Establishment and Optimization of an Aggregate Culture System of Testicular Cells from Marine Medaka, Oryzias dancena
Abstract
:1. Introduction
- We first attempted to find an efficient way to prepare the dispersed testicular cells, from which sperm cells were removed.
- Then, we evaluated their self-aggregation under the following three methods: (1) Matrigel suspension, (2) 3-LGS, and (3) culturing into ULA.
- Subsequently, the effects of media composition on size and number of testicular aggregates were investigated to find optimal culture conditions.
- Finally, we tested if this culture condition is able to produce fertilizable sperm from the cultured testicular aggregates.
2. Materials and Methods
2.1. Fish
2.2. Cell Culture Media
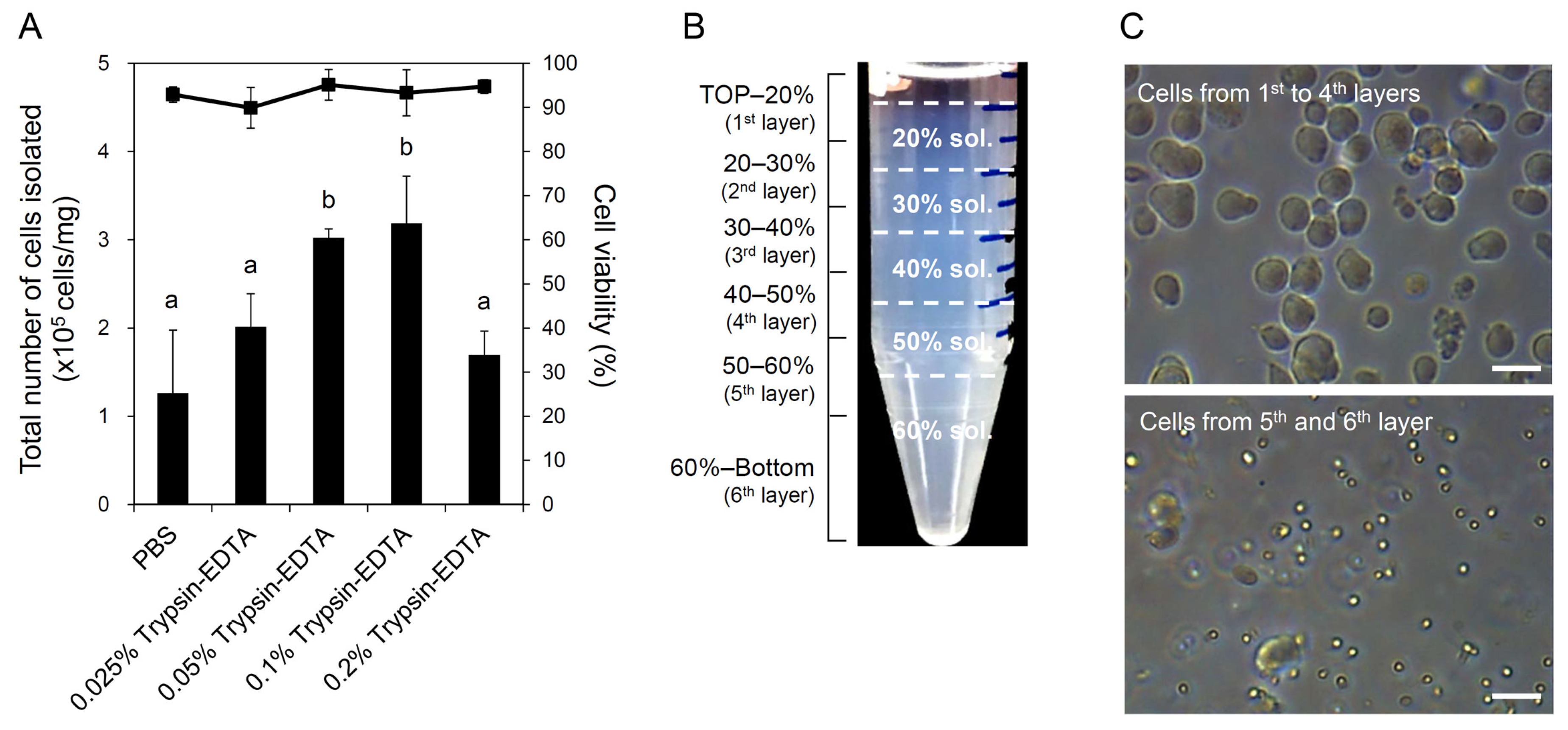
2.3. Cell Isolation and Percoll Density Gradient Centrifugation
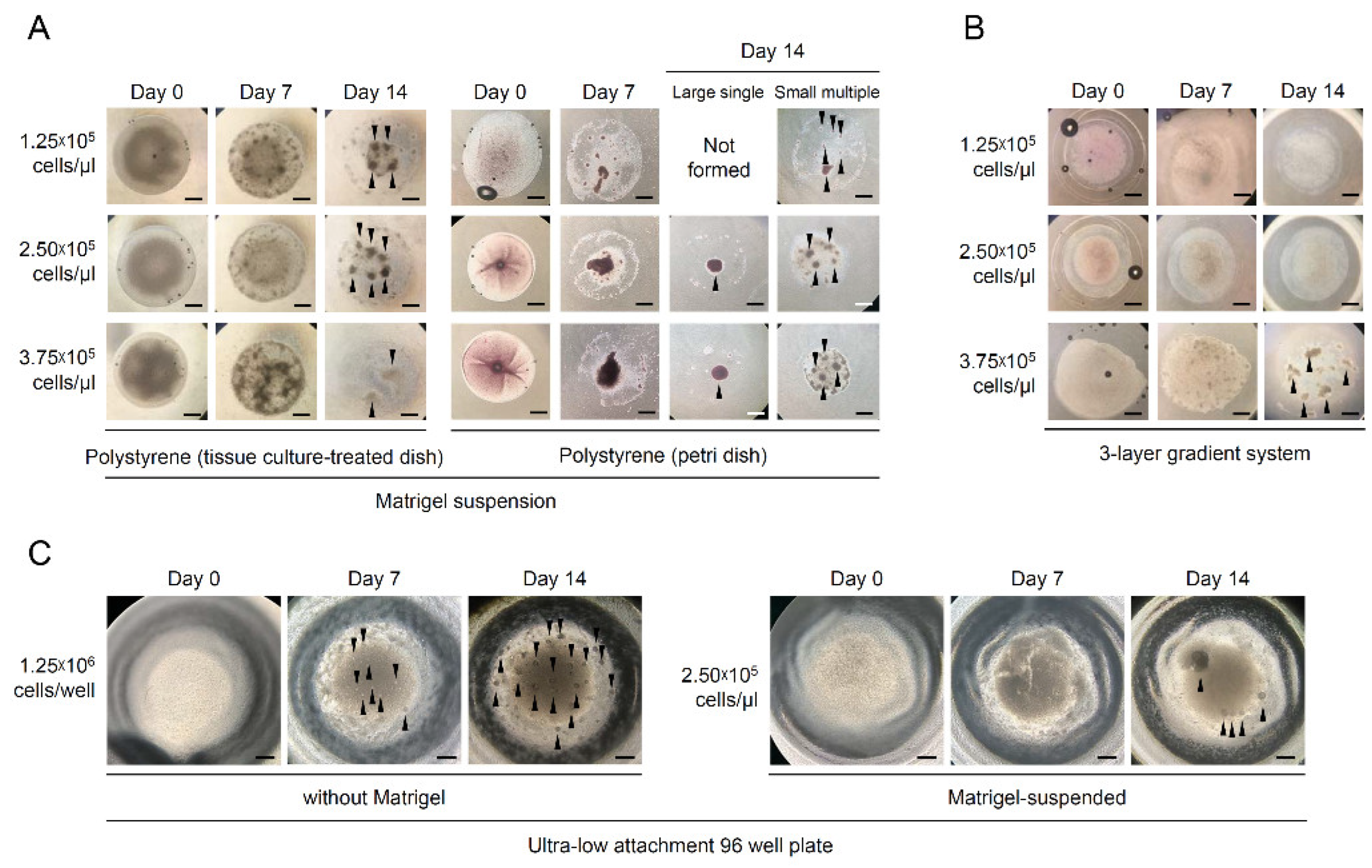
2.4. Induction of Testicular Cell Aggregates
2.5. Measurement of Number, Size, and Viability of Testicular Aggregates
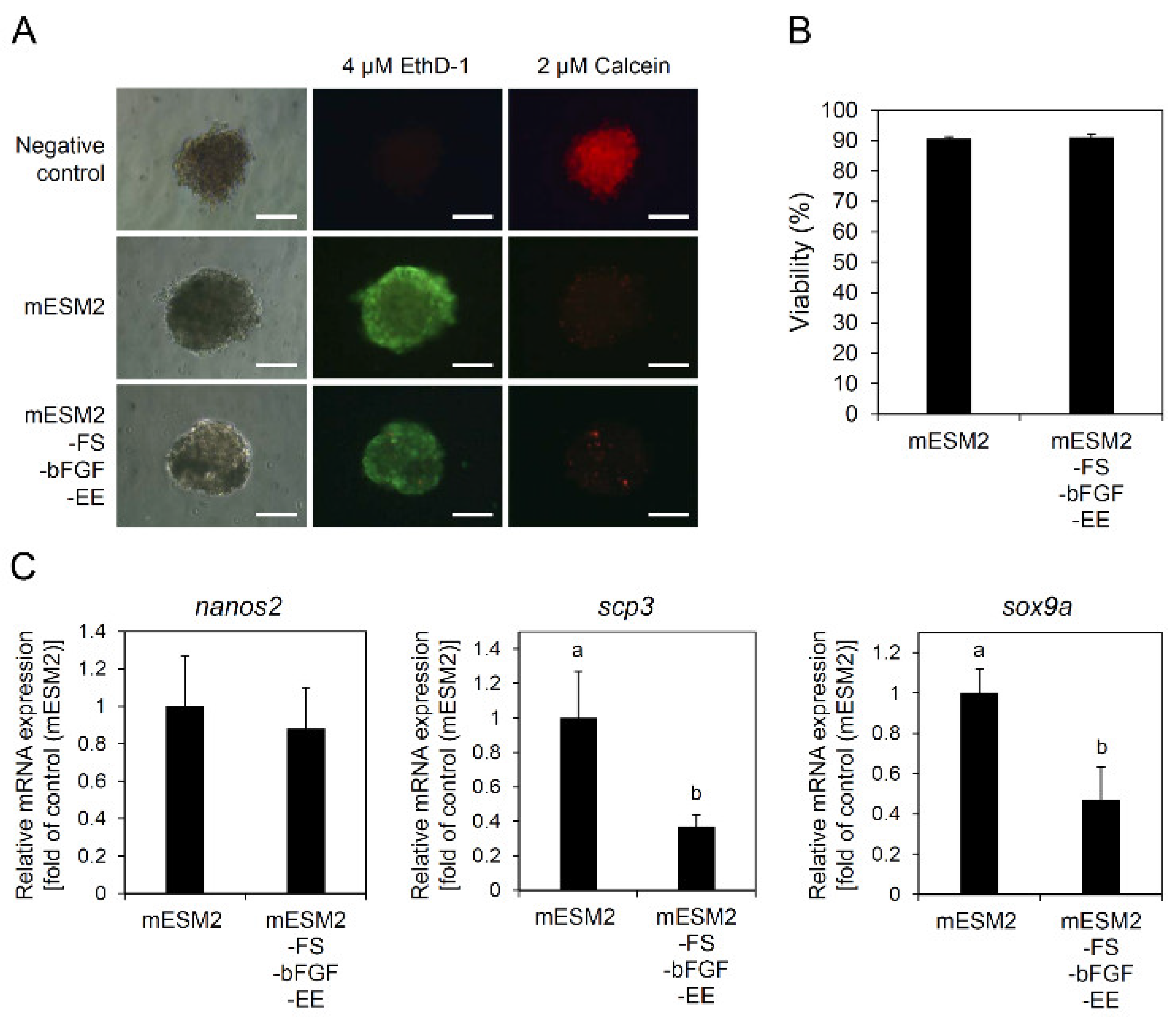
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Arifical Fertilization
2.8. Statistical Analysis
3. Results
3.1. Testicular Cell Isolation and Removal of Sperm Cells
3.2. Effects of Culture Methods on the Formation of O. dancena Testicular Aggregates
3.3. Effects of Media Types on the Number and Size of Testicular Aggregates Formed
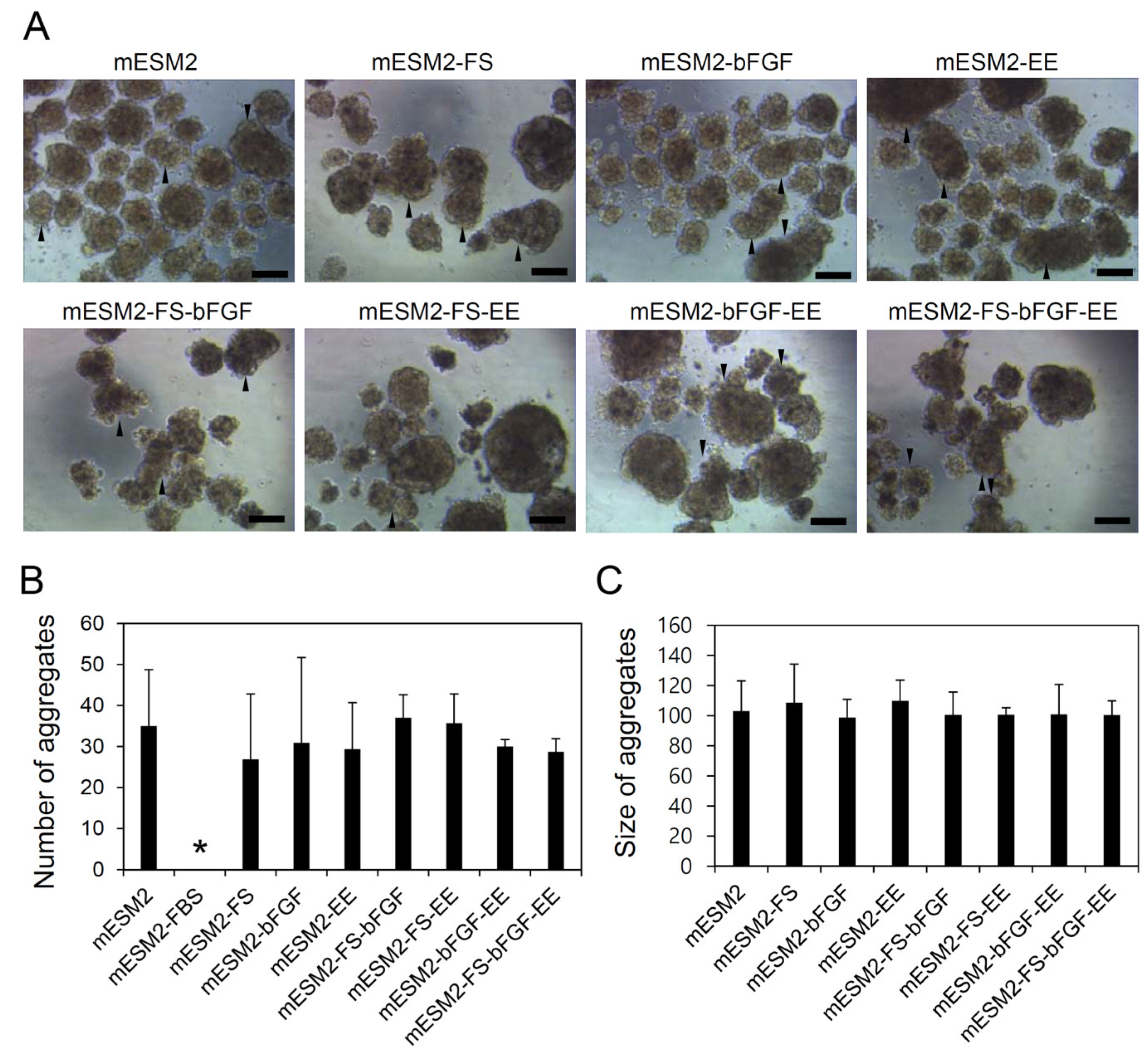
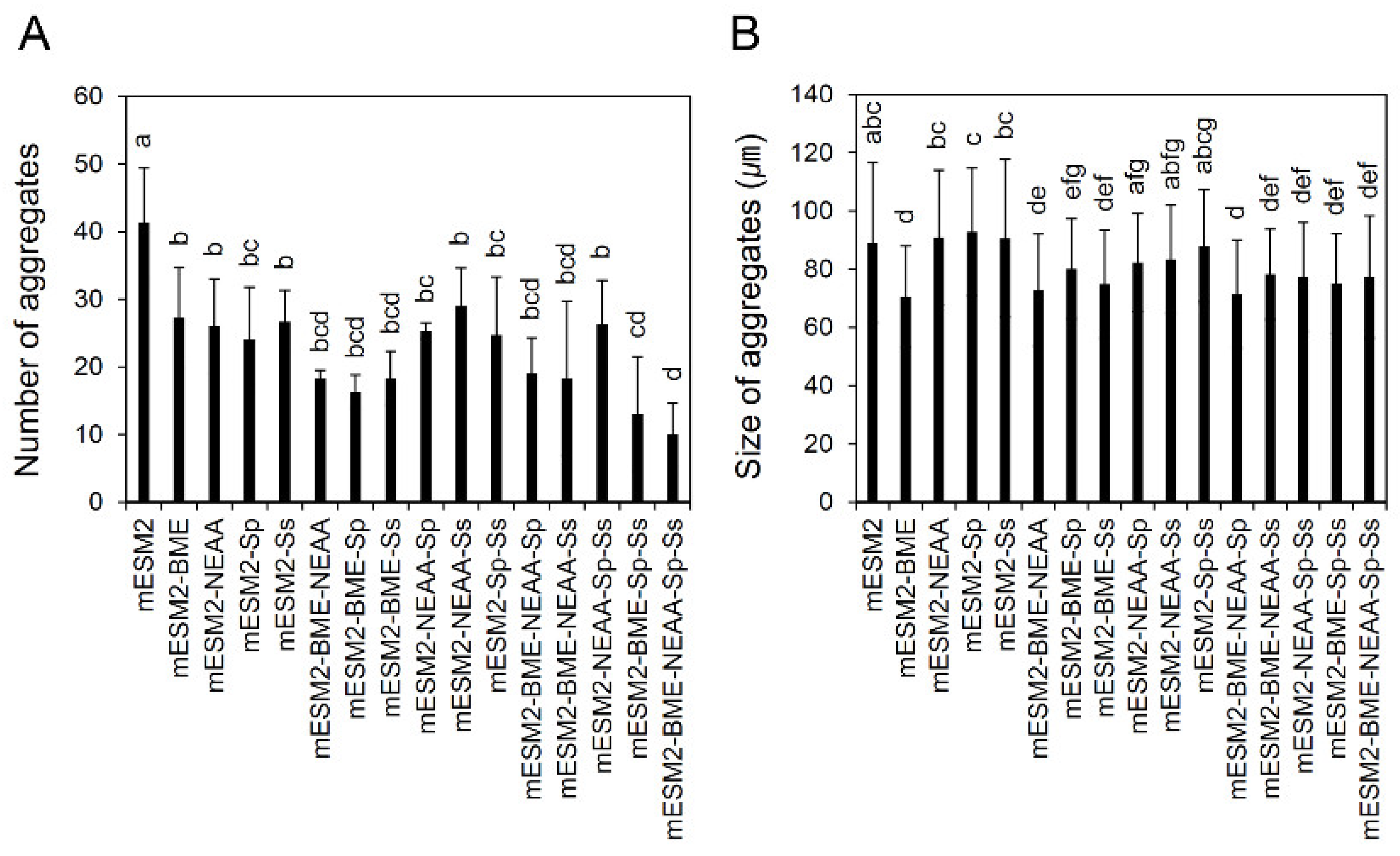
3.4. Effects of the Composition of mESM2 on the Culture of Testicular Aggregates: Part I
3.5. Effects of the Composition of mESM2 on the Culture of Testicular Aggregates: Part II
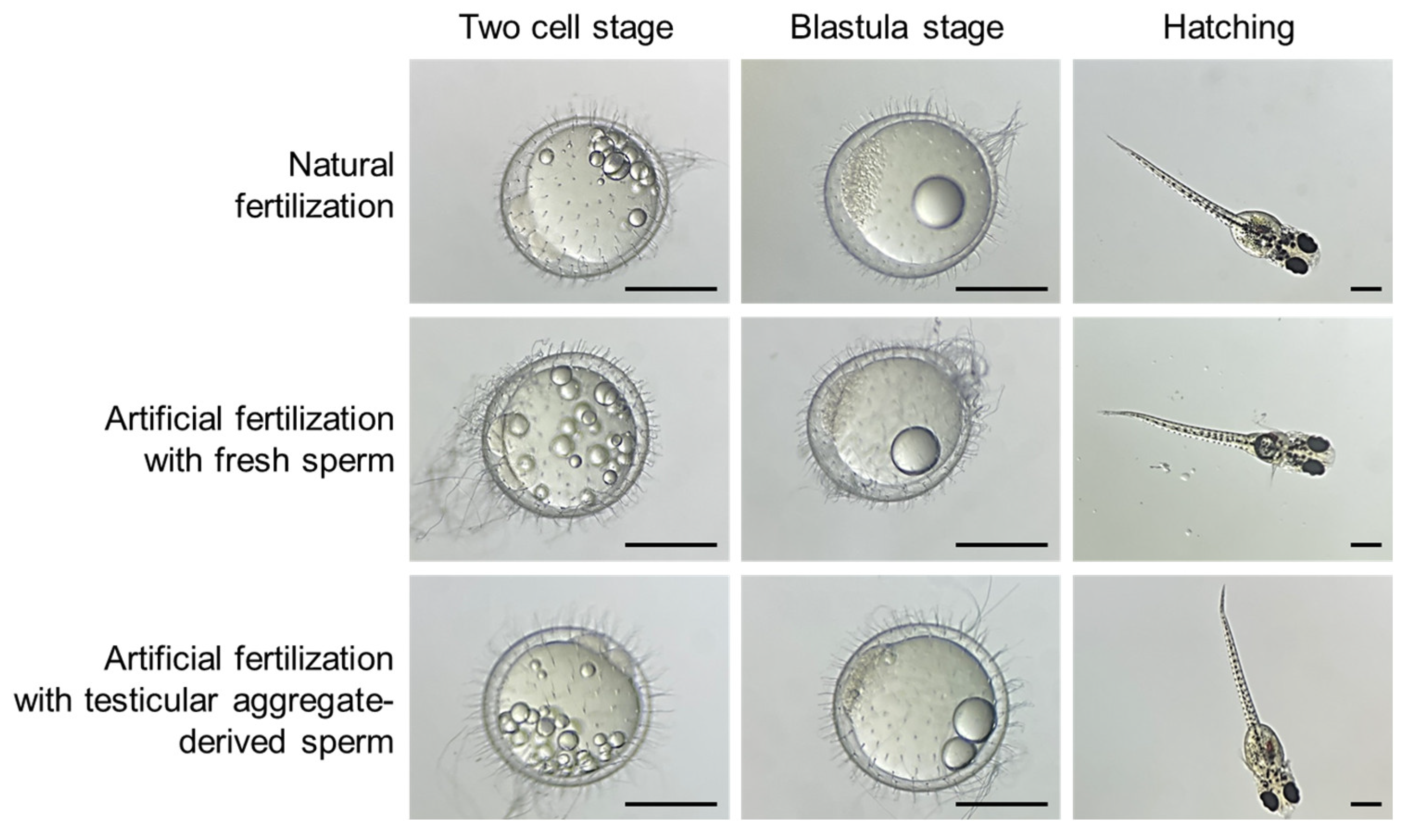
3.6. Evaluation of Fertility of the Sperms Derived from Testicular Aggregates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibtisham, F.; Honaramoo, A. Spermatogonial stem cells for the in vitro spermatogenesis and in vivo restoration of fertility. Cells 2020, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, A.A.; Ingham, P.W. Regulation of stem cell maintenance and transit amplifying cell proliferation by