Induction of Reproductive Sterility in Coho Salmon (Oncorhynchus kisutch) by an Immersion-Based Gene Silencing Technology
Abstract
1. Introduction
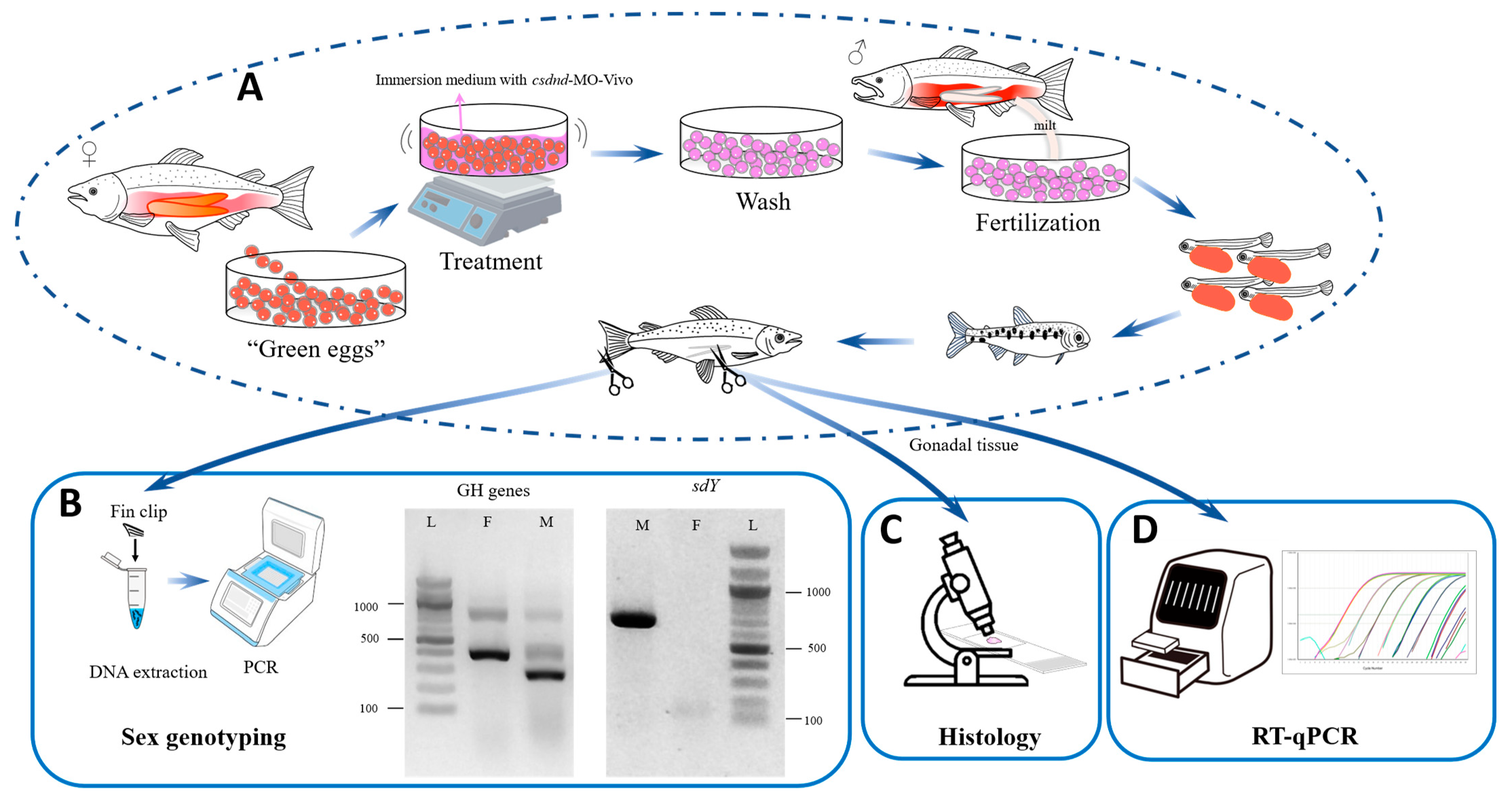
2. Materials and Methods
2.1. dnd Gene Target Region Verification and MO Design
2.2. Immersion Treatment
2.3. Animal Husbandry
2.4. Animal Sampling
2.5. Sex Genotyping
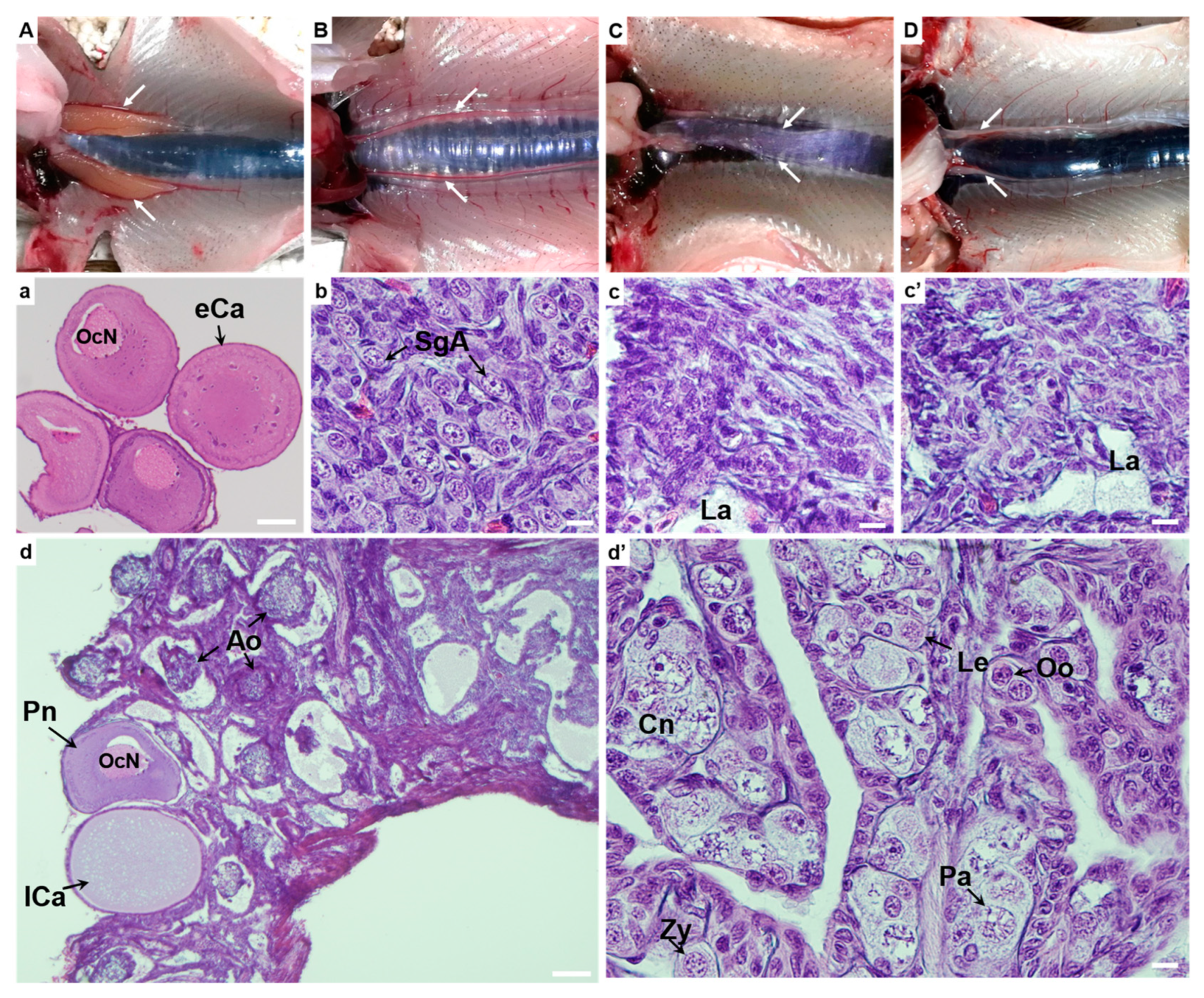
2.6. Gonad Histology
2.7. RT-qPCR Assay
3. Results
3.1. Immersion Treatment
3.2. Sex Genotyping
3.3. Dissection and Gonadal Histology
3.4. Gene Expression in Gonadal Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, S.J.; Gong, Z.; Fletcher, G.L.; Shears, M.A.; King, M.J.; Idler, D.R.; Hew, C.L. Growth Enhancement in Transgenic Atlantic Salmon by the Use of an “All Fish” Chimeric Growth Hormone Gene Construct. Bio/Technol. 1992, 10, 176–181. [Google Scholar] [CrossRef]
- Lind, C.E.; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Selective Breeding in Fish and Conservation of Genetic Resources for Aquaculture. Reprod. Domest. Anim. 2012, 47, 255–263. [Google Scholar] [CrossRef]
- Dunham, R.A. Transgenic Fish Resistant to Infectious Diseases, Their Risk and Prevention of Escape into the Environment and Future Candidate Genes for Disease Transgene Manipulation. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 139–161. [Google Scholar] [CrossRef]
