to COVID-19. The photographs were taken by expert divers, taking into account the same distance and angle for each sampling photograph. Control tiles were not photographed during the second monitoring time due to logistic issues. To measure the percentage cover of major taxon groups, the images of colonized tiles were subdivided into 16 equal squares, and four subdivisions were randomly selected for each sampling time. Two tiles of the treatment of Bioferment (F) were lost at the second time of monitoring. A total of 240 replicates of photographs of colonized tiles were analyzed using the software Photoquad v1_4 [42]. The measure of benthic groups covered in subdivisions was made using a layer-based analysis with a grid of 144 cells of 12 pixels, combined with a multiscale image segmentation with visual correction (Figure S1).
The main benthic groups, including macroinvertebrates and macroalgae, were analyzed in terms of the percentage of cover for each material among the four monitoring times. Less frequent benthic groups were summarized as “other groups” and included members of Porifera, Cnidaria, and other colonial species that were not possible to visually identify from images (Figure S2).
2.4. Data Analysis
To test differences in the percentage of cover and Shannon diversity index values of major benthic groups among the materials, monitoring times, and their interaction, univariate and multivariate permuted analyses of variance (PERMANOVA) of 999 permutations with Euclidean and Bray Curtis distance matrices, respectively, were performed using the function Adonis2 from package vegan. Factors included in the analysis were treatments of materials and control with 3 levels of variation (B, F, and Ct) and monitoring times with four levels of variation at the 4th, 8th, 13th, and 27th months of colonization time (T1, T2, T3, and T4 monitoring times). To assess significant differences from the PERMANOVA test, the heterogeneity of data dispersion was tested on major taxon cover for each factor using the betadisper function from the vegan package and pairwise comparisons using the mvpaircomp function from the biotools package. The Shannon diversity index was estimated from the mean percentage cover of benthic groups for each treatment and time of monitoring. Diversity values were also analyzed through a glm to detect differences between treatments and times of monitoring. All analyses were performed using RStudio 2023.03.0+386 software.
3. Results
Several taxon groups were recognized, including assemblages of microbes and algae (turf) and Polychaeta (mainly Sabellidae) between the dominant benthic groups in tiles, followed by green algae (Acetabularia sp.) and brown algae (Padina sp.), and less frequent groups such as sponges, ascidians, mollusks, cnidarians, and other colonial species that were not possible to identify with the pictures, gathered in “other groups”. In total, four main benthic groups remained for data analysis (i.e., turf, Polychaeta, algae, and other groups).
Polychaeta and turf groups had a marked presence in all treatments and control tiles throughout the study period. However, treatment tiles varied qualitatively from simpler to more complex surface tiles, especially in Biochar treatment during the 13th and 27th months of colonization, compared with the rest of the tiles (Figure S1). Green algae were present during the first and last periods of colonization.
The multivariate PERMANOVA test on the benthic groups covered showed significant differences among material treatments, monitoring times, and their interactions (Table 1). The factor time and the interaction treatment and time had the major variation component, explaining the 35 and 12 percent variation in the analysis, respectively (Table 1). Suggesting a temporal variation of benthic group cover according to material types.

Table 1.
Multivariate PERMANOVA on the cover of all main benthic groups.
However, 48% of the variation was not explained by the experiment, and the data of both treatment and time factors are significantly heterogeneous regarding the dispersion of the data (p < 0.05). A principal component analysis (PCA) shows the heterogeneous dispersion of the data among the materials and times (Figure S2). Therefore, significant differences in PERMANOVA tests must be taken with caution. All treatments overlap among them; however, Biochar treatment data is grouped in the center of the graphic, while Bioferment treatment and control data are scattered and peripherally distributed (Figure S2A,B). In the same line, we can see the dispersion of the monitoring time data, showing T1 tending to the left of the graphic and T2, T3, and T4 overlapping to the right, suggesting that there is a clear temporal variation of benthic colonization (Figure S2C,D), which could be more related to a local environmental variation, such as temperature, seasonality, etc.).
Treatment differences are given by the Biochar treatment, which differed from the Bioferment treatment and control tiles in terms of total benthic cover throughout the study period (p < 0.05, Figure 2). Furthermore, Biochar treatment presented the highest total benthic cover during the first period (81.23 ± 2.76, median ± SE) (Figure 2), while Bioferment and control tiles were colonized at 45.65 ± 5.43 and 47.95 ± 3.69 percent during the same period, respectively.
