Key Biofouling Organisms in Tidal Habitats Targeted by the Offshore Renewable Energy Sector in the North Atlantic Include the Massive Barnacle Chirona hameri
Abstract
:1. Introduction
1.1. Biofouling of Offshore Renewable Infrastructure
1.2. Chirona hameri
1.3. Associated Fauna
1.4. Knowledge Gaps
2. Materials and Methods
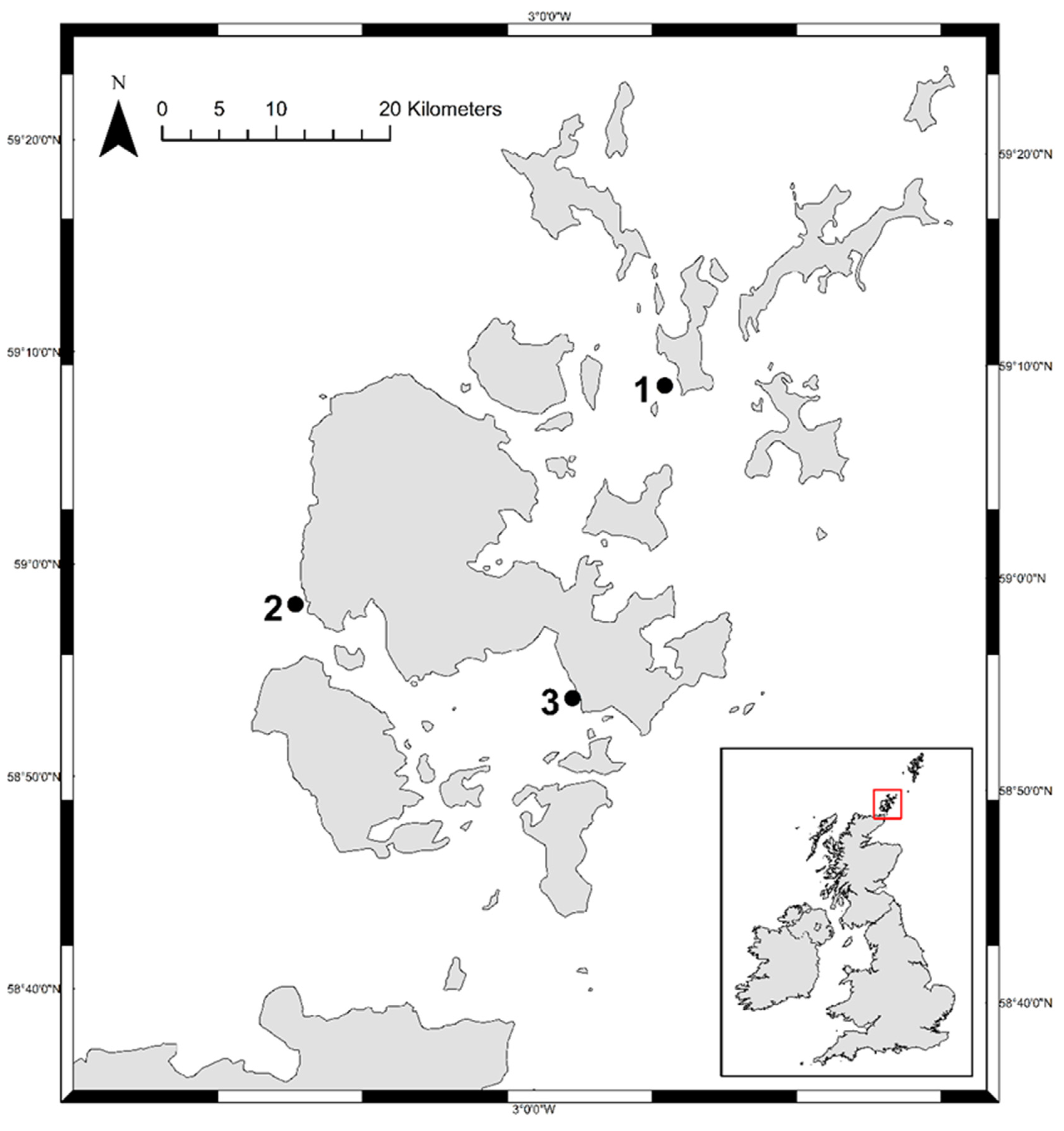
2.1. Surveys
| Site | HS (m) | Current Flow (m/s) | Water Depth (m) | Distance to Shore (km) | Nearest Port (km) |
|---|---|---|---|---|---|
| Fall of Warness | 0.9 | 2.0–4.0 | 40 | 0.58 | 6.90 |
| Billia Croo | 3.0 | 0.2–0.4 | 45 | 1.27 | 7.54 |
| Scapa Flow | 0.5 | <0.2 | 25 | 0.71 | 10.05 |
| Site | Depth | Structure | Date | Substrate | Dominant Foulant | C. ham |
|---|---|---|---|---|---|---|
| FW | 40 | TEC moorings | 01/15 | Concrete | Chirona hameri | + |
| FW | 40 | IEMP | 10/15 | HDPE | Chirona hameri | + |
| FW | 40 | IEMP | 09/17 | HDPE | Chirona hameri | + |
| FW | 40 | ADCP frame | 12/17 | HDPE/steel | Chirona hameri | + |
| FW | 3 | TEC subunit | 10/18 | Steel | Ciona intestinalis | + |
| FW | 40 | TEC subunit | 05/19 | Steel | Chirona hameri | + |
| FW | 40 | TEC moorings | 09/19 | Steel | Chirona hameri | + |
| BC | Surface | WRB | 06/15 | Steel | Alaria esculenta | - |
| BC | Surface | WRB | 02/18 | Steel | Ectopleura larynx | - |
| BC | Surface | WRB | 02/18 | Steel | Hincksia hincksia | - |
| BC | 35 | WEC moorings * | 03/18 | Fiberglass | Metridium dianthus | + |
| BC | 45 | WEC cable end | 04/18 | Steel | Chirona hameri | + |
| BC | Surface | WRB | 10/18 | Steel | Semibalanus balanoides | - |
| BC | Surface | WRB | 09/19 | Steel | Semibalanus balanoides | - |
| BC | Surface | WRB | 09/19 | Steel | Ectopleura larynx | - |
| SF | Surface | WRB | 05/15 | Steel | Amphisbetia operculata | - |
| SF | Surface | WRB | 02/18 | Steel | Petalonia fascia | - |
| SF | 25 | WEC | 06/18 | Steel | Balanus crenatus | - |
| SF | 25 | WEC moorings | 08/18 | Mixed | Balanus crenatus | - |
| SF | 25 | ADCP frame | 09/18 | HDPE/steel | Spirobranchus triqueter | - |
| SF | Surface | WRB | 10/18 | Steel | Chordaria flagelliformis | - |
| SF | Surface | WRB | 09/19 | Steel | Semibalanus balanoides | - |
2.2. Data Analysis
3. Results
3.1. Chirona hameri Dominates Biofouling Assemblages in Tidal Habitats
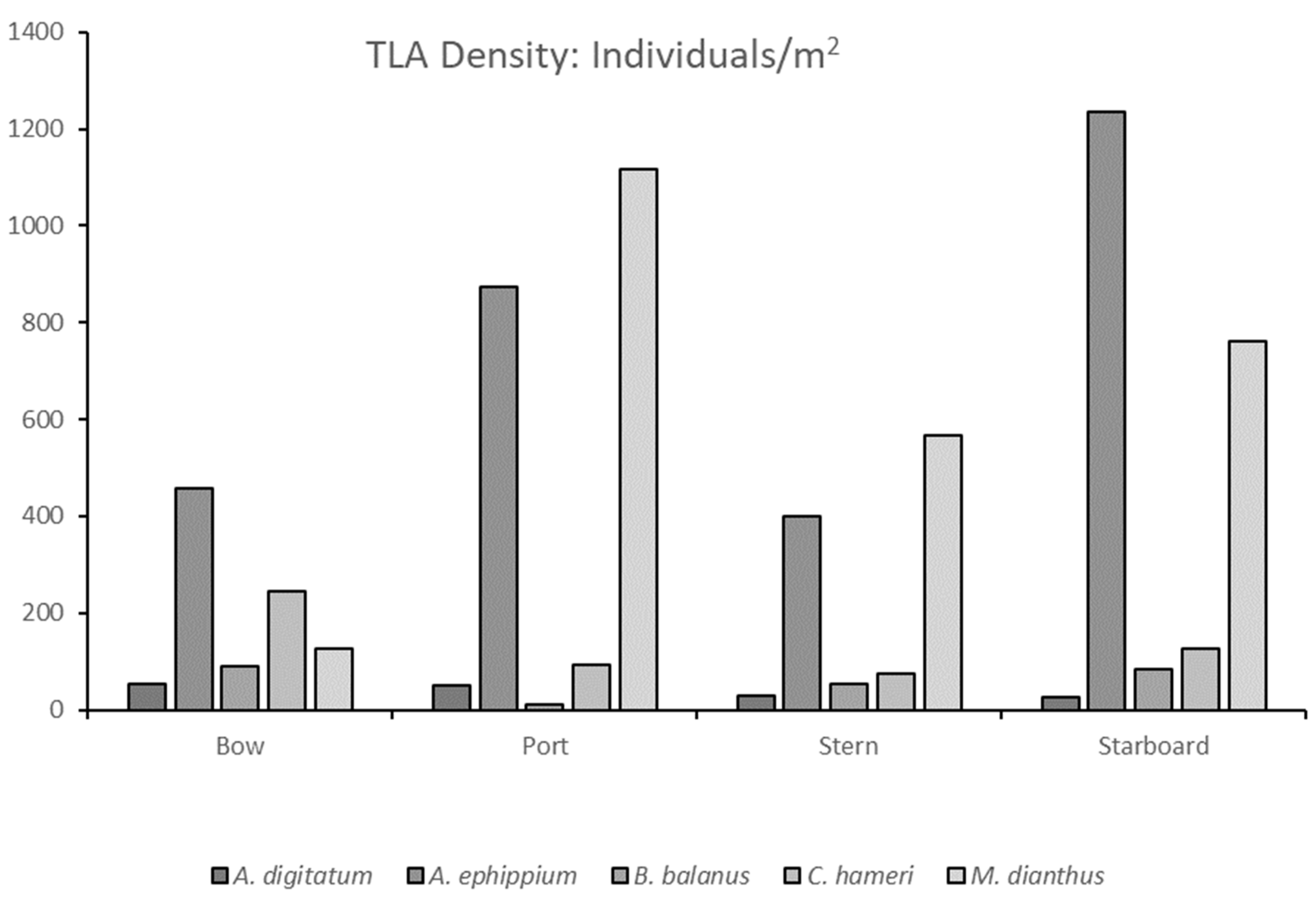
3.2. The Tether Latch Biofouling Assemblage Was Dominated by Five Organisms with Varying Abundances on Differently Orientated Vertical Faces
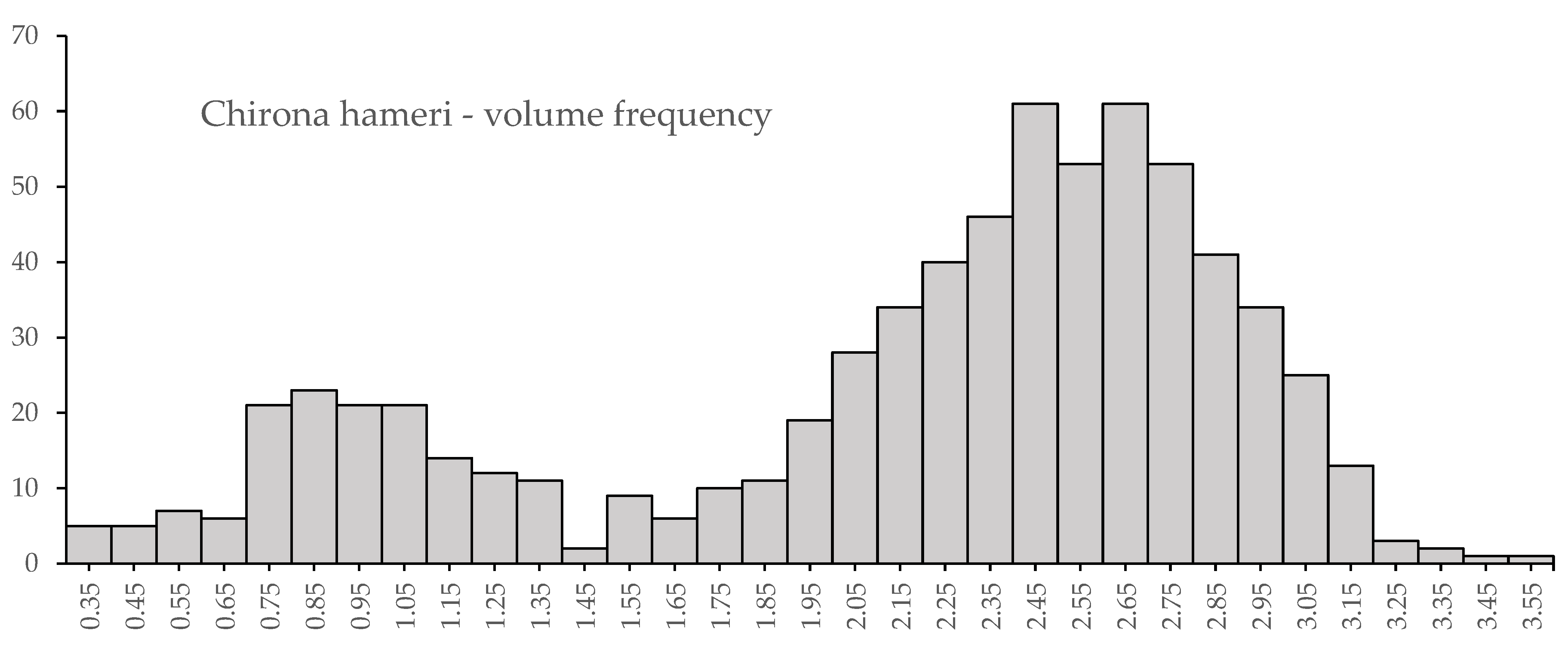
3.3. Novel Observations of Chirona hameri
