Abstract
Litter abundance and typology were investigated at different beaches and mangrove forests at nine sites on the Colombian Caribbean and Pacific coasts. Average litter abundance on the Caribbean Sea beaches (1.42 items/m2–12.21 g/m2) and in mangrove forests (1.29 items/m2–28.72 g/m2) were greater than that of the Pacific Ocean beaches (0 items/m2–0 g/m2) and mangrove forests (1.13 items/m2–79.41 g/m2). The most abundant litter material was plastic, which represented 93.61% of the total litter content. According to the Clean Coast Index, the sites analyzed in the Caribbean Sea were “Moderate” to “Extremely Dirty”, while those in the Pacific Ocean were “Clean” to “Moderate Dirty”. The Magdalena River is considered the main source of litter on the Caribbean Sea coast, while on the Pacific Ocean coast, litter is essentially associated with the mismanagement of solid wastes. This study constitutes a baseline on the litter content of beaches and mangrove forests, and is useful for establishing sound strategies for their protection, restoration and conservation.
1. Introduction
Marine litter is defined as any solid, man-made material deliberately or accidentally discharged into marine and coastal environments [1,2] and can be classified as small (1–40 mm), meso- (40.01–200 mm) and macro-litter (>200.01 mm) [3]. The origin of marine litter is mostly (80%) linked to land-based activities. Discharged items are mainly transported to marine and coastal environments through rivers and winds, or are directly abandoned on the beach by visitors. A lower percentage (20%) of litter is of marine origin and is mostly related to maritime transport, fishing, and off-shore oil extraction activities [4,5].
The transport and accumulation of litter depends on multiple factors such as oceanic and climatic conditions and beach geomorphological characteristics, among others [4,5]. Marine litter consists of different materials, including plastic, glass, wood, metal, rubber, textiles, and paper [4,6], with plastic items considered the main abundant and potential pollutants [2]. It is considered that from 6 to 10% of the annual global plastic production (ca. 315 million tons) ends up in marine environments [4,6].
It is estimated that, by 2050, 10 billion metric tons of plastic waste will be accumulated in landfills or in the natural environment, and there will be more plastic pieces (by weight) than fish in the ocean [2,5]. Overproduction, high use, and the great durability of plastics, along with inefficient waste management practices, are considered the most important reasons for the rapid increase of plastic marine litter over the last several decades [1].
The impact of marine litter on organisms is mainly related to entanglement, suffocation, and their ingestion and subsequent transfer to tissues and organs, a process that concerns the entire food chain, including humans [4,7,8]. Marine litter also favors non-native species dispersal, and the adsorption of persistent organic pollutants and heavy metals takes place on the surface of plastic items [4]. Marine litter may also affect the safety of bathers (because of cutting and sharp objects), and has serious social and economic impacts including aesthetic deterioration of coastal scenery (which produces rejective reactions of beach visitors), damages to recreational boats and fishing activities, and extra expenses needed to carry out beach clean-up operations, among others [2,6,8].
Mangrove forests constitute an ecosystem whose functions are of great ecological, aesthetic, recreational, scientific, social, economic, and cultural interest [9,10]. They are composed of unique plant species, i.e., halophytic trees and shrubs with morphological, physiological, and reproductive characteristics that allow them to survive in a critical interface among terrestrial, estuarine, and marine environments [11]. Presently, mangrove forests occupy ca. 14 million hectares worldwide, and more than two-thirds of them are found in 18 countries: Indonesia, Brazil, Australia, Mexico, Nigeria, Malaysia, Myanmar, Bangladesh, Cuba, India, Papua New Guinea, Colombia, Guinea Bissau, Mozambique, Madagascar, Philippines, Thailand, and Vietnam [11,12,13]. Colombia is the only country in South America with mangrove forests on both the Caribbean Sea and Pacific Ocean coasts. The forests cover an area of ca. 379,954 hectares, 87,230 of which are recorded along the Caribbean Sea coast where five mangrove species have been reported (i.e., Avicennia germinans and Rhizophora mangle (the two most abundant) and, secondarily, Laguncularia racemosa, Conocarpus erectus and Pelliciera rhizophorae [10]); and 292,724 ha are recorded along the Colombian Pacific coast [14,15], where nine mangrove species have been reported (i.e., those described from the Caribbean Sea coast plus Rhizophora harrisonii, R. racemosa and Mora oleifera [16]). Mangrove forests are considered a strategic ecosystem for adaptation to climate change [11,17]; however, presently most of the mangrove forests of the Colombian Caribbean Sea coast are under stress mainly due to anthropogenic activities such as: the construction of roads and tourist infrastructure; expansion of urban, agricultural and industrial frontiers; indiscriminate logging; and increased erosion linked to the emplacement of groins/breakwaters, among others [11,18]. The loss of mangrove cover on the Pacific Ocean coast is mainly related to illegal logging for energy-source purposes, and the construction of stilt houses [19,20].
Different studies have demonstrated the capacity of mangroves to act as marine litter traps due to their root structure [9,21,22], and how trapped litter affects mangrove development by undergoing degradation processes that generate microplastics able to enter the food chain, mainly at lower trophic levels, but with subsequent effects on organisms at higher trophic levels via bioaccumulation [23,24,25]. Microplastics and chemical additives used in the manufacture of plastic materials may be toxic to marine organisms [23,25]. In addition, because of their hydrophobic nature, microplastics may absorb persistent organic pollutants (e.g., polycyclic aromatic hydrocarbons and polychlorinated biphenyls), which can be released into an organism’s tissues when ingested [23].
Despite the exosystemic importance of mangrove forests and the relevant environmental impacts on them of litter (especially of plastic materials), only four studies have been carried out to investigate the impact and origin of marine litter in Colombian mangrove forests: one in the islands of Providencia and Santa Catalina after Hurricane Iota, one in the Bay of Buenaventura (in the Pacific coast), and two in Ciénaga Grande de Santa Marta (in the department of Magdalena, on the Caribbean Sea coast) [26,27,28,29].
The aim of this paper is to determine the amount, typology, and sources of litter at nine sites including beaches and mangrove forests mainly located along the department of Atlántico (in the Caribbean Sea coast)—which contains ca. 613.3 ha of mangrove forest—and the Tumaco municipality in the department of Nariño (on the Pacific Ocean coast) [30], which contains ca. 24,570 ha of mangrove forest [31]. At each site, litter was determined at different beach zones and in homogeneous mangrove zones (i.e., areas characterized by the clear predominance of specific mangrove species). The objective of this paper is the establishment of a baseline amount and typology of litter stranded/trapped at each site and the determination of possible relationships between mangrove zone location and litter abundance/typology, keeping in mind that this is of paramount importance to establish sound management actions to protect mangrove forests and their associated ecosystem services.
2. Materials and Methods
2.1. Study Area
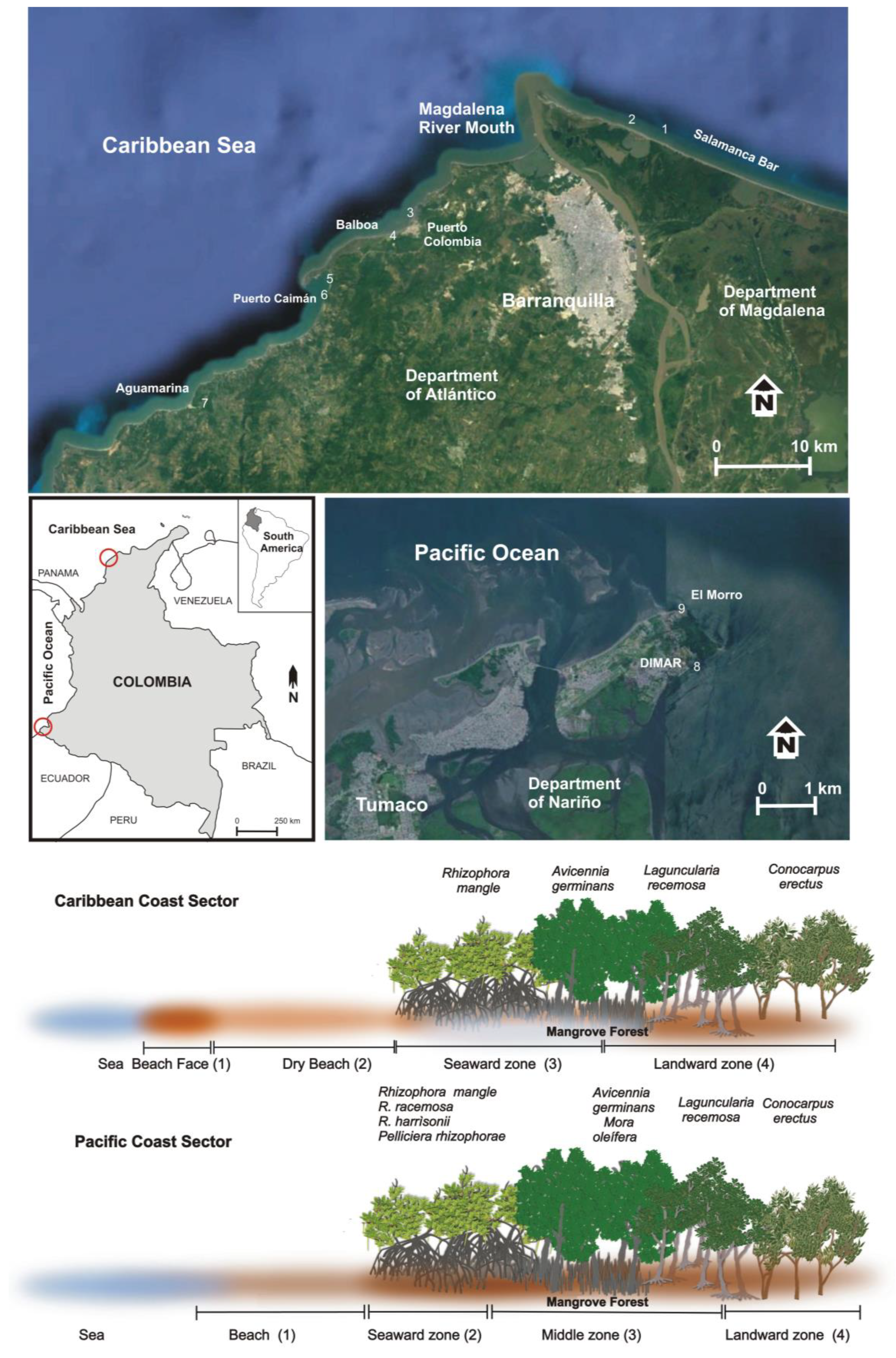
The coast of Colombia has a total length of 5548 km and includes the Caribbean Sea (2733 km) and Pacific Ocean (2815 km) coastlines [32], Figure 1. The Caribbean Sea coast borders with Panama to the southwest and Venezuela to the northeast, and constitutes a mixed, semi-diurnal microtidal (<2 m) environment, with a maximum tidal amplitude of 60 cm [33]. It is a tropical environment with seasonal variations in rainfall from the dry season (December–March) and the transitional seasonal (April–July) to the rainy season (August–November) [11]. Maximum precipitation reaches ca. 2500 mm/yr and mean annual temperature is ca. 27 °C [33]. Offshore waves essentially approach the coast from the first quadrant and are related to the trade winds (Alisios) recurrent during the December–March period [11]. Secondary refracted and diffracted wave fronts approach the coast from the fourth and third quadrants. Significant wave height, which is the average wave height of the highest one-third of the wave records, is ca. 2 m, and the average peak period is 7 s [34]. The net longshore drift has a dominant southwestward component, minor reversal to the northeast takes place during the rainy period when southerly winds dominate and set up short, high-frequency waves [35,36].