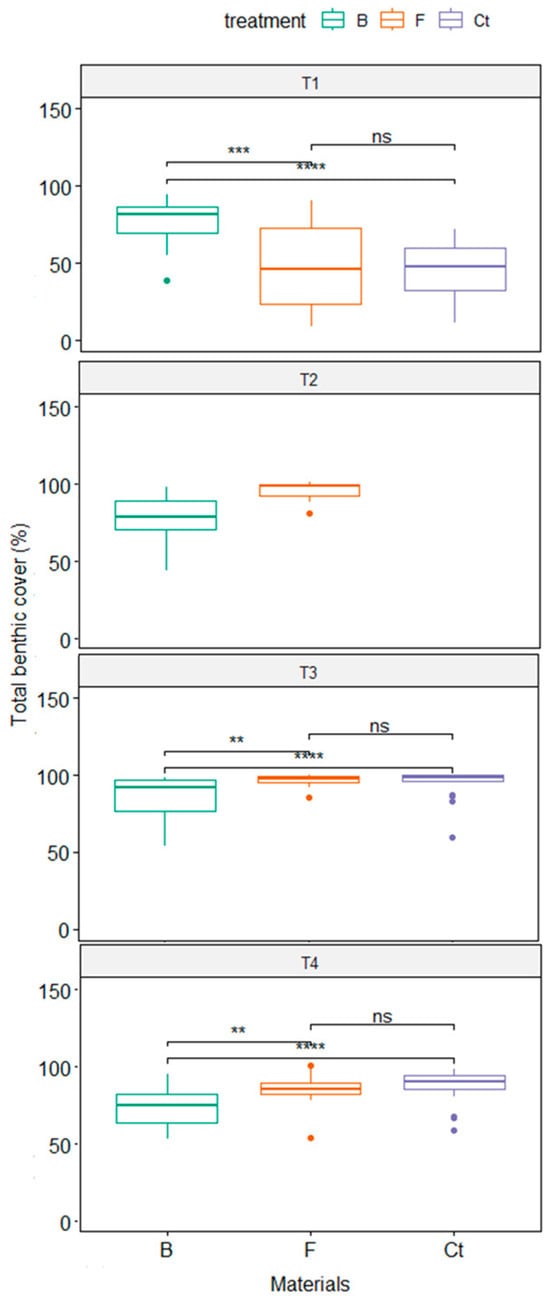
Figure 2.
Total coverage of the main groups of benthics among the material treatments. B = Biochart, F = Bioferment, and Ct = concrete as a control; T1 = fourth month, T2 = eighth month, T3 = third month, and T4 = 27th month. Pairwise T-test results legend: ns = no significant differences, ** p < 0.05, *** p < 0.005, and **** p < 0.0005.
However, the total benthic cover increased afterwards for Bioferment treatment and control tiles and maintained higher levels during the rest of the study. These materials showed the same pattern, as there is no evidence of differences between Bioferment treatment and control tiles in terms of total benthic cover (p > 0.05, Figure 2).
Besides this, the significant interaction between treatments and time suggests changes in community structure related to certain materials depending on the colonization time. This can be observed in Bioferment treatment and control tiles, which vary from a low cover in the first monitoring time to higher coverage of benthic organisms in the rest of the study and show a similar pattern of colonization, which has the Polychaeta group as a dominant component of the community structure after the first period of colonization from there on (Figure 3). In contrast, Biochar treatment showed a rapid settlement of benthic organisms with high coverage, in which turf and Polychaeta were both important components of the species composition of tiles throughout the study period (Figure 3).
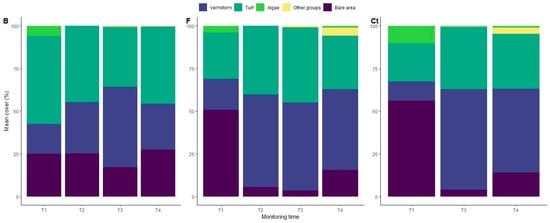
Figure 3.
Temporal variation of benthic group cover among treatments.
Moreover, univariate analyses of each benthic group showed significant variation among treatments, time, and their interactions. However, the main source of variation was related mostly to the time in all cases (p < 0.05), which could be associated with temporal variation due to changes in local environmental conditions.
