What’s at Play: Humpback Whale Interaction with Seaweed Is a Global Phenomenon
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Body Part Interactions
3.2. UAV Videos
4. Discussion
Role of Social Media
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brakes, P.; Simmonds, M.P. Whales and Dolphins: Cognition, Culture, Conservation and Human Perceptions; Routledge: Longdon, UK, 2013. [Google Scholar]
- Parra, G.J. Observations of an Indo-Pacific humpback dolphin carrying a sponge: Object play or tool use? Mammalia 2007, 71, 147–149. [Google Scholar] [CrossRef]
- McMillan, C.J.; Towers, J.R.; Hildering, J. The innovation and diffusion of “trap-feeding,” a novel humpback whale foraging strategy. Mar. Mammal Sci. 2019, 35, 779–796. [Google Scholar] [CrossRef]
- Mann, J.B. Behavioral development in wild bottlenose dolphin newborns (Tursiops sp.). Behaviour 1999, 136, 529–566. [Google Scholar]
- Deakos, M.H.; Branstetter, B.K.; Mazzuca, L.; Fertl, D.; Mobley, J.R., Jr. Two Unusual Interactions Between a Bottlenose Dolphin (Tursiops truncatus) and a Humpback Whale (Megaptera novaeangliae) in Hawaiian Waters. Aquat. Mamm. 2010, 36, 121–128. [Google Scholar] [CrossRef]
- Paulos, R.D.; Trone, M.; Kuczaj, S.A., II. Play in wild and captive cetaceans. Int. J. Comp. Psychol. 2010, 23, 701–722. [Google Scholar] [CrossRef]
- Patterson, E.M.; Mann, J. Cetacean innovation. In Animal Creativity and Innovation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 73–125. [Google Scholar]
- Kuczaj, S.; Makecha, R. The role of play in the evolution and ontogeny of contextually flexible communication. In Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication; MIT Press: Cambridge, UK, 2008; pp. 253–277. [Google Scholar]
- Hill, H.M.; Dietrich, S.; Cappiello, B. Learning to play: A review and theoretical investigation of the developmental mechanisms and functions of cetacean play. Learn. Behav. 2017, 45, 335–354. [Google Scholar] [CrossRef]
