Abstract
Harbour and grey seals rely on acoustic signals to mate, socialise and defend their territory. Previous studies have focused on their behaviour, movements and communication from the coast, leaving a knowledge gap in the offshore environments, and therefore being unable to determine the proper use they give to those areas and the risks they face around them. Acoustic data collected with a SoundTrap were analysed to assess the detectability of both species in the Malin Sea. Vocalisations were classified based on aural and visual features, as well as using non-parametric classification trees. Differences in the vocalisation rate of grey seals per diel, season and tidal state were also assessed through Generalised Linear Mixed Models, obtaining significant results, and finding similarities in the vocalisations of grey seals with the Scottish and Irish populations. A small sample of adult and pup harbour seals was detected, and differences in call type and number of detections per type were found across the seasons. These results show the importance of the area for both species, and lay the foundations for future studies, which will help to implement proper conservation measures such as Marine Protected Areas.
1. Introduction
1.1. Pinnipeds, Communication, Threats and Conservation
Pinnipeds are commonly used as indicators of ecosystem function [1,2]. Present in most aquatic environments such as Arctic and Antarctic seas, tropical seas, estuaries and continental shelves [3], pinnipeds can vocalise in water and in air, and some are also capable of performing it simultaneously above and below the water [4]. They produce a great variety of sounds that can be classified as barks, grunts, groans, snorts, growls, roars, yelps, whinnies, creaks and buzzes, among others [5]. The variability of these call types can be assessed at a species and individual level, finding differences even by age, gender, geographic location, emotional state and current social behaviour [4,6].
Nowadays, many pinniped populations are declining (e.g., Northern fur seal Callorhinus ursinus [7]; Antarctic fur seal Arctocephalus gazella [8]; Hawaiian monk seal Neomonachus schauinslandi [9]) or threatened (e.g., Australian sea lion Neophoca cinerea [10]; Caspian seal Pusa caspica [11]; Galápagos fur seal Arctocephalus galapagoensis [12]), with fisheries interactions (direct and indirect), pollution and infections being their main threats [13,14]. In addition, considering their distribution, principally in cold waters and close to continental shelf regions, and their poor adaptability to tropical conditions [14], climate change is going to play an important role in the survival of these animals.
Although the impact of noise at a large scale and the development of acoustic monitoring techniques have been more focused on cetaceans [15,16,17,18,19], some evidence of acoustic disturbance on pinnipeds has been investigated (e.g., offshore wind farms [20]; acoustic deterrents [21]; shipping [22]). Nevertheless, in order to assess the effects of anthropogenic underwater noise, and therefore protect and manage pinniped species, it is crucial to describe and understand first their vocal behaviour. Satellite telemetry [23,24,25], aerial and ground surveys [26,27], photo-identification [28,29] and recently, habitat modelling [30,31,32] have been used to identify important breeding, foraging and pupping areas. To a lesser extent, despite its advantages over the previous techniques, underwater acoustic studies have been carried out on different pinniped populations, describing the variety of calls that they produce and their associated behaviours (e.g., Weddell seal Leptonychotes weddellii [33]; grey seal Halichoerus grypus [34]; crabeater seal Lobodon carcinophagus [35]; bearded seal Erignathus barbatus [36]). However, such studies have only been performed close to haul-out sites, paying more attention to the breeding calls of the males (e.g., grey seal [37]; harbour seal Phoca vitulina [38]), and on a short-term basis. Therefore, there is a need for long-term studies focused on the use of haul-out sites and foraging areas in order to fill this knowledge gap and design effective Marine Protected Areas (MPAs) [39].
1.2. Harbour and Grey Seals
1.2.1. Distribution, Status and Protection
Harbour seals, or common seals, as well as grey seals, can be found in the Northern Hemisphere coastlines and in the shores of the North Atlantic Ocean, respectively [3,40,41,42]. Both species are protected by the EU Habitats Directive (Council Directive 92/43/EEC) (Annex II), which requires the maintenance or restoration to a Favourable Conservation Status of the species as well as the designation of Special Areas of Conservation (SAC) to protect their habitat [27,43].
In Europe, around 40% of harbour seal populations can be found in the UK, with most of them in Scotland [44], with the last estimate being 43,450 individuals in 2016 [45]. Certain colonies are increasing or remaining stable [46,47], whereas others are suffering dramatic declines [48,49]. In the Republic of Ireland, harbour seals are very common in its coastal waters, with haul-out sites covering the entire coast [44]. However, little is known about the trajectory of these populations, with the last estimate carried out in the early 2000s with approximately 2905 individuals [50,51,52,53]. Grey seal populations in the UK numbered 116,100 individuals in 2010 [54], a small increase from those obtained in previous years, and representing approximately 40% of the world population [55]. In Ireland, grey seal populations are estimated to be between 7284 and 9365 individuals, around 6% of the total number in Western Europe [44,56,57]. According to [58], it seems that these populations are following a positive trend, with an increase of 25% from the estimates carried out by [56] to the counts made by [59].
To date, nine and thirteen designated SACs with harbour seals as qualifying interests are present in the UK and the Republic of Ireland, respectively, whereas in the same order, seven and ten SACs for grey seals have been designated in such countries [60,61].
1.2.2. Feeding Ecology
Both harbour and grey seals are generalist and benthic feeders [62], preying on a wide variety of taxa [63]. Nevertheless, differences in their diets can be found at regional and seasonal scales [24,64,65,66], subject to prey availability, distribution and abundance as well as additional factors in the surroundings of the haul-out sites [67,68]. Since both species are central-place foragers, they spend most of their time foraging within 50 km of the coast [69], although distances up to 300 and 1280 km have been recorded for harbour [70] and grey seal pups [63], respectively. In general, they travel for a few days (e.g., harbour seal trips lasted 1–3 days in Scotland [71,72]; grey seal trips lasted 2–5 days in the Baltic Sea [24,73], restricted to return onshore between foraging trips [74].
1.2.3. Breeding, Nursing and Communication
Both species exhibit high site fidelity, returning to the same haul-out sites to rest, moult and reproduce [28,75,76]. During the mating season, underwater acoustic displays and dives will be performed for male–male competition and fighting, as well as for territory defence and female attraction [77,78,79,80,81,82]. Several studies around the world have described the loud vocalisations emitted by the males during these dives: a broadband, nonharmonic roar, with energy ranging from 0.05 to 4 kHz of frequency being the most displayed by harbour seals [83,84,85,86,87]; and a small repertoire of short, broadband calls described as typically less than 3 kHz, with clicks with harmonics reaching 15–30 kHz emitted by grey seals [37,88,89]. Recently, forelimb claps were observed as underwater percussive signals in grey seals, which may be attributed to a warning for potential competitors and/or a sign of fitness to females [90].
However, despite certain similarities, grey seals are found to be more vocal [37,91,92,93] and spend more time at sea below the surface [94], and their moulting and breeding seasons occur at different times of the year [75,95,96], and whereas harbour seal pups can enter the water almost immediately after birth [97], grey seal pups remain on land for around 18 days to nurse [41]. During the lactation period, mother and pup remain in constant contact [98], although female grey seals have been reported to leave their pup alone while they spend several hours in the water [99]. Therefore, vocal communication and recognition occur during the mentioned period [100,101,102,103], with pups displaying individually distinctive calls of different frequencies and harmonic patterns to attract their mother’s attention; these calls are no longer included in their vocal repertoire once weaned [99,104,105,106,107,108]. In general, female harbour seals barely vocalise, except when they are on land, in order to communicate with their offspring and/or to threaten other seals [83]. Similarly, female grey seals may use acoustic signals during certain social interactions, but they almost never do so with their offspring [99].
1.3. Research Objectives
This study is focused on the acoustic data collected with a SoundTrap in the Malin Sea across a 10-month period as part of the SeaMonitor project [109]. Such data were processed, analysed and compared with the results of new studies on seal vocalisations to (1) evaluate the acoustic detectability of these species in offshore waters; (2) describe the acoustic characteristics of harbour and grey seals present in the area; (3) assess differences in their vocalisations between day and night, seasons, tidal states and locations.
Results from the study will help to (a) shed some light on the scarce knowledge of both species; (b) determine the importance of the area and detect periods when the animals are more acoustically active as well as evaluate the possible anthropogenic disturbance; (c) develop more studies of these characteristics across their distribution; (d) lay the foundation for the implementation of a future cross-border MPA network.
2. Materials and Methods
2.1. Study Area and Data Collection
This study was carried out using data collected during the SeaMonitor project in the Malin Sea. An acoustic array system consisting of three SoundTraps ST500 HF (Ocean Instruments, Auckland, New Zealand) was deployed as part of a larger telemetry tracking array from the R.Vs. Celtic Voyager (Marine Institute) of Queen of Ulster (DAERA) at an average depth of 55 m from October 2020 to October 2022 (Figure 1). Data from March 2021 to December 2021 from the station 76 (55°37′12.9″ N 6°46′35.4″ W) were analysed during the present study. An extended use of the area by harbour and grey seals has been reported through satellite telemetry studies and survey counts in haul-outs along the coast [44,110].
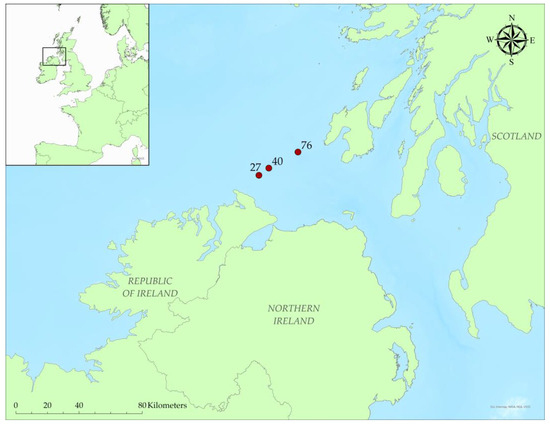
Figure 1.
Deployment sites (red dots) of acoustic receivers between the UK and the Republic of Ireland. Station ID numbers are also indicated.
Such a station was designed as a single point mooring and comprised 70 kg of sacrificial iron chain links, a VEMCO VR2AR telemetry unit, which was also used as an acoustic release, the SoundTrap, standing 2–3 m above the seafloor, and two subsurface buoys (Figure 2). SoundTrap units were set to record for 20 min every hour, at a sampling rate of 96 kHz [111].
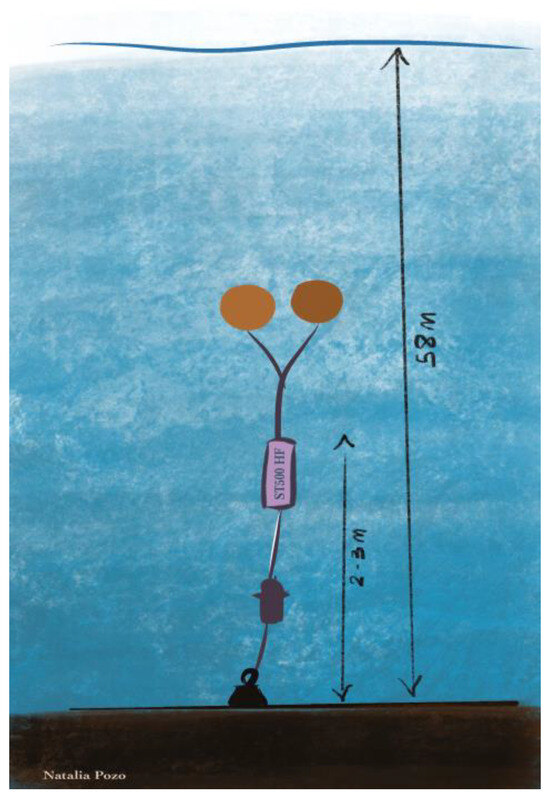
Figure 2.
