Evidence of the Intrusion of the Oceanic Lightfish (Vinciguerria nimbaria) into Korean Waters Based on High-Throughput Sequencing of Mixed Fish Eggs
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gjøsæter, J.; Kawaguchi, K. A review of the world resources of mesopelagic fish. FAO Fish. Tech. Pap. 1980, 193, 157. [Google Scholar]
- Badcock, J. Photichthyidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1984; Volume 1, pp. 318–324. [Google Scholar]
- Scott, W.B.; Scott, M.G. Atlantic fishes of Canada. Can. Bull. Fish. Aquat. Sci. 1988, 219, 731. [Google Scholar]
- Claro, R. Características generales de la ictiofauna. In Ecología de Los Peces Marinos de Cuba; Claro, R., Ed.; Instituto de Oceanología Academia de Ciencias de Cuba: La Habana, Cuba; Centro de Investigaciones de Quintana Roo: Chetumal, Mexico, 1994; pp. 55–70. [Google Scholar]
- Stéquert, B.; Ménard, F.; Marchal, E. Reproductive biology of Vinciguerria nimbaria in the equatorial waters of the eastern Atlantic Ocean. J. Fish Biol. 2003, 62, 1116–1136. [Google Scholar] [CrossRef]
- Pequeño, G.; de Peces, C. Lista sistematica revisaday comentada. Rev. Biol. Mar. 1989, 24, 1–132. [Google Scholar]
- Nakabo, T. Fishes of Japan with Pictorial Keys to the Species; Tokai University Press: Tokyo, Japan, 2013. [Google Scholar]
- Youn, C.H.; Shim, J.H.; Kim, J.J. Pisces of Korea; Haksul Information Center: Seoul, Korea, 2021; p. 2148. [Google Scholar]
- Lebourges-Dhaussy, A.; Marchal, E.; Menkès, C.; Champalbert, G.; Biessy, B. Vinciguerria nimbaria (micronekton), environment and tuna: Their relationships in the Eastern Tropical Atlantic. Oceanol. Acta 2000, 23, 515–528. [Google Scholar] [CrossRef]
- Ménard, F.; Marchal, E. Foraging behaviour of tuna feeding on small schooling Vinciguerria nimbaria in the surface layer of the equatorial Atlantic Ocean. Aquat. Living Resour. 2003, 16, 231–238. [Google Scholar] [CrossRef]
- Marchal, E.; Lebourges, A. Acoustic evidence for unusual diel behaviour of a mesopelagic fish (Vinciguerria nimbaria) exploited by tuna. ICES J. Mar. Sci. 1996, 53, 443–447. [Google Scholar] [CrossRef]
- Kendall, A.W., Jr.; Ahlstrom, E.H.; Moser, H.G. Early life history stages of fishes and their characters. In Ontogeny and Systematics of Fishes; Moser, H.G., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W., Richardson, S.L., Eds.; American Society of Ichthyologists and Herpetologists: Kansas, MO, USA, 1984. [Google Scholar]
- Leiby, M.M. Life history and ecology of pelagic fish eggs and larvae. Mar. Plankton Life Cycle Strateg. 1984, 6, 121–140. [Google Scholar]
- Houde, E.D. Fish early life dynamics and recruitment variability. Am. Fish. Soc. Symp. 1987, 2, 17–29. [Google Scholar]
- Jung, S.; Hwang, S.D.; Kim, J. Fecundity and growth-dependent mortality of Pacific anchovy (Engraulis japonicus) in Korean coastal waters. Fish. Res. 2008, 93, 39–46. [Google Scholar] [CrossRef]
- Dahlke, F.T.; Wohlrab, S.; Butzin, M.; Pörtner, H.O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 2020, 369, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.T.; Yang, J.S.; Chen, K.C.; Lee, Y.S. An Identification Guide of Marine Fish Eggs from Taiwan; Institute of Zoology, Academia Sinica and Taiwan Power Company: New Taipei, Taiwan, 2001; p. 179. [Google Scholar]