- Regan, T.; Bean, T.P.; Ellis, T.; Davie, A.; Carboni, S.; Migaud, H.; Houston, R.D. Genetic Improvement Technologies to Support the Sustainable Growth of UK Aquaculture. Rev. Aquac. 2021, 13, 1958–1985. [Google Scholar] [CrossRef]
- Muir, W.M.; Howard, R.D. Possible Ecological Risks of Transgenic Organism Release When Transgenes Affect Mating Success: Sexual Selection and the Trojan Gene Hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 13853–13856. [Google Scholar] [CrossRef]
- Karlsson, S.; Diserud, O.H.; Fiske, P.; Hindar, K. Widespread Genetic Introgression of Escaped Farmed Atlantic Salmon in Wild Salmon Populations. ICES J. Mar. Sci. 2016, 73, 2488–2498. [Google Scholar] [CrossRef]
- Tveiten, H.; Johnsen, H.K.; Jobling, M. Influence of Maturity Status on the Annual Cycles of Feeding and Growth in Arctic Charr Reared at Constant Temperature. J. Fish. Biol. 1996, 48, 910–924. [Google Scholar] [CrossRef]
- Kadri, S.; Mitchell, D.F.; Metcalfe, N.B.; Huntingford, F.A.; Thorpe, J.E. Differential Patterns of Feeding and Resource Accumulation in Maturing and Immature Atlantic Salmon, Salmo salar. Aquaculture 1996, 142, 245–257. [Google Scholar] [CrossRef]
- Aksnes, A.; Gjerde, B.; Roald, S.O. Biological, Chemical and Organoleptic Changes during Maturation of Farmed Atlantic Salmon, Salmo salar. Aquaculture 1986, 53, 7–20. [Google Scholar] [CrossRef]
- Zohar, Y. Endocrinology and Fish Farming: Aspects in Reproduction, Growth, and Smoltification. Fish. Physiol. Biochem. 1989, 7, 395–405. [Google Scholar] [CrossRef]
