Identification and Phylogenetic Analysis of Chitin Synthase Genes from the Deep-Sea Polychaete Branchipolynoe onnuriensis Genome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Next-Generation Sequencing
2.2. Data Filtering and De Novo Genome Assembly
2.3. Gene Prediction and Identification of the Chitin Synthase Gene
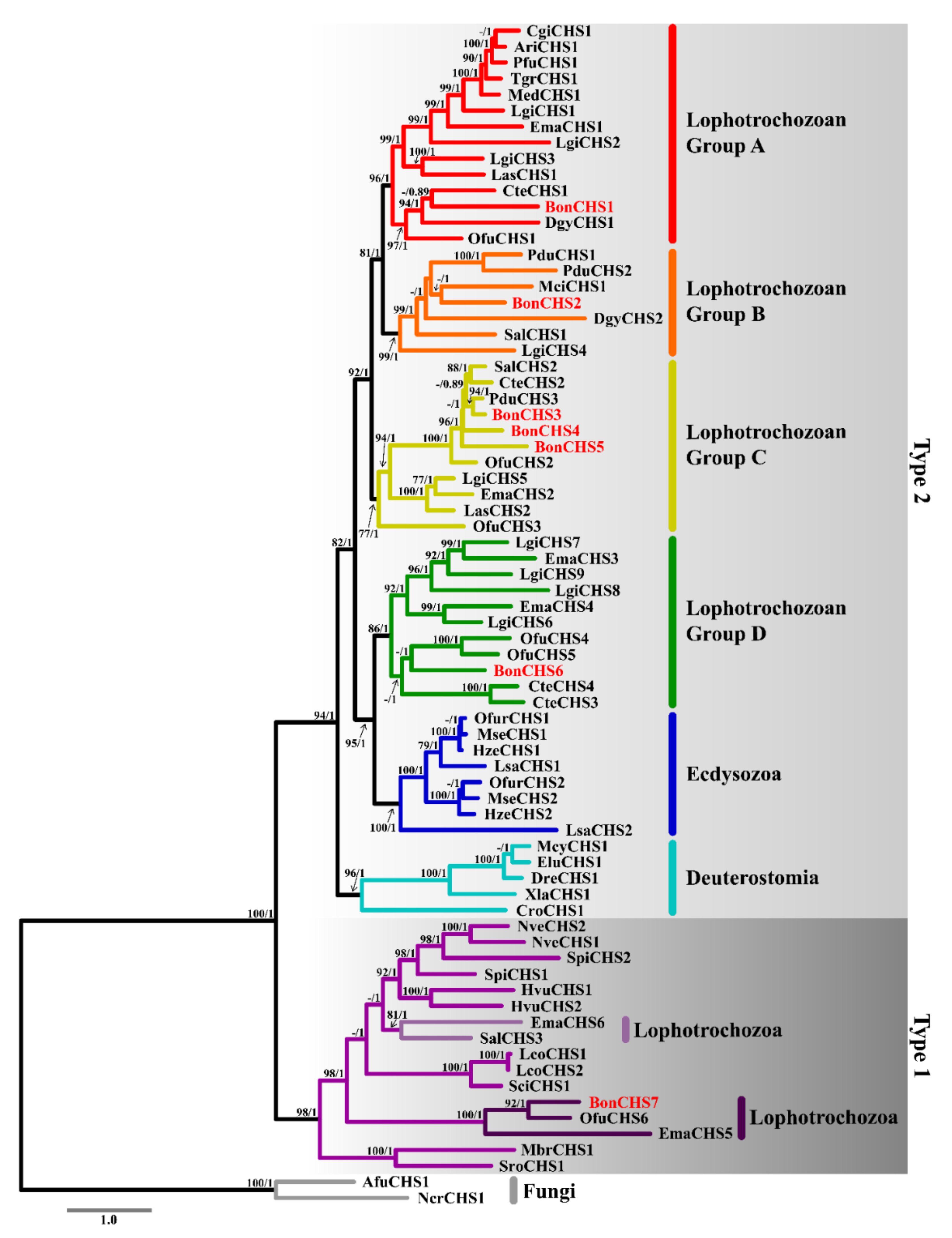
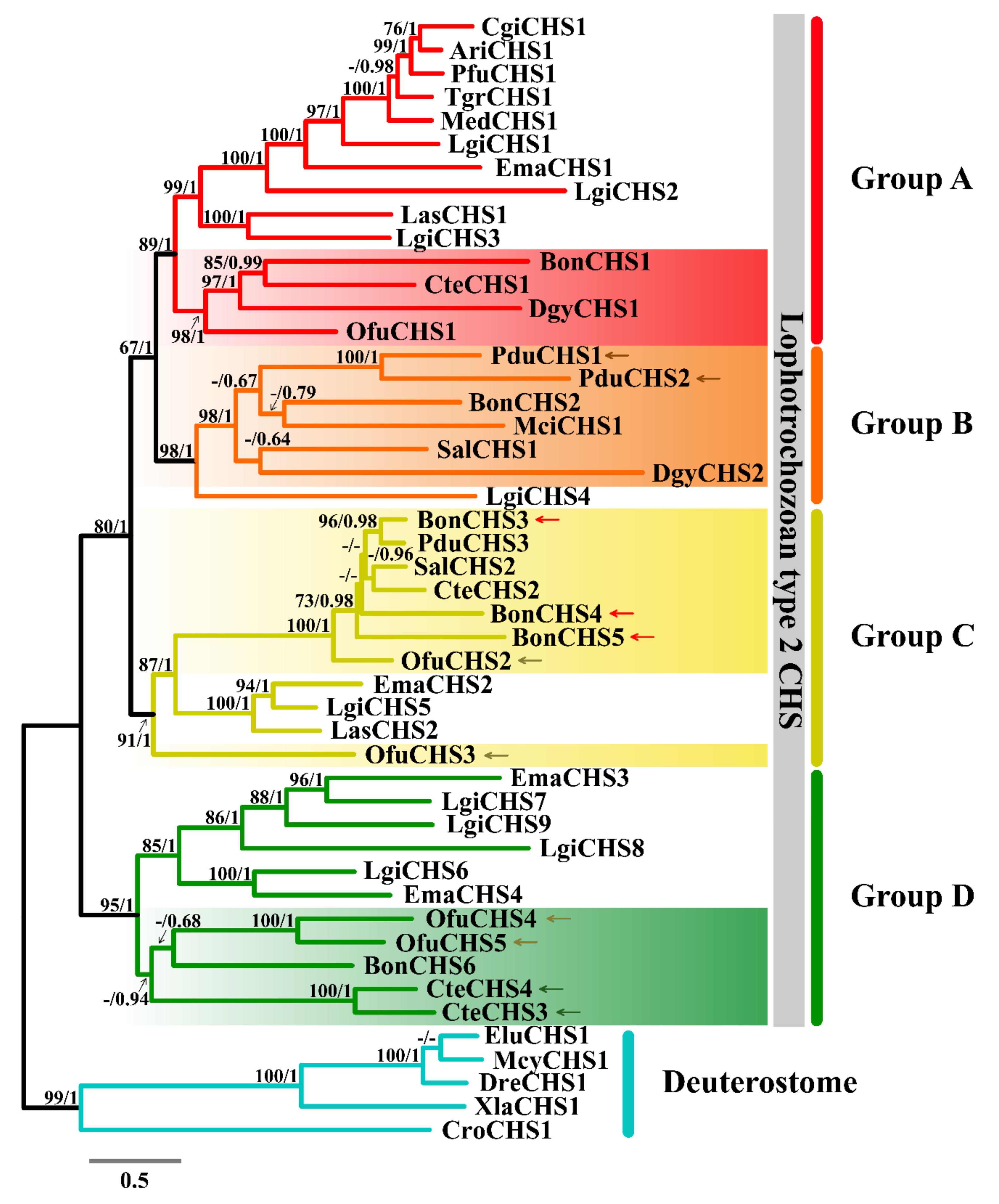
2.4. Phylogenetic Analysis of Chitin Synthase
3. Results and Discussion
3.1. Data Filtering and Genome Assembly
3.2. Gene Prediction and Chitin Synthase Search
3.3. Protein Domain Search, Identification of the GT2 Family, and Multiple Sequence Alignments
3.4. Phylogenetic Analysis of Chitin Synthase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Yuan, J.; Li, F.; Xiang, J. Chitin synthesis and degradation in crustaceans: A genomic view and application. Mar. Drugs 2021, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Uddowla, M.H.; Kim, A.R.; Park, W.; Kim, H.W. CDNAs encoding chitin synthase from shrimp (Pandalopsis japonica): Molecular characterization and expression analysis. J. Aquac. Res. Dev. 2015, 6, 298. [Google Scholar] [CrossRef] [Green Version]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef] [Green Version]
- Tharanathan, R.N.; Kittur, F.S. Chitin-the undisputed biomolecule of great potential. Crit. Reveiws Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.A.; Eyun, S. Evolutionary analysis and protein family classification of chitin deacetylases in Cryptococcus neoformans. J. Microbiol. 2020, 58, 805–811. [Google Scholar] [CrossRef]
- Xin, T.; Li, Z.; Chen, J.; Wang, J.; Zou, Z.; Xia, B. Molecular characterization of chitin synthase gene in Tetranychus cinnabarinus (Boisduval) and its response to sublethal concentrations of an insecticide. Insects 2021, 12, 501. [Google Scholar] [CrossRef]
