Correlation of the Structural Characteristics of an Artificial Oyster Reef with Its Wake Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Artificial Oyster Reef Models
2.2. Flow Analysis
2.3. Wake Volume and Evaluation Indices
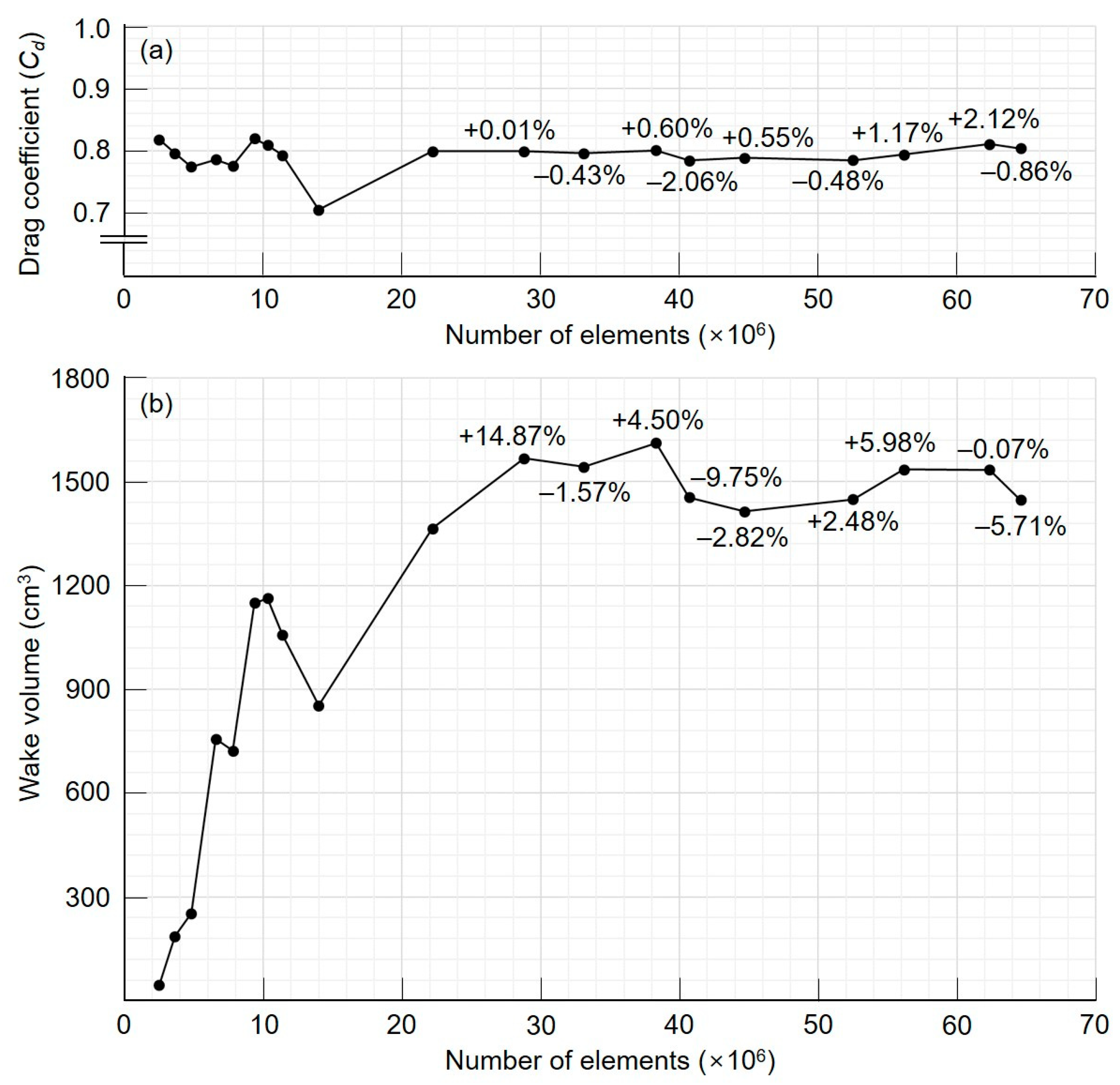
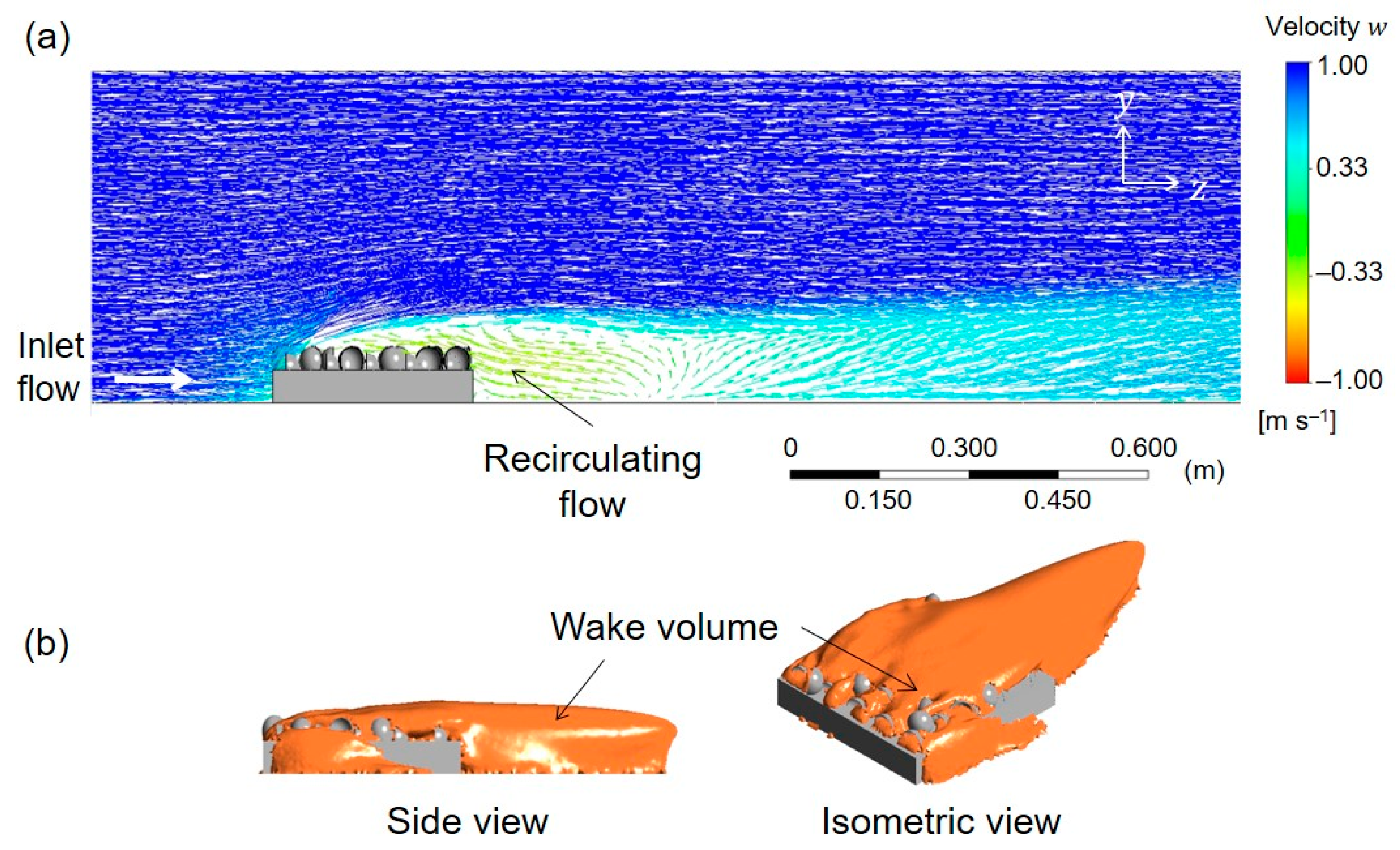
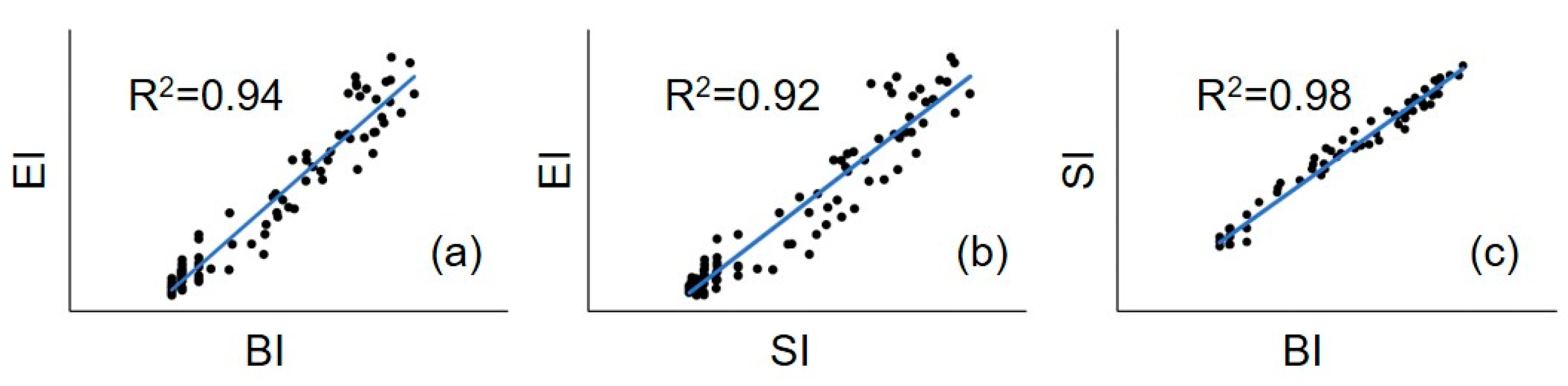
3. Results and Discussion
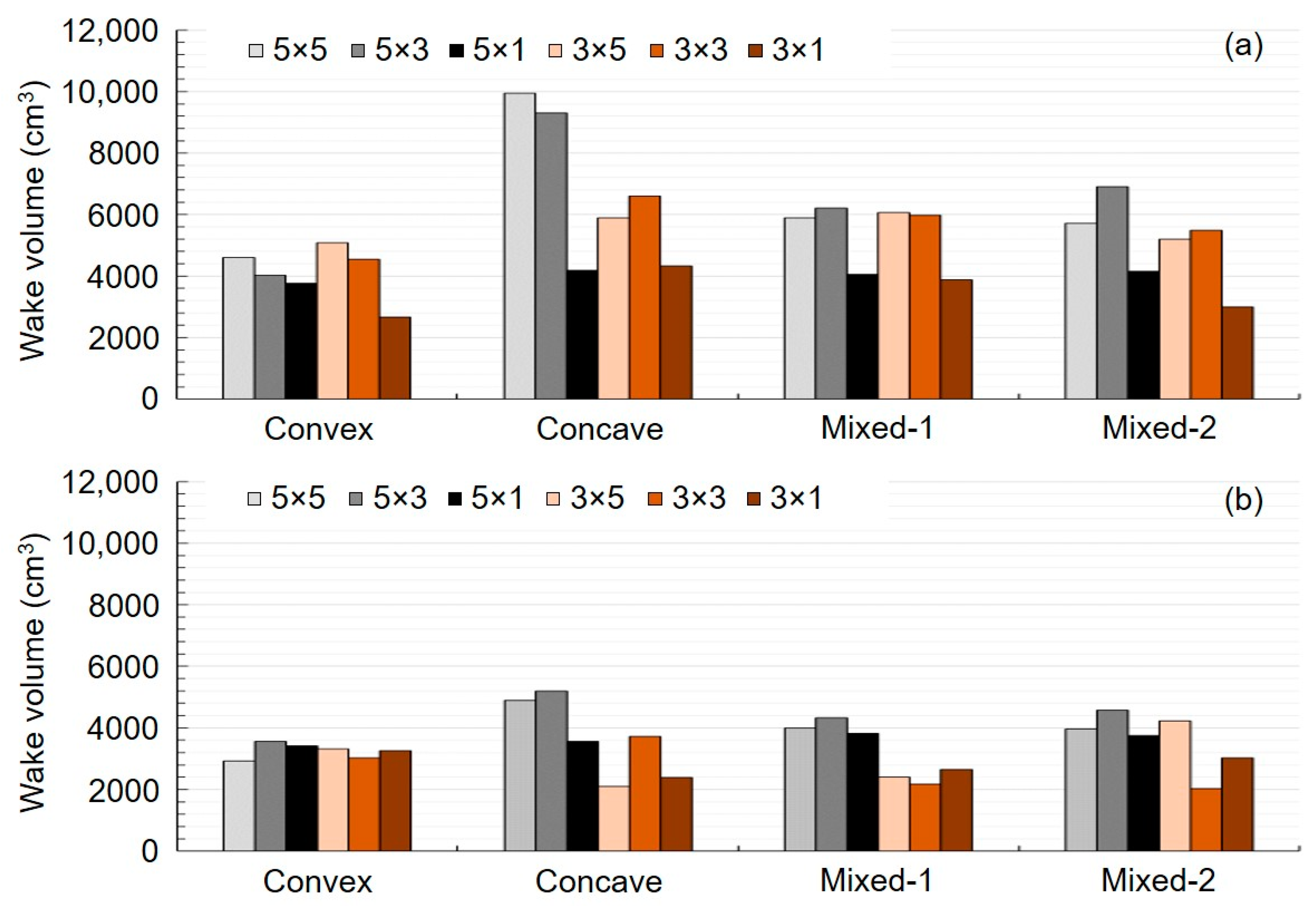
3.1. Initial AOR Model
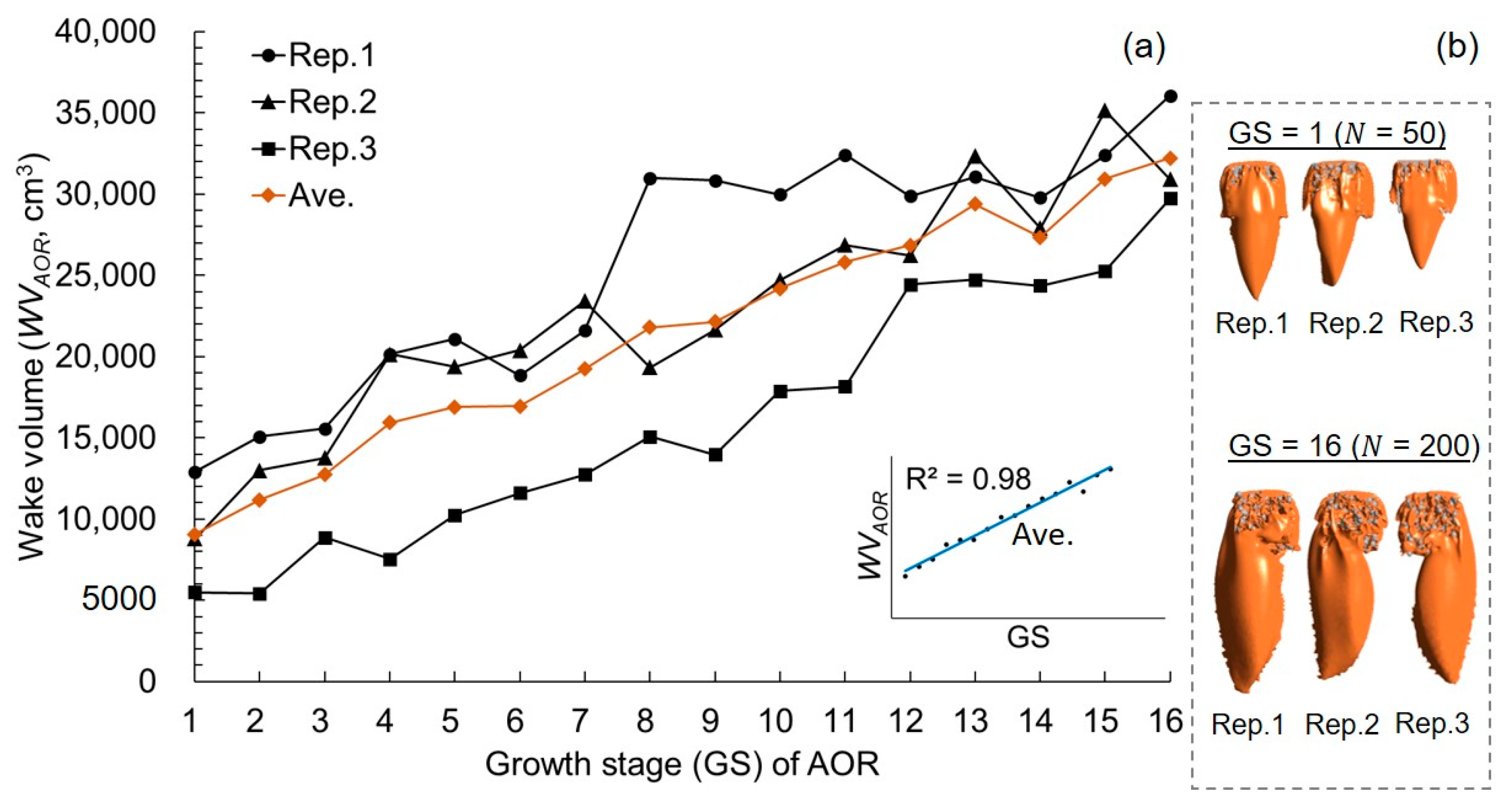
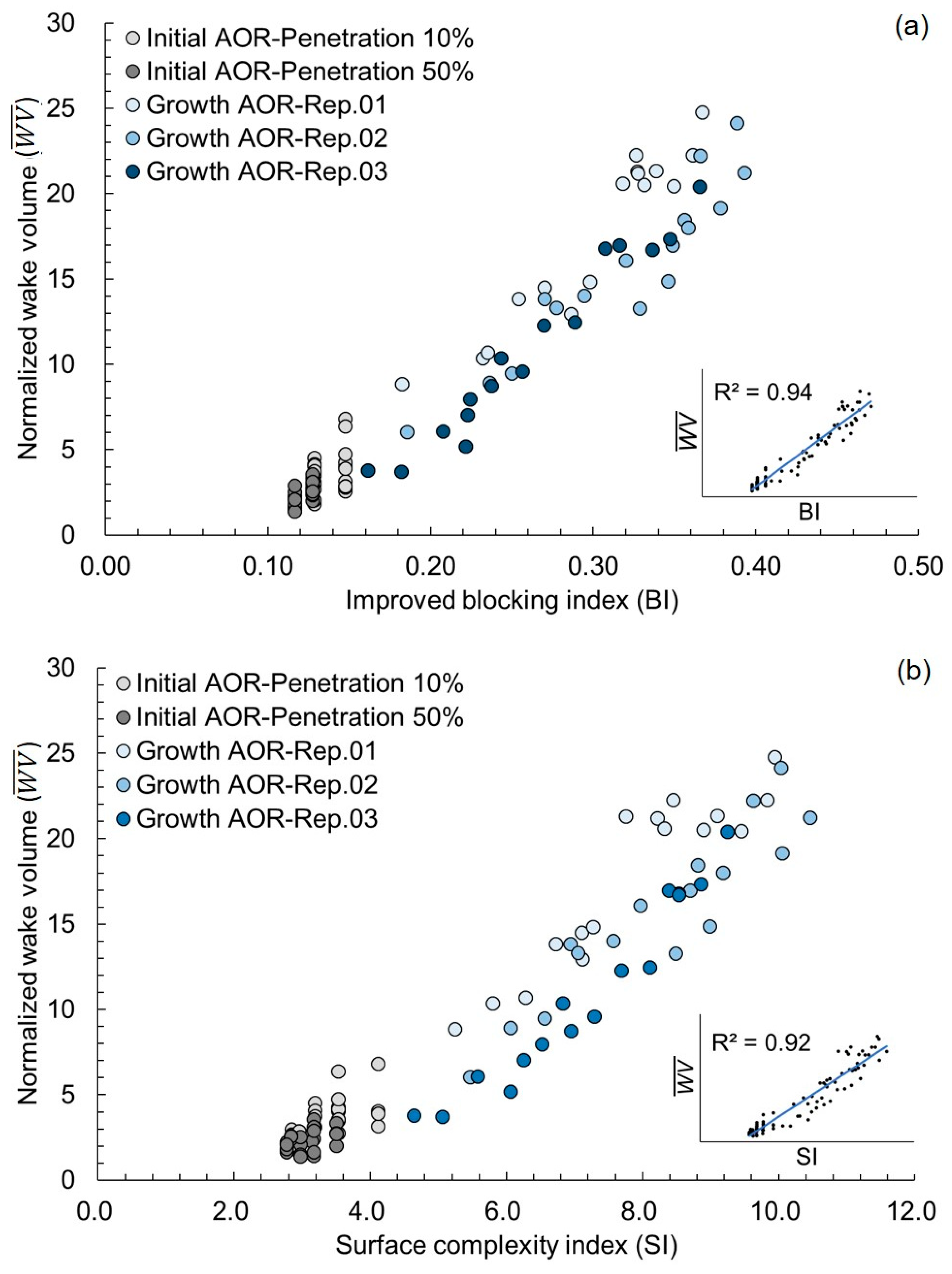
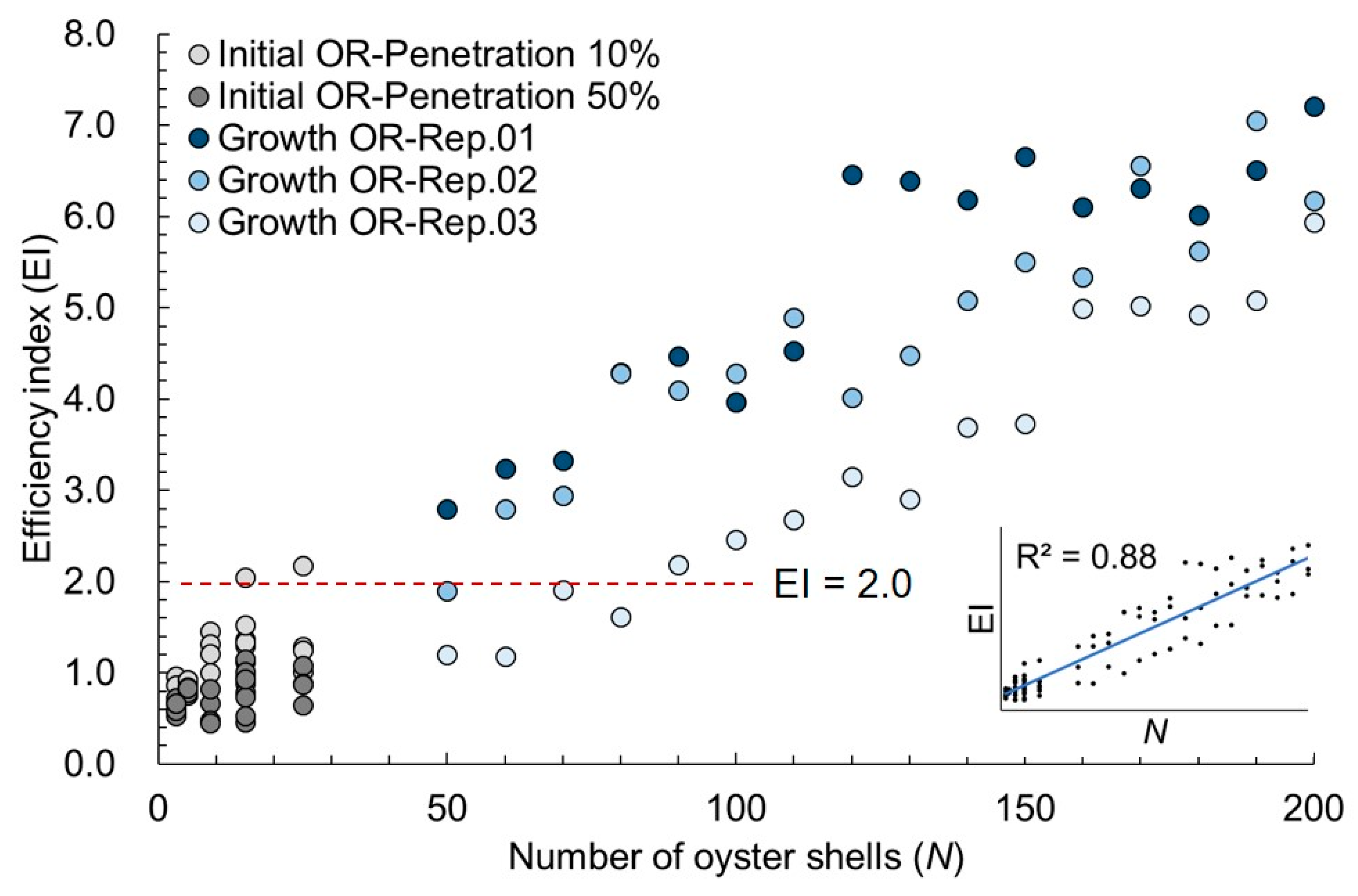
3.2. Growth AOR Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, K. Shell structure and behaviour related to cementation in oysters. Mar. Biol. 1994, 118, 89–100. [Google Scholar] [CrossRef]
- Reeves, S.E.; Renzi, J.J.; Fobert, E.K.; Silliman, B.R.; Hancock, B.; Gillies, C.L. Facilitating better outcomes: How positive species interactions can improve oyster reef restoration. Front. Mar. Sci. 2020, 7, 656. [Google Scholar] [CrossRef]
- Gillies, C.L.; Castine, S.A.; Alleway, H.K.; Crawford, C.; Fitzsimons, J.A.; Hancock, B.; Koch, P.; McAfee, D.; McLeod, I.M.; zu Ermgassen, P.S.E. Conservation status of the Oyster Reef Ecosystem of Southern and Eastern Australia. Glob. Ecol. Conserv. 2020, 22, e00988. [Google Scholar] [CrossRef]
- Sharma, S.; Goff, J.; Moody, R.M.; Byron, D.; Heck, K.L., Jr.; Powers, S.P.; Ferraro, C.; Cebrian, J. Do restored oyster reefs benefit seagrasses? An experimental study in the Northern Gulf of Mexico. Restor. Ecol. 2016, 24, 306–313. [Google Scholar] [CrossRef]
- Glenn, M.; Mathieson, A.; Grizzle, R.; Burdick, D. Seaweed communities in four subtidal habitats within the Great Bay estuary, New Hampshire: Oyster farm gear, oyster reef, eelgrass bed, and mudflat. J. Exp. Mar. Biol. Ecol. 2020, 524, 151307. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Weaver, R.J.; Stehno, A.; Bonanno, N.; Sager, A.; Kenny, A.; Zehnder, J.A.; Glasser, C.; Allen, A. Scale model design of oyster reef structures as part of a showcase living shoreline project. Shore Beach 2017, 85, 41–54. [Google Scholar]
