Experimental Characteristics of Hydrocarbon Generation from Scandinavian Alum Shale Carbonate Nodules: Implications for Hydrocarbon Generation from Majiagou Formation Marine Carbonates in China’s Ordos Basin
Abstract
:1. Introduction
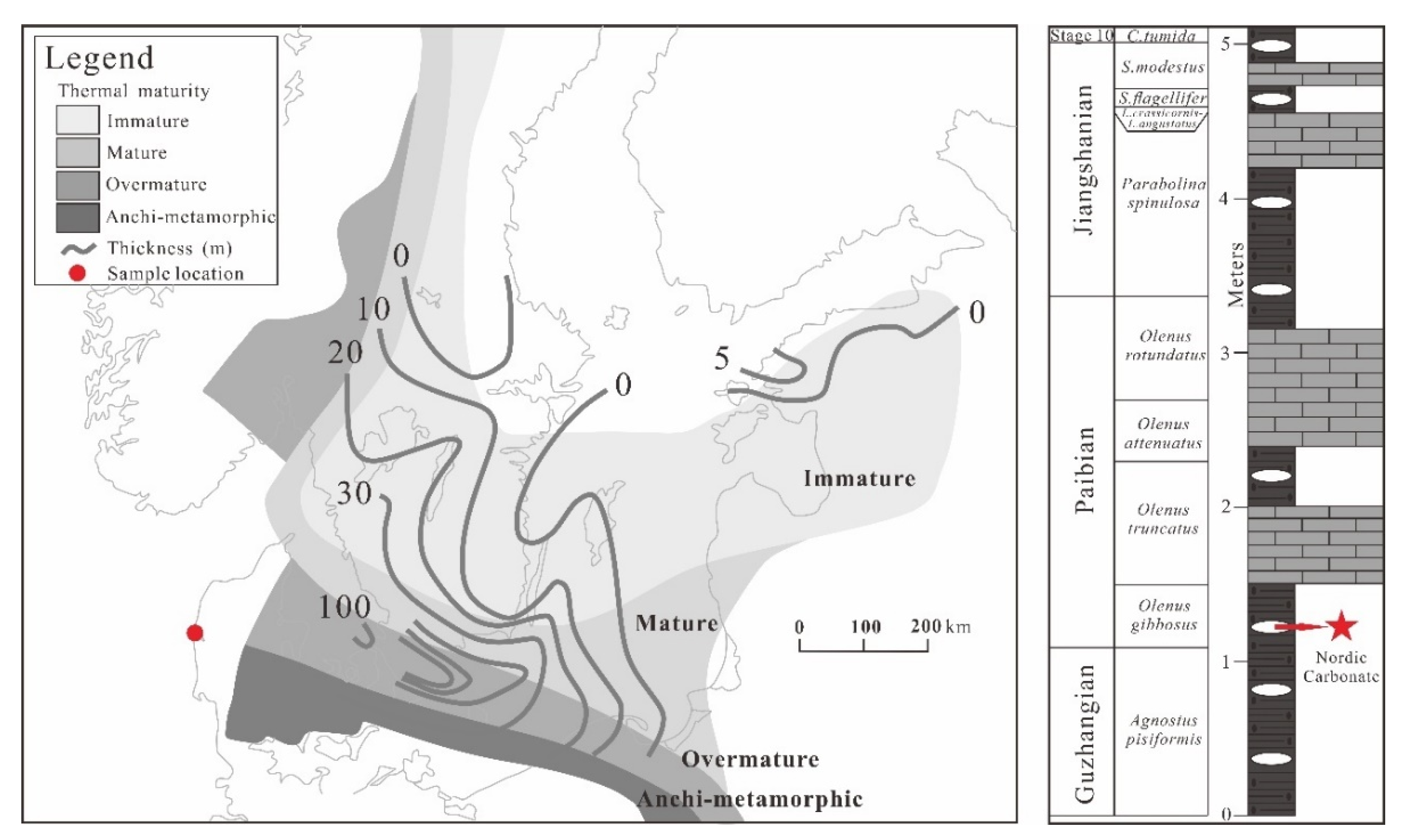
2. Geological Setting
3. Samples and Experimental Method
3.1. Samples
3.2. TOC and Rock Pyrolysis Analysis Methods
3.3. Thermal Simulation Experiment Method
4. Results
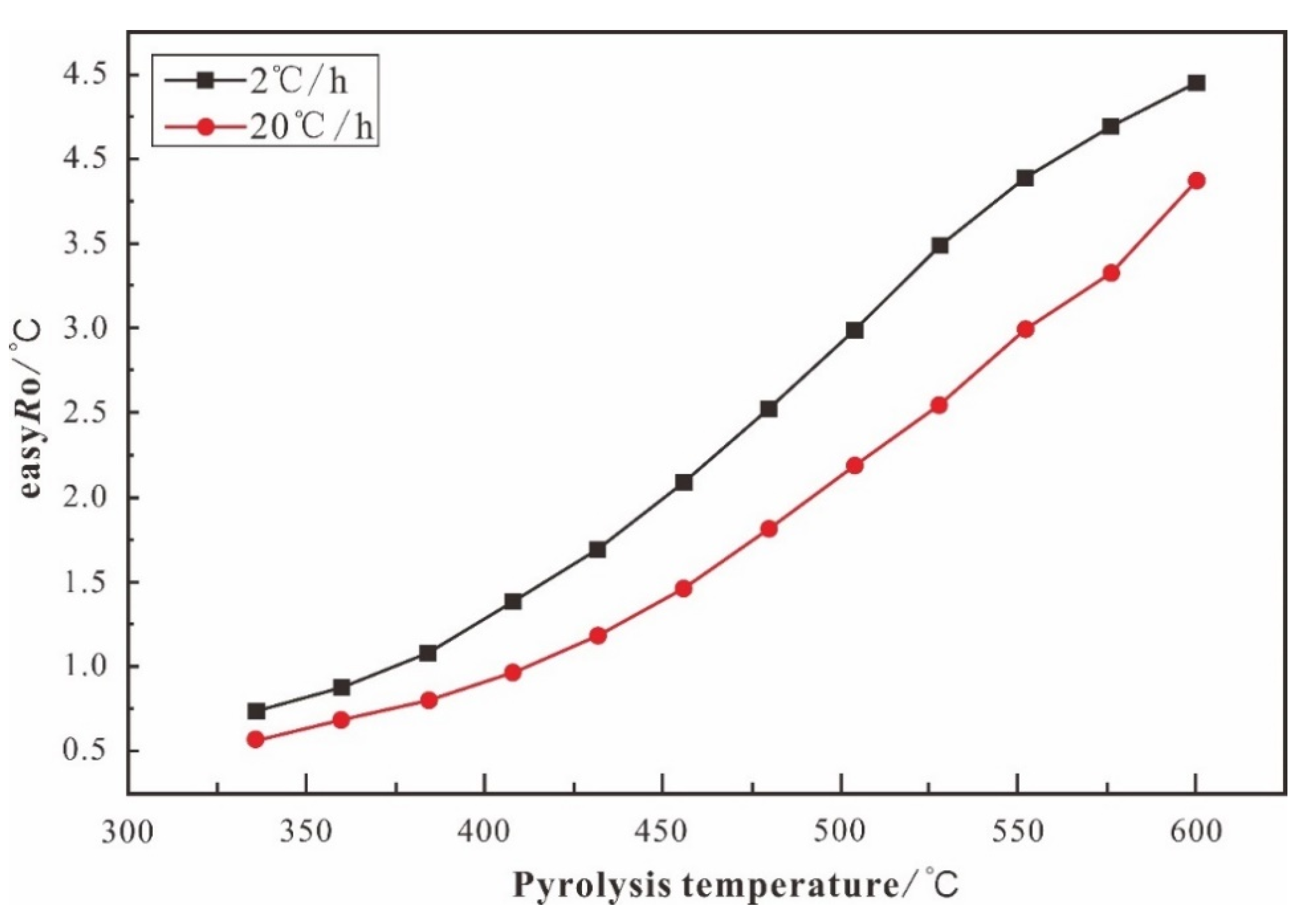
Thermal Simulation Experiment Results
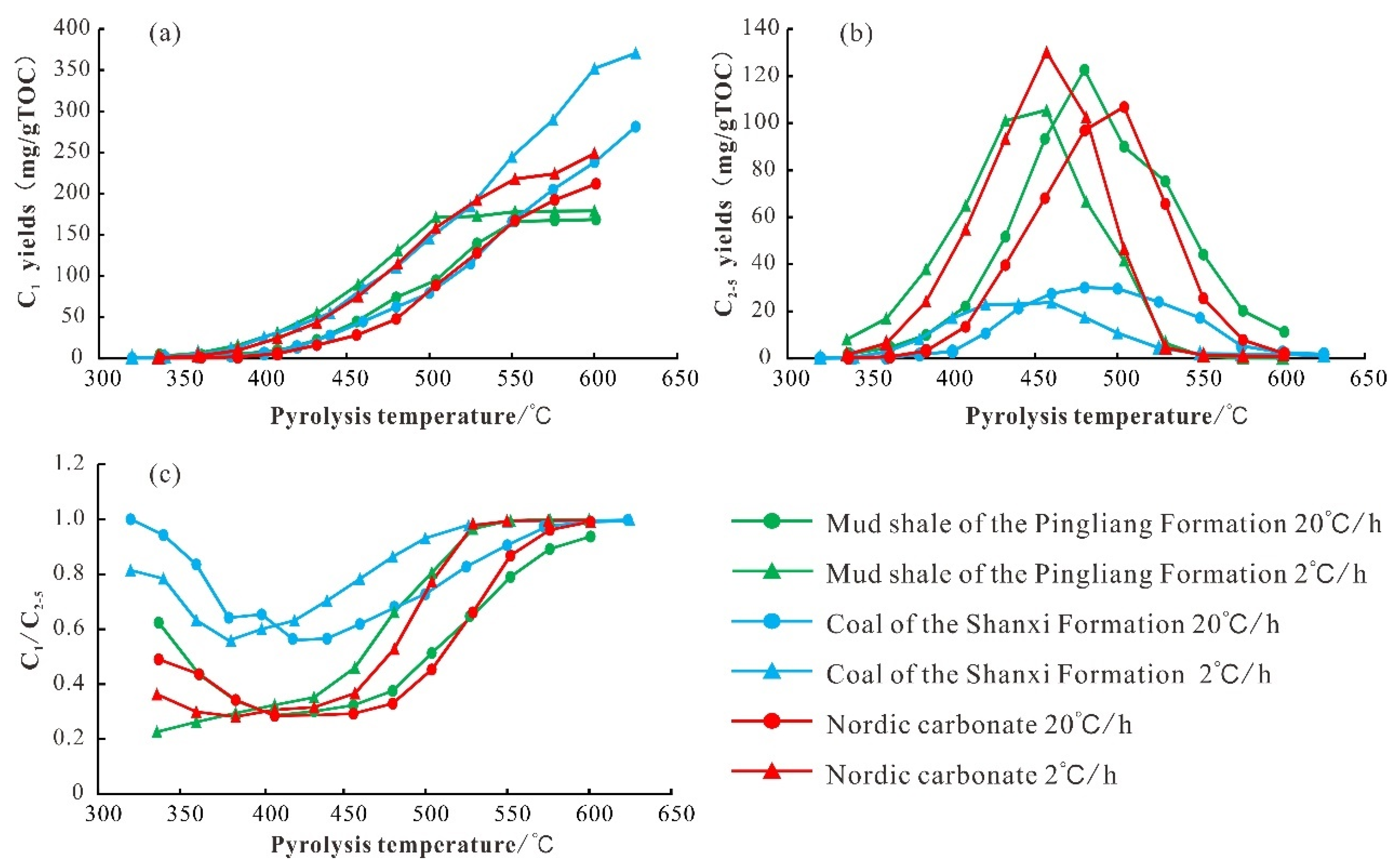
5. Comparison of Thermal Simulation Products with Mud Shale of the Pingliang Formation and Coal of the Shanxi Formation
5.1. Gaseous Product Evolutionary Characteristics
5.1.1. Pyrolysis Gaseous Hydrocarbon Characteristics
5.1.2. Pyrolysis Nonhydrocarbon Gas Characteristics
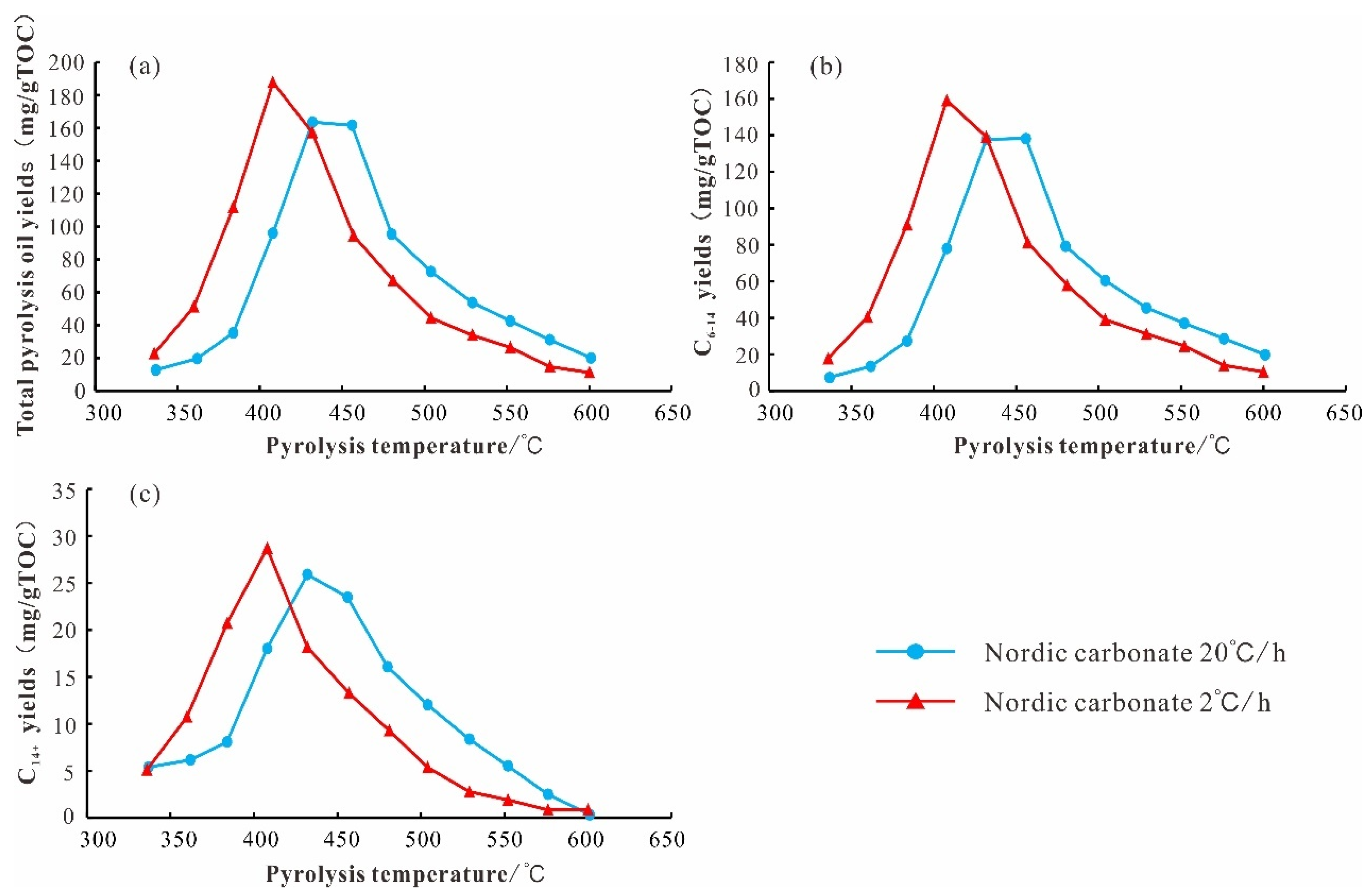
5.2. Liquid Product Evolutionary Characteristics
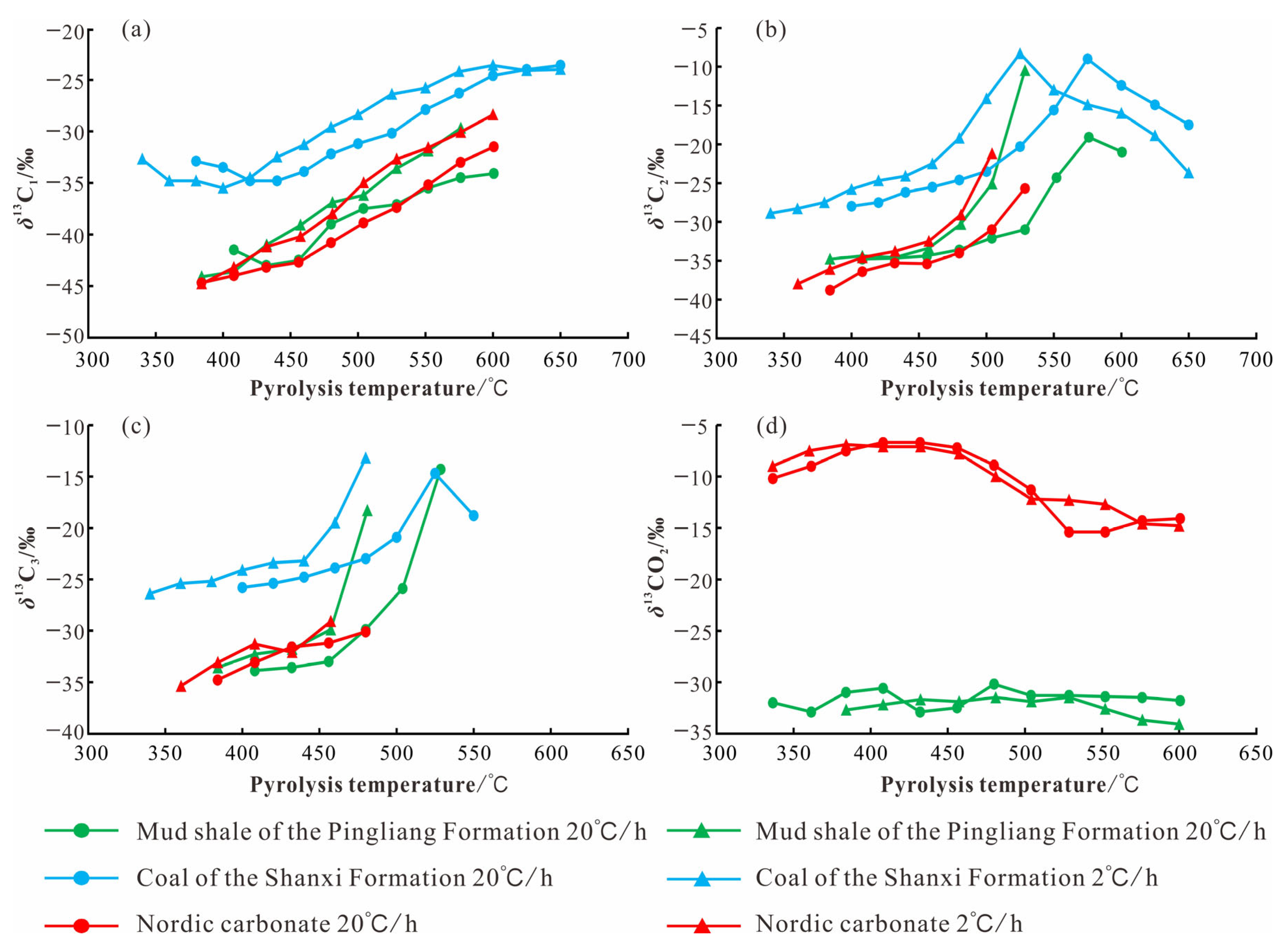
5.3. Carbon Isotope Composition
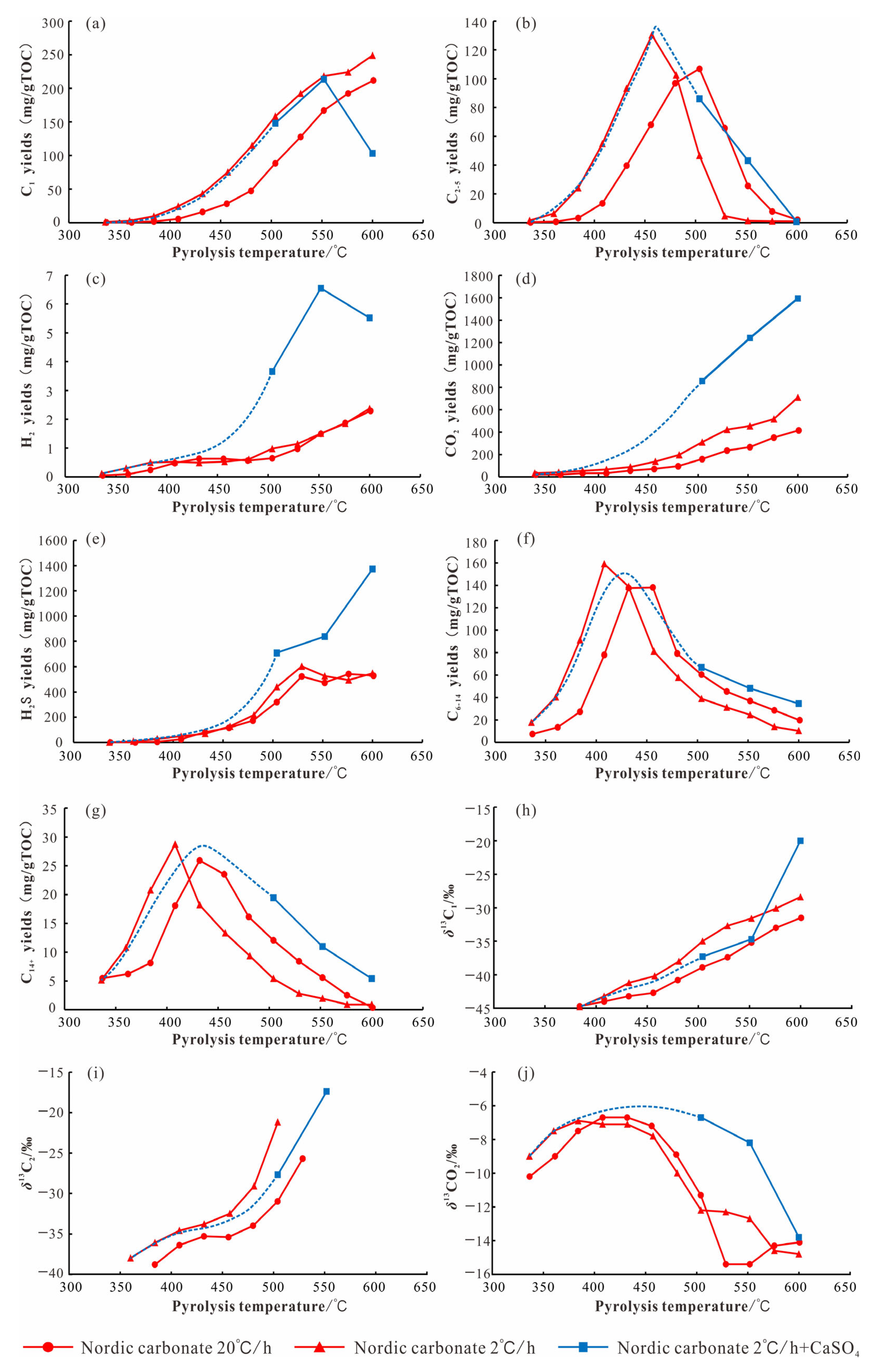
6. Organic–Inorganic Interactions: The Effect of Gypsum Rock Presence on Hydrocarbons
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Tan, X.; Li, Y.; Cao, J.; Luo, B. Occurrence and conceptual sedimentary model of Cambrian gypsum-bearing evaporites in the Sichuan Basin, SW China. Geosci. Front. 2018, 9, 1179–1191. [Google Scholar] [CrossRef]
- Radwan, A.E.; Husinec, A.; Benjumea, B.; Kassem, A.A.; Aal, A.A.; Hakimi, M.H.; Thanh, H.V.; Abdel-Fattah, M.I.; Shehata, A.A. Diagenetic overprint on porosity and permeability of a combined conventional-unconventional reservoir: Insights from the Eocene pelagic limestones, Gulf of Suez, Egypt. Mar. Pet. Geol. 2022, 146, 105967. [Google Scholar] [CrossRef]
- Jones, R.W. Comparison of carbonate and shale source rocks. In Petroleum Geochemistry and Source Rock Potential of Carbonate Rocks; Palacas, J.G., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1984; Volume 18, pp. 163–180. [Google Scholar]
- Palacas, J.G.; Anders, D.E.; King, J.D. South Florida Basin—A prime example of carbonate source rocks of petroleum. In Petroleum Geochemistry and Source Rock Potential of Carbonate Rocks; Palacas, J.G., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1984; Volume 18, pp. 71–96. [Google Scholar]
- Katz, B.J.; Dittmar, E.I.; Ehret, G.E. A geochemical review of carbonate source rocks in Italy. J. Pet. Geol. 2000, 23, 399–424. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, J.; Wei, X.; Xu, W.; Sun, L.; Liu, J.; Zhao, Y.; Gao, J.; Zhang, Y. Natural gas accumulation and models in Ordovician carbonates, Ordos Basin, China. Pet. Explor. Dev. 2014, 41, 393–403. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Shen, Y.; Ji, W.; Wang, Q. Rock fabric of tight sandstone and its influence on irreducible water saturation in Eastern Ordos Basin. Energy Fuels 2023, 37, 3685–3696. [Google Scholar] [CrossRef]