- Ikeda, T.; Hirai, A.; Tabata, S.; Onishi, Y.; Mito, S. An Atlas of Early Stage Fishes in Japan, 2nd ed.; Okiyama, M., Ed.; Tokai University Press: Tokyo, Japan, 2014; p. 108. [Google Scholar]
- Oh, J.; Kim, S. Morphological and molecular characterization of separated pelagic eggs from Lophius litulon (Lophiiformes; Lophiidae). J. Fish Biol. 2015, 86, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Oh, J.; Kim, S. Genetic identification of eggs from four species of Ophichthidae and Congridae (Anguilliformes) in the northern East China Sea. PLoS ONE 2018, 13, e0195382. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Kim, J.K.; Ryu, J.H. First report on the occurrence of eggs of the small yellow croaker Larimichthys polyactis from Chilsan-do Island, Jeollanam-do, Korea. Korean J. Fish. Aquat. Sci. 2020, 53, 650–655. [Google Scholar]
- Han, S.H.; Kim, M.J.; Song, C.B. Molecular identification and distribution pattern of fish eggs collected around Jejudo Island. Korean J. Ichthyol. 2015, 27, 284–292. [Google Scholar]
- Harada, A.E.; Lindgren, E.A.; Hermsmeier, M.C.; Rogowski, P.A.; Terrill, E.; Burton, R.S. Monitoring spawning activity in a southern California marine protected area using molecular identification of fish eggs. PLoS ONE 2015, 10, e0134647. [Google Scholar] [CrossRef]
- Choi, H.Y.; Chin, B.S.; Park, G.S.; Kim, S. Evidence of intrusion of a rare species, Peristedion liorhynchus, into Korean waters based on high-throughput sequencing of the mixed fish eggs. Korean J. Ichthyol. 2022, 34, 8–15. [Google Scholar] [CrossRef]
- Vihtakari, M. GgOceanMaps: Plot Data on Oceanographic Maps Using ‘ggplot2’. R Package Version 1.3.7. Available online: https://mikkovihtakari.github.io/ggOceanMaps/ (accessed on 6 December 2022).
- Illumina, I. 16S Metagenomic Sequencing Library Preparation. Preparing 16S ribosomal RNA gene amplicons for the illumina MiSeq system. Microb. Genom. 2013, 1, 28. [Google Scholar]
- Leray, M.; Yang, J.Y.; Meyer, C.P.; Mills, S.C.; Agudelo, N.; Ranwez, V.; Boehm, J.T.; Machida, R.J. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: Application for characterizing coral reef fish gut contents. Front. Zool. 2013, 10, 34. [Google Scholar] [CrossRef]
- Smith, M.M. , Heemstra, P.C. Smiths’ Sea Fishes; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Bushnell, B.; Rood, J.; Singer, E. BBMerge—Accurate paired shotgun read merging via overlap. PLoS ONE 2017, 12, e0185056. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Wangensteen, O.S.; Chapman, D.D.; Boussarie, G.; Buddo, D.; Guttridge, T.L.; Hertler, H.; Mouillot, D.; Vigliola, L.; Mariani, S. Environmental DNA reveals tropical shark diversity in contrasting levels of anthropogenic impact. Sci. Rep. 2017, 7, 16886. [Google Scholar] [CrossRef] [PubMed]
- Boussarie, G.; Bakker, J.; Wangensteen, O.S.; Mariani, S.; Bonnin, L.; Juhel, J.-B.; Kiszka, J.J.; Kulbicki, M.; Manel, S.; Robbins, W.D.; et al. Environmental DNA illuminates the dark diversity of sharks. Sci. Adv. 2018, 4, eaap9661. [Google Scholar] [CrossRef]
- Kim, G.; Song, Y. Identification of freshwater fish species in Korea using environmental DNA technique from the experiment at the freshwater fish ecological learning center in Yangpyeong, Gyeonggi Do. J. Environ. Impact Assess. 2021, 30, 1–12. [Google Scholar]