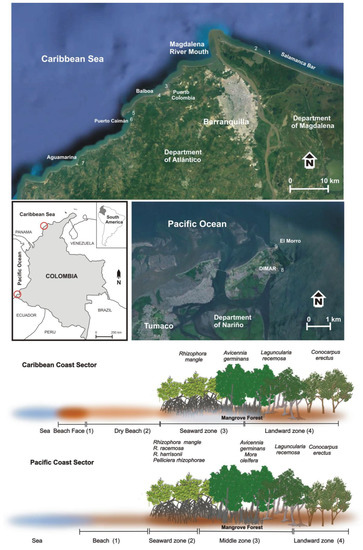
Figure 1.
Location map showing the position of the nine studied sites. The investigated beaches and mangrove forest zones are also presented, as well as the typical zoning of mangrove species along the Caribbean Sea and the Pacific Ocean coasts.
The Colombian Pacific Ocean coast borders Panama to the north and Ecuador to the south [37]. This region is characterized by heavy rainfall, numerous rivers, and luxuriant vegetation [38]. The climate of the Pacific Ocean coast is humid tropical, dominated by the migration of the Intertropical Convergence Zone and its interaction with the high relief of the Western Ridge of the Colombian Andes. The average temperature is 26 °C with minimum values of about 14 °C. Rainfall reaches a maximum of ca. 10,000 mm/year in the central zone, and decreases towards the north and south to ca. 3000 mm/year [38]. The tide is mixed semi-diurnal with a mesotidal range, i.e., average amplitude between 2 and 4 m. Predominant winds come from the south and from the west to the southwest along the central and southern parts of the Pacific Ocean coast. Significant waves are 0.5–1.5 m high during calm weather and 2.5–3.5 m high during storm periods [38].
The Caribbean Sea of Colombia has ca. 87,230 ha of mangrove forests, with the largest extensions in the Ciénaga Grande de Santa Marta (department of Magdalena), El Canal del Dique, the Archipelagos of Nuestra Señora del Rosario and San Bernardo (Bolívar), the bay of Barbacoas, and the mouth of the Sinú River (department of Córdoba) [39]. The investigated sites are located in the departments of Atlántico and Magdalena, Figure 1.
Along the 64.5 km long coastline of the department of Atlántico [11,40], mangrove forests recorded important losses over recent decades, i.e., their cover decreased from 1184 ha in 1998 to 613 ha in 2005 because of the emplacement of human settlements linked to tourism and urban developments, illegal logging, the modification of fresh water supplies, and pollution problems [11,41]. Sites investigated in this paper are close to the Magdalena River mouth (Figure 1) that drains a basin of 257,430 km2, corresponding to 724 municipalities representing 80% of the Colombian population [11]. The river transports to the coast large amounts of wastes and pollutants [42].
The Pacific Ocean coast of Colombia has ca. 292,724 ha of mangrove forest, with the largest extensions found at the Jurado River mouth, in the Gulf of Tribugá, in the southern area of the department of Chocó, and, especially, at the departments of Valle del Cauca and Nariño, close to Ecuador [43]. In the Nariño department, where the investigated sites are located, 72,262 ha of mangrove forest has been recorded [44]. It is mainly affected by sewage and solid waste dumping, logging, and agricultural and livestock activities that generate an annual loss of 0.05% of coverage [44,45]. In Tumaco municipality, the main impacts on mangrove forests are related to urban growth, tourism, port traffic, the development of shrimp farming, and the discharge of urban and industrial wastewaters [31]. Unfortunately, Tumaco is an area where different illegal armed groups converge and has one of the highest rates of violence in the country [46]; for this reason it was not possible to investigate a large number of sites in this area.
2.2. Sampling Sites Description
We investigated seven sites along the departments of Magdalena and Atlántico (on the Caribbean Sea coast of Colombia) and two in the department of Nariño (on the Pacific Ocean coast of Colombia) in order to obtain a baseline on litter content and amount at each site and at the different zones of each site (Figure 1 and Figure 2). In order to predominantly investigate litter related to marine inputs, i.e., not linked with bathers or other recreational beach activities—which can vary from place to place making the comparison among different sites unrealistic,—all sites but one (Puerto Colombia) were selected in rural and remote areas [47]. The site surveyed at Puerto Colombia, a village area, was in a coastal sector not frequented by beachgoers and can be considered as rural. In relation to the Pacific Ocean coast, the sampling included coastal sites close to the urban perimeter of Tumaco but far from direct anthropic disturbances, i.e., they were essentially influenced by the local dynamics of waves, currents and tides.

Figure 2.
Images of the beaches/mangrove forests studied: (a) Salamanca A; (b) Salamanca B; (c) Puerto Colombia; (d) Balboa; (e) Puerto Caimán A; (f) Puerto Caimán B; (g) Aguamarina; (h) Complejo DIMAR (at high tide); (i) Complejo DIMAR (at low tide); (j) El Morro (at high tide); (k) El Morro (at low tide); (l) Litter trapped in a pneumatophore at Salamanca A; (m) The “Christmas tree” effect due to litter trapped in a plant of Rhizophora sp. at El Morro.
Each site investigated usually included the beach and different backing zones of mangrove forests (Figure 1). In places, the beach was nonexistent due to erosion by waves. Litter observation was carried out along transects 10 m in width (in the alongshore direction) [24,29] landward extended from the shoreline, according to the specific characteristics of each site, i.e., the cross-shore dimension investigated varied between sites (from 10 to 124 m). At each site, litter content was analyzed at different zones (i.e., parts of beach and mangrove environments): namely, at the beach face (that coincided in the Caribbean Sea coast with the strandline because it is a microtidal environment), on the dry beach, and in homogeneous zones of mangrove forests, i.e., zones characterized by the clear predominance of a specific mangrove species. Concerning the mangrove forests, at each sampled zone the following aspects/parameters were characterized: mangrove species, relative density of trees (“high”—tree coverage >75%; “moderate”—51–75%; and “low”—≤50%), presence/absence of pneumatophores and seedlings, and presence of other plant species (Table 1) [21,24,48].

Table 1.
Characteristics of beaches and mangrove forests at each sampled site.
2.3. Marine Littler Characterization
Surveys were carried out in December and January 2022 on the Caribbean Sea coast, and in August 2022 on the Pacific Ocean coast. At each site two observers covered the whole beach and mangrove forest zones by moving along 1 m separated transects parallel to the shoreline. All litter items larger than 2 cm in the longest linear dimension, ensuring the inclusion of bottle caps and cigarette butts as recommended by UNEP/MAP [49], were collected and brought to the laboratory. There, items were carefully washed to remove adhered sediments, dried under natural conditions, and weighed. Litter items were identified by assigning them to diverse categories according to their composition [50,51]. The amount of litter was expressed as number of items per linear meter (items/m) and square meter of beach/mangrove forest zone (items/m2), and as weight of items per linear meter (g/m) and per square meter (g/m2).
To determine the level of cleanliness of each zone, the Clean Coast Index (CCI), developed by Alkalay et al. [52] was calculated according to the following expression:
The index reflects the total number of items/m2, which is the result of the product of the width of each beach/mangrove zone (10 m in this case) and the zone length, i.e., the cross-shore dimension of each zone that varied between sites according to the specific geomorphological characteristics and vegetation cover typology and continuity. Consistent with the CCI index calculation [52], a coefficient K = 20 was used in equation (1) to make sure that the value of the resulting index did not fall between 0 and 1. CCI varies from “Very Clean” (0–2), “Clean” (2–5), “Moderate Dirty” (5–10) and “Dirty” (10–20) to “Extremely Dirty” (>20) [52,53,54].
2.4. Statistical Approach
To compare litter composition among coastal sites, a nonparametric multi-dimensional scaling analysis (nMDS) was performed. The objective of the nMDS analysis is to group data points into classes of similar points based on a series of variables, i.e., to represent the original position of data in multidimensional space as accurately as possible using a reduced number of dimensions (typically two) that can be easily plotted and visualized. The nMDS relies on rank orders (distances) for ordination, and this technique is based on a similarity and dissimilarity matrix. Previous to the analysis, the dataset was normalized, i.e., the number of items per zone was converted into items/m2 and transformed to the square root. Analysis was made using the Bray–Curtis dissimilarity index [55]. In the nMDS plots, litter categories with a Pearson correlation > 0.7 (when item numbers were considered) and 0.8 (for item weights) were represented as vectors from a central point in a 2D space plot. Orientation of the vectors is related to the composition of the most important items. Analysis was conducted using PRIMER V.6 (Plymouth Marine Laboratory, UK). Very few coastal sites were removed from the nMDS analysis because they contained very few items or no litter. Cluster analysis was carried out as a complementary technique. This is a statistical method used with the goal of sorting different objects or data points into groups in such a manner that the degree of association between two objects is high if they belong to the same group and low if they belong to different groups. This helps to discover structures in data without explaining why those structures exist. The treatment of the data and the index for calculating dissimilarity were those used for the previous technique.
3. Results
Overall, beach litter was represented by small- and medium sized-fresh items that presented low abrasion and discoloration, whereas items in mangrove forests often presented larger sizes and showed poor conservation states. Considering all the zones investigated along the seven Caribbean Sea and two Pacific Ocean sites (Figure 1 and Figure 2), a total of 2664 items (or 86 kg), belonging to 59 litter groups, were counted in an area of 3500 m2, with an average abundance per zone of 0.761 items/m2 (or 35.8 g/m2) ranging from 0 to 6.9 items/m2 (or 16 kg). On the Caribbean Sea coast, the average amount of litter was 1.3 items/m2 (or 22.56 g/m2), while on the Pacific Ocean coast this was 0.3 items/m2 (68.1 g/m2). A total amount of 36 kg of litter was collected at the seven Caribbean Sea sites, while 50 kg were collected at the two Pacific Ocean sites. Litter abundance and weight per zone and site surveyed are presented in Table 2.

