Abstract
The design of an artificial reef (AR) module for improving the fishing productivity of cephalopod molluscs in the Ares-Betanzos estuary (Galicia, NW Spain) is addressed in this study. At the time of deciding on a suitable AR design, it is first necessary to assess how the different marine species use ARs so that it is possible to define the complexity of the design: its size and shape, as well as the number of nest cavities it should present and the dimensions of these cavities. Thus, two different cubic modules are proposed, both with an edge of 1500 mm. One of them can be considered as the standard design, while the other has been modified to include four open cylindrical holes. Several tools are employed to assess both proposals. Moreover, a CFD (computational fluid dynamics) model is performed. The results suggest that the flow in the interior of the tubes provides a suitable environment for cephalopod molluscs, given that circulation is produced, guaranteeing nutrient renewal. As further contributions, the present work determines how the capture of cephalopods and other species in Galician fish markets has evolved and reviews the habitat preferred by cephalopods in Galicia. It also proposes and compares two AR modules.
1. Introduction
Pollution and overfishing threaten marine habitats, leading to their degradation, which in turn makes it difficult to exploit their resources [1]. By way of example, cephalopods, a species of great importance all over the world [2], particularly in Galicia (NW Spain) [3], have experienced a major decline due to continued overfishing [4,5]. While public administrations have developed strategies to curtail biomass loss in marine ecosystems, studies show that those strategies are not always sufficient [5,6]. Consequently, new solutions, such as installing artificial reefs (ARs) specifically designed for certain marine species [5,7,8,9], must be proposed to protect marine ecosystems and to promote artisanal fishing.
In this context, the concentration of animal life around an AR mainly depends on the following four factors: (i) the aggregation behaviour of the species, (ii) the availability of shelter and nutrients, (iii) the tendency of the species to concentrate close to a solid object that breaks the uniformity of the environment, and (iv) the distance between the module and the coast [10,11]. The colonisation and evolution of AR benthic communities are directly related to the module design and, in particular, to its capacity to increase the available habitat, which is affected by the number of cavities and their sizes, as well as the AR design and orientation [10,12,13,14,15]. Therefore, Carral et al. [5] proposed the installation of ARs in the Ares-Betanzos estuary (Galicia, NW Spain) with the aim of reversing the decline in artisanal fishing. This study tries to go one step further by proposing specific AR designs to improve the breeding habitat of cephalopod molluscs in the same estuary.
1.1. The Problem of Cephalopods in Galicia: Evolution of Artisanal Fishing
Cephalopods play a crucial role in Galicia [16,17,18,19]. The species with the greatest socio-economic impacts on the Iberian Peninsula are the common octopus (Octopus vulgaris), cuttlefish (Sepia officinalis), and squid (Loligo vulgaris) [20].
Figure 1, Figure 2, Figure 3 and Figure 4 provide an analysis of how cephalopod artisanal fishing evolved in all the Galician fish markets for the 2006–2021 period. The data are based on the official statistics provided by Pesca de Galicia [21]. It is necessary to clarify that, despite the efforts of the local administrations, limited information is available on the relative abundance of Galician marine ecosystems [22]. Furthermore, Figure 1, Figure 2, Figure 3 and Figure 4 only include artisanal fishing. In other words, the figures derived from recreational fishing, as well as other catches that do not reach fish markets, were excluded.
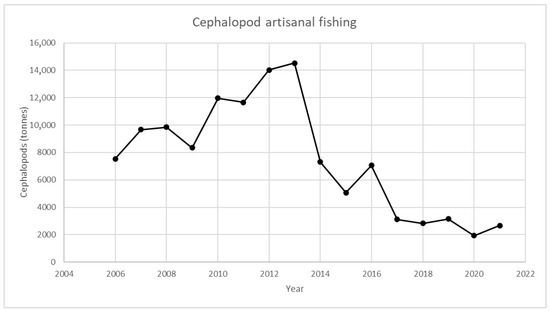
Figure 1.
Figure 1. Cephalopod landing in Galician fish markets. Source: own based on Pesca de Galicia [21].
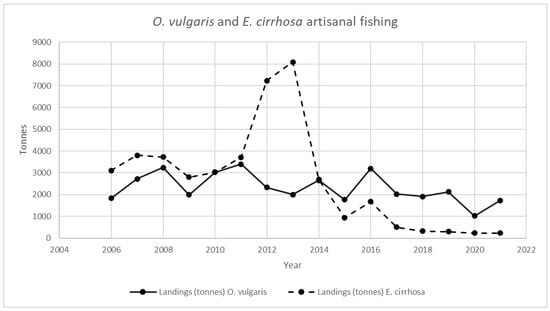
Figure 2.
O. vulgaris and E. cirrhosa landing in Galician fish markets. Source: own based on Pesca de Galicia [21].
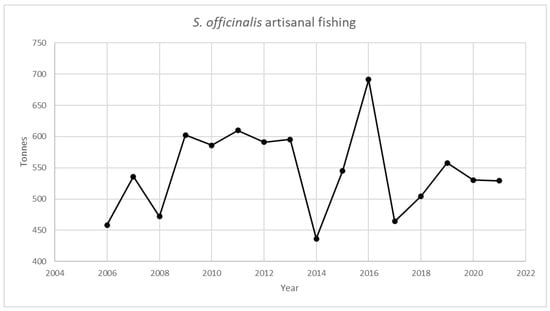
Figure 3.
S. officinalis landing in Galician fish markets. Source: own based on Pesca de Galicia [21].
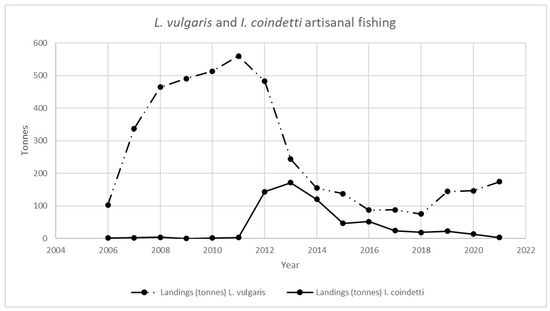
Figure 4.
L. vulgaris and I. coindetti landings in Galician fish markets. Source: own based on Pesca de Galicia [21].
If the figures for 2013 and 2021 are compared (Figure 1 and Figure 2), the landing of certain species has been on a downward trend, resulting in an 82% reduction in cephalopods. Furthermore, if the year 2021 is compared with the average number of tonnes for the whole period under study, the reduction is close to 65%. Figure 1 shows the landings in tonnes of cephalopods (O. vulgaris, Eledone cirrhosa, S. officinalis, L. vulgaris, and Illex coindetti) in all fish auctions throughout Galicia. There are two clearly differentiated stages. In the first one, from 2006 to 2013, landings experienced a continuous increase, with the exceptions of 2009 and 2011. However, the latter period is not significant, since 2010 and 2011 present nearly the same results. Nevertheless, from 2013 onwards, the trend is the opposite. The volume of landings decreased, showing how recovery efforts were unsuccessful. In fact, the highest value was reached in 2013 (14,500 tonnes), while the lowest one was recorded in 2020 (2000 tonnes).
In Figure 2, the reader can find the landing evolution for both O. vulgaris and E. cirrhosa in Galician fish markets for the same time period. O. vulgaris decreased by 25% if 2021 is compared with the average value for the 2006–2021 period, while E. cirrhosa experienced an even greater decline (about 90%).