- Checkley, D.M., Jr.; Ortner, P.B.; Settle, L.R.; Cummings, S.R. A continuous, underway fish egg sampler. Fish. Oceanogr. 1997, 6, 58–73. [Google Scholar] [CrossRef]
- Lelièvre, S.; Verrez-Bagnis, V.; Jérôme, M.; Vaz, S. PCR-RFLP analyses of formalin-fixed fish eggs for the mapping of spawning areas in the Eastern Channel and Southern North Sea. J. Plankton Res. 2010, 32, 1527–1539. [Google Scholar] [CrossRef]
- Fox, C.J.; Taylor, M.; Dickey-Collas, M.; Fossum, P.; Kraus, G.; Rohlf, N.; Munk, P.; van Damme, C.J.G.; Bolle, L.J.; Maxwell, D.L.; et al. Mapping the spawning grounds of North Sea cod (Gadus morhua) by direct and indirect means. Proc. R. Soc. B: Biol. Sci. 2008, 275, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Chow, S.; Otake, T.; Kurogi, H.; Mochioka, N.; Miller, M.J.; Aoyama, J.; Kimura, S.; Watanabe, S.; Yoshinaga, T.; et al. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat. Commun. 2011, 2, 179. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Choi, H.C.; Kim, S.; Oh, H.J.; Youn, S.H. Discovery of pelagic eggs of two species from the rare mesopelagic fish genus Trachipterus (Lampriformes: Trachipteridae). J. Mar. Sci. Eng. 2022, 10, 637. [Google Scholar] [CrossRef]
- Kim, J.Y. Relationship Between Anchovy, Engraulis japonica, egg and larval density and environmental factors in the eastern waters of Korea. Korean J. Fish. Aquat. Sci. 1992, 25, 495–500. [Google Scholar]
- Kim, S.; Yoo, J.M. Distribution of Eggs and Larvae of Maurolicus muelleri in the Thermal Front of the Korea Strait. Korean J. Ichthyol. 1999, 11, 62–71. [Google Scholar]
- Song, C.-U.; Choi, H.; Jeon, M.-S.; Kim, E.-J.; Jeong, H.G.; Kim, S.; Kim, C.-G.; Hwang, H.; Purnaningtyas, D.W.; Lee, S.; et al. Zooplankton diversity monitoring strategy for the urban coastal region using metabarcoding analysis. Sci. Rep. 2021, 11, 24339. [Google Scholar] [CrossRef]
- Kimmerling, N.; Zuqert, O.; Amitai, G.; Gurevich, T.; Armoza-Zvuloni, R.; Kolesnikov, I.; Berenshtein, I.; Melamed, S.; Gilad, S.; Benjamin, S.; et al. Quantitative species-level ecology of reef fish larvae via metabarcoding. Nat. Ecol. Evol. 2018, 2, 306–316. [Google Scholar] [CrossRef]
- Duke, E.M.; Burton, R.S. Efficacy of metabarcoding for identification of fish eggs evaluated with mock communities. Ecol. Evol. 2020, 10, 3463–3476. [Google Scholar] [CrossRef]
- Chase, J.W.; Dawid, I.B. Biogenesis of mitochondria during Xenopus laevis development. Dev. Biol. 1972, 27, 504–518. [Google Scholar] [CrossRef]
- Pikó, L.; Taylor, K.D. Amounts of mitochondrial DNA and 132 abundance of some mitochondrial gene transcripts in early mouse embryos. Dev. Biol. 1987, 123, 364–374. [Google Scholar] [CrossRef]
- Artuso, L.; Romano, A.; Verri, T.; Domenichini, A.; Argenton, F.; Santorelli, F.M.; Petruzzella, V. Mitochondrial DNA metabolism in early development of zebrafish (Danio rerio). Biochim. Biophys. Acta BBA Bioenerg. 2012, 1817, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y. Diagnosis and Change Prediction of Spawning Areas along the Coasts of the Korean Peninsula Using Pelagic Fish Eggs. Ph.D. Thesis, University of Science and Technology, Daejeon, Republic of Korea, 2021; p. 415. [Google Scholar]
- FishBase. World Wide Web Electronic Publication. Froese, R., Pauly D., Eds. Available online: http://www.fishbase.org (accessed on 6 December 2022).