- Styles, R. Flow and turbulence over an oyster reef. J. Coast. Res. 2015, 31, 978–985. [Google Scholar] [CrossRef]
- Kitsikoudis, V.; Kibler, K.M.; Walters, L.J. In-situ measurements of turbulent flow over intertidal natural and degraded oyster reefs in an estuarine lagoon. Ecol. Eng. 2020, 143, 105688. [Google Scholar] [CrossRef]
- Hitzegrad, J.; Brohmann, L.; Pfennings, K.; Hoffmann, T.K.; Eilrich, A.K.; Paul, M.; Welzel, M.; Schlurmann, T.; Aberle, J.; Wehrmann, A.; et al. Oyster reef surfaces in the central Wadden Sea: Intra-reef classification and comprehensive statistical description. Front. Mar. Sci. 2022, 9, 808018. [Google Scholar] [CrossRef]
- Humphries, A.T.; La Peyre, M.K.; Decossas, G.A. The effect of structural complexity, prey density, and “predator-free space” on prey survivorship at created oyster reef mesocosms. PLoS ONE 2011, 6, e28339. [Google Scholar] [CrossRef] [PubMed]
- Walles, B.; Mann, R.; Ysebaert, T.; Troost, K.; Herman, P.M.J.; Smaal, A.C. Demography of the ecosystem engineer Crassostrea gigas, related to vertical reef accretion and reef persistence. Estuar. Coast. Shelf Sci. 2015, 154, 224–233. [Google Scholar] [CrossRef]
- Loke, L.H.L.; Chisholm, R.A. Measuring habitat complexity and spatial heterogeneity in ecology. Ecol. Lett. 2022, 25, 2269–2288. [Google Scholar] [CrossRef] [PubMed]
- Windle, A.E.; Puckett, B.; Huebert, K.B.; Knorek, Z.; Johnston, D.W.; Ridge, J.T. Estimation of intertidal oyster reef density using spectral and structural characteristics derived from unoccupied aircraft systems and structure from motion photogrammetry. Remote Sens. 2022, 14, 2163. [Google Scholar] [CrossRef]
- Howie, A.H.; Bishop, M.J. Contemporary oyster reef restoration: Responding to a changing world. Front. Ecol. Evol. 2021, 9, 689915. [Google Scholar] [CrossRef]