Similarly, Figure 3 includes the evolution for S. officinalis. Finally, Figure 4 shows the same information for the L. vulgaris and I. coindetti species. The former shows a decline of close to 70% if 2011 and 2021 are compared, while the latter shows a decrease of 98% when the figures for 2014 and 2021 are contrasted.
There are several reasons for this decline in the landing of these and other species. For example, overexploitation, the discharge of pollutants into the sea, the proliferation of certain biotoxins and bacteria, the appearance of invasive species and, in general, pollution (climate change, acidification of seas, among other environmental impacts).
1.2. Natural Breeding Habitats for Cephalopods
The natural habitat preferred by each species must meet several conditions to ensure (i) stocks are improved, (ii) a protective refuge is provided for adult individuals with reproductive capacity, (iii) the biomass in adjacent areas increases due to larval dispersal, and (iv) recruitment occurs to allow juveniles to grow [23]. These objectives should also be met in ARs [10].
In general, cephalopods are very sensitive to environmental changes [24], temperature being a key factor for the biological processes of these species. Thus, migrations take place in the quest for conditions more favourable to their development [25]. Nevertheless, some species (O. vulgaris and L. vulgaris) show greater adaptability to changing environmental conditions [26].
In the particular case of O. vulgaris, various physical and biotic factors condition its density and distribution patterns. The most important ones are (i) temperature [27], (ii) seafloor characteristics as well as the availability and type of shelter [28,29,30,31], and (iii) both nutrient supply and fishing pressure [32,33]. In fact, temperature changes hinder the survival of O. vulgaris in deep waters and, according to Avendaño et al. [24], its exploitation should be limited to depths not exceeding 30 m.
In Galicia, it is observed that O. vulgaris lives at depths of between 5 and 21 m [16,27,34] along rocky bottoms with cavities of varying sizes and shapes to protect it from predators and competitors as well as to facilitate egg-laying [30,35]. O. vulgaris needs hard structures on soft substrates or penetrable areas on hard substrates. The diameter of the natural cavities ranges from 17 to 19 cm. Significant differences between the size and the cavity diameter of O. vulgaris were not found. There are also linked cavities, which can be used for several generations as “permanent cavities” [34]. Most of the cavities are found at depths of 5-10 m; the distance between cavities is close to 260 m2 (3.38 individuals per 1000 m2) [34]. At greater depths (15–21 m), scavenging species (starfish (Asterias rubens) and sea urchins (Paracentrotus lividus), among others) appear in all cavities. Used by females to cover the entrance of the cavities, stones and shells are common in O. vulgaris habitats. Guerra et al. [34] stated that half of the cavities could be permanent: narrow and deep holes whose walls are reinforced with shells.
Female O. vulgaris, taking advantage of warm temperatures in spring and summer, move to the coast, looking for sheltered places to lay their eggs, away from fishing areas [36,37,38,39]. After egg-laying, females fix the eggs to a common trunk or cordon at the base of the peduncles by using a sticky substance, generating clusters of variable shape and size, which are placed on the cavity ceiling [27]. Females then aerate, clean, and care for the eggs until they hatch. At this time, the female intermittently opens and closes the cavity to facilitate the exit of groups of hatchlings [40]. While the laying process may extend over days or even weeks [27], the breeding period can last for up to five months [27,41], and the hatching process can take a month and a half [24,27,42].
On the Galician coasts, the cavities used by O. vulgaris are at temperatures ranging from 12.9 to 19 °C [27]. The number of female egg chains can be equal to 160, and the total number of spawned eggs can exceed 200,000. The embryonic development time during the breeding period depends on the (i) laying date, (ii) temperature, (iii) position of the chain in the egg group, and (iv) position of the egg in the chain. Embryos in the basal and upper–middle parts of the egg chain are generally less developed than those occupying other positions. On the other hand, dissolved oxygen is essential to the embryonic development of egg chains [27,43,44], since large egg masses tend to obstruct the flow with less flow through them.
Regarding S. officinalis, the following factors should be taken into account at the time of defining an adequate breeding habitat in Galicia: (i) the type of substrate, since females carefully select where they lay their eggs, (ii) the type of zone, (iii) the depth, (iv) the temperature, and (v) the season [3,16]. S. officinalis also prefers rocky bottoms, with an abundance of Gorgonians where females lay their eggs (between 3500 and 6000) creating clusters similar to black grapes [3,45]. The presence of phanerogams also facilitates the survival of the species [3]. The laying process occurs close to the coast [46], with depths varying from 8 to 13 m. Occasionally, egg-laying can happen at depths of up to 25 m. The breeding period lasts for 40 to 45 days, with optimum temperatures varying from 12.5 to 14.75 °C [20]. The spawning season runs from December to April.
In the case of L. vulgaris, it is possible to find eggs fixed onto the ceiling of a rocky cavity [16]. This animal also has a predilection for artificial substrates, such as mooring lines or abandoned fishing nets. The egg-laying process, in clusters of eggs, is similar to that of S. officinalis [20]. Spawning activity occurs throughout the year [23], although most of the laying happens during the spring. Eggs are only found at depths of 18 to 50 m. The depth is closely related to the temperature. In other words, during the months with high temperatures, it is possible to find eggs at depths of 40–50 m, while in cold months, eggs only appear in shallow waters at depths of about 18 m [23]. The breeding period lasts between 40 and 45 days at 12–14 °C, 30 days at 17 °C, and 25 days at 22 °C [20]. Depending on their own size, females lay 3500 to 6000 eggs [20].
1.3. Objectives and Key Innovative Aspects
The main objective of this article is to design an artificial reef module specifically adapted to the needs of cephalopod molluscs in the Ares-Betanzos estuary. More specifically, this aim is divided into the following subobjectives:
- Defining suitable AR geometries to improve primary production by increasing autotrophic sources of substrate and nutrient circulation so that these reefs can function as a refuge and provide space for recruitment with available nest cavities.
- Performing a CFD analysis of the water flows (upwelling) generated in both the nest cavities and the vertical surfaces of the previously defined designs.
- Establishing a new tool for assessing the extent to which each design favours nutrient circulation: the Nest Cavity Circulation (NCC) index. The NCC is partially based on the results obtained in the CFD analysis.
- Comparing the AR designs by applying two indicators: the NCC index and the existing AREIT (AR Ecosystem Index Transformation) tool.
This work complements other studies conducted by the same research team with the objective of designing AR modules according to sustainability criteria [17,18,19,47,48].
2. Materials and Methods
Figure 5 shows a flowchart with the general steps followed in this study. The first phase was to identify the main characteristics that breeding habitats have to present for the most relevant cephalopod molluscs in Galicia. To this end, a state-of-the-art review was carried out (Section 1.2). After that, two AR designs, specifically adapted to the needs of cephalopod molluscs in Galicia, were proposed. Both modules were studied through a CFD analysis in the third stage. Finally, both designs were compared by means of two different indicators: the new NCC and existing AREIT indices.
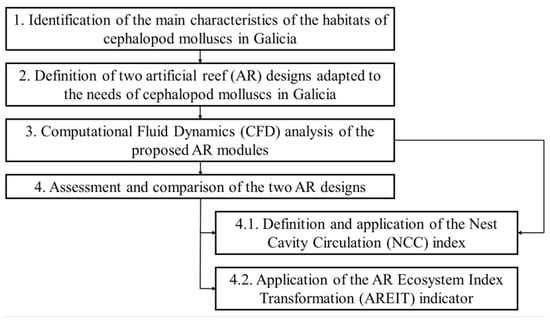
Figure 5.