Furthermore, the Shannon diversity index of the main benthic groups in Biochar treatment differed from other treatments when pairwise comparisons were made, as well as the third time of monitoring differed from the other times of monitoring (Table S1, Figure 4). During the first and last periods of colonization, diversity values were higher, whereas they were lower during the second and third periods of colonization for almost all the treatments. Thus, the diversity of the main benthic groups shows a U-shaped trend, which was less marked for Biochar treatment (Figure 4).
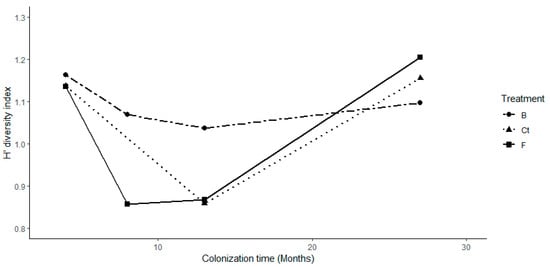
Figure 4.
Temporal variation of the Shannon diversity index of the main benthic groups among treatments.
4. Discussion
The assessment of successional stages is necessary to understand long-term ecological dynamics, predict changes in the ecological community, and assess the benefit of restoration programs instead of applying a static evaluation that could mask a failed restoration [43]. Furthermore, the monitoring of sessile or sedentary benthic organisms in hard bottoms brings more possibilities for study and reveals changes in the community structure of benthic habitats, as its stability permits observation of the development of colonization and spatial heterogeneity compared to water columns and soft bottoms [44]. Sessile organisms are more susceptible to impacts because of their low capacity to escape, making them more accessible to monitor marine environmental changes [44].
In the present study, the observations of the benthic community on tiles showed significant differences between materials and periods considered. Biochar presented a significantly higher benthic cover than the Bioferment treatment and control tiles, while this coverage rate was reversed over time, with the two last treatments not significantly different throughout the study period. In this sense, Biochar differences were maintained throughout the study period.
The succession of benthic organisms in Bioferment treatment and control tiles was given through a shift in taxon group cover at the eighth month (second period of colonization), with a rapid increase in Polychaeta group cover remaining the dominant group in both treatments until the final period of colonization. For Bioferment treatment, Polychaeta cover increased 4.6 times in four months, from 10.54 ± 0.63 (median ± standard error % cover) for the first period of colonization to 48.4 ± 1.13% and 49.68 ± 0.65% for the second and third periods, respectively, and to 43.82 ± 0.85% for the fourth period. Similarly, in control tiles, the Polychaeta cover increased more than seven times in 4 months, changing from 7.98 ± 0.44% to 58.16 ± 0.48% for the third period (control tiles were not sampled during the second period, T2) and to 51.01 ± 0.49%. In this sense, Bioferment treatment and control tiles presented a higher total benthic cover at the end of the study compared with Biochar treatment.
Conversely, Biochar treatment did not increase the total benthic cover during the second period of colonization, nor did experiments with the shift of taxon group cover. Although Polychaeta cover increased for all tiles, it did not surpass the turf community cover in the case of Biochar treatment. In this sense, while the Biochar treatment did not achieve a higher total benthic cover in the final period (T4), this treatment displayed a buffer effect on taxon group cover shifts through the successional process. Following the Polychaeta group cover, it only increased two times in a 4-month period, from 16.3 ± 0.45% for the first period to 31.87 ± 0.57% and 41.39 ± 0.77% for the second and third periods, respectively, and decreasing to 22.35 ± 0.82% for the fourth period (Figure 3).
Biochar treatment also showed a steady trend in diversity index values, compared with Bioferment treatment and control tiles, as well as higher diversity indexes throughout the study period, apart from the last monitoring period (T4). Restoration ecology has been based conceptually on the succession process, as it suggests there is a pathway to a desired restoration stage [45], which occurs through the colonization of pioneer or opportunistic species until it achieves a steady state or climax. Hence, through the two years of colonization, Biochar treatment showed that it may be an accurate technology for underwater artificial substrates and an effective material for marine organisms’ colonization in a more stable way.
Other studies found that richness is usually similar between different colonization substrates at a determined locality; however, it could differ in terms of the abundance of species or functional groups depending on the material nature [13], agreeing with our study findings. In general, all the main techniques for coral restoration report similar average survival and growth rates, so decisions on what techniques to use should be based on local conditions, cost, availability of materials, and appropriateness based on stated objectives [46]. Generally, it is assumed that the type of material will determine the development of the benthic community [47].