- O’Beirn, F.X.; Luckenbach, M.W.; Nestlerode, J.A.; Coates, G.M. Toward design criteria in constructed oyster reefs: Oyster recruitment as a function of substrate type and tidal height. J. Shellfish Res. 2000, 19, 387–395. [Google Scholar]
- Soniat, T.M.; Finelli, C.M.; Ruiz, J.T. Vertical structure and predator refuge mediate oyster reef development and community dynamics. J. Exp. Mar. Biol. Ecol. 2004, 310, 163–182. [Google Scholar] [CrossRef]
- McAfee, D.; McLeod, I.M.; Boström-Einarsson, L.; Gillies, C.L. The value and opportunity of restoring Australia’s lost rock oyster reefs. Restor. Ecol. 2020, 28, 304–314. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Lengkeek, W.; Bergsma, J.H.; Coolen, J.W.P.; Didderen, K.; Dorenbosch, M.; Driessen, F.M.F.; Kamermans, P.; Reuchlin-Hugenholtz, E.; Sas, H.; et al. Return of the native facilitated by the invasive? Population composition, substrate preferences and epibenthic species richness of a recently discovered shellfish reef with native European flat oysters (Ostrea edulis) in the North Sea. Mar. Biol. Res. 2018, 14, 590–597. [Google Scholar] [CrossRef]
- La Peyre, M.K.; Humphries, A.T.; Casas, S.M.; La Peyre, J.F. Temporal variation in development of ecosystem service from oyster reef restoration. Ecol. Eng. 2014, 63, 34–44. [Google Scholar] [CrossRef]
- Fivash, G.S.; Stüben, D.; Bachmann, M.; Walles, B.; van Belzen, J.; Didderen, K.; Temmink, R.J.M.; Lengkeek, W.; van der Heide, T.; Bouma, T.J. Can we enhance ecosystem-based coastal defense by connecting oysters to marsh edges? Analyzing the limits of oyster reef establishment. Ecol. Eng. 2021, 165, 106221. [Google Scholar] [CrossRef]