Flowchart of the main steps followed in this study.
2.1. Proposed Artificial Reef Designs for the Embryonic Development of Cephalopod Molluscs in Galicia
Among key parameters for an AR to succeed in its purpose [10,49,50,51,52,53,54,55] are the design, spatial distribution, and the number and characteristics of the holes or nest cavities. The reefs that are the most complex in terms of geometry tend to be the most species-rich [10,54,56].
The free volume in most of the existing artificial reef modules is between 70 and 98% of the total volume [57,58]. The ideal size for a cavity may be between 150 and 1500 mm [59,60]. On the other hand, most species avoid closed cavities with a single opening. However, cephalopods are an exception [10]. In general, cubic modules with cylindrical holes or cavities ranging in size from 200 to 400 mm at a depth of 700 mm provide a stable substrate for the attachment of eggs, being suitable for the survival of hatchling cephalopods.
Consequently, a suitable reef module design for cephalopods could have either a cubic or a cylindrical geometry [10,14,60,61,62,63] with cubic (200–700 mm) or cylindrical cavities (200–1000 mm diameter) [10,13,59,60,62]. Each cavity should also present one or two cylindrical openings [10,60,61,62,63]. Table 1 summarises, in a qualitative way, the extent to which each of these possibilities impacts the egg-laying and embryonic development of different cephalopods.

Table 1.
Impact of each of the module design characteristics on the egg-laying and embryonic development of different cephalopods.
Two cubic modules with an edge of 1500 mm are proposed. The cubic shape was selected with the characteristics of the Ares-Betanzos estuary in mind. This estuary presents a stable current direction [64]; the cubic AR shape can be positioned according to the prevailing current direction. It is worth mentioning that, in other places where the current direction is variable, a cylindrical AR shape would provide omnidirectionality. On the other hand, the size and shape of the module are not particularly decisive factors for attracting cephalopod molluscs, as long as the AR unit provides adequate circulation of nutrients, nest cavities with the appropriate characteristics for the targeted species, and surfaces with adequate solar energy collection. In fact, size and shape are usually conditioned by transport and installation requirements. One of the AR modules can be considered as the standard or starting design (Figure 6), as proposed in previous works [12,65,66]. The other, which is the modified one, includes four cylindrical, open L-shaped holes with diameters of 300 mm, as seen in Figure 6. The differences between both designs are indicated in red in this figure. The modified design was proposed in view of the cephalopods’ needs for substrates and nest cavities to facilitate the development of their life cycle. In both cases, the size (1500 × 1500 × 1500 mm) and shape of the concrete modules were proposed, taking into account possible transport, construction, and logistical limitations. Both modules have an interior vertical hole with a diameter of 600 mm, which is linked to two horizontal holes, 250 and 450 mm in diameter. Moreover, nest cavities with diameters of 200 and 300 mm have been added to make space for specific organisms. At the time of proposing a module design, it is important to take into account that the number and size of nest cavities must not compromise the structural integrity of the AR [65].
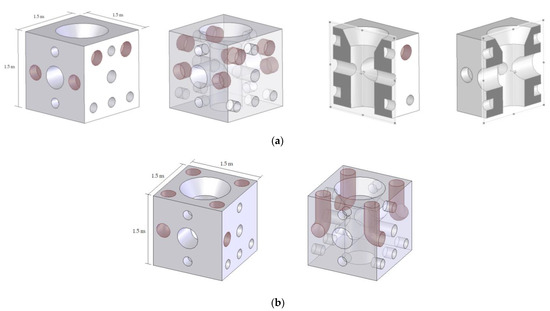
Figure 6.
AR proposals: (a) standard design and (b) modified design for cephalopods.
Two sides of the modules have smooth surfaces, providing verticality in a way that favours the upwelling effect, the movement of nutrients from deep layers to shallower ones. All holes serve as shelters and, at the same time, facilitate water currents, which are also necessary for embryonic development, particularly for L. vulgaris [10].
2.2. Computational Fluid Dynamics (CFD) Analysis
As indicated previously, a CFD model was carried out to study the AR from a hydrodynamic point of view. The CFD model was performed through the open software OpenFOAM. The model is based on the RANS (Reynolds-averaged Navier–Stokes) equations. The standard k-ε was employed as a turbulence model. Water was considered a Newtonian, incompressible, and single-phase fluid. The temporal performance was established as steady. Taking these aspects into account, the governing equations of the conservation of mass and momentum are those illustrated by Equations (1) and (2), respectively. The pressure–velocity coupling was treated through the SIMPLE (Semi Implicit Methods Pressure Linked Equations) algorithm. A second-order upwind scheme was used to discretize the governing equations.
In the equations above, ui and uj represent the velocity components for x, y, and z, ρ is the density, p is the pressure, and τij is the viscous stress tensor.
The computational domain and boundary conditions corresponding to the standard design are shown in Figure 7a. A 20,000 × 5000 × 4000 mm domain was employed with the AR inside. The computational domain and boundary conditions corresponding to the modified design are shown in Figure 7b. Since the modified design is not symmetrical, a 20,000 × 5000 × 8000 mm domain was employed. The current velocity was modelled as an inlet boundary condition. A value of 0.05 m/s was set according to typical values in this estuary [64]. The reef and seafloor were modelled as no-slip surfaces and the lateral and upper surfaces as free-slip.
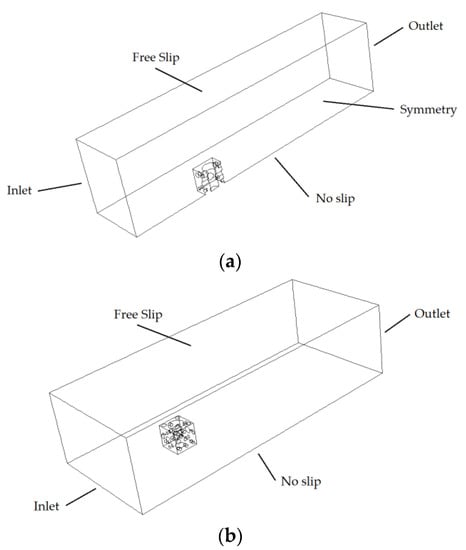
Figure 7.
Computational domain and boundary conditions; (a) standard design; (b) modified design.
The standard design was validated with experimental results for a towing tank [66].
Regarding the orientation with respect to the current velocity, the following two options were considered.
- -
- Orientation A: 250 mm diameter hole parallel to the current velocity.
- -
- Orientation B: 450 mm diameter hole parallel to the current velocity.
As is indicated in Section 2.3, the effects of the upwelling, back eddy, and circulation (inside the AR) were considered. In order to quantify these effects, the following parameters were employed.
- -
- Upwelling volume (Vupwelling): volume of the vortex formed above the AR where the x-component of the velocity is negative.
- -
- Back eddy volume (Vbackeddy): volume of the vortex formed behind the AR where the x-component of the velocity is negative.
- -
- Average flow velocity inside the AR (va): average flow velocity considering only the volume inside the AR.
A mesh independence analysis was carried out. The mesh independence analysis consisted of comparing these parameters using different meshes. Three meshes were tested for the standard AR design: mesh 1 (with around 1.4 × 106 elements depending on orientation A or orientation B), mesh 2 (with around 2.2 × 106 elements depending on orientation A or orientation B), and mesh 3 (with around 2.9 × 106 elements depending on orientation A or orientation B). Since the symmetry was not applied in the modified AR design, another three meshes with approximately double the elements were also tested for the modified AR design. Regarding the near-wall cells, a boundary layer with five layers was employed with a layer growth rate of 1.2. The size of the first layer provided y+ values of between 30 and 300 for all cases analysed. The results are summarised in Table 2. As can be seen, the results obtained with these meshes were too similar, showing their adequacy. According to this, mesh 3 was selected for the present work.