- Zakrzewski, A.; Weigert, A.; Helm, C.; Adamski, M.; Adamska, M.; Bleidorn, C.; Raible, F.; Hausen, H. Early divergence, broad distribution, and high diversity of animal chitin synthases. Genome Biol. Evol. 2014, 6, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Veronico, P.; Gray, L.J.; Jones, J.T.; Bazzicalupo, P.; Arbucci, S.; Cortese, M.R.; di Vito, M.; de Giorgi, C. Nematode chitin synthases: Gene structure, expression and function in Caenorhabditis elegans and the plant parasitic nematode Meloidogyne artiellia. Mol. Genet. Genom. 2001, 266, 28–34. [Google Scholar] [CrossRef]
- Weiss, I.M.; Schönitzer, V.; Eichner, N.; Sumper, M. The chitin synthase involved in marine bivalve mollusk shell formation contains a myosin domain. FEBS Lett. 2006, 580, 1846–1852. [Google Scholar] [CrossRef] [Green Version]
- Vortsepneva, E.; Tzetlin, A. General morphology and ultrastructure of the radula of Testudinalia testudinalis (O.F. Müller, 1776) (Patellogastropoda, Gastropoda). J. Morphol. 2019, 280, 1714–1733. [Google Scholar] [CrossRef]
- Agbaje, O.B.A.; Shir, I.B.; Zax, D.B.; Schmidt, A.; Jacob, D.E. Biomacromolecules within bivalve shells: Is chitin abundant? Acta Biomater. 2018, 80, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hoon, S.; Guerette, P.A.; Wei, W.; Ghadban, A.; Hao, C.; Miserez, A.; Waite, J.H. Infiltration of chitin by protein coacervates defines the squid beak mechanical gradient. Nat. Chem. Biol. 2015, 11, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picken, L.; Lotmar, W. Oriented protein in chitinous structures. Nature 1950, 165, 599–600. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.M.; Deleury, E.; Davies, G.J.; Henrissat, B. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 2003, 328, 307–317. [Google Scholar] [CrossRef]
- Braden, L.; Michaud, D.; Igboeli, O.O.; Dondrup, M.; Hamre, L.; Dalvin, S.; Purcell, S.L.; Kongshaug, H.; Eichner, C.; Nilsen, F.; et al. Identification of critical enzymes in the Salmon louse chitin synthesis pathway as revealed by RNA interference-mediated abrogation of infectivity. Int. J. Parasitol. 2020, 50, 873–889. [Google Scholar] [CrossRef]