TGF- β signaling in Dorsophila spermatogenesis. Curr. Biol. 2003, 13, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, Y.; Kobayashi, T.; Kato, M.; Hirabayashi, M.; Hochi, S. Characteristics of rat round spermatids differentiated from spermatogonial cells during co-culture with Sertoli cells, assessed by flow cytometry, microinsemination and RT-PCR. Theriogenology 2006, 65, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Tatagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In Vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg, J.B.; Wistuba, J.; Luetjens, C.M.; Elhija, M.A.; Huleihel, M.; Lunenfeld, E.; Gromoll, J.; Nieschlag, E.; Schlatt, S. Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J. Androl. 2008, 29, 312–329. [Google Scholar] [CrossRef]
- Reuter, K.; Ehmcke, J.; Stukenborg, J.B.; Simoni, M.; Damm, O.S.; Redmann, K.; Schlatt, S.; Wistuba, J. Reassembly of somatic cells and testicular organogenesis in vitro. Tissue Cell 2014, 46, 86–96. [Google Scholar] [CrossRef]
- Alves-Lopes, J.P.; Soder, O.; Stukenborg, J.B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 2017, 130, 76–89. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Kim, H.; Lee, S.J.; Gye, M.C. In Vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 2006, 27, 2845–2853. [Google Scholar] [CrossRef]
- Cham, T.C.; Ibtisham, F.; Fayaz, M.A.; Honaramooz, A. Generation of a highly biomimetic organoid, including vasculature, resembling the native immature testis tissue. Cells 2021, 10, 1696. [Google Scholar] [CrossRef]
- Higaki, S.; Shimada, M.; Kawamoto, K.; Todo, T.; Kawasaki, T.; Tooyama, I.; Fujioka, Y.; Sakai, N.; Takada, T. In Vitro differentiation of fertile sperm from cryopreserved spermatogonia of the endangered endemic cyprinid honmoroko (Gnathopogon caerulescens). Sci. Rep. 2017, 7, 42852. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.W.; Mo, C.Y.; Dong, M.D.; Jia, K.T.; Liu, W.; Yi, M.S. Production of functional sperm from in vitro-cultured premeiotic spermatogonia in a marine fish. Zool. Res. 2022, 43, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Hong, N.; Hong, Y. Derivation and characterization of haploid embryonic stem cell cultures in medaka fish. Nat. Protoc. 