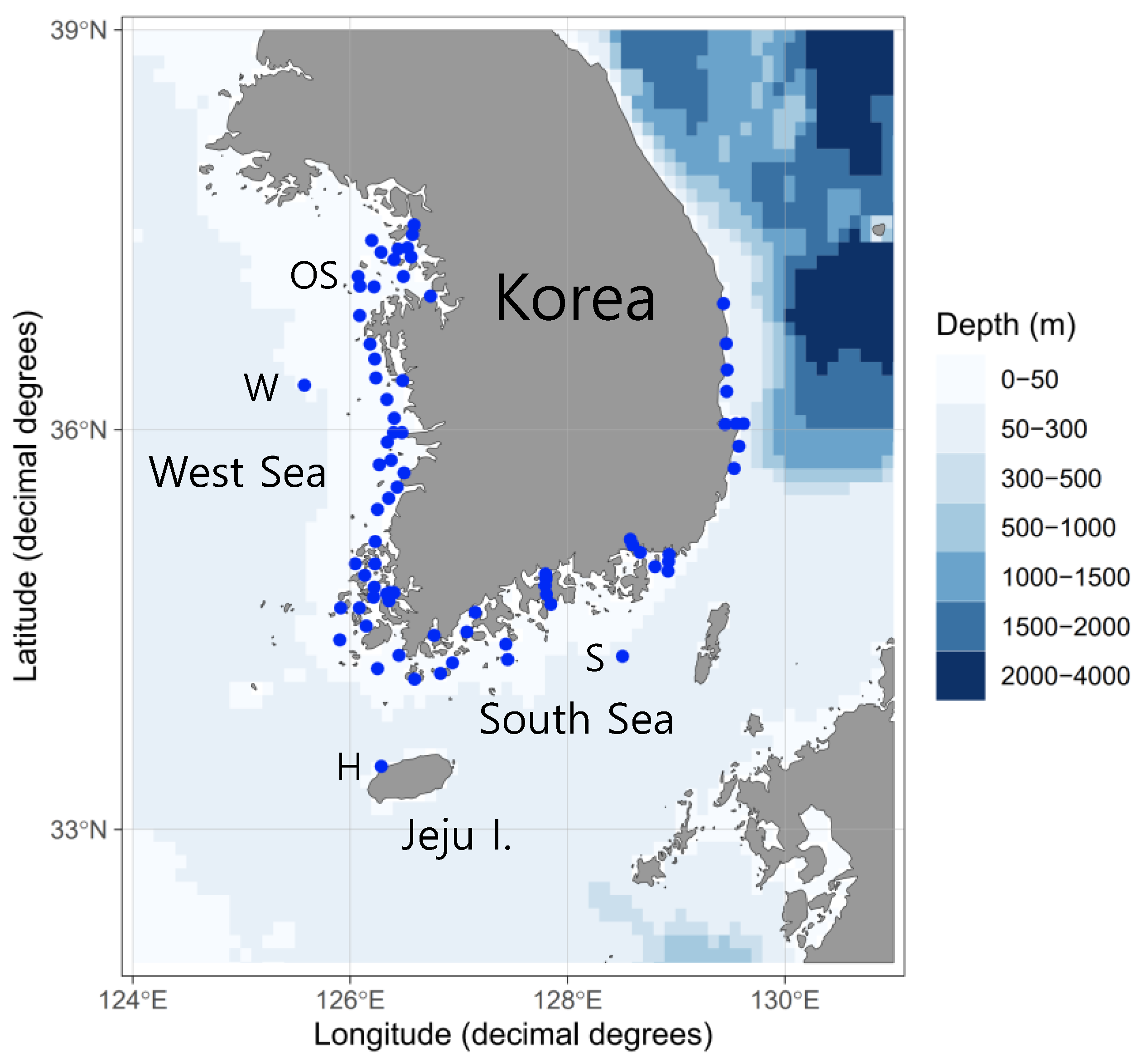

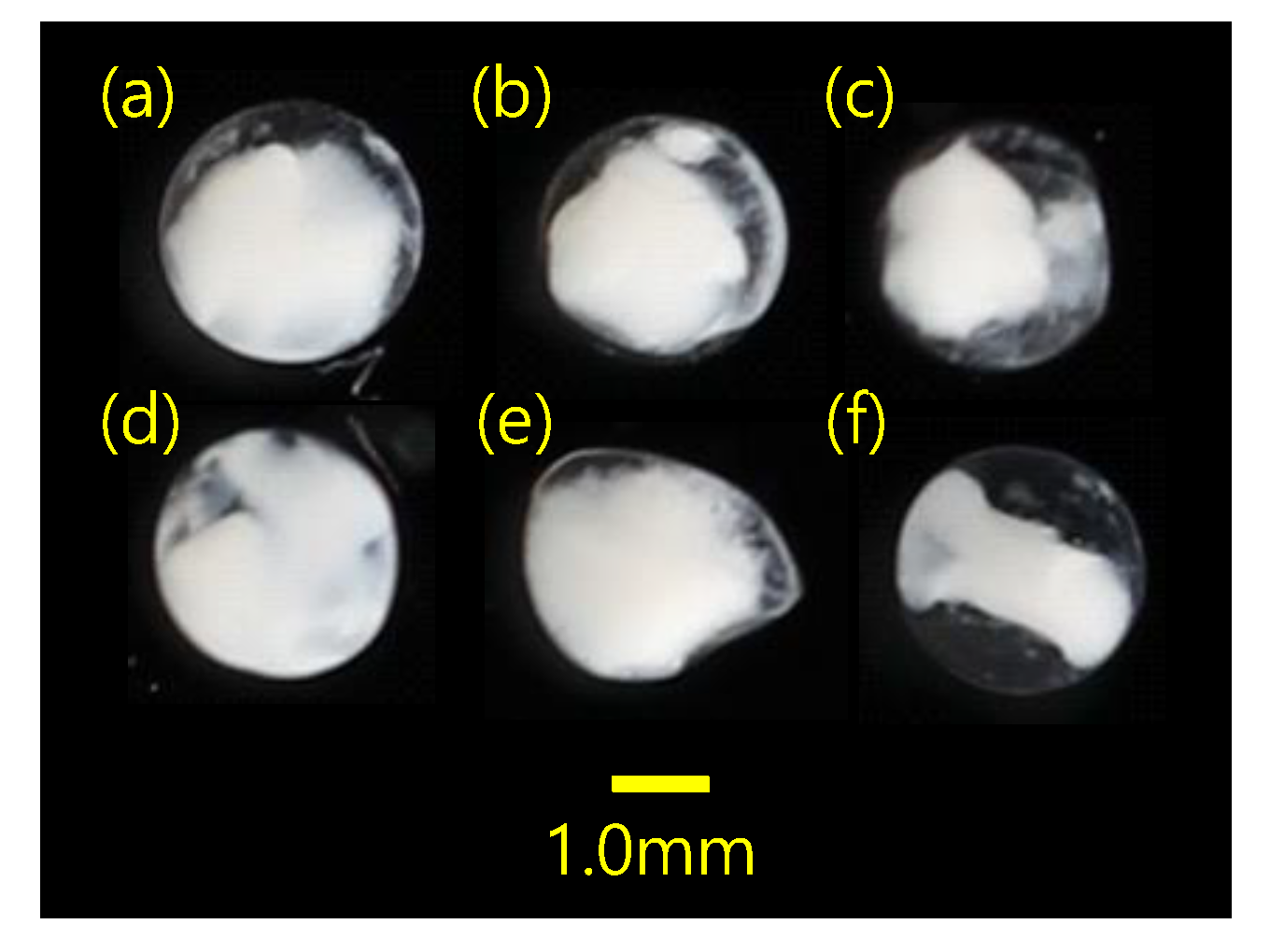
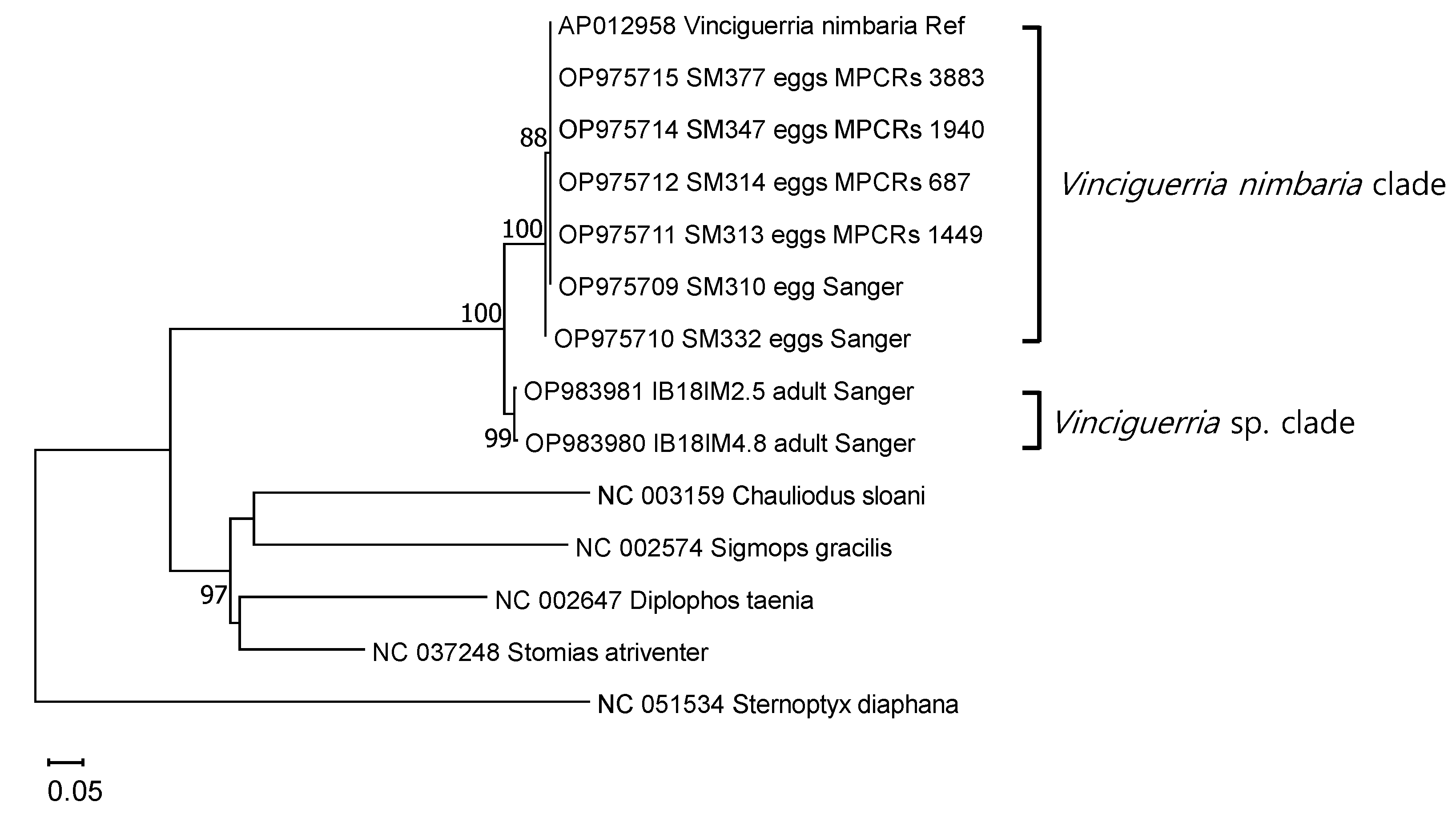
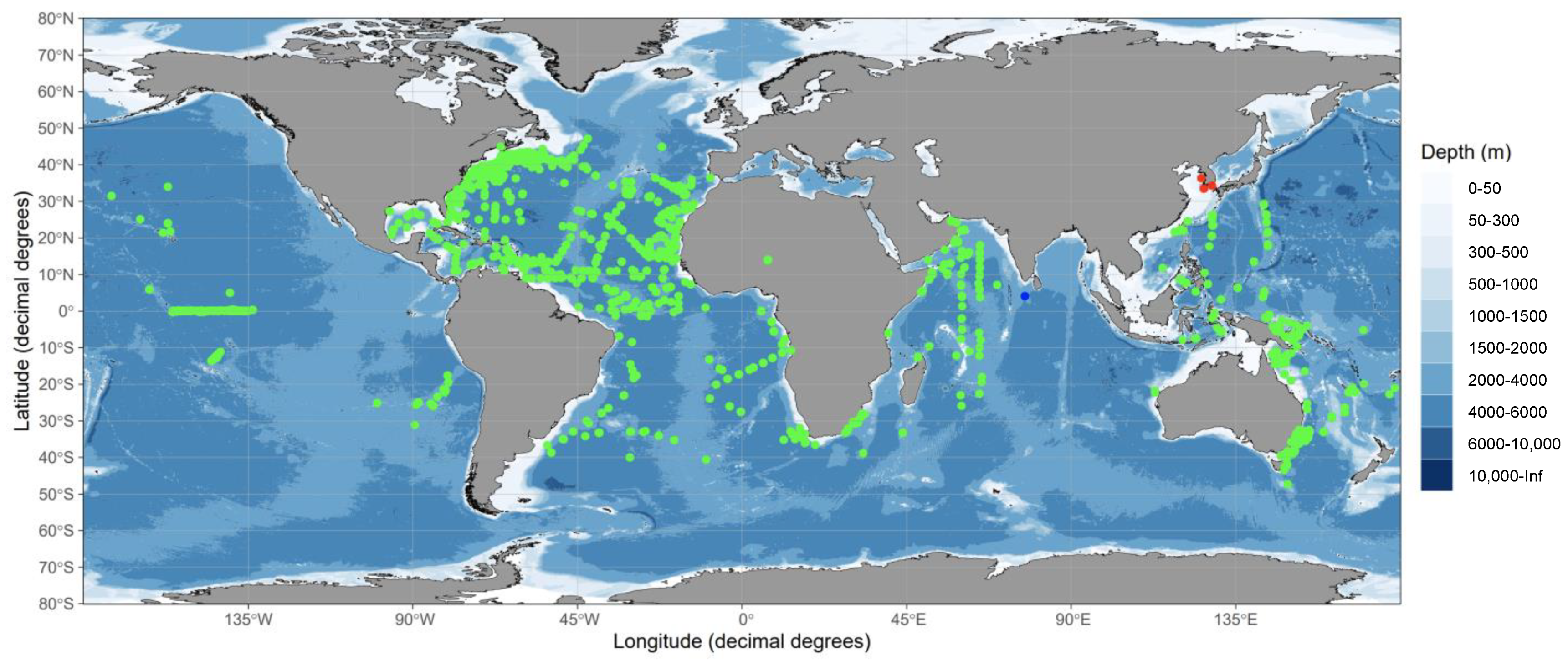
| Survey Area | Sampling Date | Sampling Gears | Number of Stations | Sampling Times | Number of Eggs | Total Sequence Reads |
|---|---|---|---|---|---|---|
| H: Hallim of Jeju Is. | 26 May 2021 | Mouth diameter, 60 or 80 cm; Mesh size, 300 μm | 1 | 5 | 892 | 1,198,048 |
| S: EEZ of the southern Korean waters | 30 May 2021 | 1 | 16 | 107 | 4,337,922 | |
| W: EEZ of the western Korean waters | 18 May 2021 | 1 | 15 | 114 | 4,025,376 | |
| 5 August 2021 | 1 | 15 | 111 | 3,616,582 | ||
| OS: Ongjin-Sungap Is. of the western Korean waters | 14 May 2021 | 1 | 11 | 42 | 2,575,872 | |
| 3 August 2021 | 1 | 27 | 584 | 7,532,516 | ||