- Huang, D.; Xiong, C.; Yang, J.; Xu, Z.; Wang, K. Gas source discrimination and natural gas genetic types of central gas field in Ordos Basin. Nat. Gas Ind. 1996, 16, 95. [Google Scholar]
- Yang, H.; Liu, X.; Zhang, D. Main controlling factors of gas pooling in Ordovician marine carbonate reservoirs in the Ordos Basin and advances in gas exploration. Nat. Gas Ind. 2013, 33, 1–12. [Google Scholar]
- Wang, J.; Jia, H.; Sun, X.; Tao, C.; Zhang, Y.; Ma, L.; Wang, F.; Jiang, H. Comprehensive evaluation on origin and source of natural gas in the Paleozoic in Fuxian Area, Ordos Basin. Nat. Gas Geosci. 2022, 33, 1476–1484. [Google Scholar]
- Dai, J.; Li, J.; Luo, X.; Zhang, W.; Hu, G.; Ma, C.; Guo, J.; Ge, S. Stable carbon isotope compositions and source rock geochemistry of the giant gas accumulations in the Ordos Basin, China. Org. Geochem. 2005, 36, 1617–1635. [Google Scholar] [CrossRef]
- Liang, D.; Guo, T.; Chen, J.; Bian, L.; Zhao, J. Some progresses on studies of hydrocarbon generation and accumulation in marine sedimentary regions, Southern China (Part 1): Distribution of four suits of regional marine source rocks. Mar. Orig. Pet. Geol. 2008, 13, 1–16. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Li, Z.; Zhang, C.; Cui, H.; Zhu, Z. Characteristics and genetic types of the Lower Paleozoic natural gas, Ordos Basin. Mar. Pet. Geol. 2018, 89, 106–119. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Shen, Y.; Ji, W.; Li, Z. Insight into water occurrence and pore size distribution by nuclear magnetic resonance in marine shale reservoirs, southern China. Energy Fuels 2023, 37, 319–327. [Google Scholar] [CrossRef]
- Ding, W.; Li, C.; Su, A.; He, Z. Study on the comprehensive geochemical cross section of Mesozoic marine source rocks and prediction of favorable hydrocarbon generation area in Qiangtan basin, Tibeta. Acta Petrol. Sin. 2011, 27, 878–896. [Google Scholar]
- Xie, L.; Sun, Y.; Yang, Z.; Chen, J.; Jang, A.; Zhang, Y.; Deng, C. Evaluation of Hydrocarbon Generation of the Xiamaling Formation Shale in Zhangjiakou and Its Significance to the Petroleum Geology in North China. Sci. China Ser. D Earth Sci. 2013, 43, 1436–1444. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, L.; Huang, H.; Ning, C. Carbon isotopic evolution of hydrocarbon gases generated from carbonate source rocks via thermal simulation methods. Pet. Geol. Exp. 2022, 44, 121–128. [Google Scholar] [CrossRef]
- Wang, Q. The Ordovician Source Rocks Generate Gaseous Hydrocarbon Thermal Simulation Experiment Research; University of Chinese Academy of Sciences: Beijing, China, 2014. [Google Scholar]
- Moldowan, J.M.; Seifert, W.K.; Gallegos, E.J. Relationship between petroleum composition and depositional environment of petroleum source rocks. AAPG Bull. 1985, 69, 1255–1268. [Google Scholar]