Interestingly, the evident presence of the Polychaeta taxon group could be due to aquaculture sites of Sabella spallanzanii (Gmelin, 1971) near the study area, which are reported to have better conditions to grow in depths around 15 m [48], which is like the depth implemented in our study (20 m). The consistency of differences in benthic taxon cover by treatments, however, shows a clear effect of materials on benthic marine organisms’ colonization. In turn, the colonization of “other” taxon groups appeared in the third period (27 months after submerging the tiles) in all treatments, accompanied by the algae taxon group only in the cases of Biochar treatment and control tiles.
Qualitatively, as previously mentioned, the tiles of the Biochar treatment showed more structurally complex surfaces during the last colonization period of 18 and 27 months (T3 and T4) compared with the first periods of four and eight months (T1 and T2) (Figure S1). In this sense, researching the mechanisms behind the Biochar material used as a complement in concrete structures for the colonization of marine organisms would help to understand how the apparent increase in structural complexity occurs (e.g., to know if this material additive may offer greater surface availability for colonization or make a difference in other factors such as shelter, shade, habitat, and food availability compared to other materials). This way, we could better understand how to improve biodiversity and biomass loss in degraded ecosystems while assisting marine restoration [4,49].
Also, differences detected related to materials would not be due to differences in installation conditions, as the proximity of the tiles guarantees that they had the same environmental conditions as temperature, salinity, depth, light availability, vertical orientation, and disposition facing the same direction to the current.
Biochar can sequester CO2 [20]. In addition, this material showed a more stable response than other tested materials, such as Bioferment (according to the author’s observation in the field). This could be related to the physical-chemical stability of the material [20,23,24,50], which is an important aspect for the restoration of marine ecosystems as hard materials increase coral transplant survival [51]. Materials must be durable during submersion and removable from the sea bottom, as required by some legislation in European countries (i.e., Spain or Italy).
In addition, Bioferments have several uses, from mitigators in oil spills [33], bio-hydrogen production [36], to plastic degradation [52]. Along the same lines, composting is an effective method of recycling organic solid waste, and it is the key process linking planting with recycling [35]. However, Bioferment material in the form of a liquid layer was not successful as the material dissolved in seawater (personal observations during the first monitoring time) and did not contribute to a significant increase in benthic invertebrate cover, as there were no significant differences in the percentage of cover of benthic groups in Bioferment compared to control tiles in the present experiment.
We acknowledge that, although other approaches, which could include biochemical and physical studies of colonized surfaces, could provide more information to understand the interaction between materials and colonizing organisms, the approach we decided to use, through photographs, is widely used and has brought results that contribute to interesting findings, especially when it comes to first tests on new materials for marine restoration.
Moreover, it has recently been determined that hard artificial reefs for transplantation are more effective in a couple of years than those without transplantation and other structures, despite being submerged for more than 100 years [53,54]. Hard artificial structures are usually made of concrete due to the availability and strength of this material when submerged. Materials of organic origin are more difficult to use than hard materials because their nature tends to disintegrate, reducing the survival of marine invertebrates, as occurred with coral transplants [55]. In this sense, the development of new types of hard organic-originated materials for the creation of artificial reefs as support structures for other restoration actions such as transplants, nubbins, and sea gardening, among others, is essential. The next step is understanding the economic and energy/material potential constraints to make an efficient upscaling of artificial reefs for marine restoration with new materials such as the concrete–Biochar mixture.
5. Conclusions
In this study, Biochar treatment was suitable for having rapid colonization and creating a complex surface in tiles, developing a diverse species composition. Past experiences show that the mitigation of human impacts can allow the recovery of marine populations, habitats, and ecosystems. In this context, if multiple human pressures are mitigated (such as, for example, marine pollution and climate change), the health conditions of the oceans could improve by 2050 with such growing evidence about new restoration methods and materials [56].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse12010169/s1, Figure S1: PCA showing the heterogeneous dispersion of data of benthic invertebrate groups by treatments shown in points (A) and overlapped colored frames (B); and by monitoring times in points (C) and colored frames (D); Figure S2: Images of experimental tiles of each treatment during the study periods; Table S1: Glm modelling on diversity of benthic groups (model < -glm(Shannon ~ Treatment + Time, data = diversity_cover).