- Cannon, D.; Kibler, K.; Walters, L.; Chambers, L. Hydrodynamic and biogeochemical evolution of a restored intertidal oyster (Crassostrea virginica) reef. Sci. Total Environ. 2022, 831, 154879. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D.; Kibler, K.M.; Kitsikoudis, V.; Medeiros, S.C.; Walters, L.J. Variation of mean flow and turbulence characteristics within canopies of restored intertidal oyster reefs as a function of restoration age. Ecol. Eng. 2022, 180, 106678. [Google Scholar] [CrossRef]
- Goelz, T.; Vogt, B.; Hartley, T. Alternative substrates used for oyster reef restoration: A review. J. Shellfish Res. 2020, 39, 1–12. [Google Scholar] [CrossRef]
- Walles, B.; Troost, K.; van den Ende, D.; Nieuwhof, S.; Smaal, A.C.; Ysebaert, T. From artificial structures to self-sustaining oyster reefs. J. Sea Res. 2016, 108, 1–9. [Google Scholar] [CrossRef]
- Kuykendall, K.M.; Moreno, P.; Powell, E.N.; Soniat, T.M.; Colley, S.; Mann, R.; Munroe, D.M. The exposed surface area to volume ratio: Is shell more efficient than limestone in promoting oyster recruitment? J. Shellfish Res. 2015, 34, 217–225. [Google Scholar] [CrossRef]
- La Peyre, M.; Furlong, J.; Brown, L.A.; Piazza, B.P.; Brown, K. Oyster reef restoration in the northern Gulf of Mexico: Extent, methods and outcomes. Ocean Cost. Manag. 2014, 89, 20–28. [Google Scholar] [CrossRef]
- Kim, D.; Woo, J.; Yoon, H.S.; Na, W.B. Efficiency, tranquillity and stability indices to evaluate performance in the artificial reef wake region. Ocean Eng. 2016, 122, 253–261. [Google Scholar] [CrossRef]
- Woo, J.; Kim, D.; Yoon, H.S.; Na, W.B. Efficient placement models of labyrinth-type artificial concrete reefs according to wake volume estimation to support natural submerged aquatic vegetation. Bull. Mar. Sci. 2018, 94, 1259–1272. [Google Scholar] [CrossRef]
- Wheaton, F. Review of the properties of Eastern oysters, Crassostrea virginica: Part I. Physical properties. Aquac. Eng. 2007, 37, 3–13. [Google Scholar] [CrossRef]
- Whitman, E.R.; Reidenbach, M.A. Benthic flow environments affect recruitment of Crassostrea virginica larvae to an intertidal oyster reef. Mar. Ecol. Prog. Ser. 2012, 463, 177–191. [Google Scholar] [CrossRef]