2010, 5, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Gong, S.P. Effects of the developmental stage of extract donor embryos on the culture of marine medaka Oryzias dancena embryonic stem cell-like cells. Korean J. Fish. Aquat. Sci. 2017, 50, 160–168. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(t)] method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Edmonds, M.E.; Woodruff, T.K. Testicular organoid formation is a property of immature somatic cells, which self-assemble and exhibit long-term hormone-responsive endocrine function. Biofabrication 2020, 12, 045002. [Google Scholar] [CrossRef]
- Song, H.Y.; Nam, Y.K.; Bang, I.C.; Kim, D.S. Early gonadogenesis and sex differentiation of marine medaka, Oryzias dancena (Beloniformes; Teleostei). Korean J. Ichthyol. 2009, 21, 141–148. [Google Scholar]
- Lee, D.; Ryu, J.H.; Lee, S.T.; Nam, Y.K.; Kim, D.S.; Gong, S.P. Identification of embryonic stem cell activities in an embryonic cell line derived from marine medaka (Oryzias dancena). Fish. Physiol. Biochem. 2015, 41, 1569–1576. [Google Scholar] [CrossRef]
- Choi, J.H.; Gong, S.P. Effects of temperatures and basal media on primary culture of the blastomeres derved from the embryos at blastula stage in marine medaka Oryzias dancena. J. Embryo. Transf. 2018, 33, 343–348. [Google Scholar]
- Son, M.J.; Gong, S.P. Feeder cell-dependent primary culture of single blastula-dervied embryonic cell lines from marine medaka (Oryzias dancena). In Vitro Cell. Dev. Biol. Anim. 2022, 9, 840–850. [Google Scholar] [CrossRef]
- Kang, J.H.; Ryu, J.H.; Nam, Y.K.; Gong, S.P. Effectiveness of a medaka whole testis organ culture system using an agarose gel stand for germ cell proliferation and differentiation. J. Biomater. Tissue. Eng. 2019, 9, 206–212. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, J.H.; Choi, J.K.; Gong, S.P. Improved conditions of a whole testis organ culture system in terms of spermatogonial proliferation levels in marine medaka (Oryzias dancena). In Vitro Cell. Dev. Biol. Anim. 2021, 57, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Kang, M.; Wu, X.; Ye, T. Development of a promising fish model (Oryzias melastigma) for assessing multiple responses to stresses in the marine environment. Biomed. Res. Int. 2014, 2014, 563131. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Nam, Y.K.; Bang, I.C.; Kim, D.S. Embryogenesis and early ontogenesis of a marine medaka, Oryzias dancena. Korean J. Ichthyol. 2009, 21, 227–238. [Google Scholar]
- de Barros, F.R.; Worst, R.A.; Saurin, G.C.; Mendes, C.M.; Assumpção, M.E.; Visintin, J.A. α-6 integrin expression in bovine spermatogonial cells purified by discontinuous Percoll density gradient. Reprod. Domest. Anim. 2012, 47, 887–890. [Google Scholar] [CrossRef]
- Goel, S.; Sugimoto, M.; Minami, N.; Yamada, M.; Kume, S.; Imai, H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol. Reprod. 2007, 77, 127–137. [Google Scholar] [CrossRef]
- Heidari, B.; Gifani, M.; Shirazi, A.; Zarnani, A.H.; Baradaran, B.; Naderi, M.M.; Behzadi, B.; Borjian-Boroujeni, S.; Sarvari, A.; Lakpour, N.; et al. Enrichment of undifferentiated type A spermatogonia from goat testis using discontinuous Percoll density gradient and differential plating. J. Med. Biotechnol. 2014, 6, 94–103. [Google Scholar]
- Linhartová, Z.; Rodina, M.; Guralp, H.; Gazo, I.; Saito, T.; Pšenička, M. Isolation and cryopreservation of early stages of germ cells of tench (Tinca tinca). Czech J. Anim. Sci. 2014, 59, 381–390. [Google Scholar] [CrossRef]
- Lacerda, S.; Batlouni, S.; Assis, L.; Resende, F.; Campos-Silva, S.; Campos-Silva, R.; Campos-silva, T.; Segatelli, T.; Franca, L. Germ cell transplantation in tilapia (Oreochromis niloticus). Cybium 2008, 32, 115–118. [Google Scholar]
- Strange, D.P.; Zarandi, Z.P.; Trivedi, G.; Atala, A.; Bishop, C.E.; Sadri-Ardekani, H.; Verma, S. Human testicular organoid system as a novel tool to study Zika virus pathogenesis. Emerg. Microbes. Infect. 2018, 7, 82. [Google Scholar] [CrossRef]