- Delfour, F.; Marten, K. Mirror image processing in three marine mammal species: Killer whales (Orcinus orca), false killer whales (Pseudorca crassidens) and California sea lions (Zalophus californianus). Behav. Process. 2001, 53, 181–190. [Google Scholar] [CrossRef]
- Smolker, R.; Richards, A.; Connor, R.; Mann, J.; Berggren, P. Sponge Carrying by Dolphins (Delphinidae, Tursiops sp.): A Foraging Specialization Involving Tool Use? Ethology 1997, 103, 454–465. [Google Scholar] [CrossRef]
- Connor, R.C.; Smolker, R.A.; Richards, A.F. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl. Acad. Sci. USA 1992, 89, 987–990. [Google Scholar] [CrossRef]
- Burghardt, G.M. The Genesis of Animal Play: Testing the Limits; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Owen, K.; Dunlop, R.; Donnelly, D. Seaweed interactions by humpback whales (Megaptera novaeangliae): A form of object play? Aquat. Mamm. 2012, 38, 418. [Google Scholar] [CrossRef]
- Clapham, P.J. The humpback whale: Seasonal feeding and breeding in a baleen whale. In Cetacean Societies; University of Chicago Press: Chicago, IL, USA, 2000; pp. 173–196. [Google Scholar]
- Dawbin, W.H. The seasonal migratory cycle of humpback whales. In Whales Dolphins Porpoises; University of California Press: Berkeley, CA, USA, 1966; pp. 145–170. [Google Scholar]
- Rasmussen, K.; Palacios, D.M.; Calambokidis, J.; Saborío, M.T.; Dalla Rosa, L.; Secchi, E.R.; Steiger, G.H.; Allen, J.M.; Stone, G.S. Southern Hemisphere humpback whales wintering off Central America: Insights from water temperature into the longest mammalian migration. Biol. Lett. 2007, 3, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Jackson, J. Global Review of Humpback Whales (Megaptera novaeangliae); Southwest Fisheries Centre: La Jolla, CA, USA, 2011; 212p. [Google Scholar]
- Jones, M.L.; Swartz, S.L. Gray whale: Eschrichtius robustus. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2009; pp. 503–511. [Google Scholar]