- Peters, K.E.; Moldowan, J.M.; Schoell, M.; Hempkins, W.B. Petroleum isotopic and biomarker composition related to source rock organic matter and depositional environment. Org. Geochem. 1986, 10, 17–27. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Burton, Z.F.M.; Moldowan, J.M.; Sykes, R.; Graham, S.A. Unraveling petroleum degradation, maturity, and mixing and addressing impact on petroleum prospectivity: Insights from frontier exploration regions in New Zealand. Energy Fuels 2018, 32, 1287–1296. [Google Scholar] [CrossRef]
- Burton, Z.F.M.; Moldowan, J.M.; Magoon, L.B.; Sykes, R.; Graham, S.A. Interpretation of source rock depositional environment and age from seep oil, east coast of New Zealand. Int. J. Earth Sci. 2019, 108, 1093. [Google Scholar] [CrossRef]
- Schulz, H.M.; Yang, S.; Schovsbo, N.H.; Rybacki, E.; Ghanizadeh, A.; Bernard, S.; Mahlstedt, N.; Krüger, M.; Amann-Hildebrandt, A.; Krooss, B.M.; et al. The Furongian to Lower Ordovician Alum Shale Formation in conventional and unconventional petroleum systems in the Baltic Basin–A review. Earth-Sci. Rev. 2021, 218, 103674. [Google Scholar] [CrossRef]
- Sanei, H.; Petersen, H.; Schovsbo, N.; Jiang, C.; Goodsite, M. Petrographic and geochemical composition of kerogen in the Furongian (U. Cambrian) Alum Shale, central Sweden: Reflections on the petroleum generation potential. Int. J. Coal Geol. 2014, 132, 158–169. [Google Scholar] [CrossRef]
- Meng, M.; Peng, J.; Ge, H.; Ji, W.; Li, X.; Wang, Q. Rock fabric of lacustrine shale and its influence on residual oil distribution in the Upper Cretaceous Qingshankou Formation, Songliao Basin. Energy Fuels 2023, 37, 7151–7160. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Zhang, D.; Cai, Z.; Luo, H.; Chen, X.; Wang, T.; Li, F.; Zhang, W.; Li, F. Restudy on geochemical characteristics and genesis of Jingbian Gas Field in Ordos Basin. J. Northwest Univ. Nat. Sci. Ed. 2002, 52, 943–956. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Zan, C.; Ma, J. Geochemical characteristics of Ordovician subsalt gas reservoir and their significance for reunderstanding the gas source of Jingbian Gasfield, East Ordos Basin. Nat. Gas Geosci. 2009, 20, 8–14. [Google Scholar]
- Liu, D.; Zhang, W.; Kong, Q.; Feng, Z.; Fang, S.; Peng, W. Lower Paleozoic source rocks and natural gas origins in Ordos Basin, NW China. Pet. Explor. Dev. 2016, 43, 540–549. [Google Scholar] [CrossRef]
- Fu, J.; Yu, Z.; Li, C.; Wang, W.; Huang, Z.; Wu, X.; Wang, S. New discovery and favorable areas of natural gas exploration in the 4th Member of Ordovician Majiagou Formation by Well Mitan 1 in the eastern Ordos Basin. Nat. Gas Ind. 2021, 41, 17–27. [Google Scholar] [CrossRef]
- Schenk, H.; Dieckmann, V. Prediction of petroleum formation: The influence of laboratory heating rates on kinetic parameters and geological extrapolations. Mar. Pet. Geol. 2004, 21, 79–95. [Google Scholar] [CrossRef]