Table 2.
Abundance (items/m2) and weight (g/m2) per zone and site. The four zones investigated are reported in Figure 1. Total values were calculated per site, considering the total number of items per site and the total area of each site.
3.1. Litter Abundance and Weight
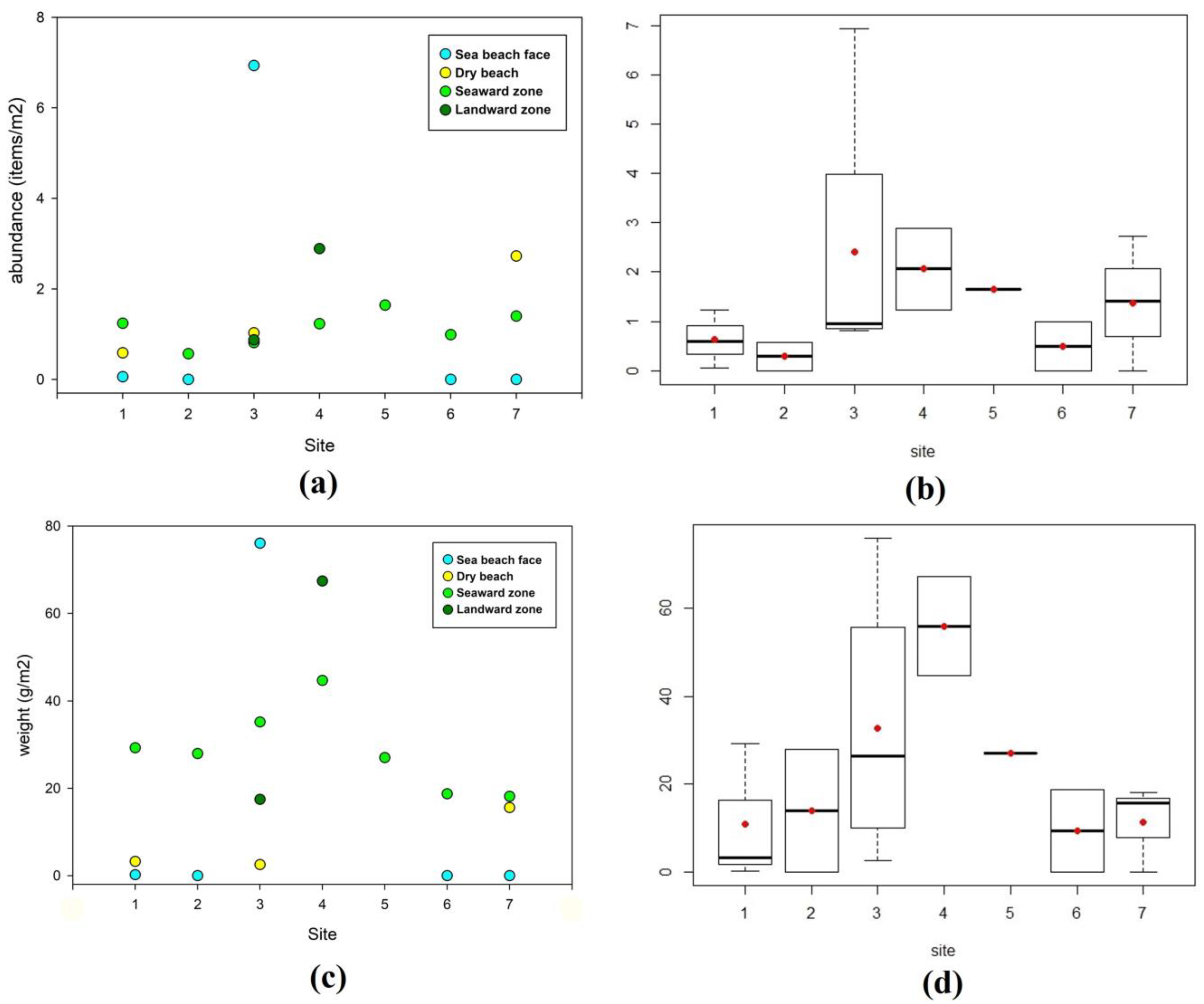
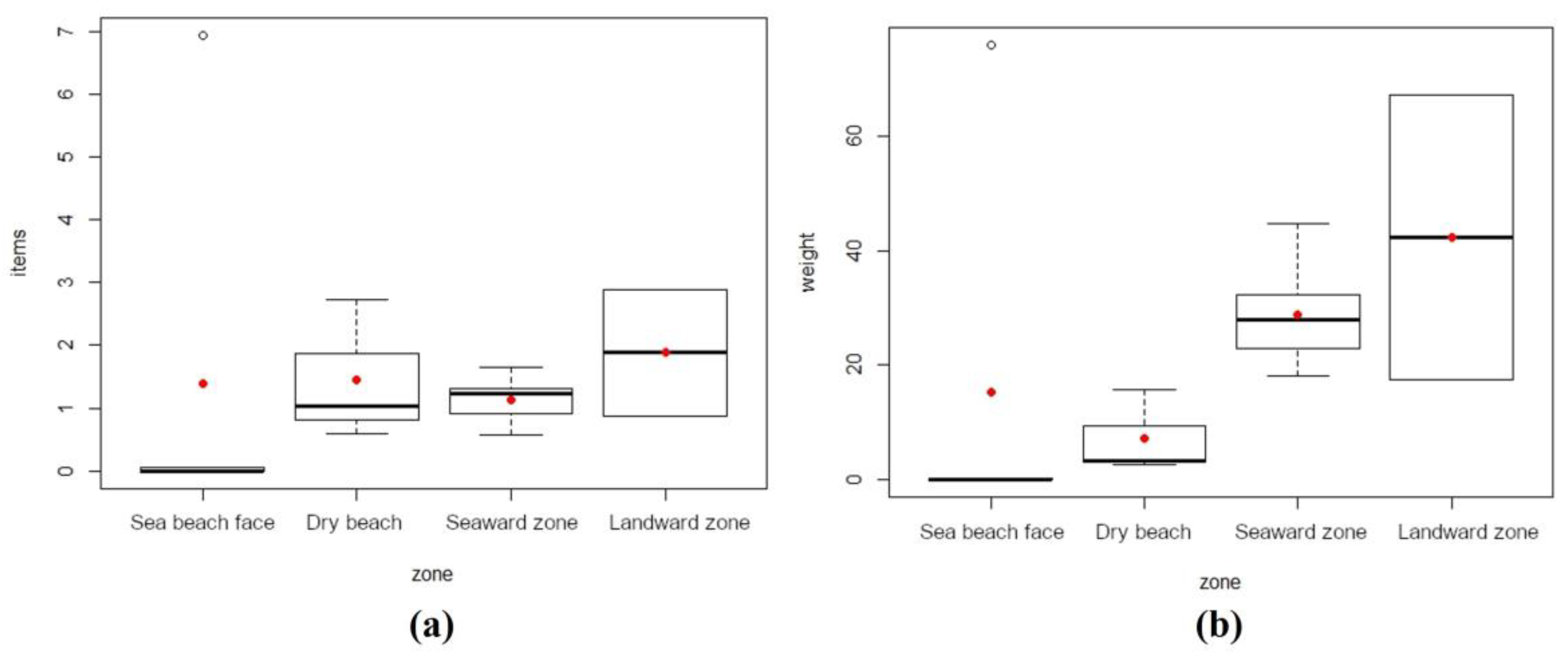
Litter abundance (items/m2) for the different zones of each site investigated on the Caribbean Sea coast is shown in detail in Figure 3a, and the mean and median values recorded at each site investigated and data dispersion (or distribution) are presented in Figure 3b. Litter amounts expressed as weight per square meter (g/m2) are presented in Figure 3c,d. Beach face zones showed the lowest amount of litter (in number of items and weight), and the highest amount was generally recorded in mangrove forest zones (Figure 4a,b). Lastly, the beach face zone of site no. 3 recorded a huge accumulation of litter constituting an outlier in both plots of Figure 4.
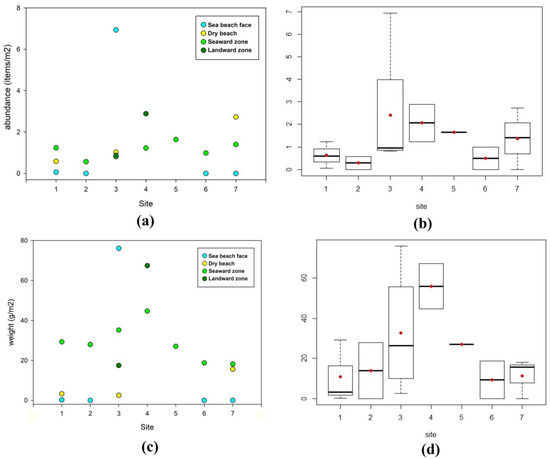
Figure 3.
Caribbean Sea coast. (a) Litter abundance (items/m2) at all beach/mangrove zones per site. (b) Box plots concerning litter abundance values (items/m2) per each site investigated; red dots represent mean values and black lines median values. (c) Weight of recollected litter items (g/m2) at all beach/mangrove zones per site. (d) Box plots concern weights of litter items (g/m2) per site.
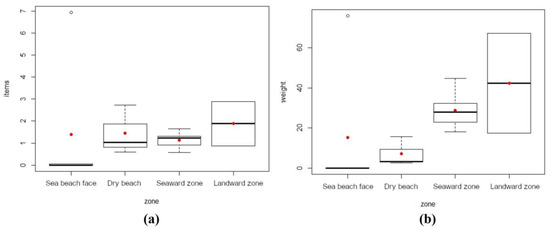
Figure 4.
Box plots concerning Caribbean Sea beach/mangrove zones. (a) Litter abundance (items/m2). (b) Weight of litter items (g/m2). Red dots represent mean values and black lines median values.