Author Contributions
Conceptualization, S.R. and M.G.-D.; methodology, S.R., N.L., X.V., M.G.-D., L.B., C.B.-V. and H.M.-C.; software, C.B.-V.; validation, S.R., H.M.-C. and C.B.-V.; formal analysis, C.B.-V.; investigation, S.R., N.L. and C.B.-V.; resources, S.R., N.L. and C.B.-V.; data curation, C.B.-V.; writing—original draft preparation, S.R. and C.B.-V.; writing—review and editing, S.R., H.M.-C. and C.B.-V.; visualization, S.R., N.L., X.V., M.G.-D., L.B., C.B.-V. and H.M.-C.; supervision, S.R. and H.M.-C.; project administration, S.R., M.G.-D. and L.B.; funding acquisition, S.R., M.G.-D. and C.B.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant numbers 88887.483420/2020-00 and 88887.758173/2022-00. This paper belongs to the project OCEAN CITIZEN (“Marine forest coastal restoration: an underwater gardening socio-ecological plan”, Horizon Europe HORIZON-MISS-2021-OCEAN-02-01, Contract Number 101093910). Additional support was provided by the EU Horizon Europe project No. 101036515, “OCEAN CITIZEN–Marine forest coastal restoration: an underwater gardening socio-ecological plan”.
Data Availability Statement
The data presented in this study is available under the prior consent of the authors in FigShare at https://figshare.com/s/2ca28c2ae88e0ebf03bb, accessed on 20 June 2023, doi:[10.6084/m9.figshare.24467968].
Acknowledgments
C.B.-V. thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship funding (No. 88887.483420/2020-00 and No. 88887.758173/2022-00). SR thanks the Conselho Nacional de Desenvolvimento Científico eTecnológico (Cnpq.Research Productivity Fellowship: 314460/2023-3). The authors also thank the director, Paolo D’Ambrosio, and all the staff of the MPA of Porto Cesareo for their availability and collaboration during data collection operations. The paper is supported by the BNP Paribas sponsorship of Underwater Gardens International. Livia Rossi, as an official English translator, corrected the grammar and vocabulary.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations. Factsheet: People and Oceans. In Proceedings of the Ocean Conference, New York, NY, USA, 5–9 June 2017; pp. 1–7. [Google Scholar]
- Rossi, S. The destruction of the ‘animal forests’ in the oceans: Towards an over-simplification of the benthic ecosystems. Ocean. Coast. Manag. 2013, 84, 77–85. [Google Scholar] [CrossRef]
- Portugal, A.B.; Carvalho, F.L.; de Macedo Carneiro, P.B.; Rossi, S.; de Oliveira Soares, M. Increased anthropogenic pressure decreases species richness in tropical intertidal reefs. Mar. Environ. Res. 2016, 120, 44–54. [Google Scholar] [CrossRef]
- Rossi, L.; Rizzo, S. Marine animal forests as Carbon immobilizers or why we should preserve these three-dimensional alive structures. In Perspectives on the Marine Animal Forests; Springer-Nature: Cham, Switzerland, 2020. [Google Scholar]
- Deudero, S.; Alomar, C. Mediterranean marine biodiversity under threat: Reviewing influence of marine litter on species. Mar. Pollut. Bull. 2015, 98, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future. First Mediterranean Assessment Report—MedECC. Available online: https://www.medecc.org/medecc-reports/climate-and-environmental-change-in-the-mediterranean-basin-current-situation-and-risks-for-the-future-1st-mediterranean-assessment-report/ (accessed on 19 October 2023).