Diagram of the mooring system of station 76. From bottom to top: anchor weights, VEMCO unit, SoundTrap and buoys.
2.2. Data Processing and Extraction
The recorded audio files were decompressed through SoundTrap Host software (version 4.0.13; available at https://www.oceaninstruments.co.nz/downloads/; accessed on 20 February 2023) and the resulting .wav files were processed in PAMGuard, version 2.02.07 [112]. To do so, data were downsampled to 5 kHz with a decimator, and a low-frequency fast Fourier transformation (FFT) module with a FFT length of 512 and FFT Hop of 256 and a whistle and moan detector with a maximum frequency of 5 kHz were selected. The processed data were then explored in PAMGuard Viewer Mode and detections were manually annotated in Raven Lite, version 2.0.4 [113], which was set to show spectrograms at FFT resolution of 2456 bands (window size), a maximum frequency of 5000 Hz and time resolution of 10 s. Spectrogram brightness and contrast were set to 71 and 74, respectively, but modified when needed depending on the file. The type of window selected was Default 1.3 Power. Different parameters were extracted per vocalisation (Table 1), following a similar approach to studies carried out on pinniped calls [34,35,83].

Table 1.
Parameters extracted from spectrograms in Raven Lite.
In order to assess the possible influence of diel (day/night), season (pre-breeding/breeding) and tides in the vocal behaviour of the species, sunrise and sunset hours were extracted per recording date (https://www.timeanddate.com, accessed on 5 December 2023) and sea surface height was downloaded from Copernicus Marine Environment Service (http://marine.copernicus.eu, accessed on 5 December 2023). In RStudio, version 4.2.2 [114], day was defined as the time span between one hour before the sunrise and two hours before the sunset, and night was defined as the time span between one hour before the sunset and two hours before the sunrise [58,115]. Sea surface height (tidal elevation) was used to calculate high and low tides with VulnToolkit R package, version 1.1.4 [116]. These values were then used to calculate and classify tidal states as high (H), low (L), ebb (E, between high and low tide) and flood (F, between low and high tide) in RStudio, following the methodology employed by [115].
2.3. Data Analysis
2.3.1. Classification of Vocalisations
Harbour and grey seal vocalisations were manually classified based on aural and visual features such as shape, frequency and call descriptions given by previous studies [34,37,83,92,108,117]. In this way, six different categories were defined for harbour seals (Figure 3) and according to those described by [83,108,117]. Ten categories were also defined for grey seals (Figure 5), following those described by [34,92] when finding similarities, and creating new ones for those that were reported for the first time. Following a similar approach described by [34], two-part calls such as “Rupe C” (Type 2C) and Type 5 as well as multiple element vocalisations like “Guttural rupe” (Type 1), “Cry” (Type 9) and “Pop” (Type 10) were selected as a single detection (Figure 5).
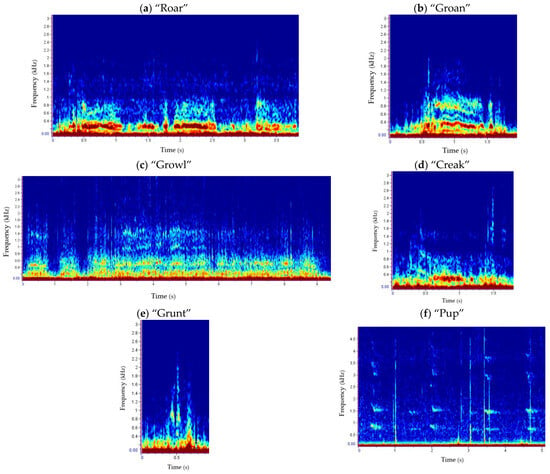
Figure 3.
Underwater repertoire of harbour seals in the Malin Sea: “roar” (a), “groan” (b), “growl” (c), “creak” (d) and “grunt” (e) calls corresponding with adult individuals, and “pup” (f) with young seals. Spectrograms show the frequency (kHz) versus time (s) per vocalisation type.
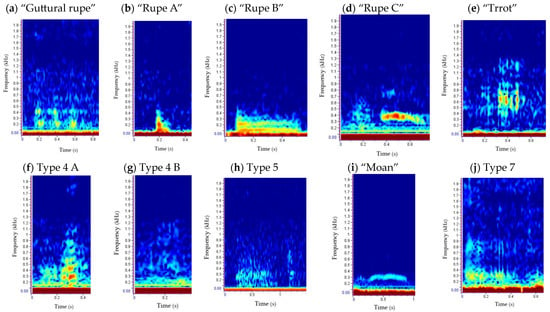
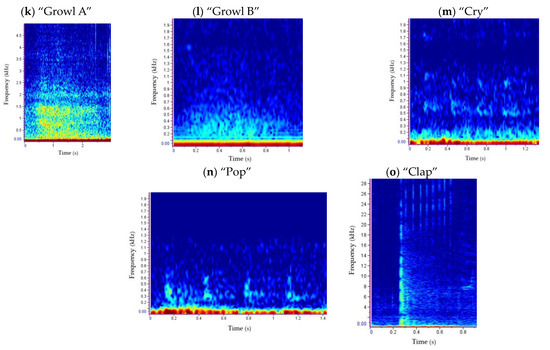
Figure 5.
Underwater repertoire of grey seals in the Malin Sea: call type 1 “guttural rupe” (a), call type 2 “rupe” (A, (b); B, (c); C, (d)), call type 3 “trrot” (e), call type 4 (A, (f); B, (g)), call type 5 (h), call type 6 “moan” (i), call type 7 (j), call type 8 “growl” (A, (k); B, (l)), call type 9 “cry” (m) and call type 10 “pop” (n). Spectrograms show the frequency (kHz) versus time (s) per vocalisation type. The spectrogram of a “clap” is also presented (o), although this is not a vocalisation.
Mean and standard deviations of each call parameter were calculated in RStudio, version 4.2.2 [114], as well as the proportion of vocalisations per call type (Table 2 and Table 3). Moreover, quantitative classifications of harbour and grey seal calls were made using non-parametric classification tree analyses [118], following the methodology used by [34,119] to classify grey and harbour seal vocalisations, respectively. Such methodology is recommended for acoustic data collected using a single recorder [120,121], where some pseudo-replication can be present, and allows for validation of the manual classifications. Classification trees were built with rpart R package, version 4.1.19 [122], using all the acoustic parameters. The trees were split into nodes using the Gini index, validated with cross-validated error values and the complexity parameter (Figure A1 and Figure A2, Table A1 and Table A2), and finally pruned using the “One Standard Error Rule” (1 SE rule) to avoid model overfitting and to ensure the best result [118].

Table 2.
Quantitative description (mean ± SD) of each harbour seal call (n = 403). Proportion of vocalisations (N) per type is also presented.

Table 3.
Quantitative description (mean ± SD) of each grey seal call (n = 8554). Proportion of vocalisations (N) per type is also presented.
2.3.2. Influence of Season, Diel and Tides
Due to the small sample obtained for harbour seals, further analyses were only performed on grey seal data. Differences in the vocalisation rate (calls·min−1) of grey seals between day and night and pre-breeding (May–August) and breeding (September–December) seasons, as well as under the four different tidal states, were also assessed. The number of calls emitted per minute was first calculated, and then data exploration and visualisation were carried out using Q-Q plots to check for normality and differences in the vocalisation rate between diel, seasons and tidal states with Wilcoxon test; bar plots and boxplots were carried out in RStudio, version 4.2.2 [114].
Generalised Linear Mixed Models (GLMMs) with Poisson distribution [123] were the chosen test statistics and were performed using lme4 R package, version 1.1-33 [124], with the vocalisation rate as the response variable, and the diel, season, tidal state and interaction between season and tide as the explanatory variables. Date was included in the model as a random factor to account for potential pseudo-replication. Such an approach was also carried out by [58], and allowed for the examination of the possible differences in the results. Model selection was based on the Akaike information criterion (AIC) and later on, collinearity, overdispersion and residual plots were inspected in order to validate the model [125,126]. Finally, pairwise comparisons between tidal states and the interaction between season and tides were conducted with emmeans R package, version 1.8.6 [127].
3. Results
3.1. Harbour Seal Vocalisations
Overall, 403 harbour seal vocalisations (333 corresponding to adult and 70 to young individuals) were detected and classified into six categories (Figure 3). Call type and number of detections per type differed across the seasons (Table A3).
The call types “roar” and “creak” (Figure 3a,d) were the least detected vocalisations, being both only recorded twice in summer (Table A3). Roars had a mean duration (±SD) of 3.36 s (±0.60) and a mean high frequency of 1022 Hz (±10) (Table 2). The beginning of these calls was characterised by a “breathing” sound, like if the animals were taking air through the mouth before vocalising. Creaks, on the other hand, presented a higher frequency range, 2460 Hz (±181) (Table 2), and their sound was similar to a rusty hinged door being opened or closed [83].
Call types “groan” and “grunt” (Figure 3b,e) showed similar mean low and high frequencies and lasted less than a second on average; however, grunts were shorter, at 0.26 s (±0.14) (Table 2). Groans sounded like moaning and were found at different frequencies, whereas grunts presented a variety of shapes and aural sounds, including “waap”, “ruurh”, “wahwap” and “hhuh”, as described by [83].
Growls (Figure 3c) were the most recorded vocalisation and only present in winter (Table A3). This type of call had different durations, with a mean of 2.30 s (±2.10) (Table 2), finding normally long growls (the longest lasted 11.55 s) right after short growls. Such vocalisations had a characteristic hoarse sound, and despite the similar shape to those described by [83,117], their mean high frequency was much higher, at 2533 Hz (±1298) (Table 2).
Harbour seal pups were also detected in summer and at the beginning of autumn (Table A3). This call type (Figure 3f) was short in duration with a mean of 0.35 s (±0.10), and a mean fundamental frequency of 686 Hz (±405). The presence of harmonics gave this call a wide frequency range (Table 2). This vocalisation sounded like a dog puppy crying very shortly, sometimes similar to a “mew”, and uttered most of the times in series of 2–4 elements, although a series of up to 47 elements was also registered.
A quantitative classification of the harbour seal calls was also performed through classification trees (Figure 4). Such a classification was only carried out with adult calls, removing harbour seal pup calls from the analysis. All acoustic parameters showed in Table 2 were included in the analysis. The misclassification rate for this tree was 12.91%.
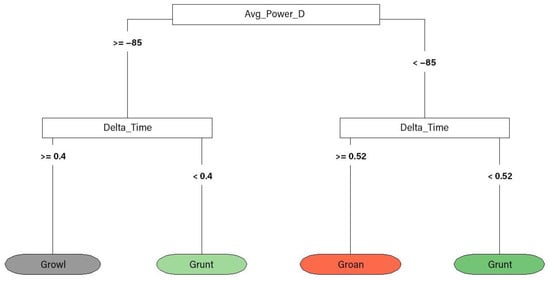
Figure 4.
Quantitative classification tree of harbour seal vocalisation types. The main factors determining splits were average power density (Avg_Power_D) and duration (Delta_Time).
3.2. Grey Seal Vocalisations
3.2.1. Call Descriptions and Classification
A total of 8554 grey seal vocalisations (1637 during the pre-breeding and 6917 during the breeding seasons) were detected and classified into ten categories (Figure 5). Call type and number of detections per type differed between day and night (Figure 6) and pre-breeding and breeding seasons (Figure 7), as well as under different tidal states (Figure A3).