- Payne, R. Swimming with Patagonia’s right whales. Natl. Geogr. 1972, 142, 576–587. [Google Scholar]
- Vallejo, A.C.; Barragán-Barrera, D.C.; Farías-Curtidor, N.; Bachmann, J.; Murillo, E.G.; Lloreda, L.A.; Murillo, Y.G. Play Behavior by a Juvenile Humpback Whale (Megaptera novaeangliae) with an Inanimate Object (Driftwood) in the Gulf of Tribugá, Colombia. Aquat. Mamm. 2022, 48, 678–683. [Google Scholar] [CrossRef]
- Shea, B.D.; Gallagher, A.J. Humpback Whale Instigates Object Play with a Lion’s Mane Jellyfish. Oceans 2021, 2, 386–392. [Google Scholar] [CrossRef]
- Apprill, A.; Mooney, T.A.; Lyman, E.; Stimpert, A.K.; Rappé, M.S. Humpback whales harbour a combination of specific and variable skin bacteria. Environ. Microbiol. Rep. 2011, 3, 223–232. [Google Scholar] [CrossRef]
- Iwasa-Arai, T.; Serejo, C.S.; Siciliano, S.; Ott, P.H.; Freire, A.S.; Elwen, S.; Crespo, E.A.; Colosio, A.C.; Carvalho, V.L.; Rodríguez-Rey, G.T. The host-specific whale louse (Cyamus boopis) as a potential tool for interpreting humpback whale (Megaptera novaeangliae) migratory routes. J. Exp. Mar. Biol. Ecol. 2018, 505, 45–51. [Google Scholar] [CrossRef]
- Fertl, D.; Newman, W.A. Barnacles. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 75–78. [Google Scholar]
- Urtizberea, F.; Detcheverry, J.; Denys, G.P. Observations of Sea Lampreys, attached to humpback whales, off Saint Pierre and miquelon archipelago (Northwestern Atlantic). Cah. Biol. Mar 2021, 62, 77–81. [Google Scholar]
- Castro, C.; Kaufman, G.; Maldini, D. A Preliminary Review of Skin Conditions and Other Body Anomalies Observed on Humpback Whales (Megaptera novaeangliae) from Ecuador; International Whaling Commission: Impington, UK, 2011. [Google Scholar]
- Van Bressem, M.-F.; Minton, G.; Collins, T.; Willson, A.; Baldwin, R.; Van Waerebeek, K. Tattoo-like skin disease in the endangered subpopulation of the Humpback Whale, Megaptera novaeangliae, in Oman (Cetacea: Balaenopteridae). Zool. Middle East 2015, 61, 1–8. [Google Scholar] [CrossRef]