- Semprucci, F.; Balsamo, M.; Appolloni, L.; Sandulli, R. Assessment of ecological quality status along the Apulian coasts (eastern Mediterranean Sea) based on meiobenthic and nematode assemblages. Mar. Biodivers. 2018, 48, 105–115. [Google Scholar] [CrossRef]
- Rinkevich, B. Ecological engineering approaches in coral reef restoration. ICES J. Mar. Sci. 2021, 78, 410–420. [Google Scholar] [CrossRef]
- Horoszowski-Fridman, Y.B.; Rinkevich, B. Restoration of the Animal Forests: Harnessing Silviculture Biodiversity Concepts for Coral Transplantation. In Marine Animal Forests; Springer: Cham, Switzerland, 2016; pp. 1313–1335. [Google Scholar] [CrossRef]
- Giangrande, A.; Gravina, M.F.; Rossi, S.; Longo, C.; Pierri, C. Aquaculture and Restoration: Perspectives from Mediterranean Sea Experiences. Water 2021, 13, 991. [Google Scholar] [CrossRef]
- Burt, J.; Bartholomew, A.; Usseglio, P.; Bauman, A.; Sale, P.F. Are artificial reefs surrogates of natural habitats for corals and fish in Dubai, United Arab Emirates? Coral Reefs 2009, 28, 663–675. [Google Scholar] [CrossRef]
- Leonard, C.; Hédouin, L.; Lacorne, M.C.; Dalle, J.; Lapinski, M.; Blanc, P.; Nugues, M.M. Performance of innovative materials as recruitment substrates for coral restoration. Restor. Ecol. 2021, 30, e13625. [Google Scholar] [CrossRef]
- Dodds, K.C.; Schaefer, N.; Bishop, M.J.; Nakagawa, S.; Brooks, P.R.; Knights, A.M.; Strain, E.M. Material type influences the abundance but not richness of colonising organisms on marine structures. J. Environ. Manag. 2022, 307, 114549. [Google Scholar] [CrossRef] [PubMed]
- Shu-Te, K.; Tsan-Chuan, H.; Kwang-Tsao, S. Experiences of Coal Ash Artificial Reefs in Taiwan. Chem. Ecol. 1995, 10, 233–247. [Google Scholar] [CrossRef]
- Collins, K.J.; Jensen, A.C.; Lockwood, A.P.M.; Turnpenny, A.W.H. Evaluation of stabilized coal-fired power station waste for artificial reef construction. Bull. Mar. Sci. 1994, 55, 1251–1262. [Google Scholar]
- Brown, L.A.; Furlong, J.N.; Brown, K.M.; La Peyre, M.K. Oyster reef restoration in the northern gulf of Mexico: Effect of artificial substrate and age on nekton and benthic macroinvertebrate assemblage use. Restor. Ecol. 2014, 22, 214–222. [Google Scholar] [CrossRef]
- Ortiz-Prosper, A.L.; Bowden-Kerby, A.; Ruiz, H.; Tirado, O.; Cabán, A.; Sanchez, G.; Crespo, J.C. Planting small massive corals on small artificial concrete reefs or dead coral heads. Bull. Mar. Sci. 2001, 69, 1047–1051. [Google Scholar]
- Ponti, M.; Fava, F.; Perlini, R.A.; Giovanardi, O.; Abbiati, M. Benthic assemblages on artificial reefs in the northwestern Adriatic Sea: Does structure type and age matter? Mar. Environ. Res. 2015, 104, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Carral, L.; Tarrío-Saavedra, J.; Barros, J.J.C.; Fabal, C.C.; Ramil, A.; Álvarez-Feal, C. Considerations on the programmed functional life (one generation) of a green artificial reef in terms of the sustainability of the modified ecosystem. Heliyon 2023, 9, e14978. [Google Scholar] [CrossRef] [PubMed]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Laili, Z.; Yasir, M.S.; Yusof, M.A.W. Leaching properties of Cs-134 from spent ion exchange resins solidified in cement-biochar matrix. AIP Conf. Proc. 2016, 1784, 040011. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J.; Feo, L.; Chow, C.L.; Lau, D. Developments and Applications of Carbon Nanotube Reinforced Cement-Based Composites as Functional Building Materials. Front. Mater. 2022, 9, 861646. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Nobre, C.; Brito, P.; Lourinho, G.; Alves, O. Preparation and Application of Biochar. no. Special Issue. Environments. 2023, pp. 1–2. Available online: https://www.mdpi.com/journal/environments/special_issues/preparation_application_biochar (accessed on 9 January 2024).