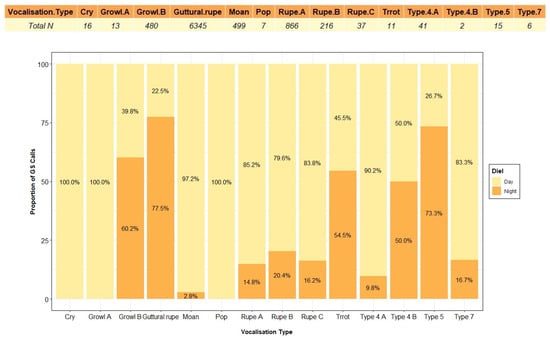
Figure 6.
Proportion of grey seal calls for each vocalisation type recorded during day and night. On the top, the total number of detections (Total N) per vocalisation type is given.
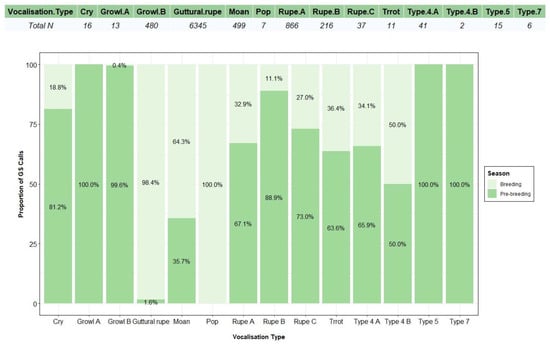
Figure 7.
Proportion of grey seal calls for each vocalisation type recorded during pre-breeding and breeding seasons. On the top, the total number of detections (Total N) per vocalisation type is given.
Call type 1 “guttural rupe” (Figure 5a) was more abundant during the night (77.5%), with most of the detections occurring during breeding season (Figure 6 and Figure 7). This multiple element vocalisation showed different shapes (Figure A4), with a mean frequency range of 262 Hz (±127) and a duration of 0.57 s (±0.14) (Table 3). Most of the detections were found in series of 3 elements; however, pulses of 1–9 elements were also recorded. Such a type showed some similarities with “guttural rup” described by [92] and types 1 and 5 defined by [34].
Call type 2 “rupe” presented three subtypes (Figure 5b–d), with all of them being most displayed during the day and pre-breeding season (Figure 6 and Figure 7). “Rupe A” was the most abundant of all subtypes, as well as the shortest in duration, at 0.14 s (±0.08) (Table 3). Such a subtype was characterised by its cone shape and “pu” sound. “Rupe B” was similar to “Rupe A” in mean frequency range, 339 Hz (±97) (Table 3) and sound; however, the main difference lay at the mid/end of the call, with the sound being more constant (“puuu”). Both subtypes were often emitted in succession. “Rupe C” was a two-part call, emitted at a higher frequency range, 434 Hz (±251); and duration, 0.36 s (±0.19) (Table 3). The first part of the vocalisation was similar to “Rupe A” in shape and sound, whereas the second part was more similar to call type 6, “moan” (Figure 5i). All these subtypes were compared to “rupe”, described by [92]; and call type 8, defined by [34].
Call type 3 “trrot” (Figure 5e) was one of the least recorded, being mostly emitted during the night and pre-breeding season (Figure 6 and Figure 7). This vocalisation presented a wide frequency range, 944 Hz (±909) (Table 3), characterised by a series of 2–3 pulses comparable to a drumbeat, and sometimes found in series of up to 40 repetitions. Such a call was compared with type 6 [34] and “trrot” [92].
Type 4 presented two subtypes (Figure 5f,g). Type 4A was more abundant during the day and pre-breeding season, whereas Type 4B was only recorded twice, once per diel and season (Figure 6 and Figure 7). With similar durations and ascending shapes, the latter subtype doubled the mean frequency range of Type 4A, with 723 Hz (±666) (Table 3). Both subtypes were similar in sound to type 4 described by [34,92].
Type 5 (Figure 5h) was only recorded in the pre-breeding season (Figure 7), occurring 73.3% of the time during the night (Figure 6). This less common vocalisation was made up of two parts, the first being constant and the second being similar to a “guttural rupe” (Figure 5a). Such a vocalisation had a mean frequency range of 477 Hz (±134), almost half of type 3, described by [34], but it was longer in duration, lasting 1.13 s (±0.38) (Table 3), like type 6, described by [92].
Call type 6 “moan” (Figure 5i) was the third most abundant, being recorded 97.2% of the time during the day and 64.3% of the time in the breeding season (Figure 6 and Figure 7). This type had a mean high frequency of 388 Hz (±111) (Table 3) due to the presence of harmonics on some occasions, and different shapes (Figure A4). With a mean duration of 0.56 s (±0.46) (Table 3), moans were often found in series, with a maximum of 238 detections recorded in 20 min. This call type was very similar in shape to moans described by [92], but shorter in duration. Some similarities were also found with type 9, presented by [34].
Type 7 (Figure 5j) was an uncommon call only detected during the pre-breeding season (Figure 7), and 83.3% of the time during the day (Figure 6). Such a vocalisation was similar in shape and low frequency to type 7, described by [34]; however, it was characterised by a “purr” resonant sound, like emitted from the throat, of longer duration 0.48 s (±0.14) (Table 3).
Call type 8 “growl” was found in two subtypes (Figure 5k,l). Growl B was the most abundant of both subtypes, found 60.2% of the time during the day and 99.6% of the time during the pre-breeding season. Growl A was only recorded during the day and pre-breeding season (Figure 6 and Figure 7). The main difference between subtypes was on the duration and the frequency range, with Growl A lasting around four times more than Growl B, 4.10 s (±1.51), and with a frequency range nine times higher, 2711 Hz (±1429) (Table 3). Another difference was in the sound, defining Growl A as a “scraping” vocalisation, similar in shape to growls described by [92], whereas Growl B could be described as a “buzzing” sound of less energy, similar to type 10, described by [92], and with different shapes (Figure A4), such as type 2 in [34].
Call type 9, “cry” (Figure 5m), was only found during the day (Figure 6) at low tide (Figure A3), and 81.2% of the time in the pre-breeding season (Figure 7). Such a short vocalisation, with a mean duration of 0.97 s (±0.99) and never described before, was emitted in series of four elements most of the time. It had a higher low frequency, 363 Hz (±50), compared to the rest of the repertoire (Table 3), sounding like a dog “crying”.
Type 10, “pop” (Figure 5n), was only recorded 7 times, all of them during the day, at low tide and breeding season (Figure 6, Figure 7 and Figure A3). Such a vocalisation was similar in shape to type 9, “cry” (Figure 5m), and “Rupe A” (Figure 5b), but different in sound, which could be described as a short “pop”. Most of the time, it was emitted in a series of four elements, at a higher rate than cries, and the sound differed in some of them. This short vocalisation, with a mean duration of 0.88 s (±0.55) and a mean high frequency of 689 Hz (±85) (Table 3), is described in this study for the first time.
“Claps” (Figure 5o), despite not being a vocalisation, and therefore being excluded from the analysis, were detected three times in this study (two of them during the pre-breeding season). Such an acoustic signal was similar to those described by [90] but generally at lower frequencies (around 2–4 kHz), with the exception of the one shown in Figure 5o.
A non-parametric classification tree of the grey seal calls was also made (Figure 8), including all acoustic parameters shown in Table 3 in the analysis. The misclassification rate for this tree was 14.33%.
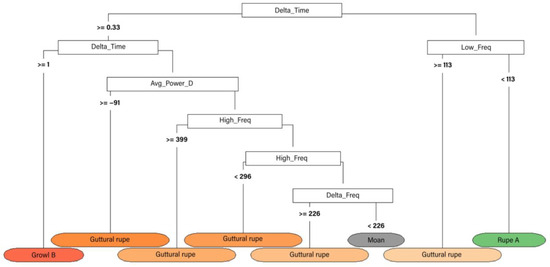
Figure 8.
Quantitative classification tree of grey seal vocalisation types. The main factors determining splits were duration (Delta_Time), low frequency (Low_Freq), average power density (Avg_Power_D), high frequency (High_Freq) and frequency range (Delta_Freq).
3.2.2. Diel, Seasonal and Tidal Patterns in the Vocalisation Rate
Means (±SD) of vocalisation rate per diel, season and tidal states were calculated. In this way, the mean grey seal vocalisation rate was higher during the day, 3.10 calls·min−1 (±3.75), than during the night, 2.78 calls·min−1 (±2.38) (Figure 9). The number of vocalisations per minute was also higher during breeding (3.14 ± 3.08 calls·min−1) than pre-breeding season (1.85 ± 2.03 calls·min−1) (Figure A5). When looking at the different tidal states, the highest vocalisation rate was recorded during low tide, with 3.34 calls·min−1 (±3.46), followed by ebb, with 3.03 calls·min−1 (±2.66); high tide, with 2.25 ± 1.78 calls·min−1; and flood tide, with 1.79 calls·min−1 (±1.14) (Figure A6).
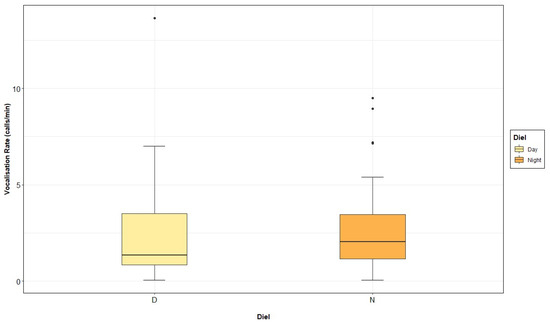
Figure 9.
Grey seal vocalisation rate (calls per minute) recorded in the Malin Sea per day and night.
Significant differences in the grey seal vocalisation rate between day and night (Figure 9; Table 4) as well as between seasons (Figure A5; Table 4) were found. The selected GLMM model also showed tidal states as a significant factor (Figure A6; Table 4), and results from the post hoc pairwise analysis displayed lower values of vocalisation rate at flood tide (Table A4). The interaction between season and tide was also significant, recording higher numbers of calls per minute during breeding and low tide periods, compared to pre-breeding ebb, high and low, and breeding ebb and high (Table 4 and Table A4; Figure 10). On the opposite side, vocalisation rate was significantly lower during pre-breeding and flood conditions, compared to the breeding ebb and high tide period and the pre-breeding low tide period (Table 4 and Table A4; Figure 10).

Table 4.
Results of the GLMM based on the AIC value. Differences in the vocalisation rate per diel, season and tidal state are shown. a Breeding was the reference category for season, day for diel, and ebb for tidal state. The asterisk (*) indicates different levels of significance for the p-value.
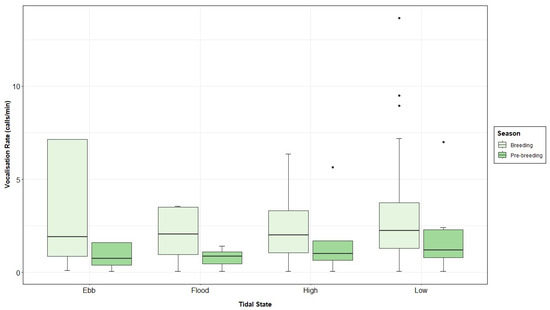
Figure 10.
Grey seal vocalisation rate (calls per minute) recorded in the Malin Sea per tidal state and season.
4. Discussion
This is the first study to report harbour and grey seal vocalisations in offshore waters. Underwater acoustic displays were detected during the period May–December 2021, and consequently analysed and classified, following and complementing previous studies on harbour [83,108,117] and grey seal [34,92] calls onshore.
4.1. Harbour Seal Vocalisations
Similarities and differences were found between the acoustic repertoires described here and those previously reported. A small sample of harbour seal detections, including adults and pups, were recorded: roars and creaks were only found during the breeding season (June–July), together with groans and grunts, which were also detected during the moulting season (August–September) but to a lesser extent. Growls were only recorded in November, accounting for 58.31% of all vocalisations (Table 2 and Table A3).