- Assis, J.; Fragkopoulou, E.; Frade, D.; Neiva, J.; Oliveira, A.; Abecasis, D.; Faugeron, S.; Serrão, E.A. A fine-tuned global distribution dataset of marine forests. Sci. Data 2020, 7, 119. [Google Scholar] [CrossRef]
- Huisman, J.M. Marine Plants of Australia; University of Western Australia Press: Perth, Australia, 2000. [Google Scholar]
- Womersley, H.B.S. The Marine Benthic Flora of Southern Australia; Australian Biological Resources Study: Adelaide, Australia, 2004. [Google Scholar]
- Wernberg, T.; Smale, D.A.; Tuya, F.; Thomsen, M.S.; Langlois, T.J.; De Bettignies, T.; Bennett, S.; Rousseaux, C.S. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial properties of fucoidans from the brown algae Fucus vesiculosus L. of the barents sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.-H.; Park, J.-H.; Yu, D.-U.; Choi, J.-I.; Choi, J.-D.; Lee, M.-S.; Kim, Y.-M. Antimicrobial activity of brown alga Eisenia bicyclis against methicillin-resistant Staphylococcus aureus. Fish. Aquat. Sci. 2011, 14, 251–256. [Google Scholar] [CrossRef]
- Morais, P.; Afonso, L.; Dias, E. Harnessing the Power of Social Media to Obtain Biodiversity Data About Cetaceans in a Poorly Monitored Area. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- McDavitt, M.T.; Kyne, P.M. Social media posts reveal the geographic range of the Critically Endangered clown wedgefish, Rhynchobatus cooki. J. Fish Biol. 2020, 97, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Pace, D.S.; Giacomini, G.; Campana, I.; Paraboschi, M.; Pellegrino, G.; Silvestri, M.; Alessi, J.; Angeletti, D.; Cafaro, V.; Pavan, G. An integrated approach for cetacean knowledge and conservation in the central Mediterranean Sea using research and social media data sources. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1302–1323. [Google Scholar] [CrossRef]