Regarding litter abundance and weight for the different zones of each site investigated along the Pacific Ocean coast (Figure 1), values ranged from 0 to 4.77 items/m2 (0−196.6 g/m2). Considering all items and zones, the two Pacific sites presented lower abundance (0.2 and 0.3 items/m2) than the Caribbean Sea sites (ranging from 0.4 to 2.1 items/m2, Table 2). In the beach zone of site no. 8, no litter was observed and the mangrove seaward zone only presented 0.08 items/m2 (or 8.3 g/m2). The mangrove middle and landward zones of site no. 8 registered the highest abundance and weights: 1.1 and 4.8 items/m2 (101.7 and 196.6 g/m2), respectively (Table 2). However, at site no. 9, the highest values were recorded in the seaward mangrove zone (0.8 items/m2 and 144.5 g/m2), but litter items had also accumulated in the middle mangrove zone (0.1 items/m2 and 25.2 g/m2, Table 2).
3.2. Litter Composition
Concerning litter composition across all whole Caribbean Sea and Pacific Ocean investigated sites, most litter items were composed of plastic materials that represented 93.61% of the total litter composition, followed by rubber (2.50%), glass (1.67%), metal (0.96%), cloth (0.96%), paper and cardboard (0.17%), unidentified materials (0.09%), and processed wood (0.03%, Table 3). Despite small differences in the percentage of materials observed between the Caribbean Sea and Pacific Ocean sites, plastic was always the predominant material (Table 3).

Table 3.
Litter proportion (%) per type of material in the Caribbean and Pacific coasts of Colombia.
Regarding plastic materials, foamed and hard plastic pieces were the most common litter groups accounting for 1039 items, i.e., 39% of the total. Other very abundant plastic litter groups included caps/lids and drinks (<2 L) that accounted for 8% in both the Caribbean Sea and Pacific Ocean coasts. Table 4 shows the 10 litter groups with the highest number of items recorded. Some litter groups such as hard plastic pieces (2.5−50 cm), drinks (<2 L), hard plastic cups, and food wrappers were common to both the Caribbean Sea and Pacific Ocean coasts (Table 4).

Table 4.
Most numerous litter items in the Caribbean Sea and Pacific Ocean coasts of Colombia.
3.3. Litter Distribution
According to the Clean Coast Index (CCI) proposed by Alkalay et al. [52] the least polluted areas (“Very Clean”) corresponded to beach face zones, as observed at the Caribbean Sea sites nos. 1, 2, 6 and 7 (Table 5). On the Pacific Ocean coast, the “Very clean” grade was also recorded, e.g., at beach and mangrove middle and seaward zones of site no. 8 and at mangrove landward zone of site no. 9. The “Clean” grade was recorded at the remaining mangrove zones of sites nos. 8 and 9 (Table 5). The remaining mangrove zones and beach face zone of site no. 3 were in very poor condition and classified as “Dirty” or “Extremely Dirty” (Table 5).

Table 5.
Clean Coast Index (CCI) proposed by Alkalay et al. [53].
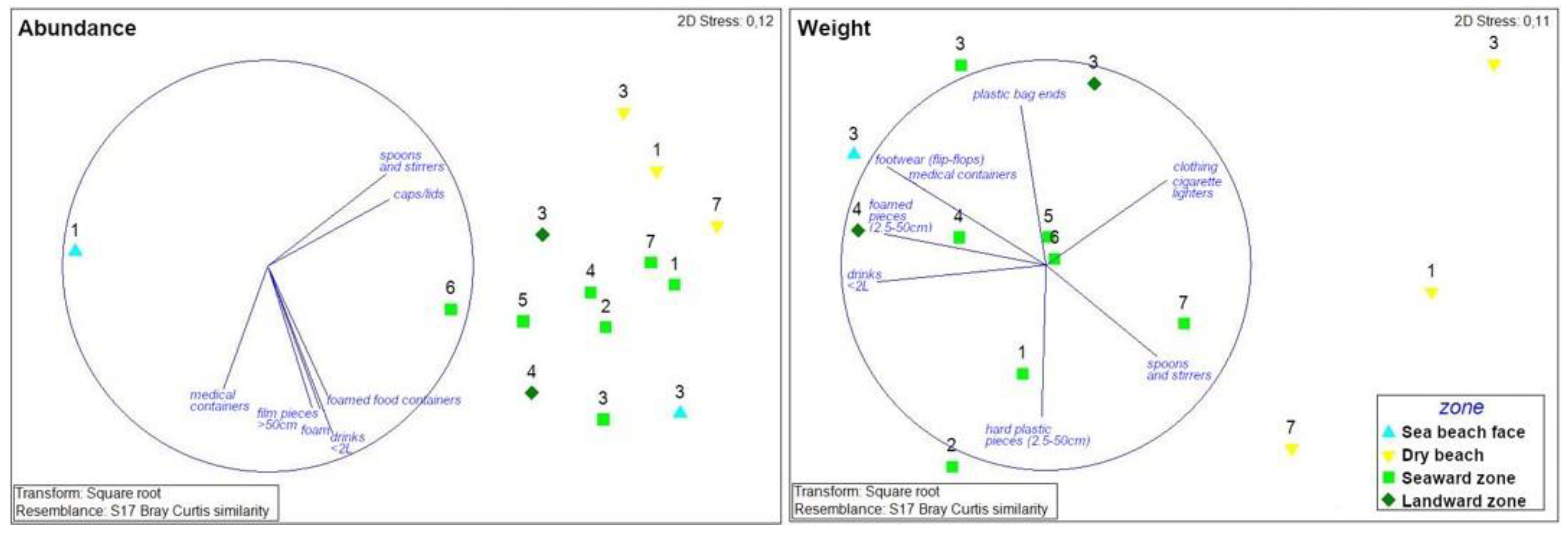
Because of the differences in tidal range between the Caribbean Sea and the Pacific Ocean coasts—i.e., sites investigated in the Pacific Ocean coast are daily flooded by the tide and this is not the case for the Caribbean Sea coast—comparison between the two coastal environments is unviable. Additionally, each type of coastline is characterized by the presence of specific plant species that can affect litter dynamics and accumulation (Figure 1 and Table 1). Therefore, statistical analyses carried out in this study only concerned the Caribbean Sea data. Considering the results of the statistical analyses, an interesting grouping among the sampled sites was observed. In terms of litter abundance and typology, yellow points (corresponding to dry beach zones) are located in a different part of the graph with respect to the green points (corresponding to zones in mangrove forests). However, dry beach zones at sites nos. 1, 3 and 7 are more similar to the nearby mangrove zones (i.e., mangroves zones of the same site) than to the corresponding mangrove zones of other sites, suggesting that there is a direct litter transfer from the dry beach zone to the adjacent mangrove zone (Figure 5).
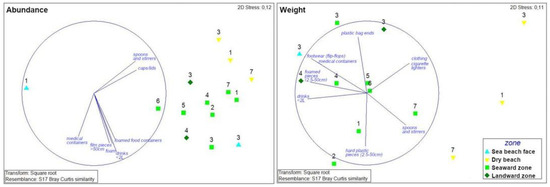
Figure 5.
Caribbean Sea coast. Nonparametric multi-dimensional scaling ordination for litter abundance and weight based on litter categories recorded at each coastal zone. Colors (cyan, yellow, light and dark green) correspond to the four zones investigated (beach face, dry beach, seaward and landward mangrove zones, respectively), while labels from 1 to 7 correspond to the sites investigated. Vector labels refer to litter categories that showed a Pearson correlation > 0.7 and 0.8 (for litter abundance expressed in number of items and weight, respectively).
There is also a clear difference among major litter groups. Vectors oriented towards beaches correspond to litter groups that are mostly found on dry beach zones and are related to beach users (spoons, stirrers and caps/lids, Figure 5). Other items are essentially observed in the mangrove forest zones, e.g., drinks (<2 L), foam, film pieces (>50 cm), etc. and are generally light, floating items. Seaward mangrove zones generally registered low amounts of litter. In order to have a better interpretation of the results, sites with no litter were not considered in the statistical analysis. The most dissimilar zone was the beach face of site no. 1, which only recorded six items. Litter composition and abundance at the beach face zone of site no. 3 were similar to values recorded in mangrove zones (Figure 5).
In terms of weight of litter items, dry beach zones are located on the right side of the graph, while mangrove zones are located on the left side (Figure 5). In mangrove zones, litter content in weight is greater than in beach zones, as shown in Figure 5. Some litter items are essentially related to beach users such as cloths, cigarette lighters, spoons and stirrers while others are especially observed in mangrove areas, such as plastic bag ends, footwear, foamed pieces, hard plastic pieces, medical containers and drinks (Figure 5).
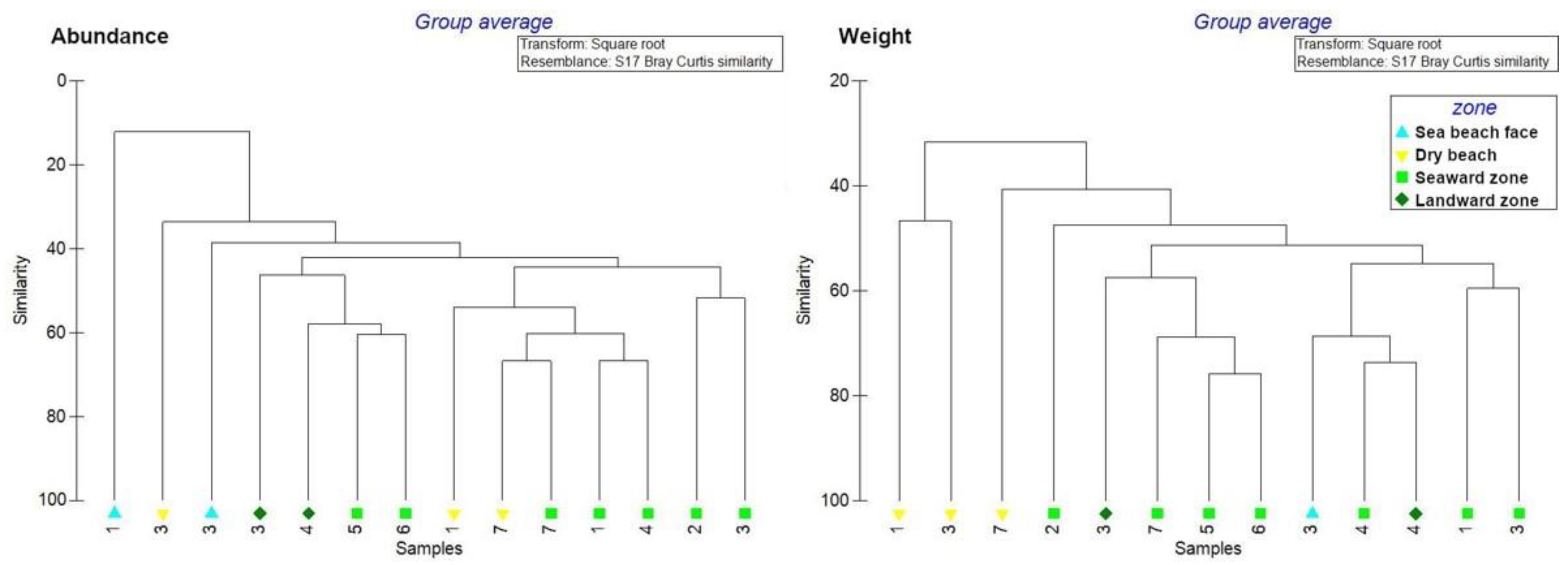
In order to better understand the results obtained from the Caribbean Sea sites, cluster analysis was performed to highlight similarities/dissimilarities between zones. In general, mangrove forest zones presented greater similarity with respect to beach zones, in terms of litter composition and weight (Figure 6).