According to [128], only male harbour seals emit underwater vocalisations, which occur mainly during the pre-breeding and breeding seasons [79,85]. Despite detecting similar calls to those described by [83] on male breeding harbour seals in California, variations in the duration, frequency range and frequency of appearance are clearly presented here. In general, calls were shorter and with lower frequencies, except growls and groans, whose frequencies were higher than those of the Californian population (Table 2). Nevertheless, such differences may rely on the fact that the individuals belong to distinct populations, receiving the influence of different evolutionary, environmental, and life history factors [129], as well as the small sample size. On the other hand, it must be noted that this species has never been reported vocalising outside of the pre-breeding and breeding seasons as found during this study. Such a behaviour, only recorded on few occasions but at high intensity, that is, accounting for most of the registered calls, may be linked to a shift in their reproductive cycle, as reported in the Wadden Sea by [130]. Therefore, females may have given birth months later than expected, or even extended their oestrus. In addition, previous studies have shown that female harbour seals perform foraging trips in late lactation, that is, when they are in oestrus [131,132], attracting males to the region. Another possibility may be a different mating tactic displayed by the males in the area, as also seen in the Moray Firth, Scotland [85], where males were observed using distinct display areas (close to haul-out sites, transit routes or foraging areas); and in Monterey Bay, California [79], where male acoustic displays were detected peaking one month before peak oestrus when in groups, and peaking during peak oestrus when alone.
Harbour seal pups were mostly detected during the summer (June–July), coinciding with the pupping season, but some vocalisations were also found in August (Table A3). The frequency range of these calls, and the way they were emitted, corresponded with the description provided by [108], giving information on the level of stress through the number of calls and their rate of emission [133]. The fact that seal pups were detected during this study lays the foundation on new research questions to be explored, since up until now, only a few studies have focused on their vocal development [134,135], plasticity [136] and rhythms [137,138,139,140] in air and in captive/controlled conditions. In the wild, studies have been conducted on their behaviour towards their mother while hauled out [98,102] and have also reported long-distance movements in offshore waters through satellite telemetry [70]. Therefore, seal pups may be vocalising throughout these offshore trips not only to grab their mother’s attention as previously thought, but also to communicate with other pups.
The classification tree built with adult calls (Figure 4) only included growls, grunts and groans in the final model based on their duration and average power density. The fact that the other categories that were excluded were only detected twice may have had an influence on the result; however, the final tree showed a good separation, similar to the manual classification reported by [83]. Further studies will allow for testing of the accuracy of these results.
4.2. Grey Seal Vocalisations
Grey seals were detected more frequently and in greater number than harbour seals, with most of the detections occurring during the breeding season. Such results agree with previous studies, which affirm that this species is more vocal [37,91,92,93]. Similarities and differences in the acoustic parameters and shape of the vocalisations detected in this study were found with those described by [92] in Scotland and [34] in the Blasket Islands, Ireland. In general, calls were shorter in duration and presented lower frequencies than those of the Scottish population. Compared to the Irish population, calls such as “guttural rupe” and “trrot” were similar in frequency and duration to types 1 and 8 and to type 6, respectively (Figure 5; Table 3).
“Guttural rupe”, previously reported as the most numerous vocalisation type during the breeding season by [34,37,92,141], accounted for 74.18% of the calls, mostly emitted during the breeding season at night (Figure 6 and Figure 7) and in accordance with these studies. Such a result reinforces the hypothesis defined by [34] regarding the important role of this call in the communication, territorial defence and mating of the species.
“Rupes” were all more abundant during the day and pre-breeding season (Figure 6 and Figure 7), accounting all subtypes for the 13.08% of the vocalisations. This call has been suggested to be uttered by females during female–male social interactions [37,92]; however, due to the characteristics of the present study, such a hypothesis cannot be confirmed here. In the Blasket Islands, this call type was also very common during the breeding season [34]. This difference in the seasonality may indicate that seals could spend more time offshore, foraging, during the day in the summer season, as reported in Canada by [142], and then move closer to the coast at night during the breeding season. Moreover, the fact that ambient and anthropogenic noise was present in the audio recordings may have covered these low-frequency vocalisations, and therefore reduced their detectability.
“Trrot” was a less common vocalisation, detected more at night during the pre-breeding season (Figure 6 and Figure 7). According to [143], this call type is produced by males during sexual and dominance interactions. Considering the low number of detections and the conditions of the study, no behaviours can be linked to it. Call types 4 and 5 were more detected in the pre-breeding season. Previously described by [92], their function remains unclear, although type 4 has been linked to “rupes”.
“Moans” were a more abundant vocalisation, recorded mostly during the day and breeding season (Figure 6 and Figure 7). Emitted by both males and females in a social interaction context [92,143], this call type was found more common during the summer by [92,141]. However, the results presented here show more similarities with those obtained by [34] in the Blasket Islands, where grey seals were observed during social interactions and emitting this call in the breeding season [144].
Almost all “growls” were recorded during the pre-breeding season, with “growl A” only found during the day and “growl B” more abundant at night (Figure 6 and Figure 7). Similar vocalisations were found by [37,92] during the breeding season, whereas [34] recorded them during both seasons, being more abundant in summer and suggesting the presence of early breeding males in the area. The difference in use between subtypes is still unknown.
Call type 7, “cry” and “pop” were reported for the first time during this study (Figure 5). Although some similarities in the shape were found with other vocalisations described by [34,92], sound and acoustic parameters did not coincide. “Type 7” and “cry” were mostly found during the pre-breeding season, whereas “pop” was only registered during the breeding season, suggesting a completely different use in communication. “Claps”, described as a mating tactic [90], were also recorded during both seasons, just like for the Irish locations.
All these calls were used to build a classification tree (Figure 8) where “guttural rupe”, “rupe A”, “moan” and “growl B” were the only ones included in the final model based on all the acoustic parameters presented in Table 3. In a similar way to the harbour seal classification analysis, the other categories may have been excluded because of their low frequency of appearance. The opposite trend may have occurred with “guttural rupes”, highly recorded during the study, and therefore with more variability that fits in the final categories of the tree. Further studies will allow an improvement of the results obtained here.
Significant differences were found in the vocalisation rate of the grey seals between day and night, producing a greater number of calls per minute during the day. These results are the opposite of those obtained in previous studies on grey [58], harbour [145] and harp seals [146], among others, which were, however, performed in coastal waters. The foraging behaviour of grey seals has been linked to oceanographic conditions and distribution of prey [142,147]. Such factors may have had an influence on the seals, being more acoustically present during the day in the area, and maybe moving towards the coast at night.
A higher number of vocalisations were recorded during the breeding season, as reported by [141] in the Gulf of Riga, finding significant differences in the vocalisation rate between the pre-breeding and breeding seasons. Such results are the opposite of those obtained by [58] in the Blasket Islands; however, the latter study was carried out in a short-term basis and from the coast, which could explain the differences. Moreover, due to the continuous monitoring of this study, the number of calls was seen to progressively decrease towards the end of the breeding season, similarly to [37].
Significant differences were also found in the vocalisation rate under the four tidal states, resulting in a higher number of vocalisations per minute at low tide and a lower number during flood tide. It is noticeable that [58] reported the opposite pattern, that is, the lowest vocalisation rate during low tide, linking this behaviour to space availability in the haul-out sites at low- and high-tide conditions [144], and/or feeding patterns [25,142,147]. The results obtained during the present study may suggest that a large number of grey seals are present in offshore waters during low tide and not necessarily resting onshore. Additional studies would help to reveal this type of behaviour. Another possibility would be linked to a higher detectability of low frequency sounds by the hydrophones during low tide conditions, since less ambient noise would be present in the recordings. A significantly higher vocalisation rate was found at low tide during the breeding season, possibly associated with a greater number of females present in the water [58,86], since mating can take place either in water or land for this species [41]. In addition, it could be suggested that the study area constitutes a travel corridor for grey seals, as seen in tracking studies [147], and therefore, males would be more acoustically active as reported in harbour seals [84]. A significantly lower vocalisation rate was recorded during the pre-breeding and flood conditions, which may be linked to foraging strategies displayed by the animals in the area [142,147,148].
4.3. Implications of the Study, Limitations and Future Recommendations
This study demonstrates that passive acoustic monitoring of seals can be feasible in offshore waters. To date, knowledge of both species has been mainly concentrated in their social behaviour at haul-out sites (e.g., through video recordings [98]), in-air and underwater vocal behaviour close to their colonies [92,112], their distribution while foraging (e.g., using GPS tags [142]), or the number of individuals present in the area (e.g., with aerial counts [44]). Nevertheless, although satellite telemetry has shown the movements of these species throughout the years, recording specimens of Irish and Scottish populations travelling between both countries [110], it is still unknown the proper use that they give to those areas and the risks that they can face around them.
Nowadays, thirty-six offshore windfarm projects are under different stages of development around Ireland as part of its commitment to net-zero carbon emissions no later than 2050 [149], and a similar trend is being followed in Scotland, with seventeen projects under development, such as the Malin Sea Wind, in order to be net zero by 2045 [150]. Furthermore, aquaculture farms and vessels can be found around the area. Since passive acoustic monitoring has been widely used to study cetaceans [17,18], the same approach can be applied to pinnipeds, which would allow for an understanding of their distribution, habitat use and communication throughout their whole range.
Several limitations were identified during the present study. Despite the long-term recordings, no vocalisations were detected from March until the end of May. This may be due to the high levels of noise recorded during that period, possibly linked to the spring tide, covering the low-frequency calls that these animals emit, or the possibility that there were no seals vocalising during those months. Moreover, the lack of visual monitoring impeded linking behaviour to the calls as well as understanding the number, age or sex of the individuals that were in fact vocalising.
As a result of the present work, future studies of these characteristics will allow for the gathering of more information on both species. As a future improvement, acoustic tags could be also used in some specimens of known sex and age in order to identify differences associated with these factors, and the vocalisations they use while travelling. Cameras would also help to record the behaviour displayed by the animals throughout their range. In the long-term, static acoustic monitoring seems a cost-effective, non-invasive method to study pinnipeds. Considering the different projects that have been focused on cetaceans, existing acoustic datasets can now be explored to search for seals. This would allow the gathering of enough data that can be used to model their distribution and habitat use, as well as to estimate their population size as an alternative option to visual surveys, which need the use of drones and more effort to collect the data. Finally, taking into account the conservation status of these species and the requirements to keep their populations and habitats in a favourable status, as demanded by the EU Habitats Directive (Council Directive 92/43/EEC) (Annex II), static acoustic monitoring studies will help to identify hotspots, key areas used by these species, and therefore design and maintain a cross-border MPA network between both countries and as a step forward to the protection of 30% of the oceans by 2030 [151]. It would also allow for regional monitoring of noise and its potential effects on these species, as required under the EU Marine Strategy Framework Directive (MSFD).
Author Contributions
Conceptualisation, Y.P.P.G. and J.O.; methodology, Y.P.P.G. and J.O.; software, Y.P.P.G.; validation, Y.P.P.G. and J.O.; formal analysis, Y.P.P.G.; investigation, Y.P.P.G., M.P. and J.O.; resources, M.P.T., M.P. and J.O.; data curation, Y.P.P.G.; writing—original draft preparation, Y.P.P.G.; writing—review and editing, M.P.T., M.P. and J.O.; visualisation, Y.P.P.G.; supervision, J.O.; project administration, J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research has received external funding by the Marine Institute under the Marine Research Programme (ref: NET/23/107) with the support of the Irish Government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The SeaMonitor consortium will make data publicly available on the project website.