- Santora, J.A.; Mantua, N.J.; Schroeder, I.D.; Field, J.C.; Hazen, E.L.; Bograd, S.J.; Sydeman, W.J.; Wells, B.K.; Calambokidis, J.; Saez, L.; et al. Habitat compression and ecosystem shifts as potential links between marine heatwave and record whale entanglements. Nat. Commun. 2020, 11, 536. [Google Scholar] [CrossRef]
- Sopinka, N. Hitch-Hiking Beaver Spotted Napping Atop Humpback Whale. Fisheries 2015, 40, 187. [Google Scholar] [CrossRef]
- Dehnhardt, G.; Hanke, F.D. Whiskers. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1074–1077. [Google Scholar]
- Bauer, G.B.; Reep, R.L.; Marshall, C.D. The tactile senses of marine mammals. Int. J. Comp. Psychol. 2018, 31, 223–240. [Google Scholar] [CrossRef]
- Murphy, C.T.; Marx, M.; Martin, W.N.; Jiang, H.; Lapseritis, J.M.; French, A.N.; Simmons, N.B.; Moore, M.J. Feeling for food: Can rostro-mental hair arrays sense hydrodynamic cues for foraging North Atlantic right whales? Anat. Rec. 2022, 305, 577–591. [Google Scholar] [CrossRef]
- Weinrich, M.T. Callosities. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2009; pp. 176–178. [Google Scholar]
- Cartwright, R.; Sullivan, M. Behavioral ontogeny in humpback whale (Megaptera novaeangliae) calves during their residence in Hawaiian waters. Mar. Mammal Sci. 2009, 25, 659–680. [Google Scholar] [CrossRef]
- Zoidis, A.M.; Lomac-MacNair, K.S.; Chomos-Betz, A.E.; Day, A.J.; Sasha McFarland, A. Effects of sex, seasonal period, and sea state on calf behavior in Hawaiian humpback whales (Megaptera novaeangliae). Aquat. Mamm. 2014, 40, 44–58. [Google Scholar] [CrossRef]
- Fertl, D.; Fulling, G. Interactions between marine mammals and turtles. Mar. Turt. Newsl. 2007, 115, 4–8. [Google Scholar]
- Pitman, R.L.; Deecke, V.B.; Gabriele, C.M.; Srinivasan, M.; Black, N.; Denkinger, J.; Durban, J.W.; Mathews, E.A.; Matkin, D.R.; Neilson, J.L. Humpback whales interfering when mammal-eating killer whales attack other species: Mobbing behavior and interspecific altruism? Mar. Mammal Sci. 2017, 33, 7–58. [Google Scholar] [CrossRef]