- Zhang, S.; Mi, J.; He, K. Synthesis of hydrocarbon gases from four different carbon sources and hydrogen gas using a gold-tube system by Fischer-Tropsch method. Chem. Geol. 2013, 349–350, 27–35. [Google Scholar] [CrossRef]
- He, K.; Zhang, S.; Mi, J.; Zhang, W. Pyrolysis involving n-hexadecane, water and minerals: Insight into the mechanisms and isotope fractionation for water-hydrocarbon reaction. J. Anal. Appl. Pyrolysis 2018, 130, 198–208. [Google Scholar] [CrossRef]
- Sweeney, J.; Burnham, A. Evaluation of a Simple Model of Vitrinite Reflectance Based on Chemical Kinetics. AAPG Bull. 1990, 74, 1559–1570. [Google Scholar]
- Li, E.; Pan, C.; Yu, S.; Jin, X.; Liu, J. Hydrocarbon generation from coal, extracted coal and bitumen rich coal in confined pyrolysis experiments. Org. Geochem. 2013, 64, 58–75. [Google Scholar] [CrossRef]
- Burnham, A.K. Kinetic models of vitrinite, kerogen, and bitumen reflectance. Org. Geochem. 2019, 131, 50–59. [Google Scholar] [CrossRef]
- Gao, D.; Guo, C.; Jiang, C.; Zhang, L.; Wang, H.; Shi, P.; Chen, Y. Hydrocarbon generation simulation of low-maturity shale in Shanxi Formation, Ordos Basin. Pet. Geol. Exp. 2018, 40, 454–460. [Google Scholar] [CrossRef]
- Xu, Y. Genetic Theory of Natural Gases and Its Application; Science Press: Beijing, China, 1994. [Google Scholar]
- Wang, Q.; Lu, H.; Greenwood, P.; Shen, C.; Liu, J.; Peng, P. Gas evolution during kerogen pyrolysis of Estonian Kukersite shale in confined gold tube system. Org. Geochem. 2013, 65, 74–82. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Q.; Hu, G.; Lv, Y.; Zhu, D.; Meng, Q.; Guo, F.; Wang, R. Mechanisms of carbon isotope fractionation in the process of natural gas generation: Geochemical evidence from thermal simulation experiment. Pet. Explor. Dev. 2020, 47, 972–983. [Google Scholar] [CrossRef]
- You, K.; Flemings, P.B.; Malinverno, A.; Collett, T.S.; Darnell, K. Mechanisms of methane hydrate formation in geological systems. Rev. Geophys. 2019, 57, 1146–1196. [Google Scholar] [CrossRef]
- Burton, Z.F.M.; Dafov, L.N. Testing the sediment organic contents required for biogenic gas hydrate formation: Insights from synthetic 3-D basin and hydrocarbon system modelling. Fuels 2022, 3, 555–562. [Google Scholar] [CrossRef]
- Burton, Z.F.M.; Dafov, L.N. Salt Diapir-Driven Recycling of Gas Hydrate. Geochem. Geophys. Geosystems 2023, 24, e2022GC010704. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wang, X.; Shi, B.; Luo, X.; Zhang, L.; Lei, Y.; Jiang, C.; Meng, Q. Carbon isotopic fractionation by desorption of shale gases. Mar. Pet. Geol. 2015, 60, 79–86. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, X.; Shi, B.; Lei, Y.; Zhao, H.; Zhao, Q.; Yin, J.; Gao, C. The 13C-depleted methane in terrigenous shale gas: A case study in the Triassic Yanchang Formation, Ordos Basin. Mar. Pet. Geol. 2022, 141, 105688. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, X.; Wang, X.; Shi, B.; Luo, X.; Zhang, L.; Lei, Y.; Jiang, C.; Liu, P. Gas geochemical evidences for biodegradation of shale gases in the Upper Triassic Yanchang Formation, Ordos Basin, China. Int. J. Coal Geol. 2017, 179, 139–152. [Google Scholar] [CrossRef]