Table 2.
Results of the mesh independence study.
2.3. AREIT and NCC Indices
Carral et al. [12] proposed a new index (AREIT: AR Ecosystem Index Transformation) to evaluate the transformation that the ecosystem can experience with the installation of an AR module. A summary of the most relevant aspects of the AREIT is included below. However, the reader is also referred to Ref. [12] for more information.
This index serves to compare different AR designs according to three transcendence aspects for the ecosystem: energy, nutrients, and habitat for settling individuals. The AREIT index incorporates three intermediate indexes: EM (Energy Modification), NM (Nutrient Modification), and HM (Habitat Modification), as shown in Equation (3) [12]. On the other hand, it is important to note that the AREIT and its three subindices are relative indicators. In other words, they serve to measure the performance of a new design in relation to a base one. Consequently, all subindices for the base design will adopt a value of 1, while the AREIT will be equal to 3. The new design will obtain values greater than 1 for the subindices if its positive impact on the ecosystem is higher than that of the base design. Values lower than 1 will be obtained otherwise. Similarly, the AREIT index for the new design will be higher than 3 if it outperforms the base design. The opposite is also possible. In this case, the standard AR module was defined as the base design.
The AREIT index presents limitations when two designs, such as the ones proposed in this study, are being compared. In particular, the index is not able to differentiate between open and closed cavities. For this reason, certain species prefer cavities with a single opening, while others prefer completely open cavities. Furthermore, it also fails to adequately assess the circulation and its potential positive impact on nutrients and the habitat. A new index is needed to fill these gaps: the one related to Nest Cavity Circulation (NCC). This index consists of two intermediate indices: NCM (Nutrient Circulation Modification) and HCM (Habitat Circulation Modification), Equation (4).
The NCM index is made up of three terms, since the hydrodynamic effect that modifies nutrient circulation is produced by three phenomena: upwelling (Ueffect) and back eddy (Beffect) effects as well as circulation inside the interior cavities (Ieffect), Equation (5). The reader should bear in mind that iron, nitrogen, and phosphorus are some of the nutrients present in marine ecosystems. They belong to the first trophic level of the marine food chain, and they move within water flows. Furthermore, these nutrients serve as food for other species that are at the bottom of the food chain. These species serve as food for other species. Consequently, it seems reasonable to assume that nutrient renewal and water circulation around and inside the AR module are correlated. The upwelling region can be defined as the one corresponding to the vortex formed above the AR. It is important to remark that the upwelling phenomenon favours the vertical exchange of seawater, increasing the transport of nutrients and also enhancing their diffusion around the AR module. In other words, this upwelling effect is likely to attract different species, including cephalopod molluscs. The back eddy region comprises the region corresponding to the vortex behind the reef. Both the upwelling and back eddy regions play roles in the delivery of nutrients.
The three effects included in Equation (5) are measured using a scale of three values: 1 (poor result), 2 (acceptable result), and 3 (optimal result). These values are assigned on the basis of the circulation results obtained through CFD analyses. Consequently, the NCM varies from 3 (the worst possible result) to 9 (the best possible performance). As indicated previously, in the particular case of the Ueffect and Beffect, the values are assigned according to the volume of the region where the x-component of the velocity is negative [67]. The volume of this region is normalised to the total volume of the module, and the following values are established for the Ueffect: 1 if the volume of the upwelling region (Vupwelling) is equal to or less than 5% of the total volume of the module (VAR), 3 if Vupwelling is equal to or greater than 10% of the value of VAR, and 2 in all other cases. Similarly, the values for Beffect are defined according to the following rules: 1 if the volume of the back eddy effect (Vbackeddy) is equal to or less than 10% the value of VAR, 3 if Vbackeddy is equal to or higher than 15% the value of VAR, and 2 in all other cases. The value for the circulation inside the interior cavities (Ieffect) is defined from the average flow velocity inside the module (va) and from the current velocity (vc). The closer va is to vc, the better the circulation of nutrients. If the average flow velocity (va) is 0.25 times the current velocity (vc) or less, the worst possible value (1) is assigned. If the average flow velocity is more than 0.25 times vc but less than half of the value adopted by the current velocity, the acceptable value (2) is given. If va is 0.5 times the current velocity or higher, the best possible result (3) is adopted.
On the other hand, the HCM index takes three aspects into consideration: (i) the number of nest cavities (NNC), (ii) their size (SNC) and (iii) their type (TNC) (open or closed), Equation (6). Analogously to the NCM, each one of these terms is assessed through a dimensionless scale of values: 1 (poor result), 2 (acceptable result), and 3 (optimal result). It is important to note that the NNC is a comparative metric. In other words, when several designs are being compared, the best one adopts a value of 3 (optimal result). The results for the other designs are assigned according to a set of rules. For instance, the AR module with the largest number of cavities adopts a value of 3 for the NNC. The other designs take a value of 1 if there are no cavities or if the number of cavities is between zero and one-third of the largest number of cavities. A value of 3 is assigned to all the designs in which the number of cavities is equal to or greater than two thirds of the largest number of holes. In all other cases, a value of 2 is given. Similarly, the SNC adopts a value of 1 if only one-third or fewer of the cavities are of a suitable size for the corresponding species, in this case cephalopod molluscs (Section 1.2 and Section 2.1). The best possible value (3) is assigned if two-thirds or more of the cavities present an appropriate size for the targeted species. A value of 2 is defined in all other cases. Regarding TNC, three values are also possible: 1 if only one-tenth or fewer of the cavities are closed, 3 if at least half of the cavities are open, and 2 if the AR design is between the previous two cases. In contrast to other subindices, the level of exigency needed to obtain acceptable and optimal results in TNC is lower. The reason for this is to obtain a balance between the species that prefer open cavities and those that prefer holes with only one opening. Therefore, the HCM varies from 3 to 9, the worst and best possible results, respectively.
3. Results and Discussion
As indicated above, two AR designs (standard and modified) were proposed and analysed in this study. The AREIT and NCC indices were calculated for both modules. Two factors must be considered. One is that the egg chain must be oxygenated, particularly in the case of O. vulgaris. A second factor is the need for current in the tubular structures of the vertical, hollow model, especially for the embryonic development of L. vulgaris. Thus, two CFD studies were carried out with the aim of observing how water circulates in these cavities. The upwelling generated on the side faces of the two module types was also studied.
3.1. AREIT Index Results
The final ARs designs presented in Section 2.1 were assessed through the AREIT index. In this case, the parameters EM and NM adopted the same value for both designs, while the parameter HM corresponding to the nest cavities was different, as shown in Table 3.

Table 3.
General information and relevant parameters for the standard and modified AR unit designs.
The reference design, that is, the standard model, adopted a value of 1 for all partial indices included in Equation (1). The modified AR unit obtained the same results with only one exception: HM took a value of 0.7. In other words, the standard design presented an AREIT index equal to 3, while the modified model obtained a value of 2.7. This suggests that the modified design is more likely than the reference one to generate a lower positive impact on the ecosystem. Nevertheless, as previously indicated in Section 2.3, the AREIT index failed at the time of considering some potential positive impacts. This is the reason why it is also necessary to estimate the NCC index for both designs. As a preliminary step, the results derived from the CFD analysis are presented in the following subsection.