- Osmond, M.; Kaufman, G. A heavily parasitized humpback whale (Megaptera novaeangliae). Mar. Mammal Sci. 1998, 14, 146–149. [Google Scholar] [CrossRef]
- Morlock, G.E.; Ziltener, A.; Geyer, S.; Tersteegen, J.; Mehl, A.; Schreiner, T.; Kamel, T.; Brümmer, F. Evidence that Indo-Pacific bottlenose dolphins self-medicate with invertebrates in coral reefs. iScience 2022, 25. [Google Scholar] [CrossRef]
- Peterson, R.S.; Bartholomew, G.A. The Natural History and Behavior of the California Sea Lion; American Society of Mammalogists: Stillwater, OK, USA, 1967. [Google Scholar]
- Fortune, S.M.E.; Koski, W.R.; Higdon, J.W.; Trites, A.W.; Baumgartner, M.F.; Ferguson, S.H. Evidence of molting and the function of “rock-nosing” behavior in bowhead whales in the eastern Canadian Arctic. PLoS ONE 2017, 12, e0186156. [Google Scholar] [CrossRef]
- Meynecke, J.-O.; Gustafon, J.; Cade, D.E. Exfoliating Whales–Sandy Bottom Contact Behaviour of Humpback Whales. J. Mar. Sci. Eng. 2023, 11, 600. [Google Scholar] [CrossRef]
- Pitman, R.L.; Durban, J.W.; Joyce, T.; Fearnbach, H.; Panigada, S.; Lauriano, G. Skin in the game: Epidermal molt as a driver of long-distance migration in whales. Mar. Mammal Sci. 2020, 36, 565–594. [Google Scholar] [CrossRef]
- Félix, F.; Haase, B.; Aguirre, W.E. Spondylitis in a humpback whale (Megaptera novaeangliae) from the southeast Pacific. Dis. Aquat. Org. 2007, 75, 259–264. [Google Scholar] [CrossRef]
- Rowntree, V.J. Feeding, distribution, and reproductive behavior of cyamids (Crustacea: Amphipoda) living on humpback and right whales. Can. J. Zool. 1996, 74, 103–109. [Google Scholar] [CrossRef]
- Cranswick, A.S.; Constantine, R.; Hendriks, H.; Carroll, E.L. Social media and citizen science records are important for the management of rarely sighted whales. Ocean Coast. Manag. 2022, 226, 106271. [Google Scholar] [CrossRef]
