Environmental Parameters Related to Hypoxia Development and Persistence in Jinhae Bay from 2011 to 2016 and Their Potential for Hypoxia Prediction
Abstract
:1. Introduction
2. Materials and Methods
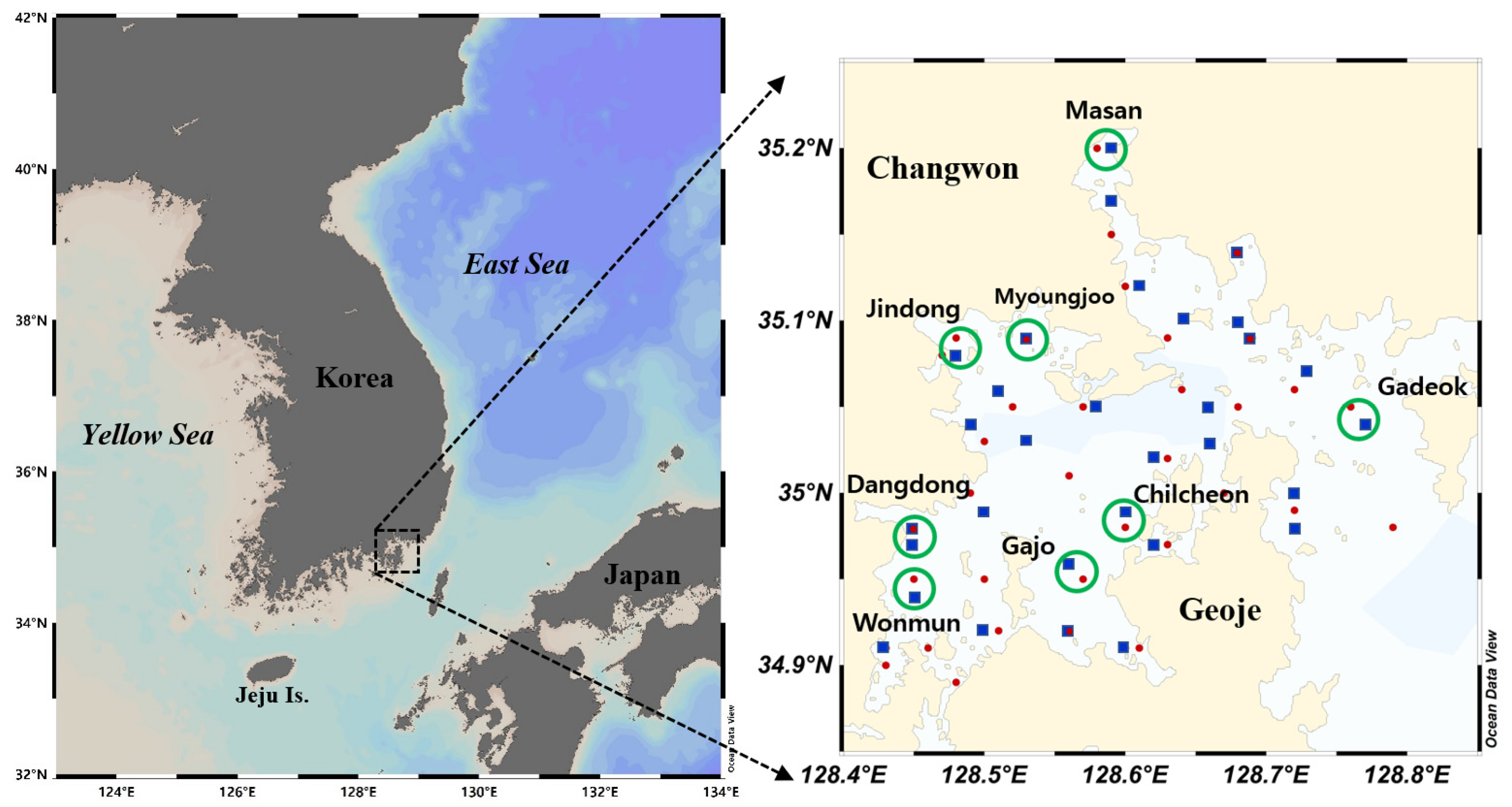
2.1. Study Area
2.2. Field Survey, Water Quality Analysis, and Meteorological Data
2.3. Hypoxic Indices
3. Results
3.1. Environmental Characteristics
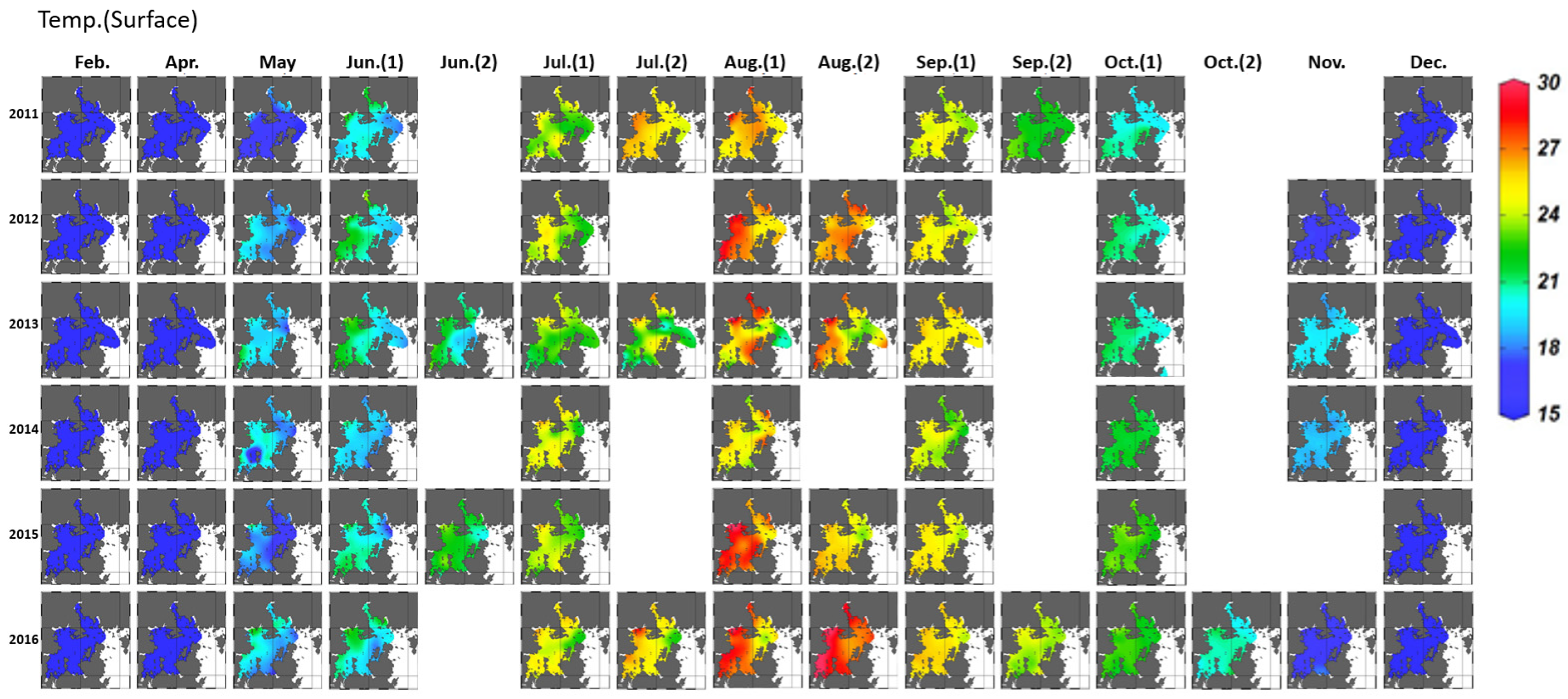
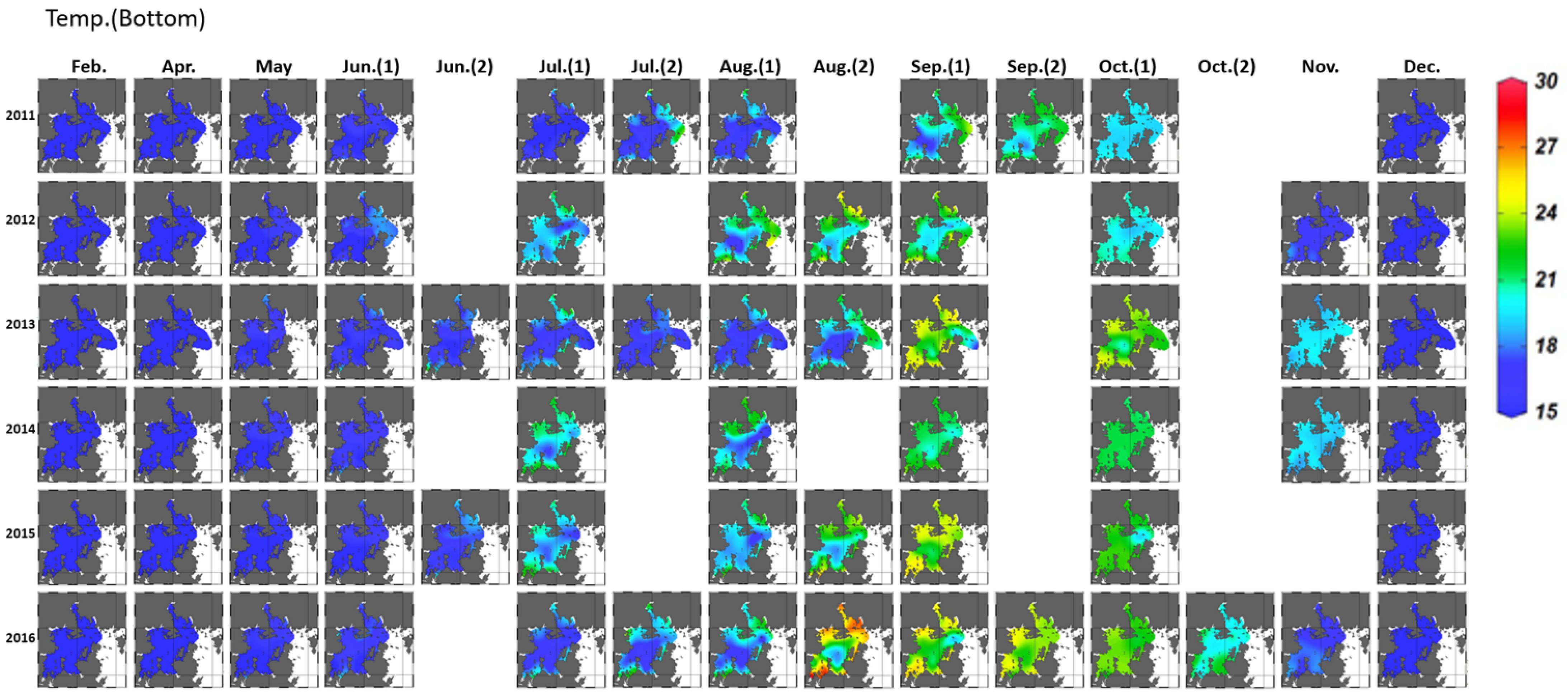
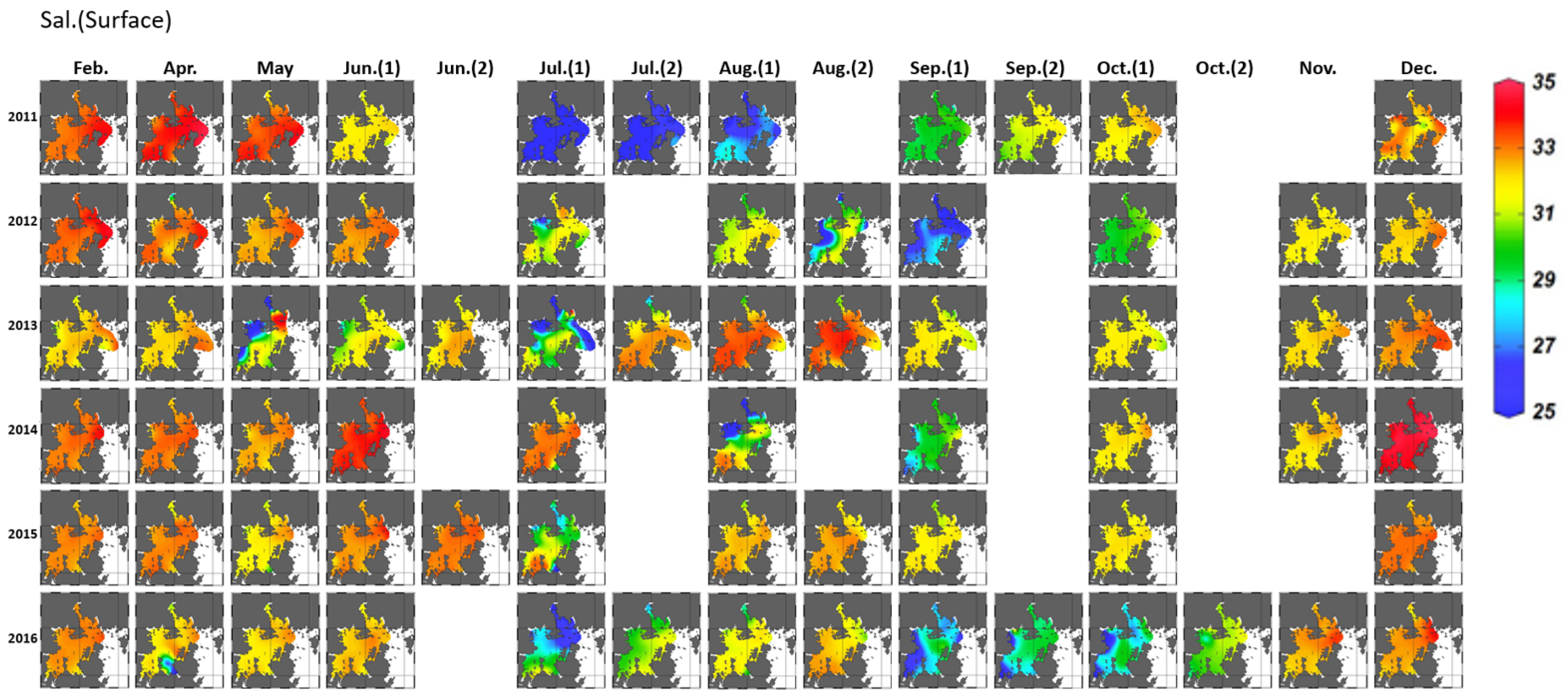
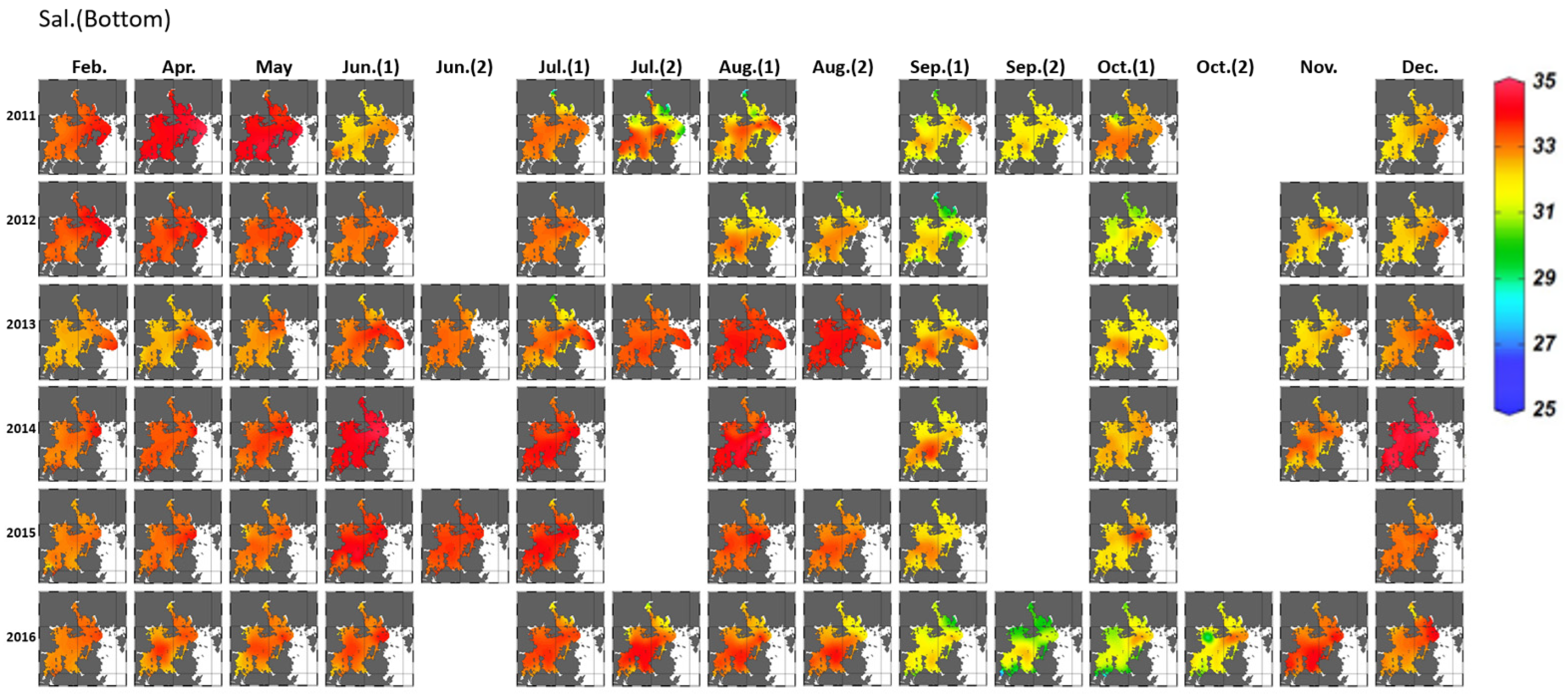
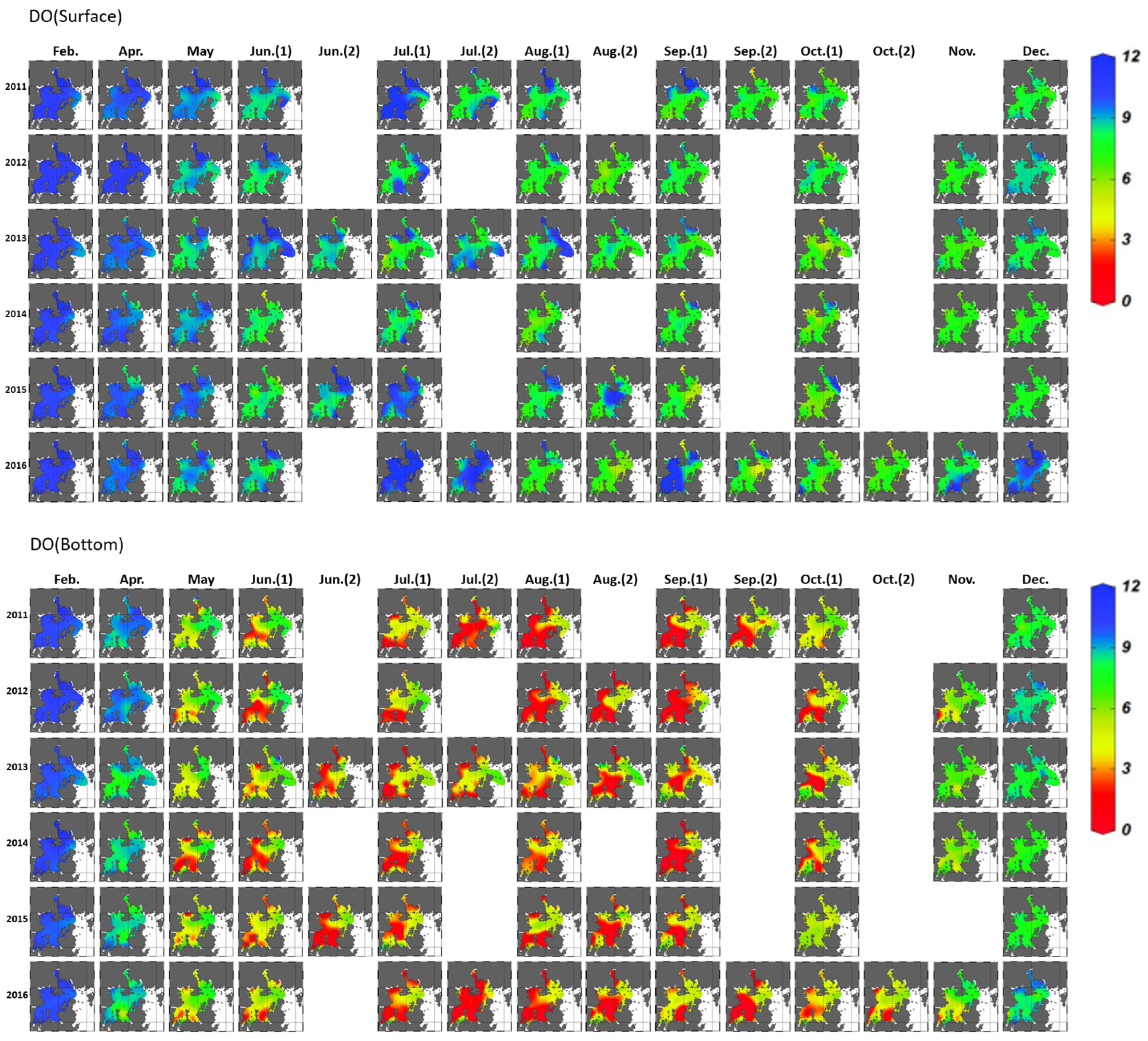
3.1.1. Variation in Seawater Quality
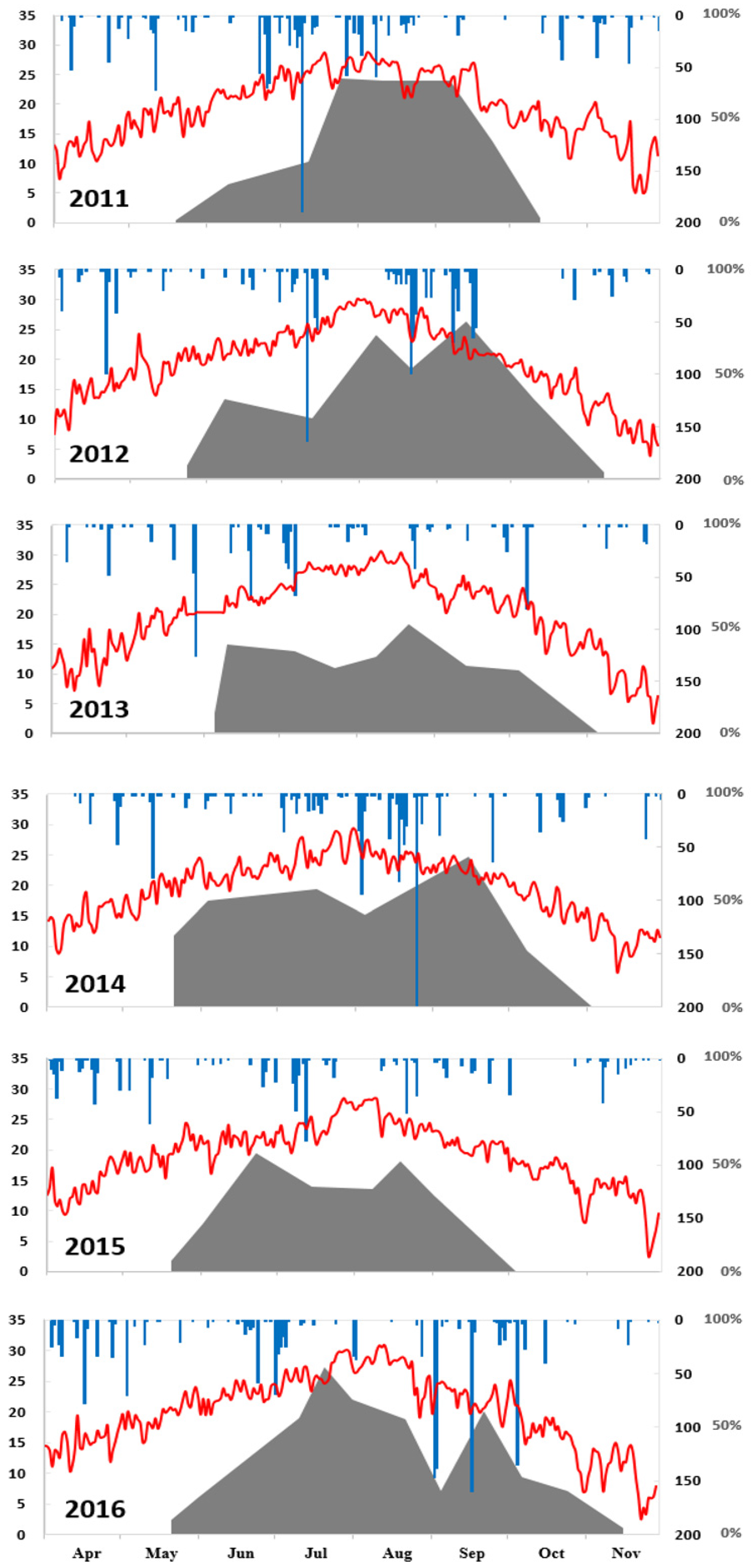
3.1.2. Meteorological Data
3.2. Characteristics of Hypoxia Development
3.2.1. Horizontal Distribution and Duration of Hypoxia Development
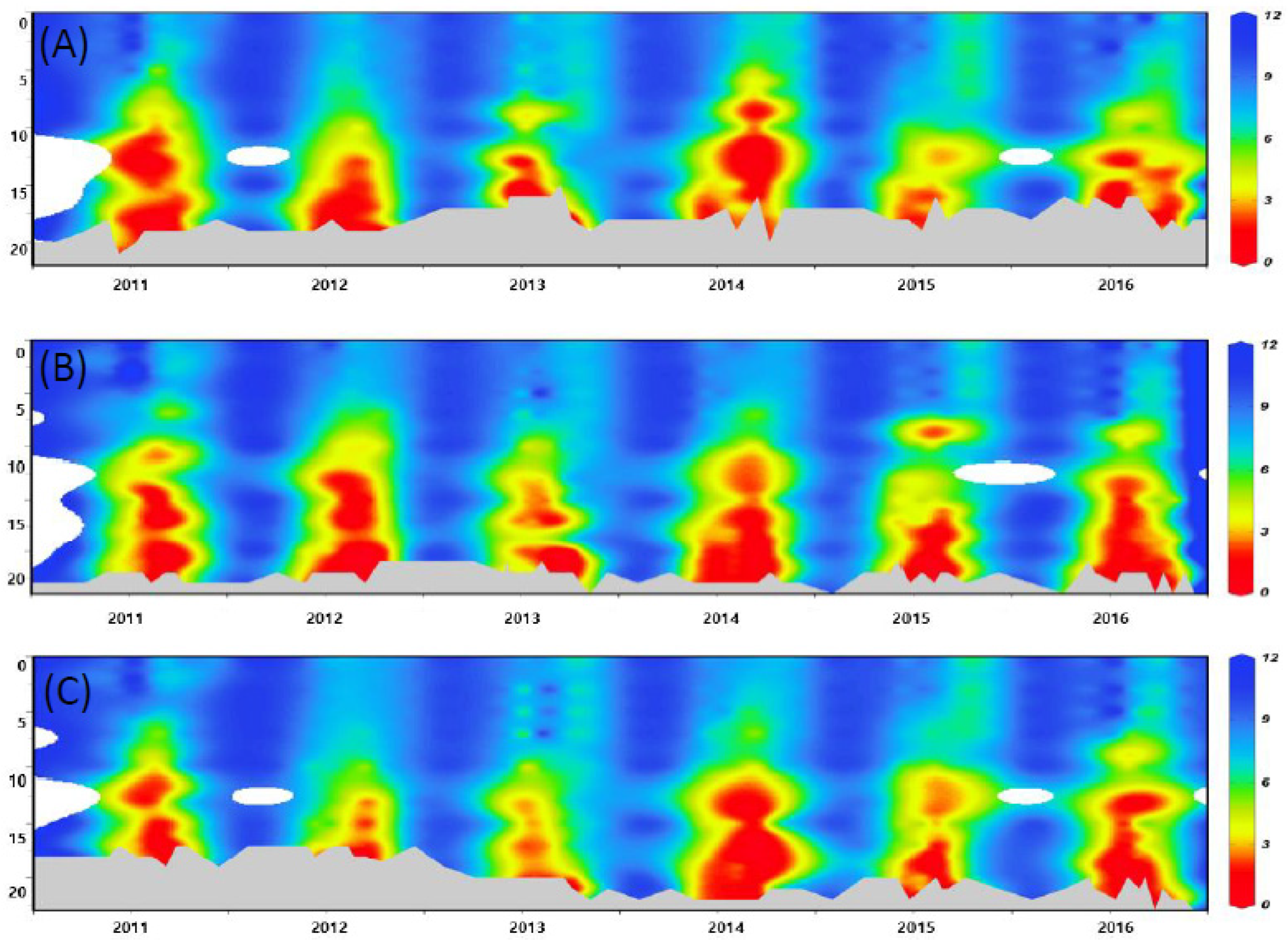
3.2.2. Vertical Distribution and Strength of Hypoxia
4. Discussion
4.1. Analysis of Factors Influencing Hypoxia
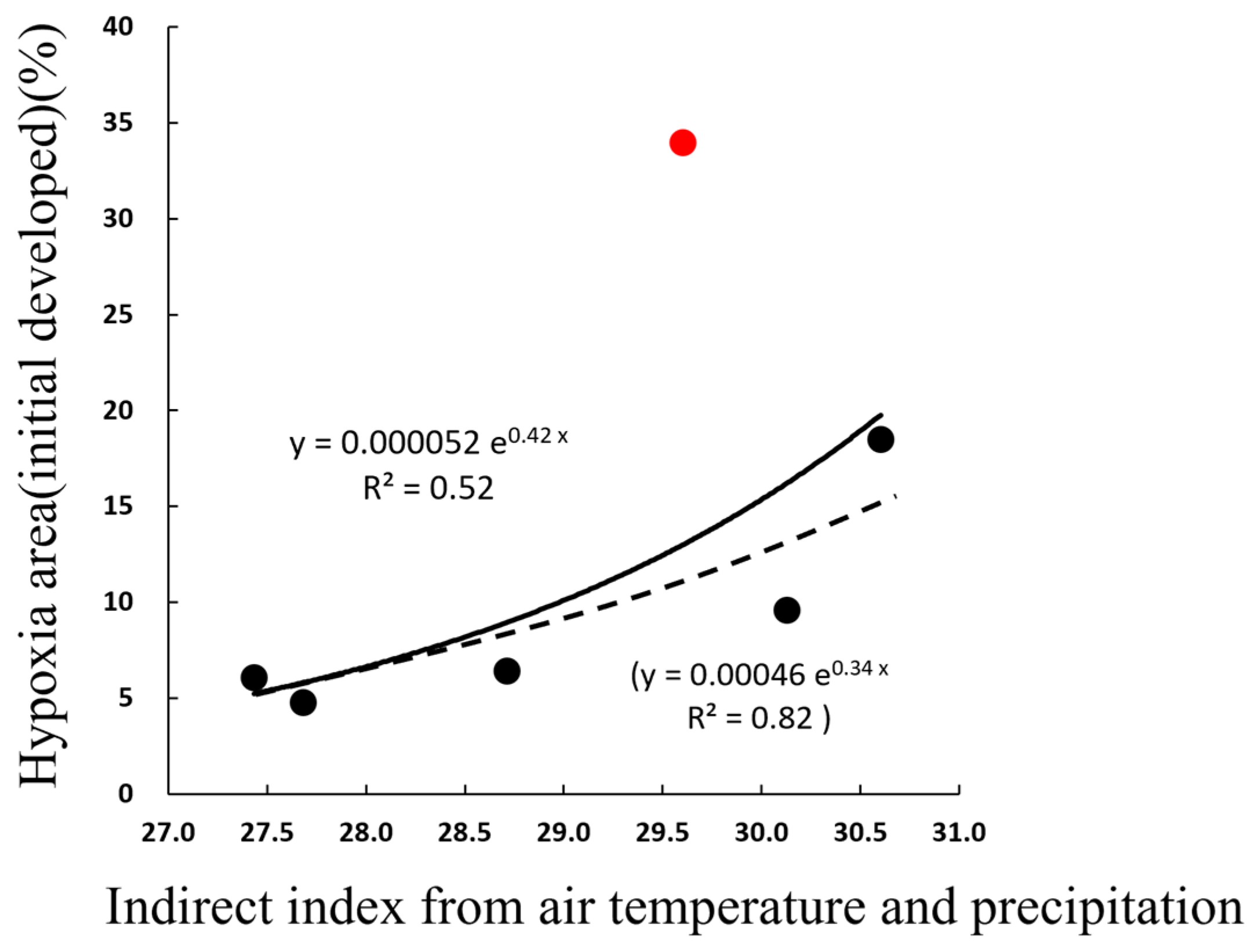
4.2. Prediction of Hypoxic Area from Weather Parameters