Acknowledgments
We would like to thank the Marine Institute for their contribution towards the cost of the present publication. All data was collected by the SeaMonitor project. SeaMonitor was funded by the EU’s INTERREG VA Programme (Environment Theme), which was managed by the Special EU Programmes Body (SEUPB), with match-funding from the Department for Agriculture, Environment and Rural Affairs in Northern Ireland and the Department of Housing, Planning and Local Government in Ireland. Project Lead was the Loughs Agency.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Cross-validation results obtained for the classification tree analysis of harbour seals.
Table A1.
Cross-validation results obtained for the classification tree analysis of harbour seals.
| CP | nsplit | rel Error | xerror | xstd | |
|---|---|---|---|---|---|
| 1 | 0.39 | 0 | 1.00 | 1.00 | 0.08 |
| 2 | 0.17 | 1 | 0.61 | 0.67 | 0.07 |
| 3 | 0.06 | 2 | 0.44 | 0.53 | 0.07 |
| 4 | 0.04 | 3 | 0.38 | 0.44 | 0.06 |
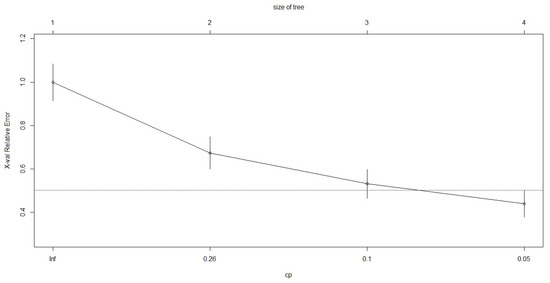
Figure A1.
Cross validation relative error plotted against complexity parameter obtained for the classification tree analysis of harbour seal calls.

Table A2.
Cross-validation results obtained for the classification tree analysis of grey seals.
Table A2.
Cross-validation results obtained for the classification tree analysis of grey seals.
| CP | nsplit | rel Error | xerror | xstd | |
|---|---|---|---|---|---|
| 1 | 0.27 | 0 | 1.00 | 1.00 | 0.02 |
| 2 | 0.09 | 1 | 0.73 | 0.74 | 0.02 |
| 3 | 0.04 | 2 | 0.64 | 0.65 | 0.02 |
| 4 | 0.01 | 3 | 0.61 | 0.62 | 0.02 |
| 5 | 0.01 | 7 | 0.55 | 0.56 | 0.02 |
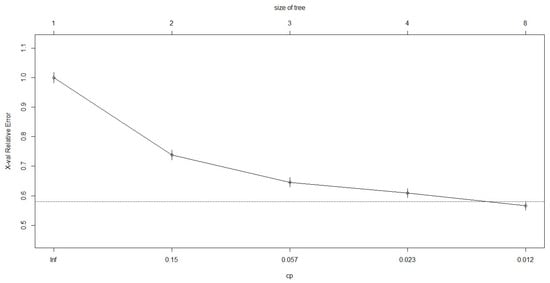
Figure A2.
Cross-validation relative error plotted against complexity parameter obtained for the classification tree analysis of grey seal calls.
Appendix B

Table A3.
Number of harbour seal underwater vocalisations detected per season: summer (May–July), autumn (August–October) and winter (November–January).
Table A3.
Number of harbour seal underwater vocalisations detected per season: summer (May–July), autumn (August–October) and winter (November–January).
| Roar | Groan | Growl | Creak | Grunt | Pup | |
|---|---|---|---|---|---|---|
| Summer | 2 | 32 | 0 | 2 | 55 | 59 |
| Autumn | 0 | 3 | 0 | 0 | 4 | 11 |
| Winter | 0 | 0 | 235 | 0 | 0 | 0 |
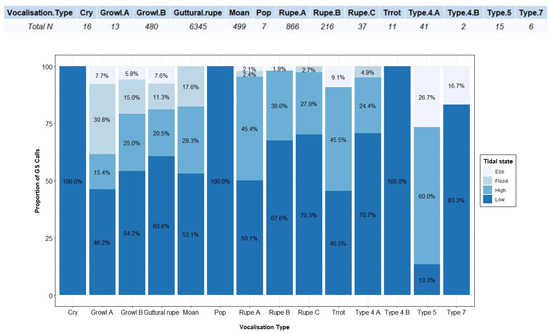
Figure A3.
Proportion of grey seal calls for each vocalisation type recorded during four tidal states: ebb, flood, high and low. On the top, the total number of detections (Total N) per vocalisation type is given.
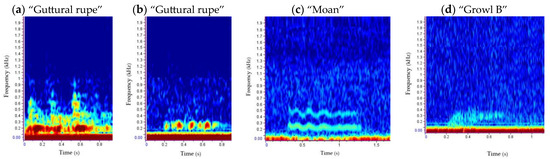
Figure A4.
Different shapes found in the underwater repertoire of grey seals in the Malin Sea. The first two (a,b) correspond to call type 1, “guttural rupe”. The third figure (c) is call type 6, “moan”, with harmonics and a wavy shape. The last figure (d) is an alternative shape to type 8, “growl B”.
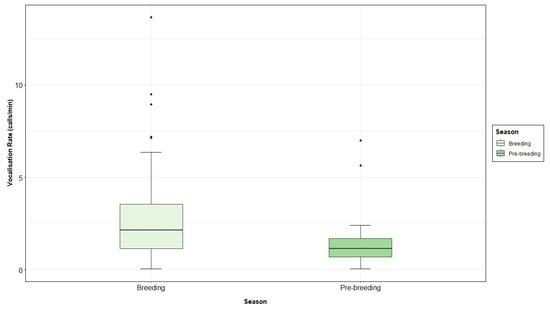
Figure A5.
Grey seal vocalisation rate (calls per minute) recorded in the Malin Sea per season.
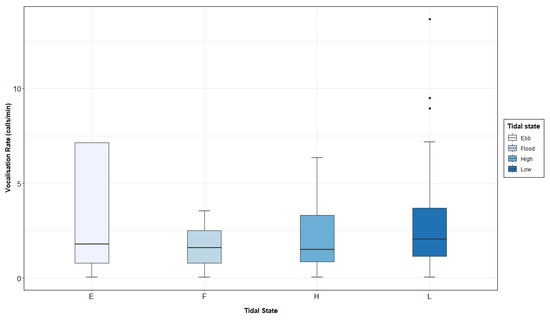
Figure A6.
Grey seal vocalisation rate (calls per minute) recorded in the Malin Sea per tidal state.

Table A4.
Results of the post hoc pairwise comparisons for tidal state and interaction between season and tidal states. Only those with significant differences in the vocalisation rate are shown.
Table A4.
Results of the post hoc pairwise comparisons for tidal state and interaction between season and tidal states. Only those with significant differences in the vocalisation rate are shown.
| Pairwise Comparison | Estimate | SE | Z Value | p Value |
|---|---|---|---|---|
| Tidal state | ||||
| Ebb-Flood | 0.29 | 0.02 | 11.82 | <0.0001 |
| Ebb-High | 0.33 | 0.02 | 15.28 | <0.0001 |
| Flood-Low | −0.31 | 0.02 | −16.83 | <0.0001 |
| High-Low | −0.35 | 0.01 | −29.95 | <0.0001 |
| Season × Tidal state | ||||
| Breeding × Ebb − Pre-breeding × Ebb | 0.54 | 0.17 | 3.14 | 0.0362 |
| Breeding × Ebb − Breeding × Flood | 0.54 | 0.009 | 63.37 | <0.0001 |
| Breeding × Ebb − Pre-breeding × Flood | 0.58 | 0.17 | 3.38 | 0.0168 |
| Breeding × Ebb − Breeding × High | 0.38 | 0.008 | 48.66 | <0.0001 |
| Breeding × Ebb − Pre-breeding × High | 0.83 | 0.17 | 4.88 | <0.0001 |
| Breeding × Ebb − Breeding × Low | −0.14 | 0.007 | −21.08 | <0.0001 |
| Breeding × Ebb − Pre-breeding × Low | 0.65 | 0.17 | 3.83 | 0.0033 |
| Pre-breeding × Ebb − Pre-breeding × High | 0.29 | 0.04 | 6.70 | <0.0001 |
| Pre-breeding × Ebb − Breeding × Low | −0.68 | 0.17 | −3.95 | 0.0020 |
| Breeding × Flood − Breeding × High | −0.17 | 0.008 | −21.40 | <0.0001 |
| Breeding × Flood − Pre-breeding × Low | −0.68 | 0.007 | −109.32 | <0.0001 |
| Pre-breeding × Flood − Breeding × High | 0.25 | 0.03 | 7.38 | <0.0001 |
| Pre-breeding × Flood − Pre-breeding × Low | −0.72 | 0.17 | −4.19 | 0.0007 |
| Breeding × High − Breeding × Low | −0.52 | 0.005 | −96.85 | <0.0001 |
| Pre-breeding × High − Breeding × Low | −0.97 | 0.17 | −5.70 | <0.0001 |
| Pre-breeding × High − Pre-breeding × Low | −0.18 | 0.02 | −7.95 | <0.0001 |
| Breeding × Low − Pre-breeding × Low | 0.79 | 0.17 | 4.65 | 0.0001 |
References
- Boyd, I.L.; Wanless, S.; Camphuysen, C.J. Top Predators in Marine Ecosystems: Their Role in Monitoring and Management; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Piatt, J.F.; Sydeman, W.J. Introduction: A modern role for seabirds as indicators. Mar. Ecol. Prog. Ser. 2007, 352, 199–204. [Google Scholar] [CrossRef]
- Bowen, W.D.; Beck, C.A.; Austin, D.A. Pinniped Ecology. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J., Kovacs, K., Eds.; Academic Press, An Imprint of Elsevier: London, UK, 2009; pp. 852–861. [Google Scholar]
- Schusterman, R.J.; Van Parijs, S.M. Pinniped vocal communication: An introduction. Aquat. Mamm. 2003, 29, 177–180. [Google Scholar] [CrossRef]
- Todd, V.L.G.; Todd, I.B.; Gardiner, J.C.; Morrin, C.N. Marine Mammal Observer and Passive Acoustic Monitoring Handbook; Pelagic Publishing: Exeter, UK, 2015. [Google Scholar]
- Mizuguchi, D.; Tsunokawa, M.; Kawamoto, M.; Kohshima, S. Underwater vocalizations and associated behavior in captive ringed seals (Pusa hispida). Polar Biol. 2016, 39, 659–699. [Google Scholar] [CrossRef]
- Gelatt, T.; Ream, R.; Johnson, D. Callorhinus ursinus. The IUCN Red List of Threatened Species 2015: e.T3590A45224953. 2015. Available online: https://www.iucnredlist.org/species/3590/45224953 (accessed on 15 February 2023).
- Hofmeyr, G.J.G. Arctocephalus gazella. The IUCN Red List of Threatened Species 2016: e.T2058A66993062. 2016. Available online: https://www.iucnredlist.org/species/2058/66993062 (accessed on 15 February 2023).
- Littnan, C.; Harting, A.; Baker, J. Neomonachus schauinslandi. The IUCN Red List of Threatened Species 2015: e.T13654A45227978. 2015. Available online: https://www.iucnredlist.org/species/13654/45227978 (accessed on 15 February 2023).
- Goldsworthy, S.D. Neophoca cinerea. The IUCN Red List of Threatened Species 2015: e.T14549A45228341. 2015. Available online: https://www.iucnredlist.org/species/14549/45228341 (accessed on 15 February 2023).
- Goodman, S.; Dmitrieva, L. Pusa caspica. The IUCN Red List of Threatened Species 2016: e.T41669A45230700. 2016. Available online: https://www.iucnredlist.org/species/41669/45230700 (accessed on 15 February 2023).
- Trillmich, F. Arctocephalus galapagoensis. The IUCN Red List of Threatened Species 2015: e.T2057A45223722. 2015. Available online: https://www.iucnredlist.org/species/2057/45223722 (accessed on 15 February 2023).