- Tian, H.; Xiao, X.; Yang, L.; Xiao, Z.; Guo, L.; Shen, J.; Lu, Y. Pyrolysis of oil at high temperatures: Gas potentials, chemical and carbon isotopic signatures. Chin. Sci. Bull. 2009, 54, 1217–1224. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, J.; Zhao, H.; Huang, J.; Liu, Y.; Wang, Y.; Xie, X.; Xu, Y. Genesis and source of natural gas in Well Mitan-1 of Ordovician Majiagou Formation, mid-eastern Ordos Basin, China. Nat. Gas Geosci. 2023, 1–26. [Google Scholar]
- Kong, Q.; Zhang, W.; Li, J.; Zan, C.L. Geochemical characteristics and genesis of Ordovician natural gas under gypsolyte in Ordos Basin. Nat. Gas Geosci. 2019, 30, 423–432. [Google Scholar] [CrossRef]
- Xia, X.; Geoffrey, S.; Ma, Q.; Tang, Y. Compositional and stable carbon isotopic fractionation during non-autocatalytic thermochemical sulfate reduction by gaseous hydrocarbons. Geochim. Cosmochim. Acta 2014, 139, 472–486. [Google Scholar] [CrossRef]
- Wei, Z.; Zou, Y.; Cai, Y.; Wang, L.; Luo, X.; Peng, P. Kinetics of oil group-type generation and expulsion: An integrated application to Dongying Depression. Bohai Bay Basin. China Org. Geochem. 2012, 52, 1–12. [Google Scholar] [CrossRef]
- Liu, W.; Teng, G.; Wang, X.; Li, M.; Hu, G.; Wang, J.; Lu, L.; Zhao, H.; Chen, Q.; Luo, H. New knowledge of hydrocarbon generating theory of organic matter in Chinese marine carbonates. Pet. Explor. Dev. 2017, 44, 155–164. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Pei, L.; Cai, Z.; Luo, H.; Wang, X.; Zhang, D.; Liu, J. Hydrocarbon generation from calcium stearate: Insights from closed-system pyrolysis. Mar. Pet. Geol. 2021, 126, 104923. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, X.; Wang, X.; Jin, Z.; Zhu, D.; Meng, Q.; Huang, S.; Liu, J.; Fu, Q. Carbon and hydrogen isotopes of methane, ethane, and propane: A review of genetic identification of natural gas. Earth-Sci. Rev. 2019, 190, 247–272. [Google Scholar] [CrossRef]
- Schoell, M. The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim. Cosmochim. Acta 1980, 44, 649–661. [Google Scholar] [CrossRef]
- Schoell, M. Genetic characterization of natural gases. AAPG Bull. 1983, 67, 2225–2238. [Google Scholar]
- James, A.T. Correlation of natural gas by use of carbon isotopic distribution between hydrocarbon components. AAPG Bull. 1983, 67, 1176–1191. [Google Scholar]
- Clayton, C. Carbon isotope fractionation during natural gas generation from kerogen. Mar. Pet. Geol. 1991, 8, 232–240. [Google Scholar] [CrossRef]
- Dai, J. Hydrocarbon isotopic characteristics of natural gas and identification of various natural gases. Nat. Gas Geosci. 1993, 4, 1–40. [Google Scholar]