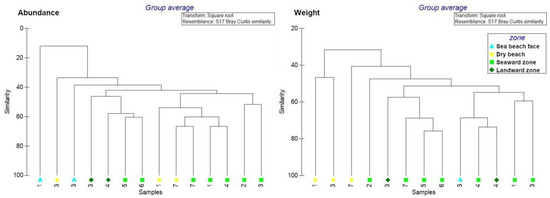
Figure 6.
Cluster analysis for abundance and weight of litter items at the Caribbean Sea sites.
On the Pacific Ocean coast, litter items mostly consisted of plastic bags, film plastic pieces (2.5–50 cm), metal fragments (2.5–50 cm), and clothing. Concerning similarities among the different zones sampled along the Pacific Ocean coast, the two sites investigated are not comparable in terms of litter composition, with site no. 8 essentially containing hard plastic pieces (2.5–50 cm), film pieces (2.5–50 cm), drinks (<2 L) and food wrappers, while site no. 9 clothing, bags and glass fragments (2.5–50 cm).
4. Discussion
The presence of litter on Colombian beaches has been extensively studied [56,57,58,59,60,61,62] and is mainly associated with tourism/recreational activities (particularly on the Caribbean Sea coast) and poor waste management practices (in the Pacific Ocean coast) [63,64], i.e., deficient beach management programs, a lack of environmental education on the part of beach users, and deficiencies in solid waste treatment and disposal services [62,63,65]. Unlike beaches, which are very dynamic due to being directly and daily exposed to wave impacts, mangrove ecosystems are usually observed in sheltered environments and present a complex network of trees, shrubs and aerial root systems (e.g., pneumatophores and prop roots) that greatly favor litter retention [21,48,66]. Therefore, the findings and lessons learnt from beaches cannot be extrapolated to strategic coastal marine ecosystems such as mangrove forests [48]. In Colombia, only a few studies exist on the impact and distribution of litter in mangrove forests (e.g., in the Ciénaga Grande de Santa Marta and Buenaventura bay, respectively on the Caribbean Sea and Pacific Ocean coasts) [26,27,28,29]. This paper is the first to deal with litter assessment and characterization corresponding to different beach zones and homogeneous mangrove forest zones.
4.1. Distribution of Litter in Beach Environments
Average litter abundance on Caribbean Sea beaches (1.42 items/m2—12.21 g/m2) and mangrove forests (1.29 items/m2–28.72 g/m2) was greater than on the Pacific Ocean coast beaches (0 items/m2–0 g/m2) and mangrove forests (1.13 items/m2–79.41 g/m2) (Table 2). This is related to the specific location of the sites studied in the Caribbean Sea, since they are very close to the Magdalena River mouth. The Magdalena is the largest river system in Colombia with a length of 1612 km and a drainage basin covering 257,438 km2 [67,68]. From an administrative point of view, this area, which hosts 80% of the total population of Colombia, belongs to 724 municipalities, 46% of which have no solid waste disposal system [65,68]. It is estimated that the Magdalena River annually deposits 16,700 tons of plastic materials into the Caribbean Sea, which eventually accumulate in the coastal and marine ecosystems of the departments of Atlántico and Magdalena [26,69]. Regarding beach litter pollution along the Department of Atlántico, Rangel-Buitrago et al. [65] confirmed the importance of the Magdalena River inputs as well as highlighting the significance of human activities related to beach tourist use. The relevance of the Magdalena River as the main source of litter items for the sites located in the Caribbean Sea coast was confirmed in this paper, since most of the items encountered i) showed a poor conservation state, i.e., abrasion, discoloration and/or other evidences of a long transport, and ii) were not commercialized in the department of Atlántico (e.g., large-sized Ron bottles, some plastic medical jars, etc.).
In dry beach zones (zone 2) observed items were often related to beach users, as spoons, stirrers and caps/lids. They were probably abandoned on nearby beaches and, then, stranded on the beaches studied in this paper by waves and currents [70]. These litter items are among the most abundant in Europe [71,72] and are also observed on Moroccan beaches frequented by beach users [73].
Beaches analyzed in this study along the Caribbean Sea coast showed an abundance of litter in accordance with Rangel-Buitrago et al. [65] and Williams et al. [60], who reported a high presence of beach litter along the coastline of the department of Atlántico. Litter abundance in this study was higher than that reported on the coast of Cádiz by Asensio-Montesinos et al. [74], with an average of 0.06 items/m2, and Nachite et al. [75] on the Mediterranean beaches of Morocco, with an average of 0.054 ± 0.036 items/m2, but was lower than the amount observed by Lavers et al. [76], who reported an average of 21.68 ± 19.01 items/m2 on the Cocos Islands (Australia).
Specifically, the zone 1 of Puerto Colombia (Figure 2c and Table 2) was the site with the highest abundance of litter, mainly plastic items (e.g., foamed plastic pieces, drinks bottles and caps), which coincides with the observations of Williams et al. [60] who reported on such a beach huge amounts of litter along with the presence of many common items plus nappies and condoms. In addition, Rangel-Buitrago et al. in several studies carried out along the Department of Atlántico [77,78], determined that Puerto Colombia was one of the sites with the highest density of plastic items. In other countries, plastic litter also accounted for more than 80% of the total beach litter amount as evidenced in Turkey [79], Italy [80], South Africa [81], Israel [82], and Morocco [75], and different authors have stated that plastics are the most common items in all coastal marine ecosystems [8,83,84]. Lastly, no litter was observed on the beaches investigated on the Pacific Ocean coast, which could be related to the mesotidal range of this coastal environment: flooding tide carries litter items landward accumulating them in correspondence of the landward limit of the tidal area, as observed in similar tidal environments by Convey et al. [85] and Asensio-Montesinos et al. [74].
4.2. Distribution of Litter in Mangrove Forests
Concerning mangrove forests, the results obtained in this paper showed that litter is observed in all zones of mangrove forests (Table 2 and Table 5).
In the Caribbean Sea mangrove forests, zone 3 generally presented the highest abundance and weight of items, followed by zones 4 and 2 (Figure 4 and Table 5). Mangrove forests studied in the Caribbean Sea were essentially located in remote places and/or close to beaches not used for bathing, and had no clean-up operations (Figure 2). In this regard, Riascos et al. [28] established that, at sites where there are no beach clean-up operations, the spatial distribution observed in marine litter likely reflects long-term spatial patterns of entanglement on mangrove roots and wrapping on the forest ground. In addition, mangrove forests studied on the Caribbean Sea coast were fronted by beaches showing a smooth slope, where the wave run-up is often able to penetrate tens of meters allowing the arrival of litter into the backing mangrove forest, as reported by Cordeiro and Costa [24] in the São Vicente Estuary (Brazil). Results obtained in this paper along the Caribbean Sea coast show that, once litter enters the mangrove from the sea, it tends to move landward (i.e., towards zone 4) during storms, whereas litter at the seaward edge of a mangrove forest (zone 3) eventually re-enters into a nearby beach zone [86]. The most abundant litter items in the Caribbean Sea mangrove forests were those with positive buoyant capacity (e.g., foamed plastic pieces, hard plastic pieces and caps/lids, Table 4). In mangrove forests, due to the low recorded hydrodynamic conditions linked to different factors such as the presence of pneumatophores and seedlings that attenuate wave energy and current turbulence, litter is retained for long periods of time and then buried (Table 1 and Figure 2l) [21,87,88,89,90]. Similar results were found in the Ciénaga Grande de Santa Marta by Garcés-Ordóñez et al. [26] who reported that many of the plastic items were found covering pneumatophores of A. germinans and L. racemosa. Along the study area, litter degradation was especially evident at Puerto Caimán B (Figure 1); this site was, until ca. 20 years ago, directly exposed to waves and currents [91]. Presently, the sampling site, which has a low canopy density which allows sunlight penetration, is located in a sheltered area protected by Puerto Velero sand spit which has been migrating southwestward during the past several decades [91]. Therefore, litter presently observed there was probably stranded ca. 20 years ago and showed evident deterioration due to the transport experienced and fragmentation caused by UV light [92].
At Tumaco municipality, on the Pacific Ocean coast of Colombia, sites investigated (Figure 2h–k) are located on an alluvial area showing rivers, canals, tidal creeks and coastal lagoons [93]. At places in such riverine and swamp environments, local inhabitants constructed stilt houses with poor or no solid waste management programs.
Waste materials are often discharged in riverbanks or directly in wetlands, rivers, and tidal creeks, and then transported away and dispersed along coastal environments by rivers and tidal action, which achieves certain relevance since it is a mesotidal environment.
In the case of Complejo DIMAR (site no. 8), the greatest abundance and weight of litter was observed in zone 4, followed by zones 3 and 2 (Table 2). Such behavior and trends in litter distribution are influenced by tidal processes [94]. As an example, in zone 4 (Table 2), a high presence of film plastic pieces was recorded (especially single-used plastics, Table 4) in the roots of Rhizophora sp. resulting in a ‘Christmas tree’ effect (Figure 2m). In this regard, Ivar do Sul et al. [87] established in the Goiana Estuary (Brazil) that the tide is apparently the main driving force controlling litter movement patterns in mangrove forests, while Yin et al. [48] determined in Penang Island (Malaysia) that lighter and more buoyant materials (e.g., plastic bottles and film plastic pieces) have a greater tendency to accumulate during rising tide in the mangrove forest landward edge.