- ANSYS Inc. ANSYS CFX-Solver Theory Guide Release 2020-R1; ANSYS Inc.: Canonsburg, PA, USA, 2020. [Google Scholar]
- Kim, D.; Jung, S.; Kim, J.; Na, W.B. Efficiency and unit propagation indices to characterize wake volumes of marine forest artificial reefs established by flatly distributed placement models. Ocean Eng. 2019, 175, 138–148. [Google Scholar] [CrossRef]
- Menter, F.R. Review of the shear-stress transport turbulence model experience from an industrial perspective. Int. J. Comput. Fluid Dyn. 2009, 23, 305–316. [Google Scholar] [CrossRef]
- Kim, D.; Jung, S.; Na, W.B. Evaluation of turbulence models for estimating the wake region of artificial reefs using particle image velocimetry and computational fluid dynamics. Ocean Eng. 2021, 223, 108673. [Google Scholar] [CrossRef]
- Ono, M.; Deguchi, I. Relation between aggregating effect of artificial fish reef and flow pattern around reef. In Coastal Structures 2003; American Society of Civil Engineers: Portland, OR, USA, 2003; pp. 1164–1175. [Google Scholar] [CrossRef]
- Miller, D.C.; Norkko, A.; Pilditch, C.A. Influence of diet on dispersal of horse mussel Atrina zelandica biodeposits. Mar. Ecol. Prog. Ser. 2002, 242, 153–167. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, D.; Na, W.B. Estimation of effective usable and burial volumes of artificial reefs and the prediction of cost-effective management. Ocean Coast. Manag. 2016, 120, 135–147. [Google Scholar] [CrossRef]
- Rouse, S.; Porter, J.S.; Wilding, T.A. Artificial reef design affects benthic secondary productivity and provision of functional habitat. Ecol. Evol. 2020, 10, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Woo, J.; Yoon, H.S.; Na, W.B. Wake lengths and structural responses of Korean general artificial reefs. Ocean Eng. 2014, 92, 83–91. [Google Scholar] [CrossRef]