(if, excluding the case of 2014, Y = 0.0004657exp (0.33738X))
X = Temp1w + ln (Precip4w) × 1.8
X = Temp1w + ln (Precip2~4w) × 1.25
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gray, J.S.; Wu, R.S.; Or, Y.Y. Effects of hypoxia and organic enrichment on the coastal marine enrichment. Mar. Ecol. Prog. Ser. 2022, 238, 249–279. [Google Scholar] [CrossRef]
- Keiyu, M.; Yokota, M. Review on the hypoxia formation and its effects on aquatic organisms. Rep. Mar. Ecol. Res. Inst. 2012, 15, 1–21. [Google Scholar]
- Saha, N.; Koner, D.; Sharma, R. Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes. Aquac. Fish. 2022, 7, 572–582. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Diaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Caballero-Alfonso, A.M.; Carstensen, J.; Conley, D.J. Biogeochemical and environmental drivers of coastal hypoxia. J. Mar. Syst. 2015, 141, 190–199. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.T.; Lim, J.H.; Yoon, J.E.; Kim, I.N. Hypoxia in Korea Coastal Waters: A Case Study of the Nutural Jinhae Bay and Artificial Shihwa Bay. Front. Mar. Sci. 2018, 5, 70. [Google Scholar] [CrossRef]