Concerning litter content in mangrove forests, the results of this study report that most litter items are composed of plastic materials, representing 93.61% of the total litter composition (Table 3 and Table 4). Such results are consistent with other studies, e.g., in São Vicente Estuary (Brazil), Cordeiro et al. [24] reported high plastic content (62.81% of total amount), with bags and food wrappers being the most numerous items. Abreo et al. [95], in Pujada Bay (Philippines), reported plastic amounts of 66.79%; Luo et al. [86] in Hong Kong, values of 70.31%; Kesavan et al. [96], in Mumbai (India), reported 62.4%; and Yin et al. [48], in Penang Island (Malaysa), observed 87.2% of plastic materials. In Colombia, Garcés-Ordóñez et al. [26] in Ciénaga Grande de Santa Marta, and Riascos et al. [28], in Buenaventura bay, recorded 84.5% and 85% of plastic content. The high abundance of film and hard plastic pieces and bags observed in the studied area coincides with those reported in the mangrove forests of Ambon Island, Indonesia, by Suyadi et al. [97] who identified plastic films and bags as the most abundant items and the landward edge of the forest as the most polluted. Kesavan et al. [96] in a mangrove forest of Mumbai, found that plastic bags were the most abundant trapped litter because they are easily carried by tidal action and wind. In turn, in Tumaco, site no. 9 is located behind a rocky arch called “El Morro” (Figure 2j,k) [98], and seawater enters through this arch during the flooding tide. In this mangrove forest, the greatest abundance of litter was recorded in zone 2 (Table 2), where a large number of plastic bags and glass fragments are observed. In contrast to the “Complejo DIMAR” (site 8), tourists are present at the “El Morro” site and probably represent the main source of litter, especially of heavy glass fragments since their accumulation occurs close to the point of discharge because such items are not very susceptible to rolling [99,100]. The ‘Christmas tree’ effect was also observed in this mangrove forest [94].
The effect of plastic litter on the mangrove soil includes different aspects linked to both the impact of items on the propagules and seedlings and the physical changes in the soil layers due to litter incorporation in first horizons, which can affect natural soil aeration, water movement and regeneration [21,27]. Other common issues observed are: (a) the formation of a barrier that can hinder the development of propagules and seedlings and limits natural root growth [27], and can alter photosynthesis, causing mortality [31,32]; (b) plastic items smothering young saplings by covering and burying smaller mangrove seedlings and, due to their weight, can be transported by waves and tide where they impact on and break young branches [95]; (c) litter preventing propagules from properly landing on the water or on the soil beneath the canopy [95]; (d) litter contamination promoting microbial colonization by pathogens and generating disease outbreaks; (e) negative effects on some species that subsist in such habitats within mangroves roots (e.g., mollusks and crustaceans) [26]; and (f) plastic fragmenting due to physical and chemical factors (e.g., high temperatures and solar ultraviolet light), generating microplastics that can be ingested by species living in the mangrove forest [23,29,101].
Finally, concerning litter abundance, results observed in this study report 1.29 items/m2 (28.72 g/m2) and 1.13 items/m2 (79.41 g/m2) for the mangrove forests of the Caribbean Sea and Pacific Ocean coasts, respectively. Although such values are close to the observations of Luo et al. [86] (who reported an average amount of 1.45 items/m2 in Hong Kong), in general, results reported in this paper showed lower values than other places around the World, e.g., Riascos et al. [28] reported in Buenaventura mangrove forests an average amount of 15.03 and 3.81 items/m2 of litter in internal and external areas, respectively; in Mumbai (India), Kesavan et al. [96] estimated average values of 8.8 items/m2; and in Ambon Island (Indonesia), Suyadi [97] estimated average values of 92 items/m2.
5. Conclusions
In Colombia, despite beaches having an important economic relevance for tourism purposes, and mangrove forests constituting a strategic ecosystem for climate change adaptation, such environments are under pressure mainly due to anthropogenic activities and urbanization processes. The results of this paper show how litter, mainly linked to land based activities, reaches beaches and mangrove forests along the Caribbean Sea coast through the Magdalena River. Once litter is stranded, it accumulates and becomes trapped in mangrove forests. Beach users constitute a secondary relevant source of pollution. In the Pacific Ocean coast of Tumaco, the mismanagement of solid waste in coastal settlements is considered the main source of litter that arrives by rivers and tidal creeks at the coast and, then, because of wind, waves, and ocean currents, enters into the mangrove forests—where it accumulates and is eventually buried. The cleaning programs that are used for beaches are not applicable to a complex ecosystem such as the mangrove forest and, therefore, strategies aimed at avoiding the entering of litter into such an environments are fundamental tools to protect them. In order to decrease the arrival of litter and especially of plastic items to beaches and mangrove forests, it is therefore mandatory to enhance the appropriate solid-waste disposal systems in many areas of Colombia and to enforce specific laws to prohibit single-use plastic items. In addition, future studies on the abundance of microplastics and their effects on mangrove forests and associated ecosystems are needed due to the large amount of plastic litter items observed. Lastly, it is necessary to work hard on environmental education at all levels, especially for the inhabitants and tourists of the coastal cities and those who live along riverbanks or in stilt houses on coastal lagoons and tidal creeks, such as in the case of Tumaco on the Pacific Ocean coast.
Author Contributions
Conceptualization, H.J.B.-A., F.A.-M. and G.A.; methodology, G.A., F.A.-M. and H.J.B.-A.; sampling, H.J.B.-A., G.R.A., N.S.L., C.J.O.-S., D.A.V.D., H.S.M. and M.A.I.-N.; formal analysis, F.A.-M. and G.A.; investigation, H.J.B.-A., H.S.M., F.A.-M. and G.A.; data curation, H.J.B.-A., F.A.-M. and G.A.; writing—original draft preparation, H.J.B.-A., F.A.-M., D.A.V.D. and G.A.; writing—review and editing, H.J.B.-A., F.A.-M., C.J.O.-S., D.A.V.D. and G.A.; project administration, H.S.M. and M.A.I.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Simón Bolívar University (Barranquilla, Colombia), project number P-03010040817.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the national park staff (Vía Parque Isla Salamanca) for their support at this sampling site. This work is a contribution to the PROPLAYAS Network, the Andalusia (Spain) Re-search Group RNM-328 and the Center for Marine and Limnological Research of the Caribbean CICMAR (Barranquilla, Colombia).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orthodoxou, D.L.; Loizidou, X.I.; Baldwin, C.; Kocareis, C.; Karonias, A.; Ateş, M.A. Seasonal and geographic variations of marine litter: A comprehensive study from the island of Cyprus. Mar. Pollut. Bull. 2022, 177, 113495. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, S.; Costa, S.; Caeiro, S. Marine litter: A review of educative interventions. Mar. Pollut. Bull. 2021, 168, 112446. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.M.; Pinheiro, L.S.; Teixeira, C.E.P.; Paiva, B.P.; Fernandes, G.M.; Brandão, D.B.; Frota, F.F.; Filho, F.J.N.S.; Schettini, C.A.F. Marine debris on a tropical coastline: Abundance, predominant sources and fate in a region with multiple activities (Fortaleza, Ceará, northeastern Brazil). Waste Manag. 2020, 108, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.T.; Rangel-Buitrago, N. Marine litter: Solutions for a major environmental problem. J. Coast. Res. 2019, 35, 648–663. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; Williams, A.T.; Costa, M.F.; de Jonge, V. Curbing the inexorable rising in marine litter: An overview. Ocean Coast. Manag. 2020, 188, 105133. [Google Scholar] [CrossRef]
- Werner, S.; Stöfen, O. Marine litter. In Handbook on Marine Environment Protection; Salomon, M., Markus, T., Eds.; Springer: Cham, Switzerland, 2018; pp. 447–461. ISBN 9789944245326. [Google Scholar]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; Williams, A.T.; Neal, W.J.; Gracia, A.; Micallef, A. Litter in coastal and marine environments. Mar. Pollut. Bull. 2022, 177, 113546. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Not, C.; Cannicci, S. Mangroves as unique but understudied traps for anthropogenic marine debris: A review of present information and the way forward. Environ. Pollut. 2021, 271, 116291. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Anfuso, G.; Chacón, S.; Badillo, M.D.; Villate, D.A.; Serrano, M.C.; Sánchez, H. Eventos naturales y actuaciones antrópicas: Impactos sobre los bosques de manglar de América del Sur. Rev. Costas 2020, 2, 211–232. [Google Scholar] [CrossRef]
- Villate, D.A.; Sánchez, H.; Portz, L.; Manzolli, R.P.; Bolívar-Anillo, H.J.; Anfuso, G. Mangrove forests evolution and threats in the Caribbean sea of Colombia. Water 2020, 12, 1113. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.; Solvik, K.; Adame, M.F.; Benson, L.; Bukoski, J.J.; Carnell, P.; Cifuentes-Jara, M.; Donato, D.; et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018, 13, 055002. [Google Scholar] [CrossRef]
- Barbier, E.B. The protective service of mangrove ecosystems: A review of valuation methods. Mar. Pollut. Bull. 2016, 109, 676–681. [Google Scholar] [CrossRef]