- Luo, Y.-J.; Takeuchi, T.; Koyanagi, R.; Yamada, L.; Kanda, M.; Khalturina, M.; Fujie, M.; Yamasaki, S.-I.; Endo, K.; Satoh, N. The Lingula genome provides insights into brachiopod evolution and the origin of phosphate biomineralization. Nat. Commun. 2015, 6, 8301. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Yang, Y.; Lan, Y.; Ip, J.C.-H.; Wong, W.C.; Kwan, Y.H.; Zhang, Y.; Han, Z.; Qiu, J.-W.; et al. Genomic signatures supporting the symbiosis and formation of chitinous tube in the deep-sea tubeworm Paraescarpia echinospica. Mol. Biol. Evol. 2021, 38, 4116–4134. [Google Scholar] [CrossRef]
- Kim, S.L.; Choi, H.; Eyun, S.; Kim, D.; Yu, O.H. A new Branchipolynoe (Aphroditiformia: Polynoidae) scale worm from the Onnuri Deep-sea hydrothermal vent field, northern Central Indian Ridge. Zool. Stud. 2022, 61, 21. [Google Scholar]
- Ryu, T.; Kim, J.G.; Lee, J.; Yu, O.H.; Yum, S.; Kim, D.; Woo, S. First transcriptome assembly of a newly discovered vent mussel, Gigantidas vrijenhoeki, at Onnuri Vent Field on the northern Central Indian Ridge. Mar. Genom. 2021, 57, 100819. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Morgenstern, B. AUGUSTUS: A web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005, 33, W465–W467. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Schultz, J.; Copley, R.R.; Doerks, T.; Ponting, C.P.; Bork, P. SMART: A web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000, 28, 231–234. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.-Z.; Li, N.-Y.; Xie, Y.-X.; Zhang, Q.; Wang, Y.; Lu, Z.-J. Identification and functional analysis of two chitin synthase genes in the common cutworm, Spodoptera litura. Insects 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Taxon (Phylum: Class) | Species | Gene Name | Type | Accession No. |
|---|---|---|---|---|
| Annelida *: Polychaeta | Owenia fusiformis | OfuCHS1 | Type 2, group A | AHX26704.1 |
| OfuCHS2 | Type 2, group C | AHX26705.1 | ||
| OfuCHS3 | Type 2, group C | AHX26706.1 | ||
| OfuCHS4 | Type 2, group D | AHX26707.1 | ||
| OfuCHS5 | Type 2, group D | AHX26713.1 | ||
| OfuCHS6 | Type 1 | AHX26703.1 | ||
| Sabellaria alveolata | SalCHS1 | Type 2, group B | AHX26717.1 | |