3.2. CFD Results
The velocity fields corresponding to both designs (standard and modified) with the two possible orientations (A and B) are shown in Figure 8 and Figure 9, respectively. Three planes are represented in these figures. A detail in the zone around the AR is also included in these figures. Regarding the standard AR, two of the planes are similar due to symmetry. All proposals offer an adequate habitat for species, promoting the circulation of nutrients. It was found that the flow in the tube interiors provides a suitable environment for cephalopods. Circulation is produced, which guarantees nutrient renewal inside the tubes, as well as a favourable hydrodynamic effect, facilitating the continued presence of nutrients around the AR. Both designs present appropriate results in terms of the circulation. However, from Figure 8 and Figure 9, it is clear that there are small differences, also reflected in the NCC index presented in the following subsection.
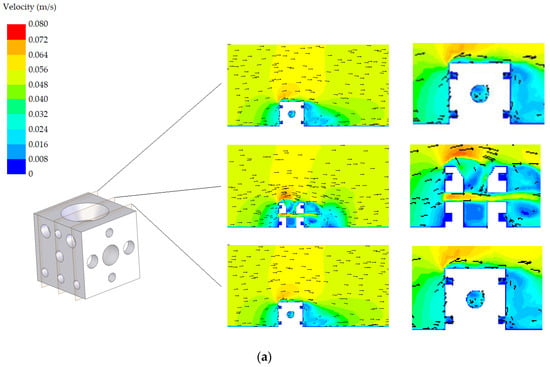
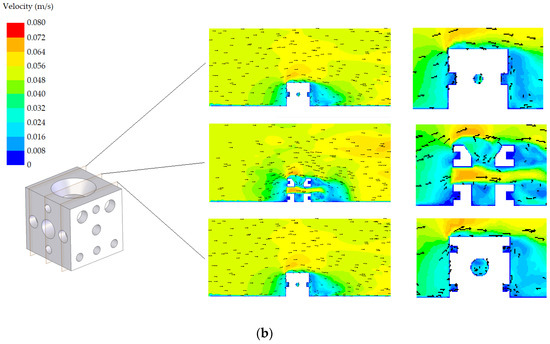
Figure 8.
Velocity field corresponding to the standard design: (a) orientation A and (b) orientation B.
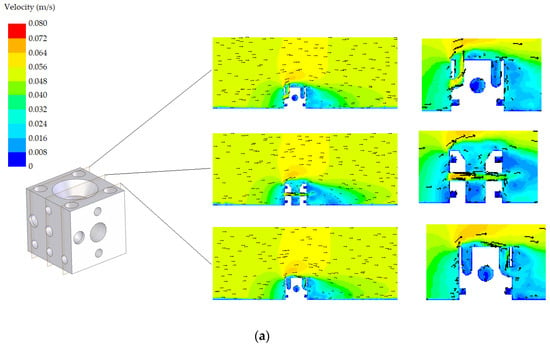
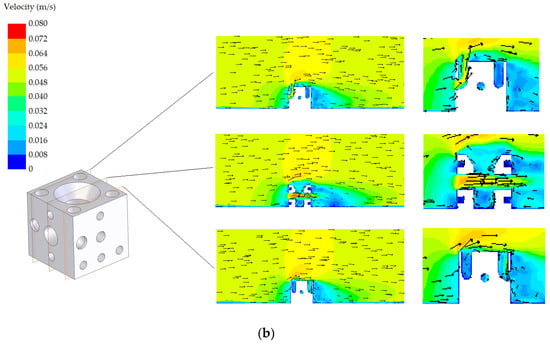
Figure 9.
Velocity field corresponding to the modified design: (a) orientation A and (b) orientation B.
3.3. NCC Index Results
The NCC index values for the two designs and orientations are summarised in Table 4. For the volume of the upwelling (Vupwelling) region, similar values were adopted for both designs and orientations (standard design and orientation A: 0.189 m3, standard design and orientation B: 0.152 m3, modified design and orientation A: 0.206 m3, modified design and orientation B: 0.165 m3). They represent between 4.5 and 6.1% of the total volume (VAR = 3.375 m3). Consequently, only values of 1 and 2 were achieved. When the same orientation was considered, the volume of the upwelling (Vupwelling) region was always higher in the modified design than in the standard one. Therefore, it is possible to say that the modified AR module is more conducive to this phenomenon, although the differences are small. In terms of the orientation, for the same design, option A always obtained higher values of Vupwelling than orientation B. The reason for this is that orientation A corresponds to the smaller main hole (250 mm) parallel to the current velocity. This promotes a higher proportion of the incoming water to be directed upwards, propitiating the upwelling effect. On the other hand, orientation B corresponds to the larger main hole (450 mm) parallel to the current velocity, and thus, a lower proportion of the upcoming water is directed upwards because it easily enters inside the AR. As for the back eddy effect, similar volumes were also obtained for both designs and orientations (standard design and orientation A: Vbackeddy = 0.619 m3, standard design and orientation B: Vbackeddy = 0.492 m3, modified design and orientation A: Vbackeddy = 0.652 m3, modified design and orientation B: Vbackeddy = 0.546 m3). These values represent between 14.58 and 19.32% of the total volume. Therefore, values of 2 and 3 were assigned according to the rules defined in Section 2.3. In this case, the trend is the same as that already discussed for the upwelling phenomenon: the modified design performs better than the standard one for a given orientation and, orientation A is more conducive to back eddy phenomenon, once a design is selected. Regarding the circulation in the interior cavities, the modified design with orientation B is the alternative with the highest average flow velocity inside the module (va = 0.031 m/s, that is, 0.62 times the current velocity). Consequently, its Ieffect subindex adopts a value of 3. If orientation A is considered, the average flow velocity decreases, taking a value of 0.022 m/s, slightly below 0.5 times the current velocity. The same trend is true for the standard design: circulation in the interior cavities is favoured when orientation B is considered, in contrast to the Ueffect and Beffect subindices. Since orientation B corresponds to the larger main hole (450 mm) parallel to the current velocity, more water enters the interior of the AR, which is expelled, mainly to the back part. This creates very intense circulation, and a high proportion of the interior of the AR has high velocities. Consequently, it is clear that the orientation with respect to the direction of the current velocity affects the nutrient circulation inside the module. Under the same orientation, the modified design always obtains higher average flow velocities than those achieved by the standard one and, consequently, better circulation exists in the interior of the module. The reason for this is that open holes promote higher velocities since they provide an inlet and an outlet. Closed holes lead to very low velocities since there is no outlet for the water to flow. On the other hand, it is important to note that the standard design with orientation B obtained a slightly higher average flow velocity than the modified design under orientation A (0.024 and 0.014 m/s, respectively). Nevertheless, these two values are so close to each other that there should not be differences in the assigned scores (both velocities are slightly below 0.5 times the current velocity, adopting a dimensionless value of 2). Consequently, the NCM index adopts a value of 7 for the modified design (orientations A and B) and for the standard module with orientation A. Nevertheless, the standard design with orientation B was the worst alternative according to this parameter.

Table 4.
NCC index results for the standard and modified designs.