- Polanía, J.; Urrego, L.E.; Agudelo, C.M. Recent advances in understanding Colombian mangroves. Acta Oecologica 2015, 63, 82–90. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Sánchez, H.; Fernandez, R.; Villate, D.; Anfuso, G. An Overview on mangrove forests distribution in Colombia: An ecosystem at risk. J. Aquat. Sci. Mar. Biol. 2019, 2, 16–18. [Google Scholar]
- Álvarez-León, R. Los manglares de Colombia y la recuperación de sus áreas degradadas: Revisión bibliográfica y nuevas experiencias. Madera Y Bosques 2003, 9, 3–25. [Google Scholar] [CrossRef]
- Osland, M.J.; Feher, L.C.; López-Portillo, J.; Day, R.H.; Suman, D.O.; Guzmán Menéndez, J.M.; Rivera-Monroy, V.H. Mangrove forests in a rapidly changing world: Global change impacts and conservation opportunities along the Gulf of Mexico coast. Estuar. Coast. Shelf Sci. 2018, 214, 120–140. [Google Scholar] [CrossRef]
- Sánchez, H.; Bolívar-Anillo, H.J.; Villate, D.; Escobar-Olaya, G.; Anfuso, G. Influencia de los impactos antrópicos sobre la evolución del bosque de manglar en Puerto Colombia (Mar Caribe colombiano). Rev. Latinoam. Recur. Nat. 2019, 15, 1–16. [Google Scholar]
- Palacios, M.L.; Cantera, J.R.; Peña, E.J. Carbon stocks in mangrove forests of the Colombian Pacific. Estuar. Coast. Shelf Sci. 2019, 227, 106299. [Google Scholar] [CrossRef]
- Palacios, M.L.; Cantera, J.R. Mangrove timber use as an ecosystem service in the Colombian Pacific. Hydrobiologia 2017, 803, 345–358. [Google Scholar] [CrossRef]
- Martin, C.; Almahasheer, H.; Duarte, C.M. Mangrove forests as traps for marine litter. Environ. Pollut. 2019, 247, 499–508. [Google Scholar] [CrossRef]
- Martin, C.; Baalkhuyur, F.; Valluzzi, L.; Saderne, V.; Cusack, M.; Almahasheer, H.; Krishnakumar, P.K.; Rabaoui, L.; Qurban, M.A.; Arias-Ortiz, A.; et al. Exponential increase of plastic burial in mangrove sediments as a major plastic sink. Sci. Adv. 2020, 6, eaaz5593. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Nor, N.H.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.A.M.M.; Costa, T.M. Evaluation of solid residues removed from a mangrove swamp in the São Vicente Estuary, SP, Brazil. Mar. Pollut. Bull. 2010, 60, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Nandhini, A.R.; Velayudhaperumal, P.; Sillanpää, M. Microplastics in mangroves and coral reef ecosystems: A review. Environ. Chem. Lett. 2022, 20, 397–416. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Castillo-Olaya, V.A.; Granados-Briceño, A.F.; Blandón, L.M.; Espinosa, L.F. Marine litter and microplastic pollution on mangrove soils of the Ciénaga Grande de Santa Marta, Colombian Caribbean. Mar. Pollut. Bull. 2019, 145, 455–462. [Google Scholar] [CrossRef]
- Garcés, O.; Bayona, M.R. Impactos de la contaminación por basura marina en el ecosistema de manglar de la Ciénaga Grande de Santa Marta, Caribe colombiano. Rev. Cienc. Mar. Y Costeras 2019, 11, 145–165. [Google Scholar] [CrossRef]
- Riascos, J.M.; Valencia, N.; Peña, E.J.; Cantera, J.R. Inhabiting the technosphere: The encroachment of anthropogenic marine litter in neotropical mangrove forests and its use as habitat by macrobenthic biota. Mar. Pollut. Bull. 2019, 142, 559–568. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Saldarriaga-Vélez, J.F.; Espinosa-Díaz, L.F. Marine litter pollution in mangrove forests from Providencia and Santa Catalina islands, after hurricane IOTA path in the Colombian Caribbean. Mar. Pollut. Bull. 2021, 168, 112471. [Google Scholar] [CrossRef]
- Del Castillo, L.; Álvares, R. Evaluación de suelos de manglar en dos localidades de la ensenada de Tumaco. Arqivos Cienc. Mar. 2011, 44, 12–20. [Google Scholar]
- Bernal, B.; Sidman, G.; Pearson, T. Assessment of Mangrove Ecosystems in Colombia and Their Potential for Emissions Reductions and Restoration; Winrock International: Arlington, VA, USA, 2017; pp. 1–29. [Google Scholar]
- Stronkhorst, J.; Levering, A.; Hendriksen, G.; Rangel-Buitrago, N.; Appelquist, L.R. Regional coastal erosion assessment based on global open access data: A case study for Colombia. J. Coast. Conserv. 2018, 22, 787–798. [Google Scholar] [CrossRef]
- Andrade, C. Cambios Recientes del Nivel del Mar en Colombia. In Deltas de Colombia: Morfodinámica y Vulnerabilidad Ante el Cambio Global; Restrepo, J., Ed.; EAFIT Universidad Press: Medellin, Colombia, 2008; pp. 103–122. [Google Scholar]
- Instituto de Investigaciones Marinas y Costeras (INVEMAR). Climatologie de la vitesse et la direction des vent pour le mar territoriale sous juridiction colombianne 8° a 19° N e 69° a 84° W. In Atlas ERS 1 et 2 et Quickscat, Colombie; INVEMAR: Santa Marta, Colombia, 2006. [Google Scholar]
- Osorio, A.F.; Mesa, J.C.; Bernal, G.R.; Montoya, R.D. Reconstrucción de cuarenta años de datos de oleaje en el mar Caribe colombiano empleando el modelo WWIIITM y diferentes fuentes de datos. Boletín Científico CIOH 2009, 56, 37–56. [Google Scholar] [CrossRef]
- Garcia, L. Clima marítimo, procesos de erosión/acreción y amenazas/vulnerabilidades por erosión: Caso de estudio de la barrera costera de Puerto Velero, Departamento del Atlántico. Master´s Thesis, Universidad del Norte, Barranquilla, Colombia, 2021; pp. 1–121. [Google Scholar]
- Guerrero, A.M.; Sánchez, R. Evaluación de la amenaza por tsunami en poblaciones del sur, centro y norte del litoral Pacífico colombiano. Boletín Científico CIOH 2019, 38, 15–40. [Google Scholar] [CrossRef]
- Correa, I.; Morton, R. Pacific Coast of Colombia. In Encyclopedia of the World’s Coastal Landforms; Bird, E., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 193–197. [Google Scholar]
- Tovilla, C.; Orihuela, D.E. Impacto del huracán Rosa sobre los bosques de manglar de la costa norte de Nayarit, México. Madera Y Bosques 2004, 10, 63–75. [Google Scholar] [CrossRef]
- Soto-Varela, Z.E.; Rosado-Porto, D.; Bolívar-Anillo, H.J.; González, C.P.; Pantoja, B.G.; Alvarado, D.E.; Anfuso, G. Preliminary microbiological coastal water quality determination along the department of Atlántico (Colombia): Relationships with beach characteristics. J. Mar. Sci. Eng. 2021, 9, 122. [Google Scholar] [CrossRef]
- Instituto de Investigaciones Marinas y Costeras (INVEMAR). Actualizacion y Ajuste del Diagnóstico y Zonificación de los Manglares de la Zona Costera del Departamento del Atlantico, Caribe Colombiano; INVEMAR: Santa Marta, Colombia, 2005; pp. 1–191. [Google Scholar]
- Botero, C.M.; Tamayo, D.; Zielinski, S.; Anfuso, G. Qualitative and quantitative beach cleanliness assessment to support marine litter management in tropical destinations. Water 2021, 13, 3455. [Google Scholar] [CrossRef]
- Casas-Monroy, O. Estado de los manglares en Colombia año 2000. In Informe del Estado de los Ambientes Marinos y Costeros en Colombia: Año 2000; Instituto de Investigaciones Marinas y Costeras (INVEMAR): Santa Marta, Colombia, 2000; pp. 48–68. [Google Scholar]
- Mejía-Rentería, J.C.; Castellanos-Galindo, G.A.; Cantera-Kintz, J.R.; Hamilton, S.E. A comparison of Colombian Pacific mangrove extent estimations: Implications for the conservation of a unique neotropical tidal forest. Estuar. Coast. Shelf Sci. 2018, 212, 233–240. [Google Scholar] [CrossRef]
- Garcés, O.; Espinosa, L. Contaminación por hidrocarburos en sedimentos de manglar del estuario del río Mira, Pacífico colombiano, afectados por derrames de petróleo crudo. Bull. Mar. Coast. Res. 2019, 48, 159–168. [Google Scholar]
- Rivera, A.; Salazar, J.; Indaburu, M.; Rubio, J.; Quiroz, F.; Vargas, J.; Palacios, J. Estudio de caso: Tumaco 2000-2015; USAID: Arlington, VA, USA, 2015; pp. 1–104.
- Williams, A.T.; Micallef, A. Beach Management, Principles & Practice; Earthscan: London, UK, 2009; pp. 1–480. ISBN 978-1-84407-435-8. [Google Scholar]
- YIN, C.S.; Chai, Y.J.; Carey, D.; Yusri, Y.; Barry, G.J. Anthropogenic marine debris accumulation in mangroves on Penang island, Malaysia. J. Sustain. Sci. Manag. 2020, 15, 36–60. [Google Scholar] [CrossRef]
- United Nations Enviroment Programme/Mediterranean Action Plan. Marine Litter Assessment in the Mediterranean; UNEP/MAP: Athens, Greece, 2015; pp. 1–86. [Google Scholar]
- Joint Research Centre of the European Commission. Guidance on Monitoring of Marine Litter in European Seas; JRC: Luxembourg, 2013; pp. 1–128. [Google Scholar]
- Cheshire, A.C.; Adler, E.; Barbière, J.; Cohen, Y.; Evans, S.; Jarayabhand, S.; Jeftic, L.; Jung, R.T.; Kinsey, S.; Kusui, E.; et al. UNEP/IOC Guidelines on Survey and Monitoring of Marine Litter; UNEP Regional Seas Reports and Studies, No. 186; IOC Technical Series No. 83; UNEP: Nairobi, Kenya, 2009; pp. 1–120. ISBN 978-92-807-3027-2. [Google Scholar]
- Alkalay, R.; Pasternak, G.; Zask, A. Clean-coast index-A new approach for beach cleanliness assessment. Ocean Coast. Manag. 2007, 50, 352–362. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Guo, H.; Lin, H.; Zhang, Y. A nationwide assessment of litter on China’s beaches using citizen science data. Environ. Pollut. 2020, 258, 113756. [Google Scholar] [CrossRef]
- Jonidi, A.; Latifi, P.; Kazemi, Z.; Kazemi, Z.; Morovati, M.; Farzadkia, M.; Torkashvand, J. Development a new index for littered waste assessment in different environments: A study on coastal and urban areas of northern Iran (Caspian Sea). Mar. Pollut. Bull. 2021, 171, 112684. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.; Curtis, T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; Velez, A.; Gracia, A.; Mantilla-Barbosa, E.; Arana, V.A.; Trilleras, J.; Arroyo-Olarte, H. Litter impacts on cleanliness and environmental status of Atlantico department beaches, Colombian Caribbean coast. Ocean Coast. Manag. 2019, 179, 104835. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; Williams, A.; Anfuso, G. Killing the goose with the golden eggs: Litter effects on scenic quality of the Caribbean coast of Colombia. Mar. Pollut. Bull. 2018, 127, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Espinosa, L.F.; Pereira, R.; Costa, M. The impact of tourism on marine litter pollution on Santa Marta beaches, Colombian Caribbean. Mar. Pollut. Bull. 2020, 160, 111558. [Google Scholar] [CrossRef]