- Diaz, J.R.; Rosenberg, R. Spreading Dead Zone and Consequences for Marine Ecosystems. Science 2008, 321, 926. [Google Scholar] [CrossRef]
- Donald, W.S.; Scott, W.N. Stratification and Bottom-Water Hypoxia in the Pamlico River Estuary. Estuaries 1992, 15, 270–281. [Google Scholar]
- Wiseman, W.J.; Rabalais, N.N.; Turner, R.E.; Dinnel, S.P.; MacNaughton, A. Seasonal and interannual variability within the Louisiana coastal current: Stratification and hypoxia. J. Mar. Syst. 1997, 12, 237–248. [Google Scholar] [CrossRef]
- Kasai, A.; Yamada, T.; Takeda, H. Flow structure and hypoxia in Hiuchi-nada, Seto Inland Sea. Estuar. Coast. Shelf Sci. 2007, 71, 210–217. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Z.; Li, Y.; Yang, Y. Physical controls of hypoxia in waters adjacent to the Yangtze Estuary: A numerical modeling study. Mar. Pollut. Bull. 2015, 97, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Djakovac, T.; Supic, N.; Aubry, F.B.; Degobbis, D.; Giani, M. Mechanisms of hypoxia frequency changes in the northern Adriatic Sea during the period 1972–2012. J. Mar. Syst. 2015, 141, 179–189. [Google Scholar] [CrossRef]