- Avila, I.; Kaschner, K.; Dormann, C. Current global risks to marine mammals: Taking stock of the threats. Biol. Conserv. 2018, 221, 44–58. [Google Scholar] [CrossRef]
- Kovacs, K.M.; Aguilar, A.; Aurioles, D.; Burkanov, V.; Campagna, C.; Gales, N.; Gelatt, T.; Goldsworthy, S.D.; Goodman, S.J.; Hofmeyr, G.J.G.; et al. Global threats to pinnipeds. Mar. Mammal Sci. 2012, 28, 414–436. [Google Scholar] [CrossRef]
- Balcomb, K.C.; Claridge, D. A mass stranding of cetaceans caused by naval sonar in the Bahamas. Bahamas J. Sci. 2001, 5, 2–12. [Google Scholar]
- Berrow, S.; Cosgrove, R.; Leeney, R.H.; O’Brien, J.; McGrath, D.; Dalgard, J.; Le Gall, Y. Effect of acoustic deterrents on the behaviour of common dolphins (Delphinus delphis). J. Cetacean Res. Manag. 2008, 10, 227–233. [Google Scholar] [CrossRef]
- Berrow, S.D.; O’Brien, J.; Meade, R.; Delarue, J.; Kowarski, K.; Martin, B.; Moloney, J.; Wall, D.; Gillespie, D.; Leaper, R.; et al. Acoustic Surveys of Cetaceans in the Irish Atlantic Margin in 2015–2016: Occurrence, Distribution and Abundance; Department of Communications, Climate Action & Environment and the National Parks and Wildlife Service (NPWS), Department of Culture, Heritage and the Gaeltacht: Dublin, Ireland, 2018; p. 348. [Google Scholar]
- Hammond, P.S.; Macleod, K.; Gillespie, D.; Swift, R.; Winship, A.; Burt, M.L.; Cañadas, A.; Vázquez, J.A.; Ridoux, V.; Certain, G.; et al. Cetacean Offshore Distribution and Abundance in the European Atlantic (CODA); Final Report; University of St Andrews: St Andrews, UK, 2009. [Google Scholar]
- Richardson, W.J.; Green, C.R., Jr.; Malme, C.I.; Thomson, D.H. Marine Mammals and Noise; Academic: San Diego, CA, USA, 1995. [Google Scholar] [CrossRef]
- Edrén, S.M.C.; Andersen, S.M.; Teilmann, J.; Carstensen, J.; Harders, P.B.; Dietz, R.; Miller, L.A. The effect of a large Danish offshore wind farm on harbor and gray seal haul-out behavior. Mar. Mammal Sci. 2010, 26, 614–634. [Google Scholar] [CrossRef]
- Findlay, C.R.; Hastie, G.D.; Farcas, A.; Merchant, N.D.; Risch, D.; Wilson, B. Exposure of individual harbour seals (Phoca vitulina) and waters surrounding protected habitats to acoustic deterrent noise from aquaculture. Aquat. Conserv. Mar. Freshw. 2022, 32, 766–780. [Google Scholar] [CrossRef]
- Jones, E.L.; Hastie, G.D.; Smout, S.; Onoufriou, J.; Merchant, N.D.; Brookes, K.L.; Thompson, D. Seals and shipping: Quantifying population risk and individual exposure to vessel noise. J. Appl. Ecol. 2017, 54, 1930–1940. [Google Scholar] [CrossRef]
- Cunningham, L.; Baxter, J.M.; Boyd, I.L.; Duck, C.D.; Lonergan, M.; Moss, S.E.; McConnell, B. Harbour seal movements and haul-out patterns: Implications for monitoring and management. Aquat. Conserv. Mar. Freshw. 2009, 19, 398–407. [Google Scholar] [CrossRef]
- McConnell, B.J.; Fedak, M.A.; Lovell, P.; Hammond, P.S. Movements and foraging areas of grey seals in the North Sea. J. Appl. Ecol. 1999, 36, 573–590. [Google Scholar] [CrossRef]
- Sharples, R.J.; Moss, S.E.; Patterson, T.A.; Hammond, P.S. Spatial Variation in Foraging Behaviour of a Marine Top Predator (Phoca vitulina) Determined by a Large-Scale Satellite Tagging Program. PLoS ONE 2012, 7, e37216. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.M.; Josephson, E.; Wood, S.A.; Murray, K.; Waring, G. Trends and Patterns of Seal Abundance at Haul-Out Sites in a Gray Seal Recolonization Zone; NOAA Technical Memorandum NMFS-NE-25. US Department of Commerce; NOAA: Woods Hole, MA, USA, 2019.
- Russell, D.J.F.; Morris, C.D.; Duck, C.D.; Thompson, D.; Hiby, L. Monitoring long-term changes in UK grey seal pup production. Aquat. Conserv. Mar. Freshw. 2019, 29, 24–39. [Google Scholar] [CrossRef]
- Gerondeau, M.; Barbraud, C.; Ridoux, V.; Vincent, C. Abundance estimate and seasonal patterns of grey seal (Halichoerus grypus) occurrence in Brittany, France, as assessed by photo-identification and capture–mark–recapture. J. Mar. Biol. Assoc. UK 2007, 87, 365–372. [Google Scholar] [CrossRef]
- Sayer, S.; Allen, R.; Hawkes, L.A.; Hockley, K.; Jarvis, D.; Witt, M.J. Pinnipeds, people and photo identification: The implications of grey seal movements for effective management of the species. J. Mar. Biol. Assoc. UK 2019, 99, 1221–1230. [Google Scholar] [CrossRef]
- Arias-del-Razo, A.; Heckel, G.; Schramm, Y.; Pardo, M.A. Terrestrial habitat preferences and segregation of four pinniped species on the islands off the western coast of the Baja California Peninsula, Mexico. Mar. Mammal Sci. 2016, 32, 1416–1432. [Google Scholar] [CrossRef]
- Reisinger, R.R.; Raymond, B.; Hindell, M.A.; Bester, M.N.; Crawford, R.J.M.; Davies, D.; de Nico Bruyn, P.J.; Dilley, B.J.; Kirkman, S.P.; Makhado, A.B.; et al. Habitat modelling of tracking data from multiple marine predators identifies important areas in the Southern Indian Ocean. Divers. Distrib. 2018, 24, 535–550. [Google Scholar] [CrossRef]
- Ventura, F.; Matthiopoulos, J.; Jeglinski, J.W.E. Minimal overlap between areas of high conservation priority for endangered Galapagos pinnipeds and the conservation zone of the Galapagos Marine Reserve. Aquat. Conserv. Mar. Freshw. 2019, 29, 115–126. [Google Scholar] [CrossRef]
- Cziko, P.A.; Munger, L.M.; Santos, N.R.; Terhune, J.M. Weddell seals produce ultrasonic vocalizations. J. Acoust. Soc. Am. 2020, 148, 3784–3796. [Google Scholar] [CrossRef]
- Pérez Tadeo, M.; Gammel, M.; O’Brien, J. First Steps towards the Automated Detection of Underwater Vocalisations of Grey Seals (Halichoerus grypus) in the Blasket Islands, Southwest Ireland. J. Mar. Sci. Eng. 2023, 11, 351. [Google Scholar] [CrossRef]
- McCreery, L.; Thomas, J.A. Acoustic Analysis of Underwater Vocalizations from Crabeater Seals (Lobodon carcinophagus): Not So Monotonous. Aquat. Mamm. 2009, 35, 490–501. [Google Scholar] [CrossRef]
- Llobet, S.M.; Ahonen, H.; Lydersen, C.; Berge, J.; Ims, R.; Kovacs, K.M. Bearded seal (Erignathus barbatus) vocalizations across seasons and habitat types in Svalbard, Norway. Polar Biol. 2021, 44, 1273–1287. [Google Scholar] [CrossRef]
- Asselin, S.; Hammill, M.O.; Barrette, C. Underwater vocalizations of ice breeding grey seals. Can. J. Zool. 1993, 71, 2211–2219. [Google Scholar] [CrossRef]
- Van Parijs, S.M.; Corkeron, P.J.; Harvey, J.; Hayes, S.A.; Mellinger, D.K.; Rouget, P.A.; Thompson, P.M.; Wahlberg, M.; Kovacs, K.M. Patterns in the vocalizations of male harbor seals. J. Acoust. Soc. Am. 2003, 113, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Cordes, L.S.; Duck, C.D.; Mackey, B.L.; Hall, A.J.; Thompson, P.M. Long-term patterns in harbour seal site-use and the consequences for managing protected areas. Anim. Conserv. 2011, 14, 430–438. [Google Scholar] [CrossRef]
- Bowen, D. Halichoerus grypus. The IUCN Red List of Threatened Species 2016: e.T9660A45226042. 2016. Available online: https://www.iucnredlist.org/species/9660/45226042 (accessed on 15 February 2023).
- Hall, A.; Thompson, D. Gray Seal. Halichoerus grypus. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J., Kovacs, K., Eds.; Academic Press, An Imprint of Elsevier: London, UK, 2009; pp. 500–503. [Google Scholar]
- Lowry, L. Phoca vitulina. The IUCN Red List of Threatened Species 2016. 2016. Available online: https://www.iucnredlist.org/species/17013/45229114 (accessed on 15 February 2023).
- Blanchet, M.; Vincent, C.; Womble, J.; Steingass, S.; Desportes, G. Harbour Seals: Population Structure, Status, and Threats in a Rapidly Changing Environment. Oceans 2021, 2, 41–63. [Google Scholar] [CrossRef]
- Carter, M.I.D.; Boehme, L.; Duck, C.D.; Grecian, W.J.; Hastie, G.D.; McConnell, B.J.; Miller, D.L.; Morris, C.D.; Moss, S.E.W.; Thompson, D.; et al. Habitat-Based Predictions of At-Sea Distribution for Grey and Harbour Seals in the British Isles; Report to BEIS, OESEA-16-76/OESEA-17-78 2020; University of St Andrews: St Andrews, UK, 2020. [Google Scholar]
- Thompson, D.; Duck, C.D.; Morris, C.D.; Russell, D.J.F. The status of harbour seals (Phoca vitulina) in the UK. Aquat. Conserv. Mar. Freshw. 2019, 29, 40–60. [Google Scholar] [CrossRef]
- Lonergan, M.; Duck, C.; Moss, S.; Morris, C.; Thompson, D. Rescaling of aerial survey data with information from small numbers of telemetry tags to estimate the size of a declining harbour seal population. Aquat. Conserv. Mar. Freshw. 2013, 23, 135–144. [Google Scholar] [CrossRef]
- Thompson, D.; Duck, C.; Lonergan, M.E. The status of harbour seals (Phoca vitulina) in the United Kingdom. NAMMCO Sci. Publ. 2010, 8, 117. [Google Scholar] [CrossRef]
- Jones, E.L.; Sparling, C.E.; McConnell, B.J.; Morris, C.D.; Smout, S. Fine-scale harbour seal usage for informed marine spatial planning. Sci. Rep. 2017, 7, 11581. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.J.; Hammond, P.S. The diet of harbour and grey seals around Britain: Examining the role of prey as a potential cause of harbour seal declines. Aquat. Conserv. Mar. Freshw. 2019, 29, 71–85. [Google Scholar] [CrossRef]
- Cronin, M.A. The status of the harbour seal (Phoca vitulina) in Ireland. NAMMCO Sci. Publ. 2010, 8, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Duck, C.; Cadhla, O.Ó.; Nairn, R.; Strong, D.; O’Keeffe, C. An assessment of population size and distribution of harbour seals in the Republic of Ireland during the moult season in August 2003. J. Zool. 2007, 273, 131–139. [Google Scholar] [CrossRef]
- Cronin, M.; Gregory, S.; Rogan, E. Moulting phenology of the harbour seal in south-west Ireland. J. Mar. Biol. Assoc. UK 2014, 94, 1079–1086. [Google Scholar] [CrossRef]
- Kavanagh, A.S.; Cronin, M.A.; Walton, M.; Rogan, E. Diet of the harbour seal (Phoca vitulina vitulina) in the west and south-west of Ireland. J. Mar. Biol. Assoc. UK 2010, 90, 1517–1527. [Google Scholar] [CrossRef]
- Thomas, L.; Russell, D.J.F.; Duck, C.D.; Morris, C.D.; Lonergan, M.; Empacher, F.; Thompson, D.; Harwood, J. Modelling the population size and dynamics of the British grey seal. Aquat. Conserv. Mar. Freshw. 2019, 29, 6–23. [Google Scholar] [CrossRef]
- Special Committee on Seals (SCOS). Scientific Advice on Matters Related to the Management of Seal Populations: 2017; Sea Mammal Research Unit, University of St Andrews: Scotland, UK, 2017; Available online: http://www.smru.st-andrews.ac.uk/files/2018/01/SCOS-2017.pdf (accessed on 16 February 2023).