4. Discussion
4.1. Fouling Assemblages in High Tidal Flow Environments
4.2. Hydrodynamic Forces and Orientation Preferences
4.3. Settlement Behaviour and Growth
4.4. Importance to the Offshore Renewable Energy Sector
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kern, F.; Rogge, K.S. The pace of governed energy transitions: Agency, international dynamics and the global Paris agreement accelerating decarbonisation processes? Energy Res. Soc. Sci. 2016, 22, 13–17. [Google Scholar] [CrossRef]
- Cooper, S.J.; Hammond, G.P. ‘Decarbonising’ UK industry: Towards a cleaner economy. Proc. Inst. Civil Eng. Energy 2018, 171, 147–157. [Google Scholar] [CrossRef]
- Scottish Government. Scottish Energy Strategy. 2023. Available online: www.gov.scot (accessed on 25 September 2023).
- Murray, R.O.H.; Gallego, A. A modelling study of the tidal stream resource of the Pentland Firth, Scotland. Renew. Energy 2017, 102, 326–340. [Google Scholar] [CrossRef]
- Offshore Wind Scotland (OWS). 2017. Available online: https://www.offshorewindscotland.org.uk/ (accessed on 25 September 2023).
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Coutts, A.; Richard, D.M.; Piola, F.; Hewitt, C.L.; Connell, S.D.; Gardner, J.P.A. Effect of vessel voyage speed on survival of biofouling organisms: Implications for translocation of non-indigenous marine species. Biofouling 2010, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Walker, J.M.; Flack, K.M.; Lust, E.E.; Schultz, M.P.; Luznik, L. Experimental and numerical studies of blade roughness and fouling on marine current turbine performance. Renew. Energy 2014, 66, 257–267. [Google Scholar] [CrossRef]
- Stringer, C.C.; Polagye, B.L. Implications of biofouling on cross-flow turbine performance. SN Appl. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Polagye, B.L.; Thomson, J. Screening for Biofouling and Corrosion of Tidal Energy Device Materials: In-Situ Results from Admiralty Inlet, Puget Sounds, Washington. Seattle (USA); National Marine Renewable Energy Center: Washington, DC, USA, 2010.
- Want, A.; Harris, R.E.; Hull, M.Q.; Long, C.L.; Porter, J.S. Sea-trial verification of a novel system for monitoring biofouling and testing anti-fouling and anti-corrosion coatings in highly energetic environments targeted by the marine renewable energy industry. Biofouling 2021, 37, 433–451. [Google Scholar] [CrossRef]
- Edyvean, R.G.J. Biodeterioration problems of North Sea Oil and gas production—A review. Int. Biodeterior. 1987, 23, 199–231. [Google Scholar] [CrossRef]