- Turner, R.E.; Rabalais, N.N.; Justić, D. Predicting summer hypoxia in the northern Gulf of Mexico: Riverine N, P, and Si loading. Mar. Pollut. Bull. 2006, 52, 139–148. [Google Scholar] [CrossRef]
- Turner, R.E.; Rabalais, N.N.; Justić, D. Predicting summer hypoxia in the northern Gulf of Mexico: Redux. Mar. Pollut. Bull. 2012, 64, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.D.; Donald, S. How climate controls the flux of nitrogen by the Mississippi River and the development of hypoxia in the Gulf of Mexico. Limnol. Oceanogr. 2007, 52, 856–861. [Google Scholar]
- Testa, J.M.; Kemp, W.M. Hypoxia-induced shifts in nitrogen and phosphorus cycling in Chesapeake Bay. Limnol. Oceanogr. 2012, 57, 835–850. [Google Scholar] [CrossRef]
- Meier, H.E.M.; Andersson, H.C.; Eilola, K.; Gustafsson, B.G.; Kuznetsov, I.; Müller-Karulis, B.; Neumann, T.; Savchuk, O.P. Hypoxia in future climates: A model ensemble study for the Baltic Sea. Geophys. Res. Lett. 2011, 38, L24608. [Google Scholar] [CrossRef]