| SalCHS2 | Type 2, group C | AHX26711.1 | ||
| SalCHS3 | Type 1 | AHX26710.1 | ||
| Dimorphilus gyrociliatus | DgyCHS1 | Type 2, group A | CAD5118528.1 | |
| DgyCHS2 | Type 2, group B | CAD5114651.1 | ||
| Platynereis dumerilii | PduCHS1 | Type 2, group B | AHX26708.1 | |
| PduCHS2 | Type 2, group B | AHX26709.1 | ||
| PduCHS3 | Type 2, group C | AHX26716.1 | ||
| Capitella teleta | CteCHS1 | Type 2, group A | ELU08572.1 | |
| CteCHS2 | Type 2, group C | ELT98539.1 | ||
| CteCHS3 | Type 2, group D | ELT92724.1 | ||
| CteCHS4 | Type 2, group D | ELT92107.1 | ||
| Annelida *: Annelida incertae sedis | Myzostoma cirriferum | MciCHS1 | Type 2, group B | AHX26714.1 |
| Mollusca *: Bivalvia | Lottia gigantea | LgiCHS1 | Type 2, group A | XP_009061726.1 |
| LgiCHS2 | Type 2, group A | XP_009061725.1 | ||
| LgiCHS3 | Type 2, group A | XP_009061724.1 | ||
| LgiCHS4 | Type 2, group B | XP_009063632.1 | ||
| LgiCHS5 | Type 2, group C | XP_009047936.1 | ||
| LgiCHS6 | Type 2, group D | XP_009066852.1 | ||
| LgiCHS7 | Type 2, group D | XP_009066854.1 | ||
| LgiCHS8 | Type 2, group D | XP_009051436.1 | ||
| LgiCHS9 | Type 2, group D | XP_009051165.1 | ||
| Mytilus edulis | MedCHS1 | Type 2, group A | CAG2205753.1 | |
| Tegillarca granosa | TgrCHS1 | Type 2, group A | AON76719.1 | |
| Atrina rigida | AriCHS1 | Type 2, group A | AAY86556.1 | |
| Pinctada fucata | PfuCHS1 | Type 2, group A | BAF73720.1 | |
| Mollusca *: Gastropoda | Elysia marginata | EmaCHS1 | Type 2, group A | GFS24687.1 |
| EmaCHS2 | Type 2, group C | GFR89942.1 | ||
| EmaCHS3 | Type 2, group D | GFR83755.1 | ||
| EmaCHS4 | Type 2, group D | GFR70591.1 | ||
| EmaCHS5 | Type 1 | GFS00558.1 | ||
| EmaCHS6 | Type 1 | GFR82903.1 | ||
| Crassostrea gigas | CgiCHS1 | Type 2, group A | XP_034323514.1 | |
| Mollusca *: Polyplacophora | Leptochiton asellus | LasCHS1 | Type 2, group A | AHX26699.1 |
| LasCHS2 | Type 2, group C | AHX26700.1 | ||
| Arthropoda: Insecta | Helicoverpa zea | HzeCHS1 | Type 2, group 1 | ADX66429.1 |
| HzeCHS2 | Type 2, group 2 | ADX66427.1 | ||