- Guo, H.; Liu, M.; Wang, Y.; Wang, Q.; Liu, J.; Peng, P. The effect of thermochemical sulfate reduction of n-pentane and condensate on the chemical and isotopic compositions of gases: Implications for the organic reactant and reaction extent. Mar. Pet. Geol. 2022, 145, 105916. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Qi, W.; Shi, S.; Jiang, F.; Zhang, C. Influence of Gypsum on the Distribution of Aromatic Molecules During Catagenesis and Its Geochemical Significance. J. Sediment. 2023, 1–13. [Google Scholar] [CrossRef]
- Zhu, X. Sedimentary Petrography; Petroleum Industry Press: Beijing, China, 2008; pp. 214–217. [Google Scholar]
- Prothero, D.R.; Schwab, F. Sedimentary Geology; Freeman: New York, NY, USA, 2004; p. 557. [Google Scholar]
- Zhao, G.; Li, S.; Liu, L. A study on characteristics and kinetics of catalytic degradation from kerogen in carbonate rocks. Chin. J. Geol. 2005, 40, 47–54. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Q.; Ma, G.; Wang, L.; Wang, X. Catalytic action of sulphate evaporates in pyrolytic gas generation of source rocks. Nat. Gas Geosci. 2009, 20, 26–31. [Google Scholar]
- Hu, M.; Cheng, Z.; Zhang, M.; Liu, M.; Song, L.; Zhang, Y.; Li, J. Effect of Calcite, Kaolinite, Gypsum, and Montmorillonite on Huadian Oil Shale Kerogen Pyrolysis. Energy Fuels 2014, 28, 1860–1867. [Google Scholar] [CrossRef]
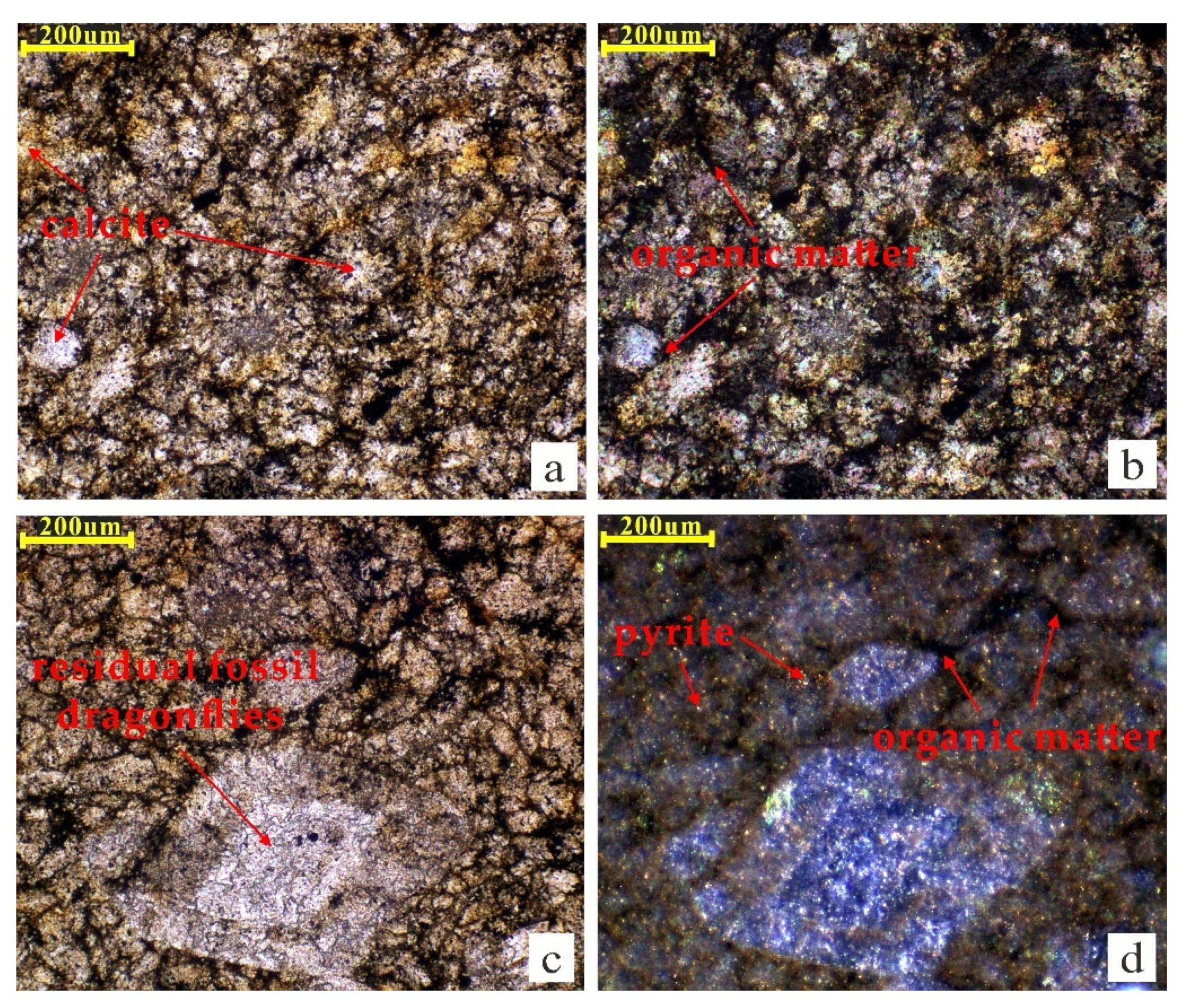

| Lithology | TOC (wt.%) | Tmax (°C) | S1 (mg/g) | S2 (mg/g) | S3 (mg/g) | HI (mg/g) | vRo (%) | Parent Material Types | Source |
|---|---|---|---|---|---|---|---|---|---|
| Nordic carbonate | 1.16 | 429 | 0.21 | 4.08 | 0.68 | 351 | 0.57 | IIB | this study |
| mud shale of Pingliang Formation | 0.86 | 443 | 0.05 | 1.34 | 0.77 | 147 | 0.80 | II | Wang et al., 2014 |
| coal of Shanxi Formation | 58.30 | 424 | 0.66 | 97.18 | / | 167 | 0.55 | III | Peng et al., 2020 |
| Heating Rate | T/ °C | easyro/% | Total Amount of G as | CH4 | C2H6 | C3H8 | iC4H10 | nC4H10 | iC5H12 | nC5H12 | H2 | CO2 | H2S | C1-5 | C6-14 | C14+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/gTOC | ||||||||||||||||
| 20 °C/h | 337 | 0.57 | 20.73 | 0.23 | 0.12 | 0.08 | 0.01 | 0.02 | 0.00 | 0.00 | 0.05 | 20.22 | 0.00 | 0.46 | 7.28 | 5.44 |
| 362 | 0.68 | 21.79 | 0.47 | 0.30 | 0.21 | 0.04 | 0.05 | 0.01 | 0.01 | 0.08 | 20.63 | 0.00 | 1.08 | 13.38 | 6.19 | |
| 384 | 0.80 | 43.12 | 1.63 | 1.29 | 1.07 | 0.22 | 0.38 | 0.08 | 0.10 | 0.24 | 33.36 | 4.75 | 4.77 | 27.22 | 8.10 | |
| 408 | 0.96 | 77.08 | 5.34 | 4.88 | 4.29 | 0.92 | 1.92 | 0.59 | 0.82 | 0.48 | 32.73 | 25.12 | 18.75 | 77.88 | 18.05 | |
| 432 | 1.19 | 193.00 | 15.89 | 14.14 | 12.89 | 2.53 | 5.88 | 1.84 | 2.38 | 0.63 | 56.40 | 80.41 | 55.56 | 137.49 | 25.91 | |
| 456 | 1.47 | 283.17 | 28.03 | 23.24 | 21.94 | 4.33 | 10.55 | 3.47 | 4.53 | 0.63 | 70.87 | 115.60 | 96.08 | 138.00 | 23.51 | |