- Cortez, J.; Levia, B.; Torres, C.G.; Parraguez, V.H.; de los Reyes, M.; Carrasco, A.; Peralta, O.A. Generation and characterization of bovine testicular organoids derived from primary somatic cell populations. Animals 2022, 12, 2283. [Google Scholar] [CrossRef]
- Ryu, J.H.; Gong, S.P. Enhanced enrichment of medaka ovarian germline stem cells by a combination of density gradient centrifugation and differential plating. Biomolecules 2020, 10, 1477. [Google Scholar] [CrossRef] [PubMed]
- Richer, G.; Baert, Y.; Goossens, E. In Vitro spermatogenesis through testis modelling: Toward the generation of testicular organoids. Andrology 2019, 8, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Topraggaleh, T.R.; Valojerdi, M.R.; Montazeri, L.; Baharvand, H. A testis-derived macroporous 3D scaffold as a platform for the generation of mouse testicular organoids. Biomater. Sci. 2019, 7, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Schartl, M. Establishment and growth responses of early medaka fish (Oryzias latipes) embryonic cells in feeder layer-free culture. Mol. Mar. Biol. Biotechnol. 1996, 5, 93–104. [Google Scholar]
- Fan, Z.; Liu, L.; Huang, X.; Zhao, Y.; Zhou, L.; Wang, D.; Wei, J. Establishment and growth responses of Nile tilapia embryonic stem-like cell lines under feeder-free condition. Develop. Growth Differ. 2017, 59, 83–93. [Google Scholar] [CrossRef]
- Ho, S.Y.; Goh, C.W.; Gan, J.Y.; Lee, Y.S.; Lam, M.K.; Hong, N.; Hong, Y.; Chan, W.K.; Shu-Chien, A.C. Derivation and long-term culture of an embryonic stem cell-like line from zebrafish blastomeres under feeder-free condition. Zebrafish 2014, 11, 407–420. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, T.; Zhao, H.; Xu, H.; Wang, W.; Liu, R.; Chen, T.; Deng, J.; Gui, J.F. Establishment of a normal medakafish spermatogonial stem cell line capable of sperm production in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 8011–8016. [Google Scholar] [CrossRef]
- Nakamura, S.; Watakabe, I.; Nishimura, T.; Toyoda, A.; Taniguchi, Y.; Tanaka, M. Analysis of medaka sox9 orthologue reveals a conserved role in germ cell maintenance. PLoS ONE 2012, 7, e29982. [Google Scholar] [CrossRef]