- Kim, D.; Woo, J.; Na, W.B.; Yoon, H.S. Flow and structural response characteristics of a box-type artificial reef. J. Korean Soc. Coast. Ocean Eng. 2014, 26, 113–119. [Google Scholar] [CrossRef]
- Lee, I.C.; Kim, D.; Jung, S.; Na, W.B. Prediction of primary physical measures for cost-effective management of artificial seaweed reefs. Mar. Technol. Soc. J. 2020, 54, 25–43. [Google Scholar] [CrossRef]
- Kim, D.; Jung, S.; Na, W.B. Two perspectives for increasing of artificial wake region. J. Fish. Mar. Sci. Educ. 2020, 32, 1623–1631. [Google Scholar] [CrossRef]
- Jung, S.; Na, W.B.; Kim, D. Rugosity and blocking indices of artificial reefs and their correlations with wake volume. Ocean Eng. 2022, 261, 112204. [Google Scholar] [CrossRef]
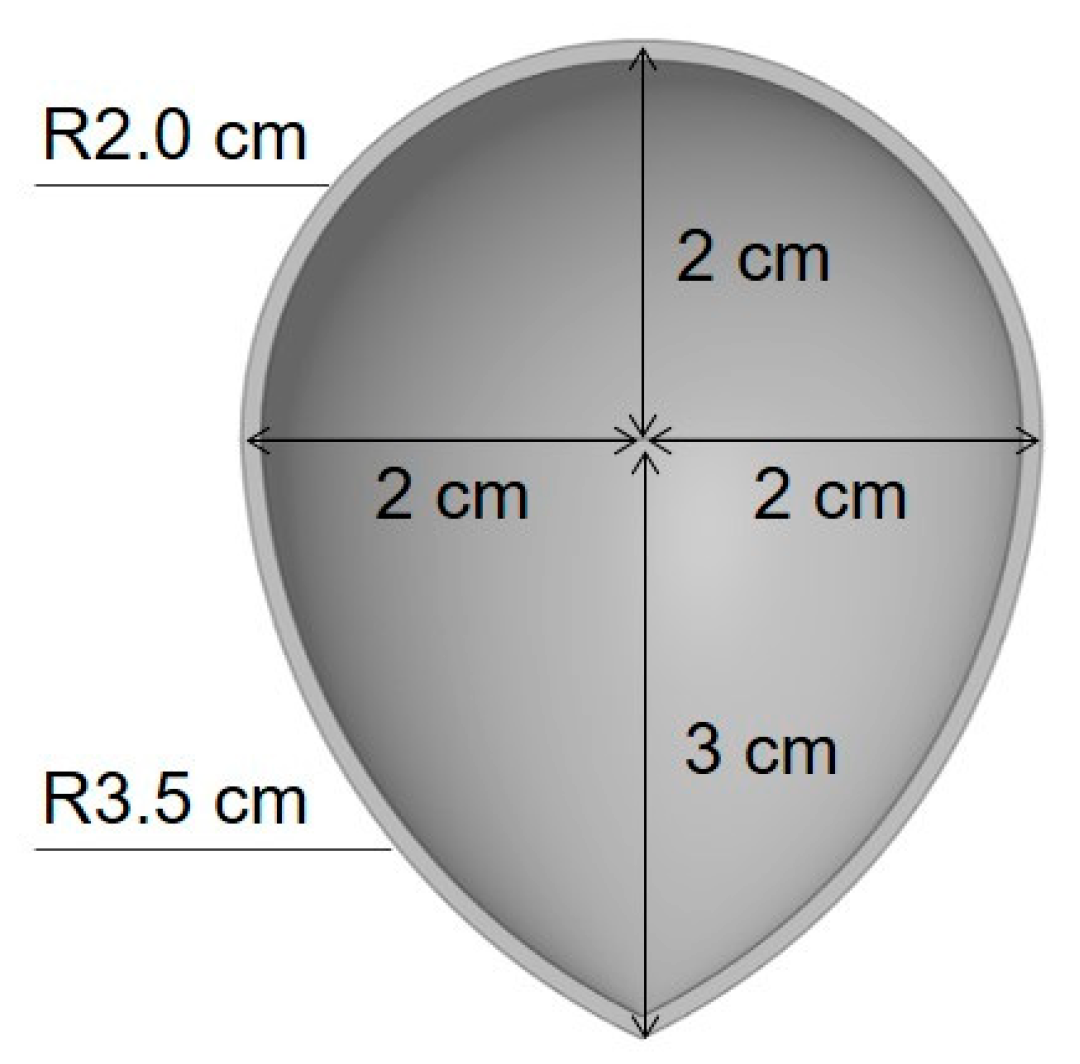
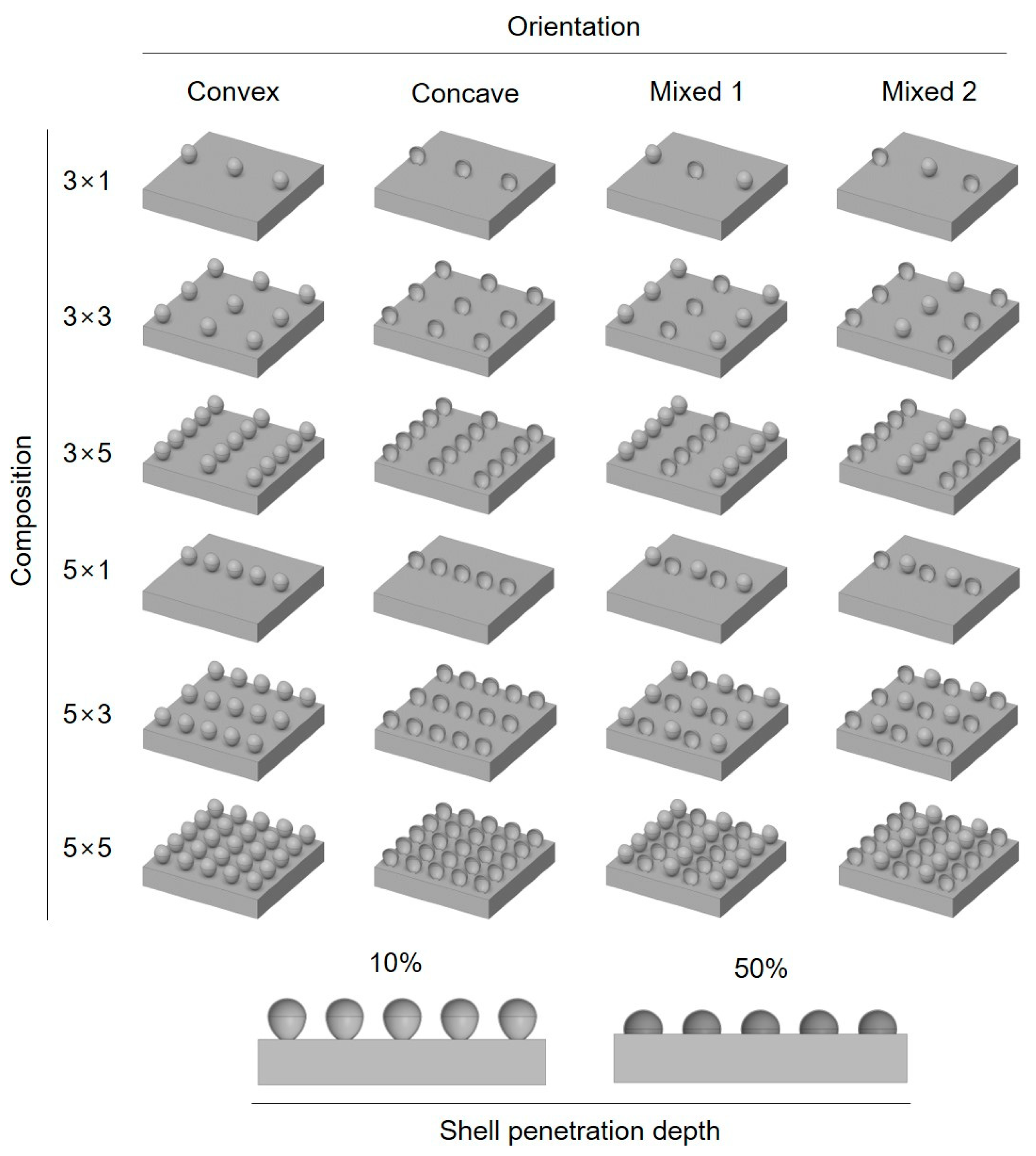



| Shell Orientation | Shell Composition | |
|---|---|---|
| Number of Oysters Penperdicular to Flow Direction (Interval) | Number of Oysters in Flow Direction (Interval) | |
| Convex Concave Mixed 1 Mixed 2 | 3 (12 cm) | 1 (–) |
| 3 (12 cm) | 3 (12 cm) | |
| 3 (12 cm) | 5 (6 cm) | |
| 5 (6 cm) | 1 (–) | |
| 5 (6 cm) | 3 (12 cm) | |
| 5 (6 cm) | 5 (6 cm) | |
| Growth Stage GS | Number of Shells | Width (cm) | Length
(cm) | Height
(cm) | Volume (cm3) | Total Surface Area (cm2) | Projection Area (cm2) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Rep. 1 | 50 | 30.66 | 30.55 | 9.97 | 4623.37 | 4906.79 | 279.60 |
| Rep. 2 | 50 | 30.35 | 30.00 | 9.80 | 4626.75 | 4978.35 | 281.46 | |
| Rep. 3 | 50 | 30.00 | 30.23 | 8.87 | 4590.10 | 4206.49 | 241.82 | |
| 2 | Rep. 1 | 60 | 30.66 | 30.55 | 13.45 | 4649.25 | 5225.37 | 355.33 |
| Rep. 2 | 60 | 30.35 | 30.00 | 14.50 | 4652.93 | 5511.11 | 358.63 | |
| Rep. 3 | 60 | 30.40 | 30.42 | 12.10 | 4613.75 | 4678.59 | 276.62 | |
| 3 | Rep. 1 | 70 | 30.66 | 30.78 | 13.45 | 4674.13 | 5926.94 | 360.21 |
| Rep. 2 | 70 | 30.69 | 30.00 | 14.50 | 4678.69 | 6035.90 | 383.25 | |
| Rep. 3 | 70 | 30.40 | 30.42 | 12.12 | 4646.65 | 5156.80 | 315.48 | |