| 480 | 1.81 | 411.44 | 47.36 | 35.10 | 33.03 | 6.70 | 14.12 | 3.60 | 4.24 | 0.57 | 94.86 | 171.87 | 144.14 | 79.20 | 16.11 | |
| 504 | 2.19 | 675.04 | 88.35 | 51.79 | 40.66 | 6.44 | 7.29 | 0.37 | 0.31 | 0.64 | 159.02 | 320.16 | 195.21 | 60.41 | 12.06 | |
| 529 | 2.54 | 952.04 | 127.64 | 45.95 | 18.20 | 1.21 | 0.26 | 0.01 | 0.01 | 0.98 | 234.81 | 522.98 | 193.28 | 45.34 | 8.39 | |
| 552 | 2.99 | 933.33 | 167.00 | 22.87 | 2.57 | 0.05 | 0.02 | 0.00 | 0.00 | 1.50 | 266.33 | 472.99 | 192.51 | 36.94 | 5.57 | |
| 576 | 3.32 | 1095.36 | 192.33 | 7.54 | 0.24 | 0.01 | 0.01 | 0.00 | 0.00 | 1.87 | 351.25 | 542.11 | 200.12 | 28.54 | 2.53 | |
| 601 | 3.87 | 1161.28 | 211.68 | 1.92 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 2.29 | 415.78 | 529.53 | 213.68 | 19.71 | 0.36 | |
| 2 °C/h | 336 | 0.74 | 34.87 | 0.82 | 0.58 | 0.49 | 0.14 | 0.15 | 0.03 | 0.04 | 0.11 | 32.51 | 0.00 | 2.25 | 17.55 | 5.13 |
| 360 | 0.88 | 56.97 | 2.68 | 2.30 | 2.10 | 0.52 | 0.85 | 0.25 | 0.28 | 0.30 | 40.40 | 7.29 | 8.98 | 40.32 | 10.78 | |
| 384 | 1.08 | 114.41 | 9.40 | 8.63 | 7.87 | 1.65 | 3.49 | 1.08 | 1.31 | 0.49 | 53.47 | 27.02 | 33.43 | 90.92 | 20.73 | |
| 408 | 1.38 | 192.50 | 24.17 | 19.58 | 18.15 | 3.47 | 8.15 | 2.45 | 2.99 | 0.52 | 65.63 | 47.39 | 78.95 | 158.92 | 28.71 | |
| 432 | 1.69 | 293.19 | 42.95 | 31.57 | 30.63 | 6.34 | 14.43 | 4.58 | 5.68 | 0.49 | 88.41 | 68.12 | 136.17 | 138.84 | 18.18 | |
| 457 | 2.09 | 468.46 | 75.07 | 49.68 | 46.61 | 9.62 | 17.42 | 3.29 | 3.60 | 0.53 | 136.36 | 126.28 | 205.29 | 81.08 | 13.29 | |
| 481 | 2.52 | 630.11 | 114.62 | 56.09 | 37.17 | 5.37 | 3.66 | 0.13 | 0.10 | 0.61 | 196.07 | 216.30 | 217.14 | 57.79 | 9.32 | |
| 504 | 2.99 | 953.60 | 158.21 | 39.14 | 6.99 | 0.32 | 0.10 | 0.01 | 0.01 | 0.97 | 309.25 | 438.61 | 204.77 | 38.88 | 5.39 | |
| 529 | 3.49 | 1222.12 | 192.05 | 4.21 | 0.31 | 0.02 | 0.01 | 0.00 | 0.00 | 1.14 | 421.81 | 602.57 | 196.60 | 31.08 | 2.81 | |
| 552 | 3.89 | 1201.07 | 217.92 | 1.21 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 1.50 | 454.06 | 526.31 | 219.20 | 24.47 | 1.94 | |
| 576 | 4.19 | 1236.05 | 223.84 | 0.93 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 1.85 | 516.13 | 493.26 | 224.80 | 13.84 | 0.88 | |
| 600 | 4.45 | 1508.19 | 248.71 | 0.83 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 2.37 | 708.38 | 547.90 | 249.55 | 10.25 | 0.89 | |
| 2 °C/h adding calcium sulfate | 504 | 2.99 | 1802.59 | 148.01 | 65.92 | 19.62 | 0.51 | 0.05 | 0.01 | 0.01 | 3.66 | 856.36 | 708.46 | 234.12 | 66.81 | 19.43 |
| 552 | 3.89 | 2341.24 | 213.34 | 42.82 | 0.25 | 0.01 | 0.01 | 0.00 | 0.00 | 6.55 | 1240.90 | 837.36 | 256.43 | 48.12 | 10.95 | |
| 600 | 4.45 | 3075.61 | 103.12 | 0.75 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.52 | 1592.90 | 1373.33 | 103.87 | 34.50 | 5.40 | |
| Heating Rate | T/°C | easyRo/% | δ13C1/‰ | δ13CCO2/‰ | δ13C2/‰ | δ13C3/‰ |