- Kubota, H.; Avarbock, M.R.; Brinste, R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef]
- Mizapour, T.; Movahedin, M.; Tengku Ibrahim, T.A.; Koruji, M.; Haron, A.W.; Nowroozi, M.R.; Rafieian, S.H. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia 2012, 44, 41–55. [Google Scholar] [CrossRef]
- Masaki, K.; Sakai, M.; Kuroki, S.; Jo, J.I.; Hoshina, K.; Fujimori, Y.; Oka, K.; Amano, T.; Yamanaka, T.; Tachibana, M.; et al. FGF2 has distinct molecular functions from GDNF in mouse germline niche. Stem Cell Rep. 2018, 10, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hai, Y.; Yao, C.; Chen, Z.; Hou, J.; Li, Z.; He, Z. Long-term culture and significant expansion of human Sertoli cells whilst maintaining stable global phenotype and AKT and SMAD1/5 activation. Cell Commun. Signal. 2015, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Bui-Marinos, M.P.; Todd, L.A.; Douglas, A.J.; Katzenback, B.A. So, you want to create a frog cell line? A guide to establishing frog skin cell lines from tissue explants. MethodsX 2022, 9, 101693. [Google Scholar] [CrossRef]
- Hewlett, G.; Opitz, G.H.; Schlumberger, H.G.; Lemke, H. Growth regulation of a murine lymphoma cell line by a 2-mercaptoethanol or macrophage-activated serum factor. Eur. J. Immum. 1977, 7, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Hess, M.W.; Cottier, H. Role of 2-mercaptoethanol in the stimulation of spleen cell culture: Increased uptake of cysteine into the TCA-soluble pool. Immun. Lett. 1980, 4, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Higaki, S.; Koyama, Y.; Shirai, E.; Yokota, T.; Fujioka, Y.; Sakai, N.; Takada, T. Establishment of testicular and ovarian cell lines from Honmoroko (Gnathopogon caerulescens). Fish. Physiol. Biochem. 2013, 39, 701–711. [Google Scholar] [CrossRef]
- Yang, H.; Tiersch, T.R. Sperm motility initiation and duration in a euryhaline fish, medaka (Oryzias latipes). Theriogenology 2009, 72, 386–392. [Google Scholar] [CrossRef]
- Miura, C.; Miura, T. Analysis of spermatogenesis using an eel model. Aqua-BioSci. Monogr. 2011, 4, 105–129. [Google Scholar] [CrossRef]