- Ji, X.; Wan, J.; Wang, X.; Peng, C.; Wang, G.; Liang, W.; Zhang, W. Mixed bacteria-loaded biochar for the immobilization of arsenic, lead, and cadmium in a polluted soil system: Effects and mechanisms. Sci. Total Environ. 2022, 811, 152112. [Google Scholar] [CrossRef]
- Khushnood, R.A.; Ahmad, S.; Savi, P.; Tulliani, J.M..; Giorcelli, M.; Ferro, G.A. Improvement in electromagnetic interference shielding effectiveness of cement composites using carbonaceous nano/micro inerts. Constr. Build Mater. 2015, 85, 208–216. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, T.; Wang, L. The biogenic reefs formed by the alien polychaete Hydroides dianthus (Serpulidae, Annelida) favor the polyp stage of Aurelia coerulea (Cnidaria, Scyphozoa) in a coastal artificial lake. Mar. Pollut. Bull. 2018, 129, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Huang, C.P.; Hsieh, S.-L.; Tsai, M.-L.; Chen, C.-W.; Dong, C.-D. Biochar derived from red algae for efficient remediation of 4-nonylphenol from marine sediments. Chemosphere 2020, 254, 126916. [Google Scholar] [CrossRef]
- Wang, Z.; Song, S.; Wang, H.; Yang, W.; Han, J.; Chen, H. Feasibility of Remediation of Heavy-Metal-Contaminated Marine Dredged Sediments by Active Capping with Enteromorpha Biochar. Int. J. Environ. Res. Public Health 2022, 19, 4944. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Rakib, R.J.; Gupta, P.K.; Sharma, P.; Garg, A.; Girard, P.; Aminabhavi, T.M. Adsorptive behavior of micro(nano)plastics through biochar: Co-existence, consequences, and challenges in contaminated ecosystems. Sci. Total Environ. 2023, 856, 159097. [Google Scholar] [CrossRef]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 2019, 27 (Suppl. S1), S1–S46. [Google Scholar] [CrossRef]
- Rafikova, G.F.; Korshunova, T.Y.; Kuzina, E.V.; Loginov, O.N. Accelerated bioconversion of cow dung into concentrated organic fertilizer using microbial composition. Int. J. Recycl. Org. Waste Agric. 2021, 10, 275–285. [Google Scholar] [CrossRef]
- Colwell, R.R.; Walker, J.D.; Cooney, J.J. Ecological Aspects of Microbial Degradation of Petroleum in the Marine Environment. CRC Crit. Rev. Microbiol. 1977, 5, 423–445. [Google Scholar] [CrossRef]
- Udebuani, A.C.; Okoli, C.I.; Nwigwe, H.C.; Ozoh, P.T.E. The Value of Animal Manure in the Enhancement of Bioremediation Processes in Petroleum Hydrocarbon Contaminated Agricultural Soils. 2012. Available online: http://www.ijat-aatsea.com (accessed on 27 June 2023).
- Yang, Q.; Zhang, S.; Li, X.; Rong, K.; Li, J.; Jiang, L. Effects of microbial inoculant and additives on pile composting of cow manure. Front. Microbiol. 2023, 13, 1084171. [Google Scholar] [CrossRef]
- Hosseinkhani, B.; Hennebel, T.; Boon, N. Potential of biogenic hydrogen production for hydrogen driven remediation strategies in marine environments. N Biotechnol. 2014, 31, 445–450. [Google Scholar] [CrossRef]
- Sandulli, R.; de Leonardis, C.; Vincx, M.; Vanaverbeke, J. Geographical and depth-related patterns in nematode communities from some Italian Marine Protected Areas. Ital. J. Zool. 2011, 78, 505–516. [Google Scholar] [CrossRef]
- Corriero, G.; Gherardi, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Musco, L.; Marzano, C.N. Inventory and distribution of hard bottom fauna from the marine protected area of porto cesareo (ionian sea): Porifera and polychaeta. Ital. J. Zool. 2004, 71, 237–245. [Google Scholar] [CrossRef][Green Version]
- Piscitelli, M.; Corriero, G.; Gaino, E.; Uriz, M.J. Reproductive cycles of the sympatric excavating sponges Cliona celata and Cliona viridis in the Mediterranean Sea. Invertebr. Biol. 2011, 130, 1–10. [Google Scholar] [CrossRef]
- Bongers, T.; Goede, R.; Korthals, G.; Yeates, G. Proposed changes of c-p classification for nematodes. Artic. Russ. J. Nematol. 1995, 3, 61–62. Available online: https://www.researchgate.net/publication/40199526 (accessed on 28 June 2023).