- Ó Cadhla, O.; Keena, T.; Strong, D.; Duck, C.; Hiby, L. Monitoring of the Breeding Population of Grey Seals in Ireland, 2009–2012; Irish Wildlife Manuals, No. 74. Department of the Arts, Heritage and the Gaeltacht; National Parks and Wildlife Service: Dublin, Ireland, 2013. [Google Scholar]
- The Intermediate Assessment 2017. Assessment of the Marine Environment in OSPAR’s Waters; OSPAR Commission: London, UK, 2017; Available online: https://oap.ospar.org/en/ospar-assessments/intermediate-assessment-2017/ (accessed on 18 February 2023).
- Pérez Tadeo, M. Factors Affecting Local Abundance and Behaviour of Grey Seals (Halichoerus grypus) and Harbour Seals (Phoca vitulina) at Two Sites on the West Coast of Ireland: Implications for Management and Conservation. Ph.D. Thesis, Atlantic Technological University, Galway, Ireland, 2022. [Google Scholar]
- Morris, C.D.; Duck, C.D. Aerial Thermal-Imaging Survey of Seals in Ireland, 2017 to 2018; Irish Wildlife Manuals, No. 111. Department of Culture, Heritage and the Gaeltacht; National Parks and Wildlife Service: Dublin, Ireland, 2019. [Google Scholar]
- JNCC (Joint Nature Conservation Committee), 2022. Available online: https://sac.jncc.gov.uk/species/ (accessed on 15 February 2023).
- NPWS (National Parks & Wildlife Service). 2022. Available online: https://www.npws.ie/marine/marine-species (accessed on 15 February 2023).
- Huon, M.; Planque, Y.; Jessopp, M.J.; Cronin, M.; Caurant, F.; Vincent, C. Fine-scale foraging habitat selection by two diving central place foragers in the Northeast Atlantic. Ecol. Evol. 2021, 11, 12349–12363. [Google Scholar] [CrossRef]
- Riedman, M. The Pinnipeds. Seals, Sea Lions, and Walruses; University of California Press: Berkeley, CA, USA, 1990. [Google Scholar]
- Breed, G.A.; Bowen, W.D.; McMillan, J.I.; Leonard, M.L. Sexual segregation of seasonal foraging habitats in a non-migratory marine mammal. Proc. R. Soc. B Biol. Sci. 2006, 273, 2319–2326. [Google Scholar] [CrossRef]
- Hammond, P.S.; Hall, A.J.; Prime, J.H. The diet of grey seals in the inner and outer Hebrides. J. Appl. Ecol. 1994, 31, 737–746. [Google Scholar] [CrossRef]
- Spitz, J.; Mariotti, L.; Ridoux, V.; Caillot, E.; Elder, J.F. The diet of harbour seals (Phoca vitulina) at the southern limit of its European distribution (Normandy, France). NAMMCO Sci. Publ. 2010, 8, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Bearhop, S.; Harrod, C.; McDonald, R. A review of spatial and temporal variation in grey and common seal diet in the United Kingdom and Ireland. J. Mar. Biol. Assoc. UK 2012, 92, 1711–1722. [Google Scholar] [CrossRef][Green Version]
- Gosch, M.; Cronin, M.; Rogan, E.; Hunt, W.; Luck, C.; Jessopp, M. Spatial variation in a top marine predator’s diet at two regionally distinct sites. PLoS ONE 2019, 14, e0209032. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; McConnell, B.J.; Smout, S.; Hammond, P.S.; Duck, C.D.; Morris, C.D.; Thompson, D.; Russell, D.J.F.; Vincent, C.; Cronin, M.; et al. Patterns of space use in sympatric marine colonial predators reveal scales of spatial partitioning. Mar. Ecol. Prog. Ser. 2015, 534, 235–249. [Google Scholar] [CrossRef]
- Bonner, W.N.; Witthames, S.R. Dispersal of common seals (Phoca vitulina) tagged in the Wash, East Anglia. J. Zool. 1974, 174, 528–531. [Google Scholar] [CrossRef]
- Thompson, P.M.; McConnell, B.J.; Tollit, D.J.; Mackay, A.; Hunter, C.; Racey, P.A. Comparative distribution, movements and diet of harbour and grey seals from the Moray Firth, N.E. Scotland. J. Appl. Ecol. 1996, 33, 1572–1584. [Google Scholar] [CrossRef]
- Thompson, P.M.; Mackay, A.; Tollit, D.J.; Enderby, S.; Hammond, P.S. The influence of body size and sex on the characteristics of harbour seal foraging trips. Can. J. Zool. 1998, 76, 1044–1053. [Google Scholar] [CrossRef]
- Sjöberg, M.; Fedak, M.A.; McConnell, B.J. Movements and diurnal behaviour patterns in a Baltic grey seal (Halichoerus grypus). Polar Biol. 1995, 15, 593–595. [Google Scholar] [CrossRef]
- Matthiopoulos, J.; McConnell, B.J.; Duck, C.; Fedak, M. Using satellite telemetry and aerial counts to estimate space use by grey seals around the British Isles. J. Appl. Ecol. 2004, 41, 476–491. [Google Scholar] [CrossRef]
- Bonner, W.N. The grey seal and common seal in European waters. Oceanogr. Mar. Biol. 1972, 10, 461–507. [Google Scholar]
- Thompson, P. Seasonal changes in the distribution and composition of common seal (Phoca vitulina) haul-out groups. J. Zool. 1989, 217, 281–294. [Google Scholar] [CrossRef]
- Boness, D.J. Activity budget of male gray seals, Halichoerus grypus. J. Mammal. 1984, 65, 291–297. [Google Scholar] [CrossRef]
- Burns, J.J.; Gol’tsev, V.N. Comparative biology of harbor seals, Phoca vitulina Linnaeus, 1758, of the Commander, Aleutian and Pribilof islands. In Soviet-American Cooperative Research on Marine Mammals; NOAA Technical Report NMFS 12; Fay, F.H., Fedoseev, G.A., Eds.; NOAA: Washington, DC, USA, 1984; Volume 1, pp. 17–24. [Google Scholar]
- Hayes, S.A.; Costa, D.P.; Harvey, J.T.; LeBouef, B.J. Aquatic mating strategies of the male Pacific harbor seal (Phoca vitulina richardii): Are males defending the hotspot? Mar. Mammal Sci. 2004, 20, 639–656. [Google Scholar] [CrossRef]
- Miksis-Olds, J.L.; Van Opzeeland, I.C.; Van Parijs, S.M.; Jones, J. Pinniped Sounds in the Polar Oceans. In Listening in the Ocean. Modern Acoustics and Signal Processing; Au, W.W.L., Lammers, M., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Twiss, S.D. Behavioural and Energetic Determinants of Individual Mating and Success in Male Grey Seals (Halichoerus grypus). Ph.D. Thesis, University of Glasgow, Glasgow, UK, 1991. [Google Scholar]
- Van Opzeeland, I.C.; Kindermann, L.; Boebel, O.; Van Parijs, S.M. Insights into the acoustic behaviour of polar pinnipeds–current knowledge and emerging techniques of study. In Animal Behaviour–New Research; Weber, E.A., Krause, L.H., Eds.; Nova Science Publishers Inc.: Hauppage, NY, USA, 2008; pp. 133–161. [Google Scholar]
- Hanggi, E.B.; Schusterman, R.J. Underwater acoustic displays and individual variation in male harbour seals, Phoca vitulina. Anim. Behav. 1994, 48, 1275–1283. [Google Scholar] [CrossRef]
- Hayes, S.A.; Kumar, A.; Daniel, P.C.; Mellinger, D.K.; Harvey, J.T.; Southall, B.L.; LeBoeuf, B.J. Evaluating the function of the male harbour seal, Phoca vitulina, roar through playback experiments. Anim. Behav. 2004, 67, 1133–1139. [Google Scholar] [CrossRef]
- Van Parijs, S.M.; Thompson, P.M.; Tollit, D.J.; Mackay, A. Distribution and activity of male harbour seals during the mating season. Anim. Behav. 1997, 54, 35–43. [Google Scholar] [CrossRef]
- Van Parijs, S.M.; Hastie, G.D.; Thompson, P.M. Geographic variation in temporal and spatial vocalization patterns of male harbour seals in the mating season. Anim. Behav. 1999, 58, 1231–1239. [Google Scholar] [CrossRef]
- Van Parijs, S.M.; Hastie, G.D.; Thompson, P.M. Individual and geographical variation in display behaviour of male harbour seals in Scotland. Anim. Behav. 2000, 59, 559–568. [Google Scholar] [CrossRef]
- Oliver, G.W. Navigation in mazes by a grey seal, Halichoerus grypus (Fabricius). Behaviour 1978, 67, 97–114. [Google Scholar] [CrossRef]
- Schevill, W.E.; Watkins, W.A.; Ray, C. Underwater sounds of pinnipeds. Science 1963, 141, 50–53. Available online: http://science.sciencemag.org/content/141/3575/50 (accessed on 8 February 2023). [CrossRef]
- Hocking, D.P.; Burville, B.; Parker, W.M.G.; Evans, A.R.; Park, T.; Marx, F.G. Percussive underwater signaling in wild gray seals. Mar. Mammal Sci. 2020, 169, 210. [Google Scholar] [CrossRef]
- Dunn, J.; Still, R.; Harrop, H. Britain’s Sea Mammals: Whales, Dolphins, Porpoises, and Seals and Where to Find Them; University Press: Princeton. NJ, USA, 2014. [Google Scholar]
- McCulloch, S. The Vocal Behaviour of the Grey Seal (Halichoerus grypus). Ph.D. Thesis, University of St. Andrews, St. Andrews, UK, 2000. [Google Scholar]
- Shapiro, A. Vocal Learning in the Grey Seal (Halichoerus grypus): An Experimental Approach. Ph.D. Thesis, University of St. Andrews, St. Andrews, UK, 2002. [Google Scholar]
- Harrison, P.J.; Buckland, S.T.; Thomas, L.; Harris, R.; Pomeroy, P.P.; Harwood, J. Incorporating movement into models of grey seal population dynamics. J. Anim. Ecol. 2006, 75, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Duck, C.; Ó Cadhla, O.; Nairn, R.; Strong, D.; O’Keeffe, C. Harbour Seal Population Assessment in the Republic of Ireland: August 2003; Irish Wildlife Manuals, No. 11. Department of Environment, Heritage and Local Government; National Parks & Wildlife Service: Dublin, Ireland, 2004. [Google Scholar]
- Cronin, M.A.; Jessopp, M.J.; Del Villar, D. Tracking Grey Seals on Irelands’ Continental Shelf; Report to National Parks & Wildlife Service, Department of Arts, Heritage, and Gaeltacht; Coastal & Marine Research Centre, University College Cork: Cork, Ireland, 2011. [Google Scholar]
- Burns, J.J. Harbor Seal and Spotted Seal. Phoca vitulina and P. largha. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J., Kovacs, K., Eds.; Academic Press, An Imprint of Elsevier: London, UK, 2009; pp. 533–542. [Google Scholar]
- Wilson, S.C.; Jones, K.A. Behaviour of harbour seal (Phoca vitulina vitulina) pups in Dundrum Bay, north-east Ireland, during transition from filial dependency to weaning. Biol. Environ. 2020, 120, 187–202. [Google Scholar] [CrossRef]
- Caudron, A.K.; Kondakov, A.A.; Siryanov, S.V. Acoustic structure and individual variation of grey seal (Halichoerus grypus) pup calls. J. Mar. Biol. Assoc. UK 1998, 78, 651–658. [Google Scholar] [CrossRef]
- McCulloch, S.; Boness, D.J. Mother-pup vocal recognition in the grey seal (Halichoerus grypus) of Sable Island, Nova Scotia, Canada. J. Zool. 2000, 251, 449–455. [Google Scholar] [CrossRef]
- McCulloch, S.; Pomeroy, P.P.; Slater, P.J.B. Individually distinctive pup vocalizations fail to prevent allo-suckling in grey seals. Can. J. Zool. 1999, 77, 716–723. [Google Scholar] [CrossRef]
- Sauvé, C.C.; Beauplet, G.; Hammill, M.O.; Charrier, I. Mother–pup vocal recognition in harbour seals: Influence of maternal behaviour, pup voice and habitat sound properties. Anim. Behav. 2015, 105, 109–120. [Google Scholar] [CrossRef]
- Smiseth, P.T.; Lorentsen, S.H. Begging and parent-offspring conflict in grey seals. Anim. Behav. 2001, 62, 273–279. [Google Scholar] [CrossRef]
- Burton, R.W.; Anderson, S.S.; Summers, C.F. Perinatal activities in the grey seal (Halichoerus grypus). J. Zool. 1975, 177, 197–201. [Google Scholar] [CrossRef]
- Davies, J.L. Observations on the grey seal Halichoerus grypus at Ramsey Island, Pembs. Proc. Zool. Soc. Lond. 1949, 119, 673–692. [Google Scholar] [CrossRef]
- Fogden, S.C.L. Mother-young behaviour at grey seal breeding beaches. J. Zool. 1971, 164, 61–92. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Wiepkema, P.R. Case study of the neonatal period of a grey seal pup (Halichoerus grypus) in captivity. Aquat. Mamm. 1988, 14, 33–38. [Google Scholar]
- Renouf, D. The vocalization of the Harbour Seal pup (Phoca vitulina) and its role in the maintenance of contact with the mother*. J. Zool. 1984, 202, 583–590. [Google Scholar] [CrossRef]
- Loughs Agency–SeaMonitor Project. 2022. Available online: https://www.loughs-agency.org/managing-our-loughs/funded-programmes/current-programmes/sea-monitor/ (accessed on 16 January 2023).