| Korean coastal waters | January–November 2021 | Mouth diameter, 80 cm; Mesh size, 300 μm | 74 | 177 | 66,994 | 56,642,638 |
| Total | 78 | 266 | 68,844 | 79,928,954 |
| Survey Area | Sampling Date | Sample Names | Number of Eggs | Net Towing Trajectory | Net Mouth Diameter (cm) | Bidirectional Raw Reads | Merged Paired Reads | MMPRs (Mean Merged Paired Reads) | MPCRs (Merged Paired Contig Reads) | Ratio of MMPRs to MPCRs (%) | Estimated Number Of V. Nimbaria Eggs | GenBank Accession Number | Remark |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hallim of Jeju Is. (33.474° N 126.2863° E) | 26 May 2021 | SM347 | 115 | vertical | 60 | 270,348 | 25,617 | 223 | 1940 | 870.9 | 9 | OP975714 | |
| SM350 | 6 | horizontal | 80 | 274,594 | 43,341 | 657 | 38 | 5.8 | 0 | ||||
| S: EEZ of the southern Korean waters (34.3085° N 128.5057° E) | 30 May 2021 | SM310 | 1 | Vertical | 60 | 315,646 | 9869 | 9869 | 9806 | 99.4 | 1 | OP975709 | SS |
| SM322 | 1 | oblique | 80 | 269,598 | 23,508 | 23,508 | 1959 | 8.3 | 0 | ||||
| SM313 | 11 | horizontal | 80 | 300,744 | 9332 | 848 | 1449 | 170.8 | 2 | OP975711 | |||
| SM314 | 15 | horizontal | 80 | 292,366 | 7290 | 486 | 687 | 141.4 | 1 | OP975712 | |||
| SM312 | 16 | horizontal | 80 | 288,270 | 12,537 | 784 | 28 | 3.6 | 0 | ||||
| OS: Ongjin-Sungap Is. of the western Korean waters (37.0782° N 126.0899° E) | 3 August 2021 | SM404 | 24 | oblique | 60 | 235,710 | 10,166 | 424 | 33 | 7.8 | 0 | ||
| SM390 | 11 | horizontal | 80 | 284,064 | 1031 | 94 | 34 | 36.3 | 0 | ||||