- Barra, T.; Bejder, L.; Dalleau, M.; Delaspre, S.; Landes, A.-E.; Harvey, M.; Hoarau, L. Social media reveal high rates of agonistic behaviors of humpback whales in response to swim-with activities off Reunion Island. Tour. Mar. Environ. 2020, 15, 191–209. [Google Scholar] [CrossRef]
- Hazen, E.L.; Friedlaender, A.S.; Thompson, M.A.; Ware, C.R.; Weinrich, M.T.; Halpin, P.N.; Wiley, D.N. Fine-scale prey aggregations and foraging ecology of humpback whales Megaptera novaeangliae. Mar. Ecol. Prog. Ser. 2009, 395, 75–89. [Google Scholar] [CrossRef]
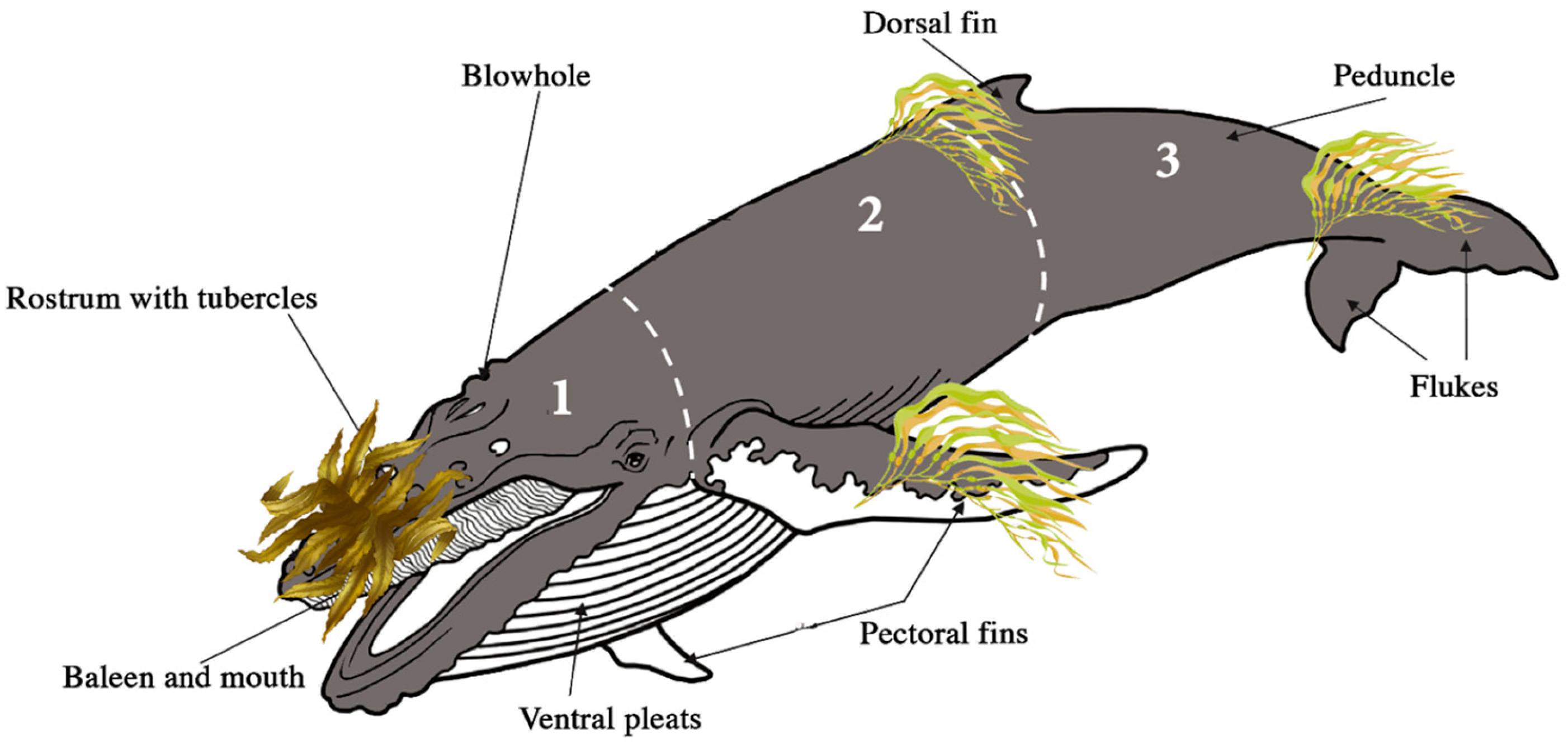
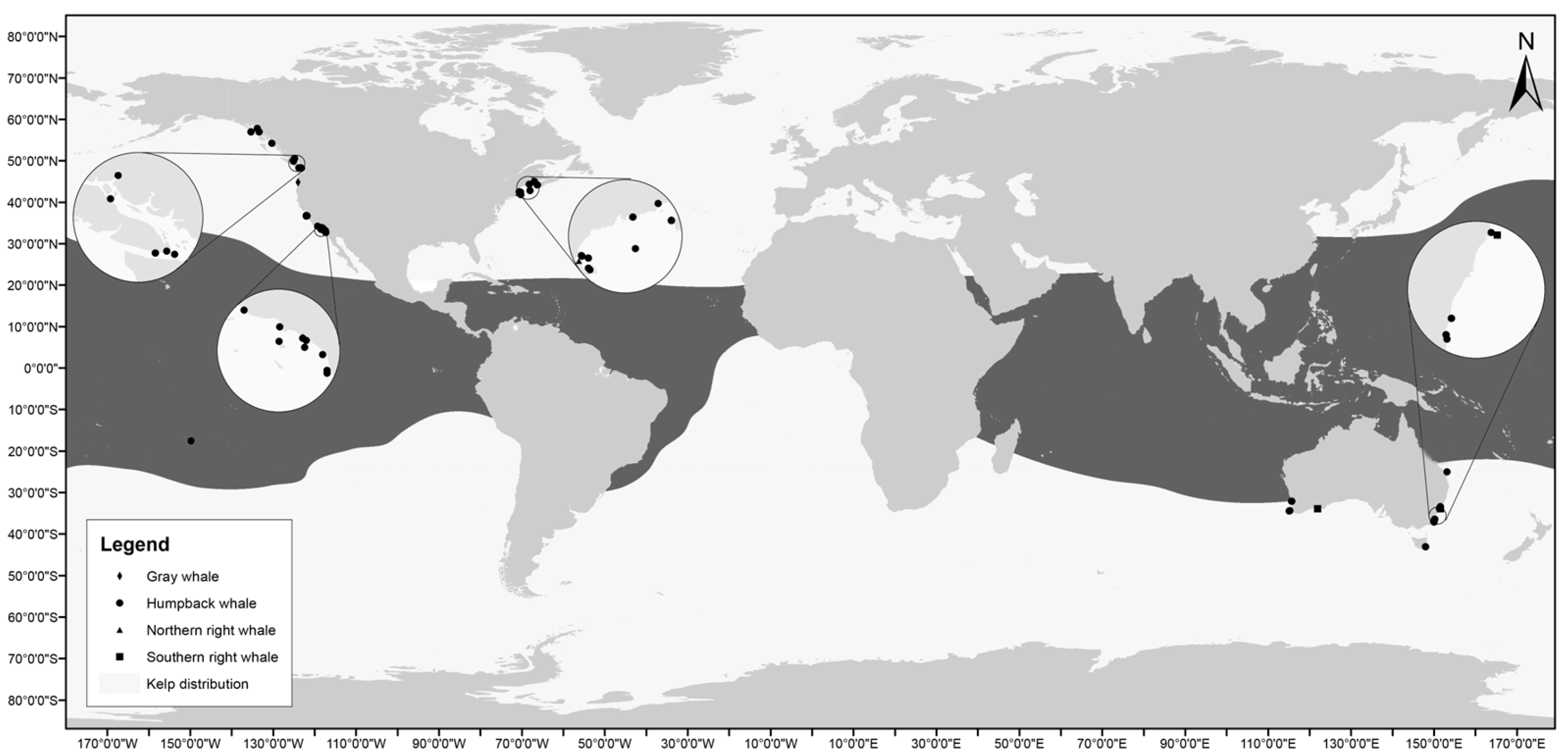
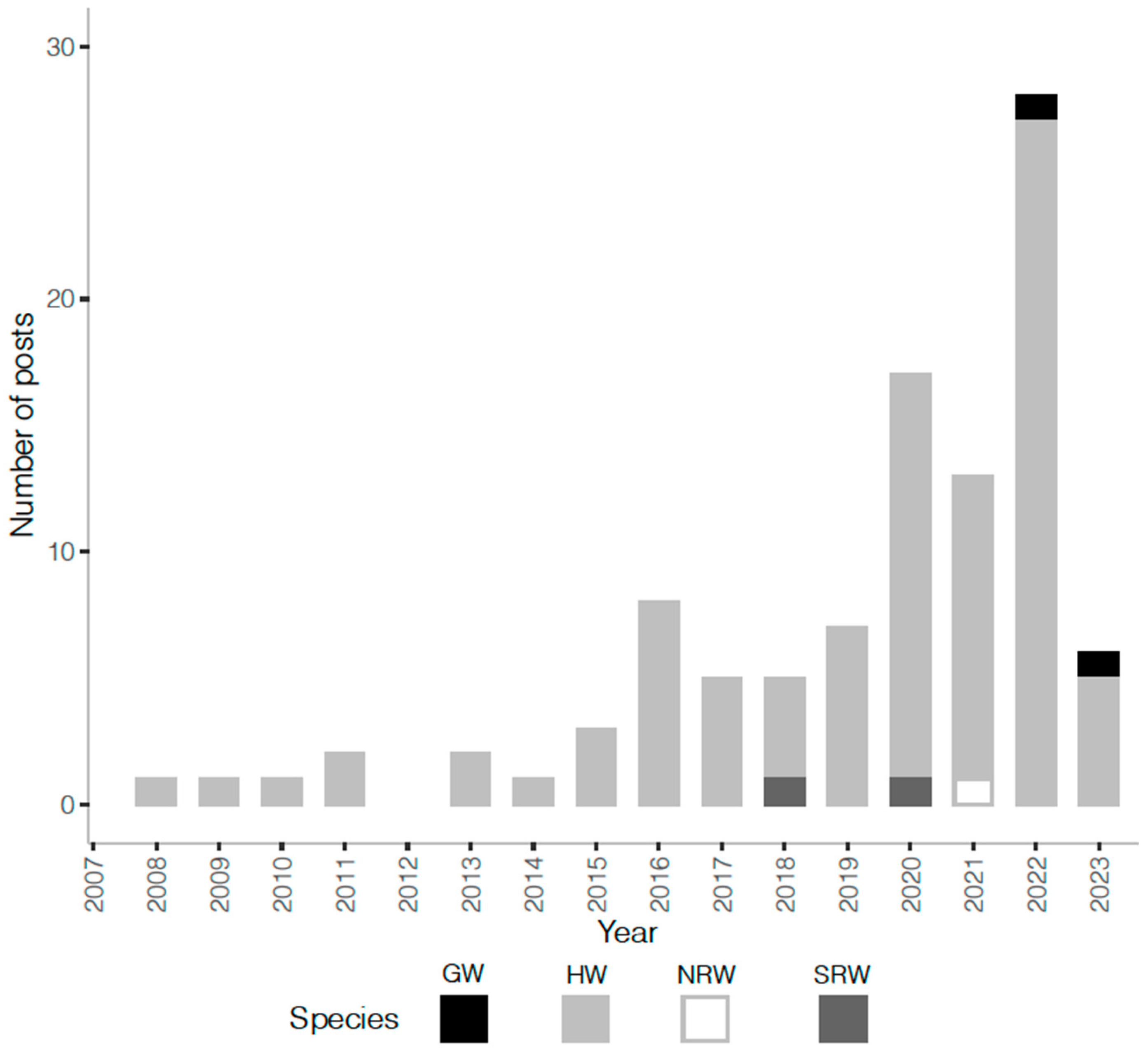

| Body Part | Seaweed Contact per Body Part | ||||
|---|---|---|---|---|---|
| HW | GW | NRW | SRW | Total | |
| Rostrum | 66 | 1 | 1 | 2 | 70 |
| Pectoral fin | 25 | 1 | - | - | 26 |
| Pectoral fin to dorsal fin *** | 22 | 1 | - | - | 23 |
| Rostrum to fin ** | 20 | - | - | - | 20 |
| Dorsal fin | 16 | - | - | - | 16 |
| Peduncle | 15 | 1 | - | - | 16 |
| Fluke | 14 | - | - | - | 14 |
| Mouth | 4 | - | - | 1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meynecke, J.-O.; Kela, H. What’s at Play: Humpback Whale Interaction with Seaweed Is a Global Phenomenon. J. Mar. Sci. Eng. 2023, 11, 1802. https://doi.org/10.3390/jmse11091802
Meynecke J-O, Kela H. What’s at Play: Humpback Whale Interaction with Seaweed Is a Global Phenomenon. Journal of Marine Science and Engineering. 2023; 11(9):1802. https://doi.org/10.3390/jmse11091802
Chicago/Turabian StyleMeynecke, Jan-Olaf, and Hilla Kela. 2023. "What’s at Play: Humpback Whale Interaction with Seaweed Is a Global Phenomenon" Journal of Marine Science and Engineering 11, no. 9: 1802. https://doi.org/10.3390/jmse11091802
APA StyleMeynecke, J.-O., & Kela, H. (2023). What’s at Play: Humpback Whale Interaction with Seaweed Is a Global Phenomenon. Journal of Marine Science and Engineering, 11(9), 1802. https://doi.org/10.3390/jmse11091802