- Feng, Y.; DiMarco, S.F.; Jackson, G.A. Relative role of wind forcing and riverine nutrient input on the extent of hypoxia in the northern Gulf of Mexico. Geophys. Res. Lett. 2012, 39, L09601. [Google Scholar] [CrossRef]
- Del Giudice, D.; Matli, V.R.R.; Obenour, D.R. Bayesian mechanistic modeling characterizes Gulf of Mexico hypoxia: 1968–2016 and future scenarios. Ecol. Appl. 2020, 30, e02032. [Google Scholar] [CrossRef]
- Schourup-Kristensen, V.; Larsen, J.; Maar, M. Drivers of hypoxia variability in a shallow and eutrophicated semi-enclosed fjord. Mar. Pollut. Bull. 2023, 188, 114621. [Google Scholar] [CrossRef]
- Roman, M.R.; Brandt, S.B.; Houde, E.D.; Pierson, J.J. Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Front. Mar. Sci. 2019, 6, 139. [Google Scholar] [CrossRef]
- Zhan, Y.; Ning, B.; Sun, J.; Chang, Y. Living in a hypoxia world: A review of the impacts of hypoxia on aquaculture. Mar. Pollut. Bull. 2023, 194, 115207. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H. Cross-sectional velocity variability and tidal exchange in a bay. Bull. Korean Fish. Tech. Soc. 1990, 26, 353–359. [Google Scholar]