|---|---|---|---|---|---|---|
| 20 °C/h | 337 | 0.57 | 0.0 | −10.2 | 0.0 | 0.0 |
| 362 | 0.68 | 0.0 | −9.0 | 0.0 | 0.0 | |
| 384 | 0.80 | −44.7 | −7.5 | −38.8 | −34.8 | |
| 408 | 0.96 | −44.0 | −6.7 | −36.4 | −33.1 | |
| 432 | 1.19 | −43.2 | −6.7 | −35.3 | −31.6 | |
| 456 | 1.47 | −42.7 | −7.2 | −35.4 | −31.2 | |
| 480 | 1.81 | −40.8 | −8.9 | −34.0 | −30.1 | |
| 504 | 2.19 | −38.9 | −11.3 | −31.0 | 0.0 | |
| 529 | 2.54 | −37.4 | −15.4 | −25.7 | 0.0 | |
| 552 | 2.99 | −35.2 | −15.4 | 0.0 | 0.0 | |
| 576 | 3.32 | −33.0 | −14.3 | 0.0 | 0.0 | |
| 601 | 3.87 | −31.5 | −14.1 | 0.0 | 0.0 | |
| 2 °C/h | 336 | 0.74 | 0.0 | −9.0 | 0.0 | 0.0 |
| 360 | 0.88 | 0.0 | −7.5 | −38.0 | −35.4 | |
| 384 | 1.08 | −44.8 | −6.9 | −36.1 | −33.1 | |
| 408 | 1.38 | −43.2 | −7.1 | −34.6 | −31.3 | |
| 432 | 1.69 | −41.2 | −7.1 | −33.8 | −32.1 | |
| 457 | 2.09 | −40.2 | −7.8 | −32.5 | −29.1 | |
| 481 | 2.52 | −38.0 | −10.0 | −29.1 | 0.0 | |
| 504 | 2.99 | −35.0 | −12.2 | −21.2 | 0.0 | |
| 529 | 3.49 | −32.7 | −12.3 | 0.0 | 0.0 | |
| 552 | 3.89 | −31.6 | −12.7 | 0.0 | 0.0 | |
| 576 | 4.19 | −30.1 | −14.6 | 0.0 | 0.0 | |
| 600 | 4.45 | −28.4 | −14.8 | 0.0 | 0.0 | |
| 2 °C/h adding calcium sulfate | 504 | 2.99 | −37.3 | −6.7 | −27.7 | 0.0 |
| 552 | 3.89 | −34.7 | −8.2 | −17.4 | 0.0 | |
| 600 | 4.45 | −20.0 | −13.8 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, Y.; Huang, J.; Shi, J.; Zhao, H.; Wang, Q.; Meng, Q. Experimental Characteristics of Hydrocarbon Generation from Scandinavian Alum Shale Carbonate Nodules: Implications for Hydrocarbon Generation from Majiagou Formation Marine Carbonates in China’s Ordos Basin. J. Mar. Sci. Eng. 2023, 11, 1616. https://doi.org/10.3390/jmse11081616
Wang Y, Xu Y, Huang J, Shi J, Zhao H, Wang Q, Meng Q. Experimental Characteristics of Hydrocarbon Generation from Scandinavian Alum Shale Carbonate Nodules: Implications for Hydrocarbon Generation from Majiagou Formation Marine Carbonates in China’s Ordos Basin. Journal of Marine Science and Engineering. 2023; 11(8):1616. https://doi.org/10.3390/jmse11081616
Chicago/Turabian StyleWang, Yiqing, Yaohui Xu, Junping Huang, Jianglong Shi, Heng Zhao, Qingtao Wang, and Qiang Meng. 2023. "Experimental Characteristics of Hydrocarbon Generation from Scandinavian Alum Shale Carbonate Nodules: Implications for Hydrocarbon Generation from Majiagou Formation Marine Carbonates in China’s Ordos Basin" Journal of Marine Science and Engineering 11, no. 8: 1616. https://doi.org/10.3390/jmse11081616
APA StyleWang, Y., Xu, Y., Huang, J., Shi, J., Zhao, H., Wang, Q., & Meng, Q. (2023). Experimental Characteristics of Hydrocarbon Generation from Scandinavian Alum Shale Carbonate Nodules: Implications for Hydrocarbon Generation from Majiagou Formation Marine Carbonates in China’s Ordos Basin. Journal of Marine Science and Engineering, 11(8), 1616. https://doi.org/10.3390/jmse11081616