- Coutts, A.D.M.; Taylor, M.D. A preliminary investigation of biosecurity risks associated with biofouling on merchant vessels in New Zealand. New Zealand J. Mar. Freshw. Res. 2004, 38, 215–229. [Google Scholar] [CrossRef]
- Shields, M.A.; Woolf, D.K.; Grist, E.P.; Kerr, S.A.; Jackson, A.C.; Harris, R.E.; Bell, M.C.; Beharie, R.; Want, A.; Osalusi, E.; et al. Marine renewable energy: The ecological implications of altering the hydrodynamics of the marine environment. Ocean Coast. Manag. 2011, 54, 2–9. [Google Scholar] [CrossRef]
- Want, A.; Crawford, R.; Kakkonen, J.; Kiddie, G.; Miller, G.; Harris, R.E.; Porter, J.S. Biodiversity characterisation and hydrodynamic consequences of marine fouling communities on marine renewable energy infrastructure in the Orkney Islands Archipelago, Scotland, UK. Biofouling 2017, 33, 567–579. [Google Scholar] [CrossRef]
- Navarrete, S.A.; Parragué, M.; Osiadacz, N.; Rojas, F.; Bonicelli, J.; Fernández, M.; Arboleda-Baena, C.; Perez-Matus, A.; Finke, R. Abundance, composition and succession of sessile subtidal assemblages in high wave-energy environments of Central Chile: Temporal and depth variation. J. Exp. Mar. Biol. Ecol. 2019, 512, 51–62. [Google Scholar] [CrossRef]
- Nall, C.R.; Schläppy, M.; Guerin, A.J. Characterisation of the biofouling community on a floating wave energy device. Biofouling 2017, 33, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Langhamer, O.; Wilhelmsson, D.; Engström, J. Artificial reef effect and fouling impacts on offshore wave power foundations and buoys—A pilot study. Estuar. Coast. Shelf Sci. 2009, 82, 426–432. [Google Scholar] [CrossRef]
- Macleod, A.K.; Stanley, M.S.; Day, J.G.; Cook, E.J. Biofouling community composition across a range of environmental conditions and geographical locations suitable for floating marine renewable energy generation. Biofouling 2016, 32, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Nall, C.R.; Guerin, A.J.; Cook, E.J. Rapid assessment of marine non-native species in northern Scotland and a synthesis of existing Scottish records. Aquat. Invasions 2015, 10, 107–121. [Google Scholar] [CrossRef]
- Cowie, P.R. Biofouling patterns with depth. In Biofouling; Blackwell: Hoboken, NJ, USA, 2010; pp. 87–99. [Google Scholar]
- Jenkins, S.R.; Martins, G.M. Succession on hard substrata. In Biofouling; Blackwell: Hoboken, NJ, USA, 2010; pp. 60–72. [Google Scholar]
- Zullo, V.A.; Marine Flora and Fauna of the Northeastern United States. In Arthropoda: Cirripedia; 1979. Available online: https://repository.library.noaa.gov/view/noaa/4908 (accessed on 25 September 2023).