- Cho, C.H. Mass mortalities of oyster due to red tide in Jinhae Bay in 1978. Korean J. Fish. Aquat. Sci. 1979, 12, 27–33. [Google Scholar]
- Kim, D.S.; Lee, C.W.; Choi, S.H.; Kim, Y.O. Long-term Changes in Water Quality of Masan Bay, Korea. J. Coast. Res. 2012, 28, 923–929. [Google Scholar] [CrossRef]
- Kwon, J.N.; Lim, J.H.; Shim, J.; Lee, J.; Choi, T.J. The long-term variations of water quality in Masan Bay, South Sea of Korea. J. Korean Soc. Mar. Environ. 2014, 19, 212–223. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.K.; Yoon, J.S. Study of Formation and Development of Oxygen Deficient Water Mass, Using Ecosystem Model in Jinhae, Masan Bay. J. Ocean Eng. Technol. 2010, 24, 41–50. [Google Scholar]
- MOF (Ministry of Oceans and Fisheries). Standard Method for the Analysis of Marine Environment. Available online: https://www.law.go.kr/admRulLsInfoP.do?admRulSeq=2000000109042 (accessed on 30 December 2020).
- NFRDI. Hypoxic Water Masses in the Coast of Korea; GMK Communication Press: Busan, Republic of Korea, 2009; 173p.