| Ostrinia furnacalis | OfurCHS1 | Type 2, group 1 | ACF53745.1 | |
| OfurCHS2 | Type 2, group 2 | ABB97082.1 | ||
| Manduca sexta | MseCHS1 | Type 2, group 1 | AAL38051.2 | |
| MseCHS2 | Type 2, group 2 | AAX20091.1 | ||
| Arthropoda: Copepoda | Lepidopterous salmonis | LsaCHS1 | Type 2, group 1 | AYN59157.1 |
| LsaCHS2 | Type 2, group 2 | AYN59158.1 | ||
| Chordata: Actinopterygii | Danio rerio | DreCHS1 | Type 2 deuterostome | AJW72838.1 |
| Esox lucius | EluCHS1 | Type 2 deuterostome | XP_010887243.2 | |
| Megalops cyprinoides | McyCHS1 | Type 2 deuterostome | XP_036403039.1 | |
| Chordata: Ascidiacea | Ciona robusta | CroCHS1 | Type 2 deuterostome | BBB15954.1 |
| Chordata: Amphibia | Xenopus laevis | XlaCHS1 | Type 2 deuterostome | XP_018120159.2 |
| Choanoflagellatea | Salpingoeca rosetta | SroCHS1 | Type 1 | EGD80959.1 |
| Monosiga brevicollis | MbrCHS1 | Type 1 | XP_001743227.1 | |
| Porifera: Calcarea | Sycon ciliatum | SciCHS1 | Type 1 | AHX26712.1 |
| Leucosolenia complicata | LcoCHS1 | Type 1 | AHX26702.1 | |
| LcoCHS2 | Type 1 | AHX26701.1 | ||
| Cnidaria: Hexacorallia | Nematostella vectensis | NveCHS1 | Type 1 | EDO41482.1 |
| NveCHS2 | Type 1 | EDO44996.1 | ||
| Stylophora pistillata | SpiCHS1 | Type 1 | PFX15170.1 | |
| SpiCHS2 | Type 1 | PFX17869.1 | ||
| Hydra vulgaris | HvuCHS1 | Type 1 | XP_004207525.2 | |
| HvuCHS2 | Type 1 | XP_012554922.1 | ||
| Fungi: Eurotiomycetes | Aspergillus fumigatus | AfuCHS1 | Fungi group | P54267.2 |
| Fungi: Sordariomycetes | Neurospora crassa | NcrCHS1 | Fungi group | P30588.2 |
| Sequencing | Number of reads before filtering | 263,730,178 |
| Mean quality score | 35.47 | |
| Percentage of ≥ Q30 (%) | 90.95 | |
| Number of bases (Gb) | 37.08 | |
| Data filtering | Number of reads after filtering | 250,683,082 |
| Assembly | Number of contigs (> 10,000 bp) | 14,816 |
| Length of N50 (bp) | 12,818 | |
| Total length of contigs (bp) | 196,561,892 | |
| Length of the largest contig (bp) | 210,881 | |
| GC content (%) | 43.71 | |