- Southward, A.J. Barnacles: Keys and Notes for the Identification of British Species (No. 57); Field Studies Council: London, UK, 2008. [Google Scholar]
- Carderelli, N.F. Barnacle cement as a dental restorative adhesive. Nat. Inst. Health Publ. 1968, 151. [Google Scholar]
- Walker, G. The histology, histochemistry and ultrastructure of the cement apparatus of three adult sessile barnacles, Elminius modestus, Balanus balanoides and Balanus hameri. Mar. Biol. 1970, 7, 239–248. [Google Scholar] [CrossRef]
- Webster, S.G. Peptidergic neurons in barnacles: An immunohistochemical study using antisera raised against crustacean neuropeptides. Biol. Bull. 1998, 195, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B. The growth rate of Balanus hameri (Ascanius). J. Mar. Biol. Assoc. 1935, 20, 57–63. [Google Scholar] [CrossRef]
- Denisenko, N.V.; Denisenko, S.G.; Lehtonen, K.K.; Andersin, A.B.; Sandler, H.R. Zoobenthos of the Cheshskaya Bay (southeastern Barents Sea): Spatial distribution and community structure in relation to environmental factors. Polar Biol. 2007, 30, 735–746. [Google Scholar] [CrossRef]
- Collie, J.S.; Hermsen, J.M.; Valentine, P.C. Recolonization of gravel habitats on Georges Bank (Northwest Atlantic). Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1847–1855. [Google Scholar] [CrossRef]
- Ferris, J.; McLellan, F.; Bastrikin, D. Preparing for facility removal—Assessment of marine growth. In Proceedings of the ASRANet International Conference on DecFommissioning of Offshore & Subsea Structures, Glasgow, Scotland, 30–31 March 2015. [Google Scholar]
- Rees, I. (Bangor University, UK). Personal communications on Chirona hameri. 2019. [Google Scholar]
- Crisp, D.J. The larval stages of Balanus hameri (Ascanius, 1767). Crustaceana 1962, 4, 123–130. [Google Scholar] [CrossRef]
- Berg, C.J.; Early, J.A.; Butman, B.; Turner, R.D. Seasonal recruitment of marine invertebrates to hard substrates on Georges Bank and the eastern continental shelf of the United States. Nautilus 1987, 101, 19–24. [Google Scholar]
- Gage, J.D.; Roberts, J.M.; Hartley, J.P.; Humphery, J.D. Potential Impacts of Deep-Sea Trawling on the Benthic Ecosystem along the Northern European Continental Margin: A Review. Am. Fish. Soc. Symp. 2005, 41, 503–517. [Google Scholar]
- Kolbasov, G.A. Subclass Cirripedia (Superorder Thoracica). In Invertebrates of Eurasian Arctic Seas and Adjacent Deep Waters; University of Alaska: Fairbanks, AK, USA, 2009; p. 49. [Google Scholar]
- Southgate, T.; Myers, A.A. Mussel fouling on the Celtic Sea Kinsale field gas platforms. Estuar. Coast. Shelf Sci. 1985, 20, 651–659. [Google Scholar] [CrossRef]
- Kerckhof, F. Barnacles (Cirripedia, Balanomorpha) in Belgian waters, an overview of the species and recent evolutions, with emphasis on exotic species. Bull. Kon. Belg. Inst. Natuurwet. Biol. 2002, 72 (Suppl. S93), 93–104. [Google Scholar]
- Pilsbry, H.A. The sessile barnacles (Cirripedia) contained in the collections of the U.S. National Museum; including a monograph of the American species. Bull. United States Natl. Mus. 1916, 93, 1–366. [Google Scholar]
- Brunel, P.; Bossé, L.; Lamarche, G. Catalogue des invertébrés marins de l’estuaire et du golfe du Saint-Laurent. Spec. Publ. Fish. Aquat. Sci. 1998, 126, 405. [Google Scholar]
- Walker, G. The biochemical composition of the cement of two barnacle species, Balanus hameri and Balanus crenatus. J. Mar. Biol. Assoc. United Kingd. 1972, 52, 429–435. [Google Scholar] [CrossRef]
- Forteath, G.N.R.; Picken, B.; Ralph, R.; Williams, J. Marine Growth Studies on the North Sea Oil. Mar. Ecol. Prog. Ser. 1982, 8, 61–68. [Google Scholar] [CrossRef]
- Sousa, W.P. Disturbance in marine intertidal boulder fields: The nonequilibrium maintenance of species diversity. Ecology 1979, 60, 1225–1239. [Google Scholar] [CrossRef]
- Austen, M.C.; Lambshead, P.J.; Hutchings, P.A.; Boucher, G.; Snelgrove, P.V.; Heip, C.; King, G.; Koike, I.; Smith, C. Biodiversity links above and below the marine sediment–water interface that may influence community stability. Biodivers. Conserv. 2002, 11, 113–136. [Google Scholar] [CrossRef]
- Orbital Marine Power (OMP). 2023. Available online: https://orbitalmarine.com/technology-development/orbital-o2 (accessed on 25 September 2023).
- Simec Atlantis Energy (SAE). 2023. Available online: https://simecatlantis.com/projects/meygen/ (accessed on 25 September 2023).