- Ferland, J.; Gosselin, M.; Starr, M. Environmental control of summer primary production in the Hudson Bay system: The role of stratification. J. Mar. Syst. 2011, 88, 385–400. [Google Scholar] [CrossRef]
- He, B.; Dai, M.; Zhai, W.; Guo, X.; Wang, L. Hypoxia in the upper reaches of the Pearl River estuary and its maintenance mechanisms: A synthesis based on multiple year observations during 2000–2008. Mar. Chem. 2014, 167, 13–24. [Google Scholar] [CrossRef]
- Huang, Y.; An, S. Weak hypoxia enhanced denitrification in a dissimilatory nitrate reduction to ammonium(DNRA)-dominated shallow and eutrophic coastal waterbody, Jinhae Bay, South Korea. Front. Mar. Sci. 2022, 9, 897474. [Google Scholar] [CrossRef]
- Park, Y.; Cha, J.; Song, B.; Huang, Y.; Kim, S.; Kim, S.; Jo, E.; Fortin, S.; An, S. Total microbial activity and sulfur cycling microbe changes in response to the development of hypoxia in a shallow estuary. Ocean Sci. J. 2020, 55, 165–181. [Google Scholar] [CrossRef]
- Keeling, R.F.; Körtzinger, A.; Gruber, N. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2010, 2, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, F.; Cozzi, S. Seasonal dynamics and long-term trend of hypoxia in the coastal zone of Emilia Romagna (NW Adriatic Sea, Italy). Sci. Total Environ. 2016, 541, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N.; Turner, R.E.; Sen Gupta, B.K.; Boesch, D.F.; Chapman, P.; Murrell, M.C. Hypoxia in the northern Gulf of Mexico: Does the science support the plan to reduce, mitigate, and control hypoxia? Estuaries Coasts 2007, 30, 753–772. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Linker, L.; Hinson, K. Influence of wind strength and duration on relative hypoxia reductions by opposite wind directions in an estuary with an asymmetric channel. J. Mar. Sci. Eng. 2016, 4, 62. [Google Scholar] [CrossRef]



| Temperature | Salinity | pH | Dissolved Oxygen | Ammonia | Nitrate | Nitrite | Phosphate | Silicate | Chlorophyll a | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | 1 | −0.327 * | 0.342 * | −0.305 * | 0.037 | 0.109 | 0.116 | 0.168 | 0.131 | 0.220 |
| Salinity | 1 | −0.468 ** | −0.231 | −0.332 * | −0.796 ** | −0.466 ** | −0.141 | −0.617 ** | −0.563 ** | |
| pH | 1 | 0.528 ** | −0.253 | 0.110 | 0.034 | −0.375 ** | −0.021 | 0.306 * | ||
| Dissolved Oxygen | 1 | −0.413 ** | 0.161 | −0.166 | −0.576 ** | −0.035 | 0.388 ** | |||
| Ammonia | 1 | 0.475 ** | 0.325 * | 0.853 ** | 0.550 ** | −0.174 | ||||
| Nitrate | 1 | 0.496 ** | 0.349 * | 0.861 ** | 0.406 ** | |||||
| Nitrite | 1 | 0.389 ** | 0.506 ** | 0.204 | ||||||
| Phosphate | 1 | 0.500 ** | −0.155 | |||||||
| Silicate | 1 | 0.415 ** | ||||||||
| Chlorophyll a | 1 |
| Temperature | Salinity | pH | Dissolved Oxygen | Ammonia | Nitrate | Nitrite | Phosphate | Silicate | Chlorophyll a | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | 1 | −0.512 ** | −0.266 | −0.739 ** | 0.514 ** | 0.195 | 0.402 ** | 0.596 ** | 0.321 * | 0.022 |
| Salinity | 1 | 0.105 | 0.300 * | −0.436 ** | −0.457 ** | −0.453 ** | −0.389 ** | −0.052 | 0.033 | |
| pH | 1 | 0.616 ** | −0.266 | −0.456 ** | −0.106 | −0.429 ** | −0.429 ** | −0.500 ** | ||
| Dissolved Oxygen | 1 | −0.470 ** | −0.346 * | −0.285 * | −0.625 ** | −0.660 ** | −0.184 | |||
| Ammonia | 1 | 0.414 ** | 0.302 * | 0.844 ** | 0.554 ** | −0.047 | ||||
| Nitrate | 1 | 0.475 ** | 0.491 ** | 0.428 ** | 0.087 | |||||
| Nitrite | 1 | 0.379 ** | 0.322 * | −0.082 | ||||||
| Phosphate | 1 | 0.639 ** | −0.078 | |||||||
| Silicate | 1 | −0.028 | ||||||||
| Chlorophyll a | 1 |
| January | February | March | April | May | June | July | August | September | October | November | December | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Air Temperature (°C) | Average (’90–’10) | 2.9 | 4.8 | 8.7 | 14.0 | 18.3 | 21.7 | 25.2 | 26.4 | 22.9 | 17.6 | 10.9 | 5.1 |
| 2011 | −1.1 | 5 | 7.1 | 12.9 | 17.9 | 21.9 | 25.6 | 25.7 | 23 | 16.5 | 13.1 | 3.8 | |
| 2012 | 2.2 | 2.1 | 7.9 | 14 | 19.1 | 21.8 | 26.0 | 27.5 | 21.9 | 16.8 | 9.4 | 1.7 | |
| 2013 | 1.3 | 3.6 | 9.6 | 12.5 | 18.7 | 22.4 | 27.0 | 28.0 | 23.3 | 18.3 | 10 | 4.7 | |
| 2014 | 4.0 | 5.4 | 9.6 | 14.5 | 19.4 | 22 | 25.2 | 24.6 | 22.3 | 17.2 | 11.6 | 2.9 | |
| 2015 | 3.6 | 4.6 | 8.9 | 13.9 | 19.3 | 21.2 | 24.1 | 25.4 | 20.9 | 16.4 | 11.8 | 5.5 | |
| 2016 | 0.6 | 4.1 | 8.9 | 14.7 | 19.4 | 22.5 | 26.3 | 27.3 | 22.2 | 17.4 | 9.8 | 5.4 | |
| Rainfall (mm, monthly sum) | Average (’90–’10) | 31.7 | 48.1 | 77.0 | 129.8 | 161.6 | 208.4 | 328.5 | 313.0 | 164.8 | 54.9 | 44.7 | 23.3 |
| 2011 | 0.0 | 74.7 | 26.9 | 149.6 | 147 | 238 | 414.1 | 198.5 | 27.9 | 91.5 | 141.2 | 4.6 | |
| 2012 | 5.2 | 11.0 | 101.4 | 217.4 | 39.9 | 78.9 | 334.6 | 329.6 | 269 | 37.0 | 56.2 | 79.2 | |
| 2013 | 13.7 | 71.2 | 84.9 | 90.5 | 222.4 | 148.5 | 190.1 | 82.3 | 58.5 | 83.2 | 59.6 | 5.1 | |