Regarding the HCM subindex, both modules meet the conditions to obtain the best possible value (3) for the NNC indicator. Furthermore, all the cavities present a suitable size for cephalopod molluscs, obtaining a value of 3 for SNC. However, the standard design has no open cavities (TNC = 1), whereas one out of four cavities in the modified design is open (TNC = 2). Therefore, the modified design slightly outperforms the standard one for the HCM subindex (7 and 8 points, respectively). In this case, the orientation has no influence on the HCM subindex, since this metric assesses the impact on the habitat instead of quantifying the nutrient circulation.
If the NCC index is analysed, it is possible to say that both AR designs obtain remarkable results, since they present values close to the maximum (18) and far from the worst possible result (6). Nevertheless, in contrast to the AREIT index, in this case, the modified design was found to be slightly superior to the standard module. The selection between orientations A and B for the modified design will depend on whether the aim is to promote the circulation in the interior cavities (orientation B, Ieffect = 3) or the upwelling phenomenon (orientation A, Ueffect = 2).
Finally, it should be noted that the NCC results showed no significant differences for the two designs. This is a reasonable outcome, as the two modules differ only in the number and type of cavities. Nevertheless, the NCC appears to be sensitive enough to quantify such small differences. Greater differences would be obtained when comparing more disparate designs (for example, the proposed AR modules and a cylindrical one).
4. Conclusions
An analysis of the landings of cephalopod molluscs in Galicia showed the decline in catches in recent years. This is the reason why, after analysing the evolution of artisanal fishing in the last five years, the installation of ARs was proposed. Two different AR designs were considered in this work with the objective of imitating certain natural characteristics of the reproductive habitat for cephalopod mollusc species.
The starting point was the basic geometry of the AR module, which was based on the principles contained in ecosystem ecology and developed in Ref. [16]. From this cubic module, two AR modules were proposed: the so-called standard and the one designed under the consideration of multiplying the open-type vertical cavities.
Both designs were compared according to the existing AREIT index. This indicator served to estimate the potential positive transformation of the ecosystem once the modules had been installed. The results obtained show that both designs lead to the same level of positive impact on the ecosystem in terms of energy (EM) and nutrients (NM). Furthermore, both designs also achieved notable results in terms of habitat modification. However, in this case, the impact was not exactly the same, since their values for the HM subindex were not equal (different number of nest cavities).
Nutrient circulation was also analysed in both AR designs in all cavity types. A CFD model was applied for such a purpose. Finally, a new index was proposed and applied. In particular, it was the NCC (nest cavity circulation) indicator, which overcomes the main limitations of the AREIT.
According to the results obtained, the two proposed modules—the standard and modified ones—could ensure the development and growth of cephalopod molluscs in Galicia and specifically in the Ares-Betanzos estuary, since they act in a similar way. The first of the possibilities, the standard module, leads to a higher number of cavities, with a higher AREIT index, while the modified design favours circulation within cavities, with a higher NCC index.
Finally, this study presents some limitations that are linked to possible future developments. One option is to repeat this study varying water currents and tidal conditions. Other possibility is to analyse other parameters that also condition the habitats of the cephalopod mollusc in Galicia, such as the pH of the reef base material.
Author Contributions
Conceptualisation, M.J.R.G.; M.I.L.G.; J.J.C.B. and L.C.C.; methodology, J.J.C.B. and L.C.C.; validation, M.I.L.G., J.J.C.B. and L.C.C.; formal analysis, M.I.L.G., J.J.C.B. and L.C.C.; investigation, M.J.R.G., M.I.L.G., J.J.C.B. and L.C.C.; resources, M.J.R.G., M.I.L.G., J.J.C.B. and L.C.C.; writing—original draft preparation, M.J.R.G., M.I.L.G., J.J.C.B. and L.C.C.; writing—review and editing, J.J.C.B. and L.C.C.; visualisation, M.I.L.G., J.J.C.B. and L.C.C.; supervision, M.I.L.G., J.J.C.B. and L.C.C.; project administration, M.I.L.G., J.J.C.B. and L.C.C.; funding acquisition, L.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Xunta de Galicia under the grant CN-10MMA003CT. This study was also funded through the collaboration agreement INV07520 between Xunta de Galicia, Universidade da Coruña, and the Universidade da Coruña Foundation (FUAC) to give continuity to the previous project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the financial support from the regional government of Galicia, Xunta de Galicia, Universidade da Coruña, and the Universidade da Coruña Foundation (FUAC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Boyle, P.R.; Rodhouse, P. Cephalopods: Ecology and Fisheries; Blackwell Pub: Calgary, AB, USA, 2005. [Google Scholar]
- Guerra, A.; Hernández-Urcera, J.; Garci, M.E.; Sestelo, M.; Regueira, M.; Gilcoto, M.; González, A.F. Spawning habitat selection by the common cuttlefish Sepia officinalis in the Cíes Islands (Northwest Spain). Fish. Res. 2016, 183, 44–54. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L. World squid fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- Carral, L.; Alvarez-Feal, J.C.; Tarrio-Saavedra, J.; Rodriguez Guerreiro, M.J.; Fraguela, J.A. Social interest in developing a green modular artificial reef structure in concrete for the ecosystems of the Galician rías. J. Clean. Prod. 2018, 172, 1881–1898. [Google Scholar] [CrossRef]
- Hutchings, J.A. Collapse and recovery of marine fishes. Nature 2000, 406, 882–885. [Google Scholar] [CrossRef]
- Carral, L.; Jesús Rodríguez, M.; Fraguela, J.Á.; Díaz, V.; Álvarez, J.C.; Ferreño, S. Módulo para Formación de Arrecifes Artificiales. ES2579027, 31 January 2017. Available online: https://patentados.com/2016/modulo-para-la-formacion-de-arrecifes.1 (accessed on 27 May 2023).