- Hallam, M.G.; Heaf, N.J.; Wooton, L.R. Dynamics of Marine Structures: Methods of Calculating the Dynamic Response of Fixed Structures Subject to Wave and Current Action; CIRIA Underwater Engineering Group: London, UK, 1978. [Google Scholar]
- Yang, S.H.; Ringsberg, J.W.; Johnson, E.; Hu, Z. Biofouling on mooring lines and power cables used in wave energy converter systems—Analysis of fatigue life and energy performance. Appl. Ocean Res. 2017, 65, 166–177. [Google Scholar] [CrossRef]
- Ashton, G.V.; Boos, K.; Shucksmith, R.; Cook, E.J. Rapid assessment of the distribution of marine non-native species in marinas in Scotland. Aquat. Invasions 2006, 1, 209–213. [Google Scholar] [CrossRef]
- Quoceant Ltd. Mooring & Connections: Overview, Description & Functional Specifications. Wave Energy Scotl. Libr. 2016, 81, 62. [Google Scholar]
- European Marine Energy Centre (EMEC). 2023. Available online: www.emec.org.uk (accessed on 25 September 2023).
- Boland, J.M. The horizontal zonation of two species of intertidal barnacle in South Africa. South Afr. J. Mar. Sci. 1997, 18, 49–61. [Google Scholar] [CrossRef]
- Mangiafico, S.S.; Summary and Analysis of Extension. Program Evaluation in R, Version, 1. 2016. Available online: http://rcompanion.org/handbook/ (accessed on 25 September 2023).
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; p. 192. [Google Scholar]
- Digby, P.G.N.; Kempton, R.A. Multivariate Analysis of Ecological Communities; Chapman and Hall: London, UK, 1987. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, C.; Atlar, M.; Callow, M.E.; Candries, M.; Milne, A.; Townsin, R.L. The development of foul release coatings for sea going vessels. Proc. Inst. Mar. Eng. Sci. Tech. B J. Mar. Des. Oper. 2003, 4, 11–23. [Google Scholar]
- Barnes, H.; Powell, H.T. The development, general morphology and subsequent elimination of barnacle populations, Balanus crenatus and B. balanoides, after a heavy initial settlement. J. Anim. Ecol. 1950, 19, 175–179. [Google Scholar] [CrossRef]
- Crisp, D.J. Territorial behaviour in barnacle settlement. J. Exp. Biol. 1961, 38, 429–446. [Google Scholar] [CrossRef]
- Bertness, M.D.; Gaines, S.D.; Yeh, S.M. Making mountains out of barnacles: The dynamics of acorn barnacle hummocking. Ecology 1998, 79, 1382–1394. [Google Scholar] [CrossRef]
- Larsson, A.I.; Jonsson, P.R. Barnacle larvae actively select flow environments supporting post-settlement growth and survival. Ecology 2006, 87, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Viola, S.M.; Page, H.M.; Zaleski, S.F.; Miller, R.J.; Doheny, B.; Dugan, J.E.; Schroeder, D.M.; Schroeter, S.C. Anthropogenic disturbance facilitates a non-native species on offshore oil platforms. J. Appl. Ecol. 2018, 55, 1583–1593. [Google Scholar] [CrossRef]
- Langhamer, O. Artificial reef effect in relation to offshore renewable energy conversion: State of the art. Sci. World J. 2012, 2012, 386713. [Google Scholar] [CrossRef]
- Firth, L.B.; Browne, K.A.; Knights, A.M.; Hawkins, S.J.; Nash, R. Eco-engineered rock pools: A concrete solution to biodiversity loss and urban sprawl in the marine environment. Environ. Res. Lett. 2016, 11, 094015. [Google Scholar] [CrossRef]