| 4 | Rep. 1 | 80 | 30.66 | 30.78 | 16.40 | 4694.84 | 6344.33 | 389.63 |
| Rep. 2 | 80 | 31.51 | 30.00 | 17.50 | 4704.36 | 6553.99 | 425.60 | |
| Rep. 3 | 80 | 30.63 | 30.42 | 12.12 | 4671.32 | 5646.30 | 339.27 | |
| 5 | Rep. 1 | 90 | 30.94 | 31.24 | 16.80 | 4720.66 | 6862.75 | 417.85 |
| Rep. 2 | 90 | 31.51 | 31.84 | 17.69 | 4730.41 | 7075.31 | 436.84 | |
| Rep. 3 | 90 | 32.51 | 30.42 | 12.83 | 4697.92 | 6181.34 | 362.12 | |
| 6 | Rep. 1 | 100 | 30.94 | 33.37 | 18.42 | 4744.21 | 7339.73 | 443.11 |
| Rep. 2 | 100 | 31.51 | 31.84 | 17.71 | 4756.25 | 7594.81 | 463.90 | |
| Rep. 3 | 100 | 32.51 | 31.47 | 12.83 | 4725.18 | 6672.37 | 364.56 | |
| 7 | Rep. 1 | 110 | 32.42 | 33.37 | 19.29 | 4770.46 | 7863.62 | 482.67 |
| Rep. 2 | 110 | 31.51 | 32.34 | 20.05 | 4781.96 | 8113.50 | 503.89 | |
| Rep. 3 | 110 | 32.51 | 31.90 | 16.01 | 4751.47 | 7201.67 | 386.06 | |
| 8 | Rep. 1 | 120 | 32.42 | 33.37 | 21.81 | 4796.22 | 8383.68 | 529.98 |
| Rep. 2 | 120 | 31.51 | 32.34 | 22.07 | 4808.13 | 8644.26 | 517.64 | |
| Rep. 3 | 120 | 32.73 | 34.51 | 16.01 | 4778.98 | 7706.13 | 397.70 | |
| 9 | Rep. 1 | 130 | 32.42 | 33.37 | 21.81 | 4820.80 | 8891.89 | 530.86 |
| Rep. 2 | 130 | 31.51 | 32.34 | 22.07 | 4833.87 | 9156.26 | 545.31 | |
| Rep. 3 | 130 | 32.73 | 34.51 | 16.06 | 4805.34 | 8234.50 | 419.21 | |
| 10 | Rep. 1 | 140 | 33.87 | 33.37 | 21.81 | 4846.33 | 9401.96 | 538.54 |
| Rep. 2 | 140 | 32.15 | 34.55 | 22.07 | 4859.06 | 9666.91 | 560.63 | |
| Rep. 3 | 140 | 32.73 | 34.51 | 16.06 | 4835.33 | 8680.43 | 440.96 | |
| 11 | Rep. 1 | 150 | 33.87 | 34.52 | 21.81 | 4870.18 | 9875.24 | 552.31 |
| Rep. 2 | 150 | 32.15 | 35.80 | 22.07 | 4882.69 | 10,134.06 | 572.38 | |
| Rep. 3 | 150 | 32.73 | 34.76 | 19.40 | 4862.30 | 9217.09 | 471.87 | |
| 12 | Rep. 1 | 160 | 33.87 | 34.52 | 21.81 | 4896.19 | 10,397.32 | 561.01 |
| Rep. 2 | 160 | 32.43 | 35.80 | 22.07 | 4908.55 | 10,655.81 | 581.27 | |