- Carral, L.; Alvarez-Feal, C.; Rodríguez-Guerreiro, M.J.; Vargas, A.; Arean, N.; Carballo, R. Methodology for positioning a group of green artificial reef based on a database management system, applied in the estuary of Ares-Betanzos (Nw Iberian Peninsula). J. Clean. Prod. 2019, 233, 1047–1060. [Google Scholar] [CrossRef]
- Becker, A.; Taylor, M.; Folpp, H.; Lowry, M. Revisiting an artificial reef after 10 years: What has changed and what remains the same? Fish. Res. 2022, 249, 106261. [Google Scholar] [CrossRef]
- Herrera Pérez, R.B. Dinámica de las Comunidades Bentónicas de los Arrecifes Artificiales de Arguineguín (Gran Canaria) y Lanzarote. Ph.D. Thesis, Facultad de Ciencias del Mar, Puerto Real, Spain, 1998. [Google Scholar]
- Seaman, W.; Sprague, L.M. Artificial Habitats for Marine and Freshwater Fisheries; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Carral, L.; Lamas, M.I.; Cartelle Barros, J.J.; López, I.; Carballo, R. Proposed conceptual framework to design artificial reefs based on particular ecosystem ecology traits. Biology 2022, 11, 680. [Google Scholar] [CrossRef]
- Allemand, W.J.; Debernardi, D. Artificial reefs in the Principality of Monaco: Protection and enhancement of coastal zones. In Artificial Reefs in European Seas; Kluver Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 151–166. [Google Scholar]
- Barnabé, G.; Charbonnel, E.; Marinaro, J.Y.; Ody, D.; Francour, P. Artificial reefs in France: Analysis, assessments and prospects. In Artificial Reefs in European Seas; Jensen, A.C., Collins, K.J., Lockwood, A.P.M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 167–184. [Google Scholar]
- Costa Monteiro, C.; Neves Santos, M. Portuguese artificial reefs. In Artificial Reefs in European Seas; Kluver Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 249–261. [Google Scholar]
- Guerra, A.; Garci, M.E.; Hernández-Uicera, J.; González, A. Hábitats para la Puesta de Cefalópodos en el Parque Nacional Marítimo Terrestre de las Islas Atlánticas de Galicia; Sociedad de Ciencias de Galicia: Pontevedra, Spain, 2013. [Google Scholar]
- Carral, L.; Camba Fabal, C.; Lamas Galdo, M.I.; Rodríguez-Guerreiro, M.J.; Cartelle Barros, J.J. Assessment of the materials employed in green artificial reefs for the Galician estuaries in terms of circular economy. Int. J. Environ. Res. Public Health 2020, 17, 8850. [Google Scholar] [CrossRef]
- Camba, C.; Mier, J.L.; Carral, L.; Lamas, M.I.; Álvarez, J.C.; Díaz-Díaz, A.M.; Tarrío-Saavedra, J. Erosive degradation study of concrete augmented by mussel shells for marine construction. J. Mar. Sci. Eng. 2021, 9, 1087. [Google Scholar] [CrossRef]
- Carral, L.; Lamas Galdo, M.I.; Buenhombre, J.L.M.; Barros, J.J.C.; Naya, S.; Tarrio-Saavedra, J. Application of the residuals from purification of bivalve molluscs in Galician to facilitate marine ecosystem resiliency through artificial reefs with shells—One generation. Sci. Total Environm. 2023, 856, 159095. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A. Mollusca, Cephalopoda. In Fauna Ibérica; Museo Nacional de Ciencias Naturales (CSIC): Madrid, Spain, 1992; Volume 1. [Google Scholar]
- Consellería do Mar da Xunta de Galicia. Official statistics for the Galician Fishing Activity; Pesca de Galicia: Galicia, Spain, 2021. [Google Scholar]
- Villasante, S. Sostenibilidad de las Pesquerías Artesanales de Galicia; Netbiblo: A Coruña, Spain, 2010. [Google Scholar]
- Guerra, M.E.; Hernández-Urcera, A.; Garcí, J.; Cabanellas-Reboredo, B.; Sestelo, M.; Palmer, M.; Regueira, M.; Gilcoto, M.; Calvo-Manazza, M.; González, M.; et al. Identificación y caracterización de hábitats esenciales para tres especies de cefalópodos en los parques nacionales de las islas Atlánticas de Galicia y Cabrera. In Proyectos de Investigación en Parques Nacionales: 2012–2015; Serie Investigación en la, Red; Armengol, J., Ed.; Organismo Autónomo de Parques Nacionales, MAPAMA, Naturaleza y Parques Nacionales: Madrid, Spain, 2018; pp. 13–43. [Google Scholar]
- Avendaño, O.; Velazquez-Abunader, I.; Fernández-Jardón, C.; Ángeles-González, L.E.; Hernández-Flores, A.; Guerra, A. Biomass and distribution of the red octopus (Octopus maya) in the north-east of the Campeche Bank. J. Mar. Biol. Assoc. UK 2019, 99, 1317–1323. [Google Scholar] [CrossRef]
- Pierce, G.J.; Valavanis, V.D.; Guerra, A.; Jereb, P.; Orsi-Relini, L.; Bellido, J.M.; Katara, I.; Piatkowski, U.; Pereira, J.; Balguerias, E.; et al. A review of cephalopod–environment interactions in European seas. Hydrobiologia 2008, 612, 49–70. [Google Scholar] [CrossRef]
- Ramos, J.E.; Pecl, G.T.; Moltschaniwskyj, N.A.; Strugnell, J.M.; León, R.I.; Semmens, J.M. Body size, growth and life span: Implications for the polewards range shift of Octopus tetricus in South-Eastern Australia. PLoS ONE 2014, 9, e103480. [Google Scholar] [CrossRef]
- Garci, M.E.; Hernández-Urcera, J.; Gilcoto, M.; Fernández-Gago, R.; González, A.F.; Guerra, A. From brooding to hatching: New insights from a female Octopus vulgaris in the wild. J. Mar. Biol. Assoc. UK 2015, 96, 1341–1346. [Google Scholar] [CrossRef]
- Hartwick, B.; Ambrose, R.F.; Robinson, S.M.C. Dynamic of shallow-water population of Octopus dofeini. Mar. Biol. 1984, 82, 65–72. [Google Scholar] [CrossRef]
- Katsanevakis, G.; Verriopoulos, S. Den ecology of Octopus vulgaris Cuvier, 1797, on soft sediment: Availability and types of shelter. Sci. Mar. 2004, 68, 147–157. [Google Scholar] [CrossRef]
- Mather, J.A. Factors affecting the spatial distribution of natural populations of Octopus joubini robson. Anim. Behav. 1982, 30, 1166–1170. [Google Scholar] [CrossRef]
- Mereu, D.; Agus, M.; Cannas, B.; Cau, R.; Coluccia, A.; Cuccu, E. Mark-recapture investigation on Octopus vulgaris specimens in an area of the central western Mediterranean Sea. J. Mar. Biol. Assoc. UK 2015, 95, 131–138. [Google Scholar] [CrossRef]
- Hanlon, R.T.; Messenger, J.B. Cephalopod Behaviour; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Leite, T.S.; Haimovici, M.; Mather, J.; Oliveira, J.E.L. Habitat, distribution, and abundance of the commercial octopus (Octopus insularis) in a tropical oceanic island, Brazil: Information for management of an artisanal fishery inside a marine protected area. Fish. Res. 2009, 98, 85–91. [Google Scholar] [CrossRef]
- Guerra, A.; Hernández-Urcera, J.; Garci, M.E.; Sestelo, M.; González, A.F.; Cabanellas-Reboredo, M.; Calvo-Manazza, M.; Morales-Nin, B. Dwellers in dens on sandy bottoms: Ecological and behavioural traits of Octopus vulgaris. Sci. Mar. 2014, 78, 405–414. [Google Scholar] [CrossRef]
- Mather, J.A. Home choice and modifications by juvenile Octopus vulgaris (Mollusca: Cephalopoda): Specialized intelligence and tool use? J. Zool. 1994, 233, 359–368. [Google Scholar] [CrossRef]
- Alonso-Fernández, G.; Otero, A.; Bañón, J.; Campelos, R.; Santos, J.M.; Mucientes, J. Sex ratio variation in an exploited population of common octopus: Ontogenic shifts and spatio-temporal dynamics. Hydrobiologia 2017, 794, 1–16. [Google Scholar] [CrossRef]