- Kakkonen, J.E.; Worsfold, T.M.; Ashelby, C.W.; Taylor, A.; Beaton, K. The value of regular monitoring and diverse sampling techniques to assess aquatic non-native species: A case study from Orkney. Manag. Biol. Invasions 2019, 10, 46–79. [Google Scholar] [CrossRef]
- Want, A.; Kakkonen, J.E. A new range-extending record of the invasive sea squirt Styela clava in the north of Scotland. Mar. Biodivers. Rec. 2021, 14, 1–5. [Google Scholar]
- Shanks, A.L.; Wright, W.G. Adding teeth to wave action: The destructive effects of wave-borne rocks on intertidal organisms. Oecologia 1986, 69, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.W. Life in the maelstrom: The biomechanics of wave-swept rocky shores. Trends Ecol. Evol. 1987, 2, 61–66. [Google Scholar] [CrossRef]
- Gaylord, B. Biological implications of surf-zone flow complexity. Limnol. Oceanogr. 2000, 45, 174–188. [Google Scholar] [CrossRef]
- Schultz, M.P.; Kavanagh, C.J.; Swain, G.W. Hydrodynamic forces on barnacles: Implications on detachment from fouling-release surfaces. Biofouling 1999, 13, 323–335. [Google Scholar] [CrossRef]
- Orme, J.A.C.; Masters, I.; Griffiths, R.T. Investigation of the effect of biofouling on the efficiency of marine current turbines. In Proceedings of the MAREC 2001, International Conference on Marine Renewable Energies, Blyth, UK, 7–9 July 2001; pp. 91–99. [Google Scholar]
- Menge, B.A.; Lubchenco, J.; Bracken, M.E.S.; Chan, F.; Foley, M.M.; Freidenburg, T.L.; Gaines, S.D.; Hudson, G.; Krenz, C.; Leslie, H.; et al. Coastal oceanography sets the pace of rocky intertidal community dynamics. Proc. Natl. Acad. Sci. USA 2003, 100, 12229–12234. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.D.; Piola, R.F.; Taylor, M.D.; Hewitt, C.L.; Gardner, J.P. The effect of vessel speed on the survivorship of biofouling organisms at different hull locations. Biofouling 2010, 26, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Dalley, R.; Crisp, D.J. Conchoderma: A fouling hazard to ships underway. Mar. Biol. Lett. 1981, 2, 141–152. [Google Scholar]
- Crisp, D.J.; Stubbings, H.G. The orientation of barnacles to water currents. J. Anim. Ecol. 1957, 26, 179–196. [Google Scholar] [CrossRef]
- Wethey, D.S. Sun and shade mediate competition in the barnacles Chthamalus and Semibalanus: A field experiment. Biol. Bull. 1984, 167, 176–185. [Google Scholar] [CrossRef]
- Glasby, T.M.; Connell, S.D. Orientation and position of substrate have large effects on epibiotic assemblages. Mar. Ecol. Prog. Ser. 2001, 214, 127–135. [Google Scholar] [CrossRef]
- Lewis, J.R. The Ecology of Rocky Shores; Hodder & Stoughton: London, UK, 1964. [Google Scholar]
- Blanchette, C.A.; Thornber, C.; Gaines, S. Effects of wave exposure on intertidal fucoid algae. Proc. Calif. Isl. Symp. 2000, 5, 347–355. [Google Scholar]
- Prendergast, G.S. Settlement and behaviour of marine fouling organisms. In Biofouling; Blackwell: Hoboken, NJ, USA, 2010; pp. 30–59. [Google Scholar]