| Rep. 3 | 160 | 32.73 | 34.76 | 19.40 | 4892.84 | 9702.75 | 502.82 | |
| 13 | Rep. 1 | 170 | 33.87 | 35.42 | 21.81 | 4922.08 | 10,910.30 | 573.46 |
| Rep. 2 | 170 | 32.43 | 35.80 | 22.07 | 4934.32 | 11,171.07 | 593.24 | |
| Rep. 3 | 170 | 33.61 | 36.36 | 19.67 | 4919.47 | 10,241.49 | 530.95 | |
| 14 | Rep. 1 | 180 | 33.87 | 35.70 | 21.81 | 4947.57 | 11,420.00 | 592.16 |
| Rep. 2 | 180 | 32.43 | 35.80 | 23.78 | 4959.01 | 11,666.11 | 613.22 | |
| Rep. 3 | 180 | 33.61 | 37.47 | 22.52 | 4950.13 | 10,738.68 | 564.82 | |
| 15 | Rep. 1 | 190 | 33.99 | 35.70 | 22.69 | 4972.73 | 11,923.42 | 613.67 |
| Rep. 2 | 190 | 33.94 | 35.80 | 26.25 | 4984.80 | 12,188.37 | 658.90 | |
| Rep. 3 | 190 | 33.97 | 37.47 | 22.52 | 4976.82 | 11,272.94 | 589.31 | |
| 16 | Rep. 1 | 200 | 35.07 | 35.70 | 22.69 | 4998.45 | 12,440.46 | 643.39 |
| Rep. 2 | 200 | 33.94 | 35.80 | 26.25 | 5010.37 | 12,705.07 | 666.88 | |
| Rep. 3 | 200 | 33.97 | 37.47 | 24.59 | 5006.37 | 11,768.37 | 620.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Jung, S.; Chau, T.V.; Na, W.-B. Correlation of the Structural Characteristics of an Artificial Oyster Reef with Its Wake Region. J. Mar. Sci. Eng. 2023, 11, 775. https://doi.org/10.3390/jmse11040775
Kim M, Jung S, Chau TV, Na W-B. Correlation of the Structural Characteristics of an Artificial Oyster Reef with Its Wake Region. Journal of Marine Science and Engineering. 2023; 11(4):775. https://doi.org/10.3390/jmse11040775
Chicago/Turabian StyleKim, Minju, Somi Jung, Than Van Chau, and Won-Bae Na. 2023. "Correlation of the Structural Characteristics of an Artificial Oyster Reef with Its Wake Region" Journal of Marine Science and Engineering 11, no. 4: 775. https://doi.org/10.3390/jmse11040775
APA StyleKim, M., Jung, S., Chau, T. V., & Na, W.-B. (2023). Correlation of the Structural Characteristics of an Artificial Oyster Reef with Its Wake Region. Journal of Marine Science and Engineering, 11(4), 775. https://doi.org/10.3390/jmse11040775