- Boal, J.G. Behavioral research methods for octopuses and cuttlefishes. Vie Milieu 2011, 61, 203–210. [Google Scholar]
- García-Martínez, M.; Moya, M.C.; González, F.; Torres, M.; Farzaneh, P.; Vargas-Yañez, S. Comparative pattern of Octopus vulgaris life cycle with environmental parameters in the Northern Alboran Sea (Western Mediterranean Sea). Turk. J. Fish. Aquat. Sci. 2018, 18, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.; Guerra, A.; González, A.F. A review of reproductive strategies in cephalopods. Biol. Rev. 2001, 76, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Urcera, J.; Garci, M.E.; Roura, A.; González, A.F.; Cabanellas-Reboredo, M.; Morales-Nin, B.; Guerra, A. Cannibalistic behavior of octopus (Octopus vulgaris) in the wild. J. Comp. Psychol. 2014, 128, 427–430. [Google Scholar] [CrossRef]
- Mangold, K.; Boletzky, S. New data on reproductive biology and growth of Octopus vulgaris. Mar. Biol. 1973, 19, 7–12. [Google Scholar] [CrossRef]
- Villanueva, R.; Norman, M.D. Biology of the Planktonic Stages of Benthic Octopuses; Taylor & Francis: Abingdon, UK, 2008. [Google Scholar]
- Chung, W.S. Effects of Temperature, Salinity and Photoperiod on the Deposition of Growth Increments in Statoliths of the Oval Squid Sepioteuthis Lessoniana Lesson, 1830 (Cephalopoda: Loliginidae) during Early Stages; National Sun Yatsen University: Kaohsiung City, Taiwan, 2003. [Google Scholar]
- Strathmann, R.R.; Strathmann, M.F. Oxygen supply and limits on aggregation of embryos. J. Mar. Biol. Assoc. UK 1995, 75, 413–428. [Google Scholar] [CrossRef]
- Bloor, I.S.M.; Attrill, M.J.; Jackson, E.L. A review of the factors influencing spawning, early life stage survival and recruitment variability in the common cuttlefish (Sepia officinalis). In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2013; Volume 65, pp. 1–65. [Google Scholar]
- Lishchenko, F.; Perales-Raya, C.; Barrett, C.; Oesterwind, D.; Power, A.M.; Larivain, A.; Laptikhovsky, V.; Karatza, A.; Badouvas, N.; Lishchenko, A.; et al. A review of recent studies on the life history and ecology of European cephalopods with emphasis on species with the greatest commercial fishery and culture potential. Fish. Res. 2020, 236, 105847. [Google Scholar] [CrossRef]
- Carral, L.; Cartelle Barros, J.J.; Carro Fidalgo, H.; Camba Fabal, C.; Munín Doce, A. Greenhouse gas emissions and energy consumption of coastal ecosystem enhancement programme through sustainable artificial reefs in Galicia. Int. J. Environ. Res. Public Health 2021, 18, 1909. [Google Scholar] [CrossRef] [PubMed]
- Carral, L.; Tarrío-Saavedra, J.; Cartelle, J.J.; Camba-Fabal, C.; Ramil, A.; Álvarez-Feal, C. Design of artificial reef with a functional life limited to one generation. Heliyon 2023. [Google Scholar] [CrossRef]
- Bohnsack, J.A.; Sutherland, D.L. Artificial reef research: A review with recommendations for future priorities. Bull. Mar. Sci. 1985, 37, 11–39. [Google Scholar]
- Higo, S.; Tabata, N. On the fish gathering effect of the artificial reefs ascertained by diving observation. IV. At the sea in the west of Biro Island in the Shibushi Bay. Mem. Fac. Fish. Kagoshima Univ. 1979, 28, 107–117. [Google Scholar]
- Higo, N.; Tabata, S.; Nagashima, M.; Sakono, S. On the fish gathering effect of the artificial reefs ascertained by diving observation. VII At the off sea of Maskura City. Mem. Fac. Fish. Kagoshima Univ. 1980, 29, 51–63. [Google Scholar]
- Higo, N.; Higo, N.M. On the fish gathering effect of the artificial reefs ascertained by diving observation II. At the sea of the Satsuma Peninsula in Kagoshima Prefecture. Mem. Fac. Fish. Kagoshima Univ. 1978, 27, 117–130. [Google Scholar]
- Lemoine, H.R.; Paxton, A.B.; Anisfeld, S.C.; Rosemond, R.C.; Peterson, C.H. Selecting the optimal artificial reefs to achieve fish habitat enhancement goals. Biol. Conserv. 2019, 238, 108200. [Google Scholar] [CrossRef]
- Ogawa, Y.; Takemura, Y. Experiments on the attractiveness of artificial reefs for marine fishes III. Observations on stone bream in the outdoor tank. Bull. Tokai Reg. Fish. Res. Lab. 1966, 46, 127–135. [Google Scholar]
- Walton, J.M. Puget sound artificial reef study. State of Washington. Dep. Fish. Tech. Rep. 1979, 50, 130. [Google Scholar]
- Chang, K.H.; Lee, S.C.; Kwang-Tsao, S. Evaluation of artificial reef efficiency based on the studies of model reef fish community installed in northern Taiwan. Bull. Inst. Zool. Acad. Sin. 1977, 16, 23–36. [Google Scholar]
- Mottet, M.G. Enhancement of the marine environment for fisheries and aquaculture in Japan. Washingt. Dep. Fish. Tech. Rep. 1981, 69, 96. [Google Scholar]
- Ogawa, Y. The present status and future prospects of artificial reefs: Developmental trends of artificial reef units. In Japanese Artificial Reef Technology; Vik, S.F., Ed.; Aquabio Inc.: Belleair Bluffs, FL, USA, 1982; pp. 23–41. [Google Scholar]
- Grove, R.S.; Sonu, C.J. Review of Japanese Fishing Reef Technology; Technical Report 83-RD-137; Southern California Edison Company: Rosemead, CA, USA, 1983. [Google Scholar]
- White, A.T.; Ming, C.L.; de Silva, M.W.R.N.; Guarin, F.Y. Artificial reefs for marine habitat enhancement in Southeast Asia; Association of Southeast Asian Nations: Manila, Philippines, 1990; ISBN 971-1022-83-4. [Google Scholar]
- Bombace, G.; Fabi, G.; Fiorentini, L. Artificial Reefs in the Adriatic Sea. In Artificial Reefs in European Seas; Springer: Dordrecht, The Netherlands, 2000; pp. 31–63. [Google Scholar]
- Badalamenti, F.; D’Anna, G.; Riggio, S. Artificial reefs in the Gulf of Castellammare (North-West Sicily): A case study. In Artificial Reefs in European Seas; Springer: Dordrecht, The Netherlands, 2000; pp. 75–96. [Google Scholar]
- Relini, G. The Loano artificial reef. In Artificial Reefs in European Seas; Springer: Dordrecht, The Netherlands, 2000; pp. 129–149. [Google Scholar]
- Carral, L.; Lamas-Galdo, M.I.; Rodríguez-Guerreiro, M.J.; Vargas, A.; Álvarez-Fea, C.; López, I.; Carballo, R. Configuration methodology for a green variety reef system (AR group) based on hydrodynamic criteria—Application to the Ría de Ares-Betanzos. Estuar. Coast. Shelf Sci. 2021, 252, 107301. [Google Scholar] [CrossRef]
- Lamas Galdo, M.I.; Rodríguez Guerreiro, M.J.; Lamas Vigo, J.; Ameneiros Rodriguez, I.; Veira Lorenzo, R.; Carral Couce, J.C.; Carral Couce, L. Definition of an artificial reef unit through hydrodynamic and structural (CFD and FEM) models—Application to the Ares-Betanzos estuary. J. Mar. Sci. Eng. 2022, 10, 230. [Google Scholar] [CrossRef]
- Santiago Caamaño, L.; Lamas Galdo, M.I.; Carballo, R.; López, I.; Cartelle Barros, J.J.; Carral, L. Numerical and experimental analysis of the velocity field inside an artificial reef. Application to the Ares-Betanzos estuary. J. Mar. Sci. Eng. 2022, 10, 1827. [Google Scholar] [CrossRef]
- Zhou, P.; Gao, P.; Zheng, S. Three-dimensional numerical simulation on flow behavior behind trapezoidal artificial reefs. Ocean Eng. 2022, 266, 112899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).