- Want, A.; Porter, J. BioFREE: An international study of biofouling impacts on the marine renewable energy industry. In Proceedings of the 2018 OCEANS-MTS/IEEE Kobe Techno-Oceans (OTO), Kobe, Japan, 28–31 May 2018; pp. 1–7. [Google Scholar]
- Knight-Jones, E.W.; Crisp, D.J. Gregariousness in barnacles in relation to the fouling of ships and to anti-fouling research. Nature 1953, 171, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.; Hawkins, S.J.; Doncaster, C.P. Population consequences of mutual attraction between settling and adult barnacles. J. Anim. Ecol. 2003, 72, 941–952. [Google Scholar] [CrossRef]
- Gascoigne, J.; Lipcius, R.N. Allee effects in marine systems. Mar. Ecol. Prog. Ser. 2004, 269, 49–59. [Google Scholar] [CrossRef]
- Connell, J.H. The consequences of variation in initial settlement vs. post-settlement mortality in rocky intertidal communities. J. Exp. Mar. Biol. Ecol. 1985, 93, 11–45. [Google Scholar] [CrossRef]
- Roughgarden, J.; Iwasa, Y.; Baxter, C. Demographic theory for an open marine population with space-limited recruitment. Ecology 1985, 66, 54–67. [Google Scholar] [CrossRef]
- Jenkins, S.R. Larval habitat selection, not larval supply, determines settlement patterns and adult distribution in two chthamalid barnacles. J. Anim. Ecol. 2005, 74, 893–904. [Google Scholar] [CrossRef]
- Page, H.M. Differences in population structure and growth rate of the stalked barnacle Pollicipes polymerus between a rocky headland and an offshore oil platform. Mar. Ecol. Prog. Ser. 1986, 29, 157–164. [Google Scholar] [CrossRef]
- Denny, M.W. Wave forces on intertidal organisms: A case study. Limnol. Oceanogr. 1985, 30, 1171–1187. [Google Scholar] [CrossRef]
- Bassindale, R. British Barnacles. Synopses of the British Fauna; No. 14; Linnean Society: London, UK, 1964. [Google Scholar]
- López, D. Giant barnacle “picoroco” culture in Chile. Pp. 56–57 in: Pham, C.K., R.M.; Higgins, M. 55 De Girolamo & E. Isidro (Eds). Abstract Proceedings of the International Workshop: Developing a Sustainable Aquaculture Industry in the Azores. Arquipélago. Life Mar. Sci. 2008, 13 (Suppl. S7), 81. [Google Scholar]
- Pham, C.K.; Girolamo, M.D.; Isidro, E. Recruitment and Growth of Megabalanus azoricus (Pilsbry, 1916) on Artificial Substrates: First Steps Towards Commercial Culture in the Azores; Universidade dos Azores: Ponta Delgada, Portugal, 2011. [Google Scholar]
- Marshall, D.J.; Keough, M.J. Variable effects of larval size on post-metamorphic performance in the field. Mar. Ecol. Prog. Ser. 2004, 279, 73–80. [Google Scholar] [CrossRef]
- Wernberg, T.; Thomsen, M.S. The effect of wave exposure on the morphology of Ecklonia radiata. Aquat. Bot. 2005, 83, 61–70. [Google Scholar] [CrossRef]
- Swain, G.; Anil, A.C.; Baier, R.E.; Chia, F.S.; Conte, E.; Cook, A.; Hadfield, M.; Haslbeck, E.; Holm, E.; Kavanagh, C.; et al. Biofouling and barnacle adhesion data for fouling-release coatings subjected to static immersion at seven marine sites. Biofouling 2000, 16, 331–344. [Google Scholar] [CrossRef]