- Cavaco, J.E.; Bogerd, J.; Goos, H.; Schulz, R.W. Testosterone inhibits 11-ketotestosterone-induced spermatogenesis in African catfish (Clarias gariepinus). Biol. Reprod. 2001, 65, 1807–1812. [Google Scholar] [CrossRef]
- Leal, M.C.; de Waal, P.P.; García-López, A.; Chen, S.X.; Bogerd, J.; Schulz, R.W. Zebrafish primary testis tissue culture: An approach to study testis function ex vivo. Gen. Comp. Endocrinol. 2009, 162, 134–138. [Google Scholar] [CrossRef]
- Nóbrega, R.H.; Morais, R.D.V.d.S.; Crespo, D.; de Waal, P.P.; de França, L.R.; Schulz, R.W.; Bogerd, J. Fsh stimulates spermatogonial proliferation and differentiation in zebrafish via Igf3. Endocrinology 2015, 156, 38043817. [Google Scholar] [CrossRef] [PubMed]

| Genes | Primer Sequences (5′ > 3′) | Product Size (bp) | Accession Number |
|---|---|---|---|
| nanos2 | Forward: AAACTACACCTGTCCCATCTG | 111 | XM_036217407.1 |
| Reverse: AACTTGTAGGAGGGCAGCATC | |||
| scp3 | Forward: CAGCTGCTAGCTTTGAGGAA | 224 | XM_024295185.2 |
| Reverse: CTGAGAGAACTGCTGCATTG | |||
| sox9a | Forward: GGAGAAGTGTCACTCCGACG | 138 | XM_024272555.2 |
| Reverse: TTGTAGTCCGGACCCCTGAT | |||
| 18s rRNA | Forward: TCCAGCTCCAATAGCGATTCACC | 253 | HM347347.1 |
| Reverse: AGAACCGGAGTCCTATTCCA |
| Culture Methods | Surface Materials | Matrigel + Cell Drop Volume (μL) | Suspended Cell Concentration (Cells/μL) | Final No. of Cells Seeded (Cells) | No. of Trials | No. (%) a of Aggregate Formation | No. (%) b of Aggregate Type | |
|---|---|---|---|---|---|---|---|---|
| Large Single | Small Multiple | |||||||
| Matrigel suspension | Polystyrene (tissue-culture-treated dish) | 5 | 1.25 × 105 | 6.25 × 105 | 5 | 4 (80) | 0 (0) | 4 (100) |
| 2.50 × 105 | 1.25 × 106 | 3 | 3 (100) | 0 (0) | 3 (100) | |||
| 3.75 × 105 | 1.875 × 106 | 3 | 3 (100) | 0 (0) | 3 (100) | |||
| Matrigel suspension | Polystyrene (Petri dish) | 5 | 1.25 × 105 | 6.25 × 105 | 5 | 5 (100) | 0 (0) | 5 (100) |
| 2.50 × 105 | 1.25 × 106 | 10 | 10 (100) | 2 (20) | 8 (80) | |||
| 3.75 × 105 | 1.875 × 106 | 5 | 5 (100) | 2 (40) | 3 (60) | |||
| Matrigel suspension | Agarose gel | 5 | 1.25 × 105 | 6.25 × 105 | 4 | 0 (0) | 0 (0) | 0 (0) |