- Portz, L.; Manzolli, R.P.; Villate-Daza, D.A.; Fontán-Bouzas, Á. Where does marine litter hide? The Providencia and Santa Catalina Island problem, Seaflower reserve (Colombia). Sci. Total Environ. 2022, 813, 151878. [Google Scholar] [CrossRef]
- Williams, A.T.; Rangel-Buitrago, N.G.; Anfuso, G.; Cervantes, O.; Botero, C.M. Litter impacts on scenery and tourism on the Colombian north Caribbean coast. Tour. Manag. 2016, 55, 209–224. [Google Scholar] [CrossRef]
- Portz, L.; Portantiolo, R.; Vasquez, G.; Laiton, L.; Villate, D.; Ivar do Sul, J.A. Marine litter arrived: Distribution and potential sources on an unpopulated atoll in the Seaflower biosphere reserve, Caribbean Sea. Mar. Pollut. Bull. 2020, 157, 111323. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; Gracia, A.; Velez-Mendoza, A.; Carvajal-Florián, A.; Mojica-Martinez, L.; Neal, W.J. Where did this refuse come from? Marine anthropogenic litter on a remote island of the Colombian Caribbean sea. Mar. Pollut. Bull. 2019, 149, 110611. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Espinosa, L.F.; Pereira, R.; Issa, B.B.; Meigikos, R. Plastic litter pollution along sandy beaches in the Caribbean and Pacific coast of Colombia. Environ. Pollut. 2020, 267, 115495. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Espinosa, L.F.; Costa, M.; Salles, L.B.; Meigikos, R. Abundance, distribution, and characteristics of microplastics in coastal surface waters of the Colombian Caribbean and Pacific. Environ. Sci. Pollut. Res. 2021, 28, 43431–43442. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Buitrago, N.; Williams, A.; Anfuso, G.; Arias, M.; Gracia, A. Magnitudes, sources, and management of beach litter along the Atlantico department coastline, Caribbean coast of Colombia. Ocean Coast. Manag. 2017, 138, 142–157. [Google Scholar] [CrossRef]
- Feller, I.C.; Lovelock, C.E.; Berger, U.; McKee, K.L.; Joye, S.B.; Ball, M.C. Biocomplexity in mangrove ecosystems. Ann. Rev. Mar. Sci. 2010, 2, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, J.D.; Zapata, P.; Díaz, J.M.; Garzón-Ferreira, J.; García, C.B. Fluvial fluxes into the Caribbean Sea and their impact on coastal ecosystems: The Magdalena River, Colombia. Glob. Planet. Chang. 2006, 50, 33–49. [Google Scholar] [CrossRef]
- Higgins, A.; Restrepo, J.C.; Ortiz, J.C.; Pierini, J.; Otero, L. Suspended sediment transport in the Magdalena River (Colombia, South America): Hydrologic regime, rating parameters and effective discharge variability. Int. J. Sediment Res. 2016, 31, 25–35. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; Van Der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Halsband, C.; Herzke, D. Plastic litter in the European Arctic: What do we know? Emerg. Contam. 2019, 5, 308–318. [Google Scholar] [CrossRef]
- Addamo, A.M.; Laroche, P.; Hanke, G. Top Marine Beach Litter Items in Europe; JRC: Luxembourg, 2017; pp. 1–118. ISBN 978-92-79-87711-7. [Google Scholar]
- Williams, A.T.; Randerson, P.; Di Giacomo, C.; Anfuso, G.; Macias, A.; Perales, J.A. Distribution of beach litter along the coastline of Cádiz, Spain. Mar. Pollut. Bull. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Maziane, F.; Nachite, D.; Anfuso, G. Artificial polymer materials debris characteristics along the Moroccan Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 1–7. [Google Scholar] [CrossRef]
- Asensio-Montesinos, F.; Anfuso, G.; Ramírez, M.O.; Smolka, R.; Sanabria, J.G.; Enríquez, A.F.; Arenas, P.; Bedoya, A.M. Beach litter composition and distribution on the Atlantic coast of Cádiz (SW Spain). Reg. Stud. Mar. Sci. 2020, 34, 101050. [Google Scholar] [CrossRef]
- Nachite, D.; Maziane, F.; Anfuso, G.; Williams, A.T. Spatial and temporal variations of litter at the Mediterranean beaches of Morocco mainly due to beach users. Ocean Coast. Manag. 2019, 179, 104846. [Google Scholar] [CrossRef]
- Lavers, J.L.; Dicks, L.; Dicks, M.R.; Finger, A. Significant plastic accumulation on the Cocos (Keeling) Islands, Australia. Sci. Rep. 2019, 9, 7102. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Buitrago, N.; Castro-Barros, J.D.; Gracia, A.; Villamil, J.D.; Williams, A.T. Litter impacts on beach/dune systems along the Atlantico Department, the Caribbean Coastline of Colombia. Mar. Pollut. Bull. 2018, 137, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Buitrago, N.; Velez, A.; Mantilla-Barbosa, E.; Arroyo-Olarte, H.; Arana, V.A.; Trilleras, J.; Gracia, A.; Neal, W.J.; Williams, A.T. Plastic pollution on the Colombian central Caribbean beaches. Mar. Pollut. Bull. 2021, 162, 111837. [Google Scholar] [CrossRef] [PubMed]
- Topçu, E.N.; Tonay, A.M.; Dede, A.; Öztürk, A.A.; Öztürk, B. Origin and abundance of marine litter along sandy beaches of the Turkish Western Black Sea Coast. Mar. Environ. Res. 2013, 85, 21–28. [Google Scholar] [CrossRef]
- Munari, C.; Corbau, C.; Simeoni, U.; Mistri, M. Marine litter on Mediterranean shores: Analysis of composition, spatial distribution and sources in north-western Adriatic beaches. Waste Manag. 2016, 49, 483–490. [Google Scholar] [CrossRef]
- Chitaka, T.Y.; von Blottnitz, H. Accumulation and characteristics of plastic debris along five beaches in Cape Town. Mar. Pollut. Bull. 2019, 138, 451–457. [Google Scholar] [CrossRef]
- Pasternak, G.; Zviely, D.; Ribic, C.A.; Ariel, A.; Spanier, E. Sources, composition and spatial distribution of marine debris along the Mediterranean coast of Israel. Mar. Pollut. Bull. 2017, 114, 1036–1045. [Google Scholar] [CrossRef]
- Pawar, P.R.; Shirgaonkar, S.S.; Patil, R.B. Plastic marine debris: Sources, distribution and impacts on coastal and ocean biodiversity. PENCIL Publ. Biol. Sci. 2016, 3, 40–54. [Google Scholar]
- Morales-Caselles, C.; Viejo, J.; Martí, E.; González-Fernández, D.; Pragnell-Raasch, H.; González-Gordillo, J.I.; Montero, E.; Arroyo, G.M.; Hanke, G.; Salvo, V.S.; et al. An inshore–offshore sorting system revealed from global classification of ocean litter. Nat. Sustain. 2021, 4, 484–493. [Google Scholar] [CrossRef]
- Convey, P.; Barnes, D.K.A.; Morton, A. Debris accumulation on oceanic island shores of the Scotia Arc, Antarctica. Polar Biol. 2002, 25, 612–617. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Vorsatz, L.D.; Not, C.; Cannicci, S. Landward zones of mangroves are sinks for both land and water borne anthropogenic debris. Sci. Total Environ. 2022, 818, 151809. [Google Scholar] [CrossRef] [PubMed]
- Ivar do Sul, J.A.; Costa, M.F.; Silva-Cavalcanti, J.S.; Araújo, M.C.B. Plastic debris retention and exportation by a mangrove forest patch. Mar. Pollut. Bull. 2014, 78, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Norris, B.K.; Mullarney, J.C.; Bryan, K.R.; Henderson, S.M. The effect of pneumatophore density on turbulence: A field study in a Sonneratia-dominated mangrove forest, Vietnam. Cont. Shelf Res. 2017, 147, 114–127. [Google Scholar] [CrossRef]
- Horstman, E.M.; Dohmen-Janssen, C.M.; Narra, P.M.F.; van den Berg, N.J.F.; Siemerink, M.; Hulscher, S.J.M.H. Wave attenuation in mangroves: A quantitative approach to field observations. Coast. Eng. 2014, 94, 47–62. [Google Scholar] [CrossRef]
- Hashim, A.M.; Catherine, S.M.P.; Takaijudin, H. Effectiveness of mangrove forests in surface wave attenuation: A review. Res. J. Appl. Sci. Eng. Technol. 2013, 5, 4483–4488. [Google Scholar] [CrossRef]
- Anfuso, G.; Rangel-Buitrago, N.; Correa, I. Evolution of sandspits along the Caribbean coast of Colombia: Natural and human influences. In Sand and Gravel Spits; Randazzo, G., Jackson, D.W.T., Cooper, J.A.G., Eds.; Springer: New York, NY, USA, 2015; pp. 1–19. ISBN 9783319137162. [Google Scholar]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: An insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Montagut, E.; Cabrera, E. Situación de riesgo en la ensenada de Tumaco. Boletín Científico CCCP 1997, 6, 8–28. [Google Scholar] [CrossRef]
- Williams, A.T.; Simmons, S.L. The degradation of plastic litter in rivers: Implications for beaches. J. Coast. Conserv. 1996, 2, 63–72. [Google Scholar] [CrossRef]
- Abreo, N.A.S.; Siblos, S.K.V.; Macusi, E.D. Anthropogenic marine debris (AMD) in mangrove forests of Pujada Bay, Davao Oriental, Philippines. J. Mar. Isl. Cult. 2021, 9, 38–53. [Google Scholar] [CrossRef]
- Kesavan, S.; Xavier, K.A.M.; Deshmukhe, G.; Jaiswar, A.K.; Bhusan, S.; Sukla, S.P. Anthropogenic pressure on mangrove ecosystems: Quantification and source identification of surficial and trapped debris. Sci. Total Environ. 2021, 794, 148677. [Google Scholar] [CrossRef] [PubMed]
- Suyadi; Manullang, C.Y. Distribution of plastic debris pollution and it is implications on mangrove vegetation. Mar. Pollut. Bull. 2020, 160, 111642. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Investigaciones Marinas y Costeras (INVEMAR). Propuesta de Estandarización de los Levantamientos Geomorfológicos en la Zona Costera del Caribe Colombiano; INVEMAR: Santa Marta, Colombia, 2012; pp. 1–110. [Google Scholar]
- Gerigny, O.; Brun, M.; Fabri, M.C.; Tomasino, C.; Le Moigne, M.; Jadaud, A.; Galgani, F. Seafloor litter from the continental shelf and canyons in French Mediterranean water: Distribution, typologies and trends. Mar. Pollut. Bull. 2019, 146, 653–666. [Google Scholar] [CrossRef]
- Pace, R.; Dimech, M. Litter as a source of habitat islands on deep water muddy bottoms. Rapp. Comm. Int. Mer Médit. 2007, 38, 567. [Google Scholar]
- Maghsodian, Z.; Sanati, A.M.; Tahmasebi, S.; Shahriari, M.H.; Ramavandi, B. Study of microplastics pollution in sediments and organisms in mangrove forests: A review. Environ. Res. 2022, 208, 112725. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).