- Kiil, S.; Dam-Johansen, K.; Weinell, C.E.; Pedersen, M.S.; Codolar, S.A. Dynamic simulations of a self-polishing antifouling paint exposed to seawater. J. Coat. Technol. 2002, 74, 45–54. [Google Scholar] [CrossRef]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J.; Anderson, M.J. Seasonal and temporal aspects of recruitment and succession in an intertidal estuarine fouling assemblage. J. Mar. Biol. Assoc. United Kingd. 1994, 74, 563–584. [Google Scholar] [CrossRef]
- Bram, J.B.; Page, H.M.; Dugan, J.E. Spatial and temporal variability in early successional patterns of an invertebrate assemblage at an offshore oil platform. J. Exp. Mar. Biol. Ecol. 2005, 317, 223–237. [Google Scholar] [CrossRef]


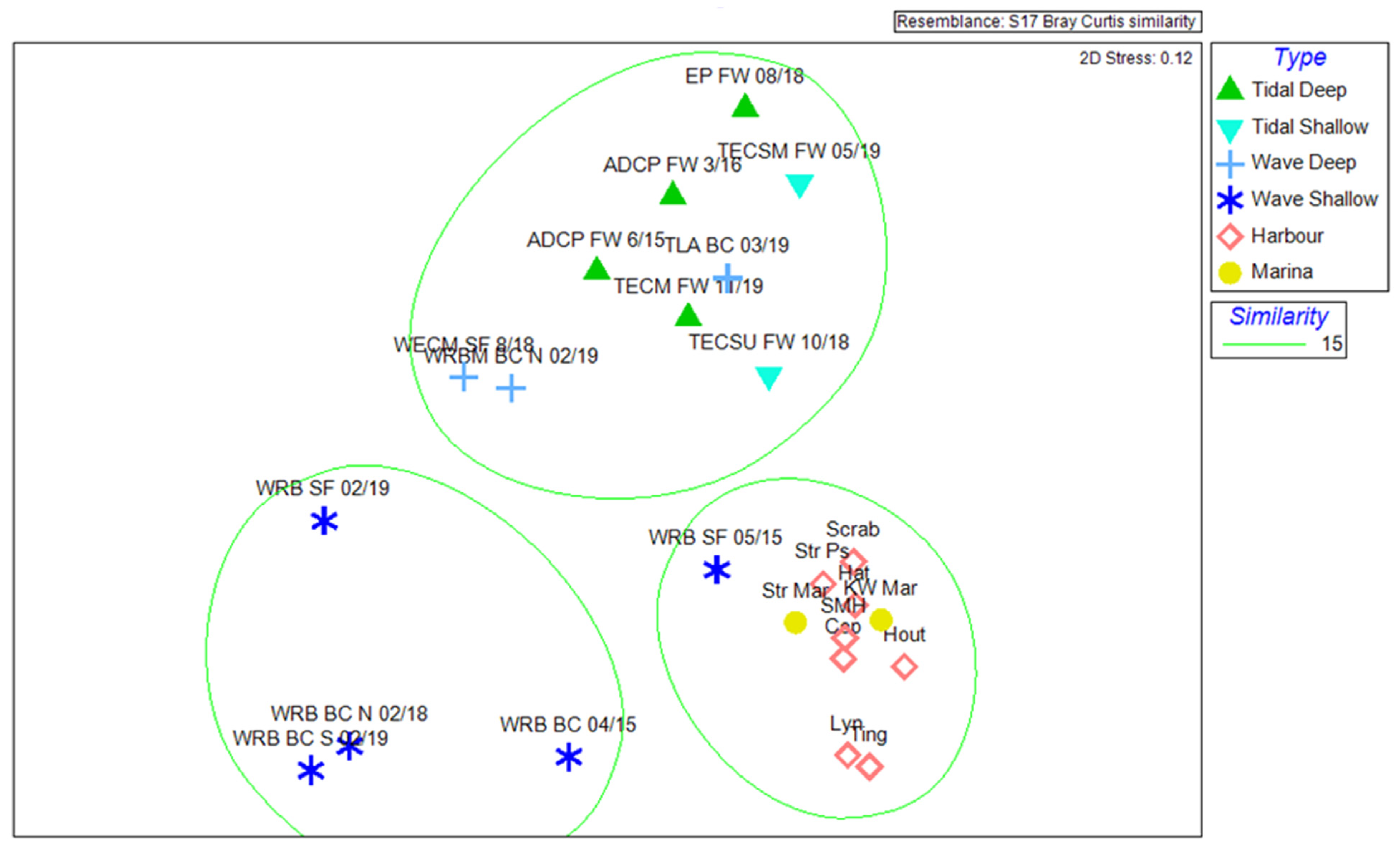
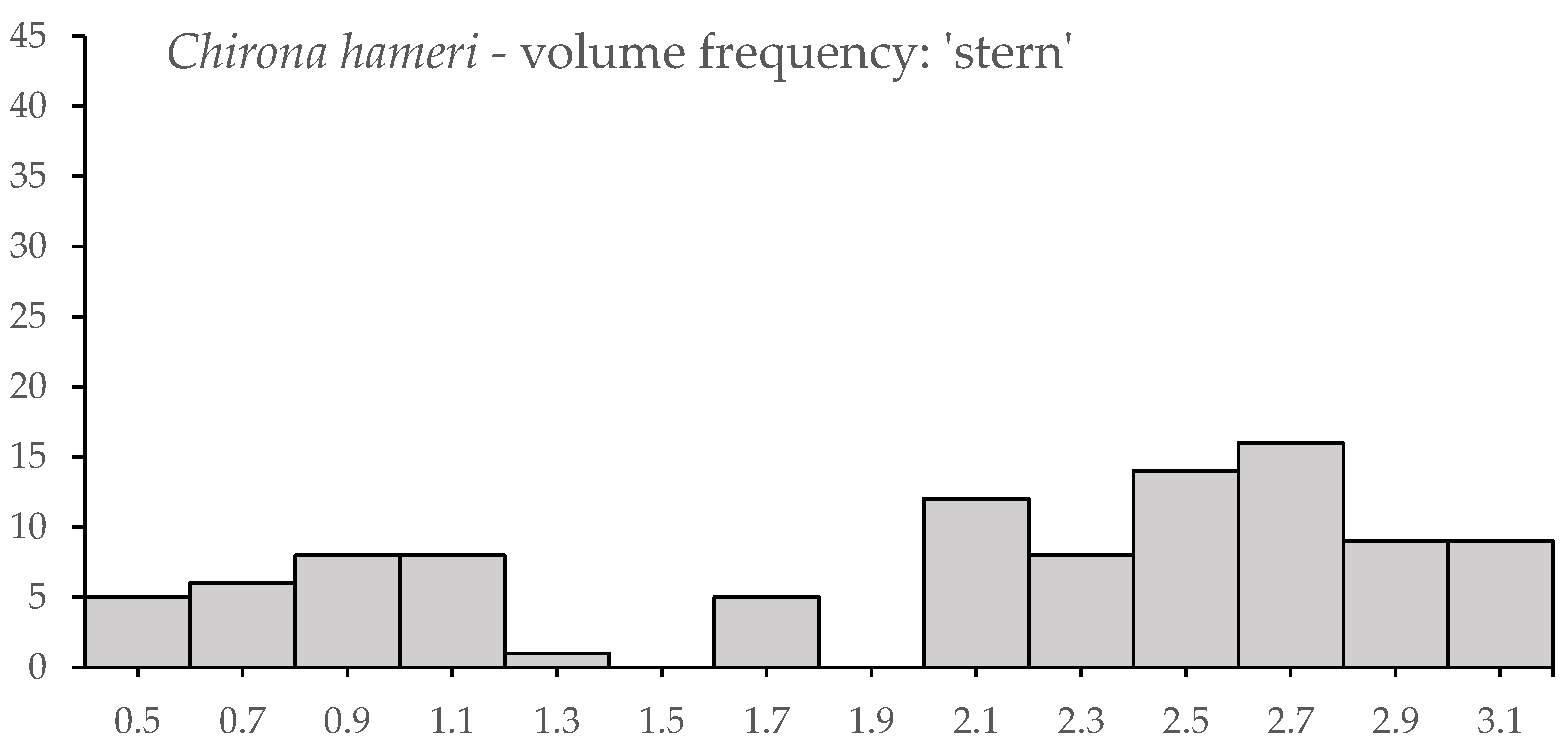
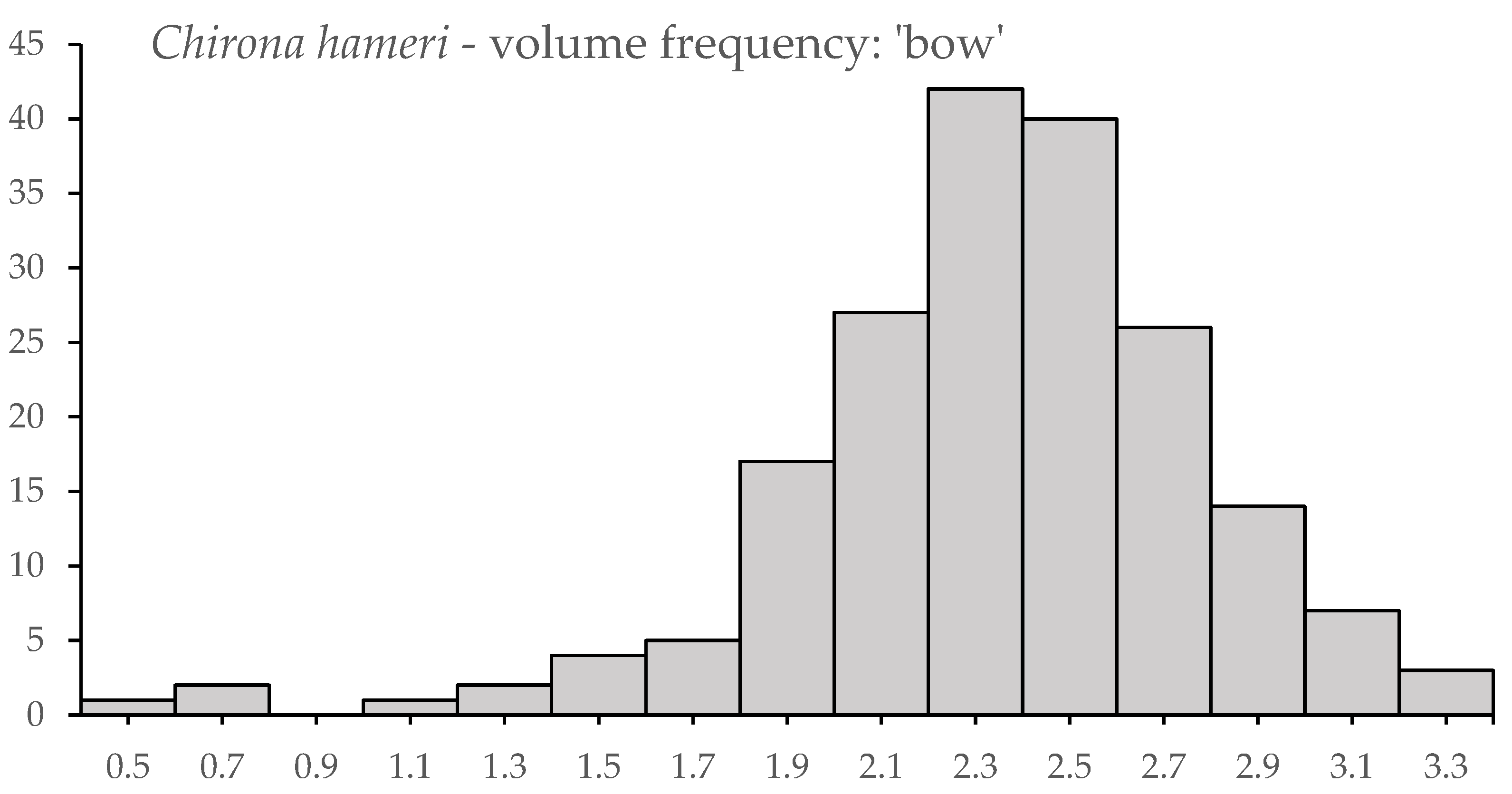

| Tidal Deep | Tidal Shallow | Wave Deep | Wave Shallow | Harbour | |
|---|---|---|---|---|---|
| Tidal Shallow | 0.48 (0.133) | ||||
| Wave Deep | 0.57 (0.029) | 0.21 (0.300) | |||
| Wave Shallow | 0.85 (0.008) | 0.69 (0.048) | 0.33 (0.125) | ||
| Harbour | 1.00 (0.006) | 0.95 (0.022) | 1.00 (0.006) | 0.81 (0.002) | |
| Marina | 1.00 (0.067) | 0.75 (0.333) | 1.00 (0.100) | 0.47 (0.143) | 0.22 (0.133) |
| Species | Type | Species | Type |
|---|---|---|---|
| Alcyonium digitatum | Dead man’s fingers | Electra pilosa | An encrusting bryozoan |
| Amphisbetia operculata | A hydroid | Metridium dianthus | Plumose anemone |
| Anomia epihippium | Saddle oyster | Mytilus edulis | Common mussel |
| Balanus balanus | An acorn barnacle | Omalosecosa ramulosa | An encrusting bryozoan |
| Caryophyllia smithii | Devonshire cup coral | Ophiothrix fragilis | Common brittlestar |
| Cellepora pumicosa | An encrusting bryozoan | Spirobranchus triqueter | Tube worm |
| Chirona hameri | An acorn barnacle | Scruparia chelata | An encrusting bryozoan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Want, A.; Goubard, A.; Jonveaux, S.; Leaver, D.; Bell, M.C. Key Biofouling Organisms in Tidal Habitats Targeted by the Offshore Renewable Energy Sector in the North Atlantic Include the Massive Barnacle Chirona hameri. J. Mar. Sci. Eng. 2023, 11, 2168. https://doi.org/10.3390/jmse11112168
Want A, Goubard A, Jonveaux S, Leaver D, Bell MC. Key Biofouling Organisms in Tidal Habitats Targeted by the Offshore Renewable Energy Sector in the North Atlantic Include the Massive Barnacle Chirona hameri. Journal of Marine Science and Engineering. 2023; 11(11):2168. https://doi.org/10.3390/jmse11112168
Chicago/Turabian StyleWant, Andrew, Audrey Goubard, Solène Jonveaux, Donald Leaver, and Michael C. Bell. 2023. "Key Biofouling Organisms in Tidal Habitats Targeted by the Offshore Renewable Energy Sector in the North Atlantic Include the Massive Barnacle Chirona hameri" Journal of Marine Science and Engineering 11, no. 11: 2168. https://doi.org/10.3390/jmse11112168
APA StyleWant, A., Goubard, A., Jonveaux, S., Leaver, D., & Bell, M. C. (2023). Key Biofouling Organisms in Tidal Habitats Targeted by the Offshore Renewable Energy Sector in the North Atlantic Include the Massive Barnacle Chirona hameri. Journal of Marine Science and Engineering, 11(11), 2168. https://doi.org/10.3390/jmse11112168