| W: EEZ of the western Korean waters (36.3343° N 125.5795° E) | 18 May 2021 | SM332 | 6 | oblique | 80 | 274,508 | 1551 | 259 | 1439 | 556.7 | 6 | OP975710 | SS |
| SM331 | 3 | horizontal | 60 | 311,818 | 1084 | 361 | 85 | 23.5 | 0 | ||||
| 5 August 2021 | SM377 | 3 | oblique | 80 | 259,636 | 13,600 | 4533 | 3883 | 85.7 | 1 | OP975715 | ||
| SS, Sanger sequencing | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Chin, B.-s.; Wang, S.-y. Evidence of the Intrusion of the Oceanic Lightfish (Vinciguerria nimbaria) into Korean Waters Based on High-Throughput Sequencing of Mixed Fish Eggs. J. Mar. Sci. Eng. 2023, 11, 257. https://doi.org/10.3390/jmse11020257
Kim S, Chin B-s, Wang S-y. Evidence of the Intrusion of the Oceanic Lightfish (Vinciguerria nimbaria) into Korean Waters Based on High-Throughput Sequencing of Mixed Fish Eggs. Journal of Marine Science and Engineering. 2023; 11(2):257. https://doi.org/10.3390/jmse11020257
Chicago/Turabian StyleKim, Sung, Byung-sun Chin, and Soon-young Wang. 2023. "Evidence of the Intrusion of the Oceanic Lightfish (Vinciguerria nimbaria) into Korean Waters Based on High-Throughput Sequencing of Mixed Fish Eggs" Journal of Marine Science and Engineering 11, no. 2: 257. https://doi.org/10.3390/jmse11020257
APA StyleKim, S., Chin, B.-s., & Wang, S.-y. (2023). Evidence of the Intrusion of the Oceanic Lightfish (Vinciguerria nimbaria) into Korean Waters Based on High-Throughput Sequencing of Mixed Fish Eggs. Journal of Marine Science and Engineering, 11(2), 257. https://doi.org/10.3390/jmse11020257