- Duran, A.; Collado-Vides, L.; Palma, L.; Burkepile, D.E. Interactive effects of herbivory and substrate orientation on algal community dynamics on a coral reef. Mar. Biol. 2018, 165, 156. [Google Scholar] [CrossRef] [PubMed]
- Trygonis, V.; Sini, M. PhotoQuad: A dedicated seabed image processing software, and a comparative error analysis of four photoquadrat methods. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 99–108. [Google Scholar] [CrossRef]
- Boerema, A.; Geerts, L.; Oosterlee, L.; Temmerman, S.; Meire, P. Ecosystem service delivery in restoration projects: The effect of ecological succession on the benefits of tidal marsh restoration. Ecol. Soc. 2016, 21, 2–11. [Google Scholar] [CrossRef]
- Carlos García-Gómez, J. A Guide on Environmental Monitoring of Rocky Seabeds in Mediterranean Marine Protected Areas and Surrounding Zones; RAC/SPA: Tunis, Tunisia, 2015. [Google Scholar]
- Young, T.P.; Petersen, D.A.; Clary, J.J. The ecology of restoration: Historical links, emerging issues and unexplored realms. Ecol. Lett. 2005, 8, 662–673. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Ceccarelli, D.; Babcock, R.C.; Bayraktarov, E.; Cook, N.; Harrison, P.; Hein, M.; Shaver, E.; Smith, A.; Stewart-Sinclair, P.J.; et al. Coral Restoration in a Changing World: A Global Synthesis of Methods and Techniques; James Cook University: Cairns, Australia, 2018. [Google Scholar]
- Burt, J.; Bartholomew, A.; Bauman, A.; Saif, A.; Sale, P.F. Coral recruitment and early benthic community development on several materials used in the construction of artificial reefs and breakwaters. J. Exp. Mar. Biol. Ecol. 2009, 373, 72–78. [Google Scholar] [CrossRef]
- Pierri, C.; Fanelli, G.; Giangrande, A. Experimental co-culture of low food-chain organisms, Sabella spallanzanii (Polychaeta, Sabellidae) and Cladophora prolifera (Chlorophyta, Cladophorales), in Porto Cesareo area (Mediterranean Sea). Aquac. Res. 2006, 37, 966–974. [Google Scholar] [CrossRef]
- Seaman, W. Artificial habitats and the restoration of degraded marine ecosystems and fisheries. Hydrobiologia 2007, 580, 143–155. [Google Scholar] [CrossRef]
- Venkatachalam, C.D.; Sekar, S.; Sengottian, M.; Ravichandran, S.R.; Bhuvaneshwaran, P. A critical review of the production, activation, and morphological characteristic study on functionalized biochar. J. Energy Storage 2023, 67, 107525. [Google Scholar] [CrossRef]
- Mwaura, J.M.; Murage, D.; Karisa, J.F.; Otwoma, L.M.; Said, H.O. Artificial reef structures and coral transplantation as potencial tools for enhancing locally-managed inshore reefs: A case study from Wasani Island, Kenya. WIO J. Mar. Sci. 2022, 21, 83–94. [Google Scholar] [CrossRef]
- Meyer Cifuentes, I.E.; Öztürk, B. Exploring microbial consortia from various environments for plastic degradation. Methods Enzymol. 2021, 648, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Bracho-Villavicencio, C.; Matthews-Cascon, H.; Rossi, S. Artificial Reefs around the World: A Review of the State of the Art and a Meta-Analysis of Its Effectiveness for the Restoration of Marine Ecosystems. Environments 2023, 10, 121. [Google Scholar] [CrossRef]
- Knoester, E.G.; Rienstra, J.J.; Schürmann, Q.J.F.; Wolma, A.E.; Murk, A.J.; Osinga, R. Community-managed coral reef restoration in southern Kenya initiates reef recovery using various artificial reef designs. Front. Mar. Sci. 2023, 10, 1152106. [Google Scholar] [CrossRef]
- Ferse, S.C.A. Poor Performance of Corals Transplanted onto Substrates of Short Durability. Restor. Ecol. 2010, 18, 399–407. [Google Scholar] [CrossRef]
- Duarte, C.M.; Agusti, S.; Barbier, E.; Britten, G.L.; Castilla, J.C.; Gattuso, J.-P.; Fulweiler, R.W.; Hughes, T.P.; Knowlton, N.; Lovelock, C.E.; et al. Rebuilding marine life. Nature 2020, 580, 39–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).