- Russell, D.J.F.; McConnell, B. Seal At-Sea Distribution, Movements and Behaviour; Report to DECC, URN: 14D/085; SMRU, Scottish Oceans Institute, University of St Andrews: St Andrews, UK, 2014. [Google Scholar]
- O’Brien, J.; (Atlantic Technological University, Galway, Ireland). Personal communication, 2023.
- Gillespie, D.; Mellinger, D.K.; Gordon, J.; McLaren, D.; Redmond, P.; McHugh, R.; Trinder, P.; Deng, X.Y.; Thode, A. PAMGUARD: Semi-automated, open source software for real-time acoustic detection and localization of cetaceans. J. Acoust. Soc. Am. 2009, 125, 2547. [Google Scholar] [CrossRef]
- Lisa, K.; Yang Center for Conservation Bioacoustics at the Cornell Lab of Ornithology. Raven Lite: Interactive Sound Analysis Software, Version 2.0.4; The Cornell Lab of Ornithology: Ithaca, NY, USA, 2023; Available online: https://ravensoundsoftware.com/ (accessed on 18 October 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 11 January 2023).
- O’Brien, J. Cetacean Presence at the Ocean Energy Test Site Spiddal: As Determined through Land-Based Visual Monitoring and Static Acoustic Monitoring Using PODs; Report Prepared for the Marine Institute: Galway, Ireland, 2013. [Google Scholar]
- Hill, T.D.; Anisfeld, S.C. VulnToolkit: Analysis of Tidal Datasets. 2021. Available online: https://CRAN.R-project.org/package=VulnToolkit (accessed on 9 May 2023).
- Nikolich, K.A. The Vocal Breeding Behaviour of Harbour Seals (Phoca vitulina) in Georgia Strait, Canada: Temporal Patterns and Vocal Repertoire. Master’s Thesis, Western Washington University, Bellingham, WA, USA, 2015. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.G. Classification and Regression Trees; Chapman and Hall: London, UK, 1984. [Google Scholar] [CrossRef]
- Nikolich, K.; Frouin-Mouy, H.; Acevedo-Gutiérrez, A. Quantitative classification of harbor seal breeding calls in Georgia Strait, Canada. J. Acoust. Soc. Am. 2016, 140, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Frouin-Mouy, H.C.; Hammill, M.O. In-air and underwater sounds of hooded seals during the breeding season in the Gulf of St. Lawrence. J. Acoust. Soc. Am. 2021, 150, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.C.; Castellote, M.; Berchok, C.L. Beluga whale (Delphinapterus leucas) vocalizations and call classification from the eastern Beaufort Sea population. J. Acoust. Soc. Am. 2015, 137, 3054–3067. [Google Scholar] [CrossRef]
- Therneau, T.M.; Atkinson, B.; Ripley, M.B. Rpart: Recursive Partitioning and Regression Trees (Version 4.1.19). 2022. Available online: http://CRAN.R-project.org/package=rpart (accessed on 25 May 2023).
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means (Version 1.8.6). 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 25 May 2023).
- Ralls, K.; Fiorelli, P.; Gish, S. Vocalizations and vocal mimicry in captive harbor seals, Phoca vitulina. Can. J. Zool. 1985, 63, 1050–1056. [Google Scholar] [CrossRef]
- Stirling, I.; Thomas, J.A. Relationships between underwater vocalizations and mating systems in phocid seals. Aquat. Mamm. 2003, 29, 227–246. [Google Scholar] [CrossRef]
- Reijnders, P.J.; Brasseur, S.M.; Meesters, E.H. Earlier pupping in harbour seals, Phoca vitulina. Biol. Lett. 2010, 6, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Boness, D.J.; Bowen, W.D.; Oftedal, O.T. Evidence of a maternal foraging cycle resembling that of otariid seals in a small phocid, the harbour seal. Behav. Ecol. Sociobiol. 1994, 34, 95–104. [Google Scholar] [CrossRef]
- Thompson, P.M.; Miller, D.; Cooper, R.; Hammond, P.S. Changes in the distribution and activity of female harbour seals during the breeding season: Implications for their lactation strategy and mating patterns. J. Anim. Ecol. 1994, 63, 24–30. [Google Scholar] [CrossRef]
- Perry, E.A.; Renouf, D. Further studies of the role of harbor seal (Phoca vitulina) pup vocalizations in preventing separation of mother pup pairs. Can. J. Zool. 1988, 66, 934–938. [Google Scholar] [CrossRef]
- de Reus, K. Talking Seals: Vocal Development in Eastern Atlantic Harbour Seal Pups Phoca vitulina vitulina. Master’s Thesis, Royal Veterinary College, University of London, London, UK, 2017. [Google Scholar]
- Khan, C.B.; Markowitz, H.; McCowan, B. Vocal development in captive harbor seal pups, Phoca vitulina richardii: Age, sex, and individual differences. J. Acoust. Soc. Am. 2006, 120, 1684–1694. [Google Scholar] [CrossRef]
- Torres Borda, L.; Jadoul, Y.; Rasilo, H.; Salazar Casals, A.; Ravignani, A. Vocal plasticity in harbour seal pups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200456. [Google Scholar] [CrossRef]
- Anichini, M.; de Reus, K.; Hersh, T.A.; Valente, D.; Salazar-Casals, A.; Berry, C.; Keller, P.E.; Ravignani, A. Measuring rhythms of vocal interactions: A proof of principle in harbour seal pups. Phil. Trans. R. Soc. B 2023, 378, 20210477. [Google Scholar] [CrossRef]
- Ravignani, A. Spontaneous rhythms in a harbor seal pup calls. BMC Res. Notes 2018, 11, 3. [Google Scholar] [CrossRef]
- Ravignani, A. Timing of antisynchronous calling: A case study in a harbor seal pup (Phoca vitulina). J. Comp. Psychol. 2019, 133, 272. [Google Scholar] [CrossRef] [PubMed]
- Ravignani, A.; Kello, C.T.; de Reus, K.; Kotz, S.A.; Dalla Bella, S.; Méndez-Aróstegui, M.; Rapado-Tamarit, B.; Rubio-Garcia, A.; de Boer, B. Ontogeny of vocal rhythms in harbor seal pups: An exploratory study. Curr. Zool. 2019, 65, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Prawirasasra, M.S.; Mustonen, M.; Klauson, A. The Underwater Soundscape at Gulf of Riga Marine-Protected Areas. J. Mar. Sci. Eng. 2021, 9, 915. [Google Scholar] [CrossRef]
- Nowak, B.V.R.; Bowen, W.D.; Whoriskey, K.; Lidgard, D.C.; Mills Flemming, J.E.; Iverson, S.J. Foraging behaviour of a continental shelf marine predator, the grey seal (Halichoerus grypus), is associated with in situ, subsurface oceanographic conditions. Mov. Ecol. 2020, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J. Description and Probable Behavioral Significance of Grey Seal (Halichoerus grypus) Vocalizations. Master’s Thesis, University of Rhode Island, Providence, Rhode Island, 1974. [Google Scholar]
- Pérez Tadeo, M.; Gammell, M.; O’Brien, J. Assessment of anthropogenic disturbances due to ecotourism on a Grey Seal (Halichoerus grypus) colony in the Blasket Islands SAC, SW Ireland and recommendations on best practices. Aquat. Mamm. 2021, 47, 268–282. [Google Scholar] [CrossRef]
- Nikolich, K.; Frouin-Mouy, H.; Acevedo-Gutiérrez, A. Clear diel patterns in breeding calls of harbor seals (Phoca vitulina) at Hornby Island, British Columbia, Canada. Can. J. Zool. 2018, 96, 1236–1243. [Google Scholar] [CrossRef]
- Terhune, J.M.; Ronald, K. Examining harp seal behavioural patterns via their underwater vocalizations. Appl. Anim. Ethol. 1976, 2, 261–264. [Google Scholar] [CrossRef]
- Cronin, M.; Pomeroy, P.; Jessopp, M. Size and seasonal influences on the foraging range of female grey seals in the northeast Atlantic. Mar. Biol. 2013, 160, 531–539. [Google Scholar] [CrossRef]
- Beck, C.A.; Bowen, W.D.; Iverson, S.J. Seasonal changes in buoyancy and diving behaviour of adult grey seals. J. Exp. Biol. 2000, 203, 2323–2330. [Google Scholar] [CrossRef]
- Climate Action and Low Carbon Development (Amendment) Bill (2021); Department of the Environment, Climate and Communications: Dublin, Ireland, 2021.
- Climate Change (Emissions Reduction Targets) (Scotland) Act (2019); The Stationery Office: London, UK, 2019.
- EU Biodiversity Strategy for 2030; European Commission: Brussels, Belgium, 2020.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).