| Gene prediction | Number of predicted genes | 353,344 |
| Database Type | Top Genes | Length (aa) | E-Value | Identified Group | Gene Name | Species | |
|---|---|---|---|---|---|---|---|
| Type 1 | g58373.t1 | 838 | 0 | Type 1 | BonCHS7 | Owenia fusiformis | |
| g38534.t1 | 321 | 2 × 10−51 | Group D | ||||
| Type 2 | Group A | g45117.t1 | 733 | 2 × 10−135 | Group A | BonCHS1 | O. fusiformis |
| g38534.t1 | 321 | 1 × 10−127 | Group D | ||||
| g120019.t1 | 304 | 7 × 10−91 | Group C | ||||
| g91735.t1 | 755 | 1 × 10−83 | Group B | ||||
| Group B | g38534.t1 | 321 | 2 × 10v120 | Group D | Sabellaria alveolata | ||
| g45117.t1 | 733 | 2 × 10−106 | Group A | ||||
| g91735.t1 | 755 | 4 × 10−95 | Group B | BonCHS2 | |||
| g120019.t1 | 304 | 5 × 10−76 | Group C | ||||
| Group C | g20614.t1 | 464 | 0 | Group C | BonCHS3 | S. alveolata | |
| g120019.t1 | 304 | 2 × 10−142 | Group C | BonCHS4 | |||
| g86068.t1 | 472 | 3 × 10−137 | Group C | BonCHS5 | |||
| g38534.t1 | 321 | 8 × 10−129 | Group D | ||||
| g45117.t1 | 733 | 3 × 10−106 | Group A | ||||
| Group D | g38534.t1 | 321 | 3 × 10−137 | Group D | BonCHS6 | O. fusiformis | |
| g45117.t1 | 733 | 2 × 10−122 | Group A | ||||
| g91735.t1 | 755 | 5 × 10−84 | Group B | ||||
| g120019.t1 | 304 | 3 × 10−69 | Group C | ||||
| Database | Query ID | Species | Database ID | Identity (%) | E-Value |
|---|---|---|---|---|---|
| NCBI | BonCHS1 | Lamellibrachia satsuma | KAI0208509.1 | 46.94 | 2 × 10−156 |
| BonCHS2 | Lamellibrachia satsuma | KAI0242735.1 | 56.45 | 1 × 10−92 | |
| BonCHS3 | Platynereis dumerilii | AHX26716.1 | 73.04 | 0 | |
| BonCHS4 | Platynereis dumerilii | AHX26716.1 | 88.09 | 8 × 10−132 | |
| BonCHS5 | Sabellaria alveolata | AHX26711.1 | 52.52 | 6 × 10−112 | |
| BonCHS6 | Homalodisca vitripennis | KAG8240581.1 | 64.47 | 1 × 10−154 | |
| BonCHS7 | Owenia fusiformis | CAH1788656.1 | 51.09 | 1 × 10−170 | |
| UniProt | BonCHS1 | Capitella teleta | R7UXD6 | 46.10 | 4.3 × 10−161 |
| BonCHS2 | Lottia gigantea | V4B948 | 44.1 | 1.7 × 10−121 | |
| BonCHS3 | Capitella teleta | R7TXS7 | 70.30 | 3 × 10−155 | |