| 2.50 × 105 | 1.25 × 106 | 4 | 0 (0) | 0 (0) | 0 (0) | |||
| 3.75 × 105 | 1.875 × 106 | 3 | 0 (0) | 0 (0) | 0 (0) | |||
| Three-layer gradient system | Matrigel | 3 | 1.25 × 105 | 3.75 × 105 | 4 | 0 (0) | 0 (0) | 0 (0) |
| 2.50 × 105 | 7.5 × 105 | 4 | 0 (0) | 0 (0) | 0 (0) | |||
| 3.75 × 105 | 1.125 × 106 | 3 | 1 (33.3) | 0 (0) | 1 (100) | |||
| Ultra-low attachment 96-well plate | Hydrophilic gel | - | - | 1.25 × 106 | 5 | 5 (100) | 0 (0) | 5 (100) |
| 5 | 2.50 × 105 | 1.25 × 106 | 8 | 3 (37.5) | 0 (0) | 3 (100) | ||
| Fertilization Method | No. of Eggs | No. (%) a of One-Cell Stage | No. (%) a of Two-Cell Stage | No. (%) a of Blastula Stage | No. (%) a of Hatched Larvae |
|---|---|---|---|---|---|
| Natural fertilization by mating | 150 | 124 (82.7) | 124 (82.7) | 114 (76.0) | 111 (74.0) |
| Artificial fertilization with fresh sperm | 180 | 107 (59.4) | 81 (45.0) | 80 (44.4) | 78 (43.3) |
| Artificial fertilization with aggregate-derived sperm | 276 | 122 (44.2) | 6 (2.2) | 4 (1.4) | 4 (1.4) |
| Unfertilization | 103 | 51 (49.5) | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Ryu, J.H.; Gong, S.P. Establishment and Optimization of an Aggregate Culture System of Testicular Cells from Marine Medaka, Oryzias dancena. J. Mar. Sci. Eng. 2023, 11, 2077. https://doi.org/10.3390/jmse11112077
Choi JH, Ryu JH, Gong SP. Establishment and Optimization of an Aggregate Culture System of Testicular Cells from Marine Medaka, Oryzias dancena. Journal of Marine Science and Engineering. 2023; 11(11):2077. https://doi.org/10.3390/jmse11112077
Chicago/Turabian StyleChoi, Jae Hoon, Jun Hyung Ryu, and Seung Pyo Gong. 2023. "Establishment and Optimization of an Aggregate Culture System of Testicular Cells from Marine Medaka, Oryzias dancena" Journal of Marine Science and Engineering 11, no. 11: 2077. https://doi.org/10.3390/jmse11112077
APA StyleChoi, J. H., Ryu, J. H., & Gong, S. P. (2023). Establishment and Optimization of an Aggregate Culture System of Testicular Cells from Marine Medaka, Oryzias dancena. Journal of Marine Science and Engineering, 11(11), 2077. https://doi.org/10.3390/jmse11112077