| 2014 | 5.7 | 20.0 | 152.5 | 102.9 | 106.5 | 45.1 | 149.2 | 665.9 | 109.8 | 101.0 | 51.5 | 15.7 | |
| 2015 | 33.4 | 31.0 | 69.5 | 196.3 | 134.0 | 77.0 | 197.5 | 116.7 | 88.9 | 40.2 | 85.2 | 41.0 | |
| 2016 | 40.5 | 73.2 | 81.6 | 263.0 | 123.7 | 108.8 | 181.8 | 110.0 | 522.7 | 215.8 | 35.1 | 136.8 | |
| Wind Speed (m/s) | Average (’90–’10) | 2.2 | 2.2 | 2.3 | 2.3 | 2.2 | 2.1 | 2.2 | 2.0 | 1.9 | 1.8 | 1.9 | 2.0 |
| 2011 | 2.6 | 1.7 | 2.2 | 2.4 | 2.1 | 2.2 | 2.2 | 1.8 | 1.8 | 1.6 | 1.5 | 2.1 | |
| 2012 | 2.2 | 2.1 | 2.1 | 2.4 | 1.9 | 1.9 | 1.9 | 2.2 | 1.7 | 1.5 | 1.9 | 2.2 | |
| 2013 | 2.0 | 2.1 | 2.3 | 2.3 | 2.0 | 1.7 | 2.3 | 1.7 | 1.6 | 1.7 | 1.7 | 1.9 | |
| 2014 | 1.8 | 2.1 | 1.9 | 1.8 | 2.0 | 1.6 | 1.4 | 2.0 | 1.5 | 1.7 | 1.5 | 1.9 | |
| 2015 | 1.9 | 1.9 | 2.1 | 2.0 | 2.0 | 1.8 | 1.8 | 1.4 | 1.5 | 1.4 | 1.3 | 1.6 | |
| 2016 | 1.9 | 1.9 | 1.7 | 1.7 | 1.7 | 1.5 | 1.4 | 1.4 | 1.1 | 1.4 | 1.6 | 1.8 |
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|
| Occurrence | early-Jun | end-May | early-Jun | end-May | end-May | end-May |
| Average in Jun–Oct (km2) | 164 | 205 | 140 | 201 | 142 | 176 |
| Maximum area (km2) | 284 | 308 | 213 | 289 | 227 | 316 |
| Period of maximum area | end-Jul | mid-Sep | end-Aug | mid-Sep | end-Jun | end-Jul |
| Duration (weeks) | 15 | 22 | 18 | 20 | 20 | 26 |
| Minimum Concentration (mg/L) | Thickness | Duration (Weeks) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
| Average | 0.47 | 0.50 | 0.52 | 0.35 | 0.67 | 0.43 | 64.9 | 71.9 | 45.0 | 111.2 | 54.7 | 96.1 | 10.5 | 19.8 | 12.9 | 18.7 | 11.4 | 17.1 |
| Gadeok | 5.29 | 5.01 | 3.95 | 5.06 | 4.76 | 4.04 | ||||||||||||
| Masan | 1.40 | 1.40 | 1.25 | 0.7 | 0.9 | 0.33 | 7 | 30 | 36 | 41 | 23 | 66 | 4 | 14 | 17 | 9 | 10 | 10 |
| Myoungjoo | 0.38 | 0.38 | 0.43 | 0.34 | 0.28 | 0.93 | 22 | 24 | 20 | 88 | 51 | 56 | 4 | 9 | 7 | 27 | 15 | 13 |
| Jindong | 0.28 | 0.33 | 0.39 | 0.32 | 1.12 | 0.48 | 81 | 60 | 17 | 64 | 33 | 60 | 11 | 18 | 2 | 18 | 10 | 21 |
| Dangdong–Wonmun 1 | 0.28 | 0.50 | 0.42 | 0.32 | 1.58 | 0.44 | 91 | 124 | 42 | 119 | 48 | 93 | 15 | 29 | 19 | 20 | 10 | 15 |
| Dangdong–Wonmun 2 | 0.24 | 0.23 | 0.51 | 0.25 | 0.27 | 0.33 | 85 | 87 | 47 | 150 | 59 | 122 | 15 | 22 | 11 | 20 | 11 | 15 |
| Gajo | 0.26 | 0.17 | 0.28 | 0.26 | 0.3 | 0.29 | 99 | 132 | 84 | 170 | 98 | 153 | 15 | 29 | 19 | 20 | 15 | 21 |
| Chilcheon | 0.43 | 0.49 | 0.39 | 0.27 | 0.27 | 0.22 | 68 | 46 | 70 | 147 | 71 | 124 | 8 | 18 | 13 | 17 | 10 | 24 |
| Hypoxic Area | Minimum Concentration | Thickness | Stability | ||
|---|---|---|---|---|---|
| Surface Water | Temperature | 0.670 ** | −0.873 ** | 0.208 | 0.349 ** |
| Salinity | −0.185 | 0.352 ** | −0.255 | −0.878 ** | |
| DO | −0.131 | 0.282 * | 0.080 | 0.294 * | |
| Ammonia | −0.071 | −0.021 | 0.297 | 0.298 * | |
| Nitrate | −0.055 | −0.088 | 0.099 | 0.812 ** | |
| DIP | −0.081 | −0.136 | 0.082 | 0.110 | |
| DSi | −0.119 | −0.221 | 0.155 | 0.684 ** | |
| Bottom Water | Temperature | 0.328 * | − 0.725 ** | 0.077 | 0.056 |
| Salinity | −0.111 | 0.298 * | −0.065 | −0.045 | |
| DO | −0.805 ** | −0.998 ** | −0.614 ** | −0.333 * | |
| Ammonia | 0.211 | −0.480 ** | 0.499 ** | 0.313 * | |
| Nitrate | 0.131 | −0.355 * | 0.384 * | 0.669 ** | |
| DIP | 0.353 * | −0.634 ** | 0.406 * | 0.347 * | |
| DSi | 0.179 | −0.675 ** | 0.484 ** | 0.393 ** | |
| Weather Parameter | Temperature | 0.638 ** | −0.815 ** | 0.185 | 0.440 ** |
| Rainfall (4 weeks) | 0.457 ** | −0.338 * | 0.471 ** | 0.191 | |
| Wind speed | −0.010 | 0.160 | −0.131 | 0.407 ** | |
| Daylight hours | −0.268 | 0.236 | −0.065 | −0.409 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, J.; Ye, M.-J.; Kim, Y.-S.; Lim, J.-H.; Lee, W.-C.; Lee, T. Environmental Parameters Related to Hypoxia Development and Persistence in Jinhae Bay from 2011 to 2016 and Their Potential for Hypoxia Prediction. J. Mar. Sci. Eng. 2024, 12, 14. https://doi.org/10.3390/jmse12010014
Shim J, Ye M-J, Kim Y-S, Lim J-H, Lee W-C, Lee T. Environmental Parameters Related to Hypoxia Development and Persistence in Jinhae Bay from 2011 to 2016 and Their Potential for Hypoxia Prediction. Journal of Marine Science and Engineering. 2024; 12(1):14. https://doi.org/10.3390/jmse12010014
Chicago/Turabian StyleShim, JeongHee, Mi-Ju Ye, Young-Sug Kim, Jae-Hyun Lim, Won-Chan Lee, and Tongsup Lee. 2024. "Environmental Parameters Related to Hypoxia Development and Persistence in Jinhae Bay from 2011 to 2016 and Their Potential for Hypoxia Prediction" Journal of Marine Science and Engineering 12, no. 1: 14. https://doi.org/10.3390/jmse12010014
APA StyleShim, J., Ye, M.-J., Kim, Y.-S., Lim, J.-H., Lee, W.-C., & Lee, T. (2024). Environmental Parameters Related to Hypoxia Development and Persistence in Jinhae Bay from 2011 to 2016 and Their Potential for Hypoxia Prediction. Journal of Marine Science and Engineering, 12(1), 14. https://doi.org/10.3390/jmse12010014