| BonCHS4 | Capitella teleta | R7TXS7 | 87.00 | 9.3 × 10−135 | |
| BonCHS5 | Lingula unguis | A0A1S3IM62 | 48.10 | 7 × 10−109 | |
| BonCHS6 | Bombyx mori | H9J0C4 | 66.20 | 5.7 × 10−153 | |
| BonCHS7 | Lingula unguis | A0A1S3IM62 | 48.10 | 0 |
| Query ID | Species | Database ID | Enzyme Class | Identity (%) | E-Value |
|---|---|---|---|---|---|
| BonCHS1 | Macandrevia cranium | AHX26715.1 | GT2 | 42.40 | 1.54 × 10−154 |
| BonCHS2 | Myzostoma cirriferum | AHX26714.1 | GT2 | 37.02 | 1.71 × 10−141 |
| BonCHS3 | Platynereis dumerilii | AHX26716.1 | GT2 | 76.46 | 0 |
| BonCHS4 | Platynereis dumerilii | AHX26716.1 | GT2 | 88.46 | 2.10 × 10−140 |
| BonCHS5 | Sabellaria alveolate | AHX26711.1 | GT2 | 53.22 | 4.67 × 10−132 |
| BonCHS6 | Bombyx mori | AFC69002.1 | GT2 | 66.25 | 4.35 × 10−153 |
| BonCHS7 | Owenia fusiformis | AHX26703.1 | GT2 | 50.23 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Kim, S.L.; Jeong, M.-K.; Yu, O.H.; Eyun, S. Identification and Phylogenetic Analysis of Chitin Synthase Genes from the Deep-Sea Polychaete Branchipolynoe onnuriensis Genome. J. Mar. Sci. Eng. 2022, 10, 598. https://doi.org/10.3390/jmse10050598
Choi H, Kim SL, Jeong M-K, Yu OH, Eyun S. Identification and Phylogenetic Analysis of Chitin Synthase Genes from the Deep-Sea Polychaete Branchipolynoe onnuriensis Genome. Journal of Marine Science and Engineering. 2022; 10(5):598. https://doi.org/10.3390/jmse10050598
Chicago/Turabian StyleChoi, Hyeongwoo, Sang Lyeol Kim, Man-Ki Jeong, Ok Hwan Yu, and Seongil Eyun. 2022. "Identification and Phylogenetic Analysis of Chitin Synthase Genes from the Deep-Sea Polychaete Branchipolynoe onnuriensis Genome" Journal of Marine Science and Engineering 10, no. 5: 598. https://doi.org/10.3390/jmse10050598
APA StyleChoi, H., Kim, S. L., Jeong, M.-K., Yu, O. H., & Eyun, S. (2022). Identification and Phylogenetic Analysis of Chitin Synthase Genes from the Deep-Sea Polychaete Branchipolynoe onnuriensis Genome. Journal of Marine Science and Engineering, 10(5), 598. https://doi.org/10.3390/jmse10050598