- Harris, J.; Bird, D.J. Modulation of the Fish Immune System by Hormones. Vet. Immunol. Immunopathol. 2000, 77, 163–176. [Google Scholar] [CrossRef]
- Skarstein, F.; Folstad, I.; Liljedal, S. Whether to Reproduce or Not: Immune Suppression and Costs of Parasites during Reproduction in the Arctic Charr. Can. J. Zool. 2001, 79, 271–278. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.A.; Dufour, S.; Karlsen, Ø.; Norberg, B.; et al. Control of Puberty in Farmed Fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef]
- Piferrer, F.; Beaumont, A.; Falguière, J.C.; Flajšhans, M.; Haffray, P.; Colombo, L. Polyploid Fish and Shellfish: Production, Biology and Applications to Aquaculture for Performance Improvement and Genetic Containment. Aquaculture 2009, 293, 125–156. [Google Scholar] [CrossRef]
- Johnson, O.W.; Dickhoff, W.W.; Utter, F.M. Comparative Growth and Development of Diploid and Triploid Coho Salmon, Oncorhynchus kisutch. Aquaculture 1986, 57, 329–336. [Google Scholar] [CrossRef]
- Ojolick, E.J.; Cusack, R.; Benfey, T.J.; Kerr, S.R. Survival and Growth of All-Female Diploid and Triploid Rainbow Trout (Oncorhynchus mykiss) Reared at Chronic High Temperature. Aquaculture 1995, 131, 177–187. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T. Vertebral Deformities in Triploid Atlantic Salmon (Salmo salar L.) Underyearling Smolts. Aquaculture 2010, 309, 131–136. [Google Scholar] [CrossRef]
- Wargelius, A.; Leininger, S.; Skaftnesmo, K.O.; Kleppe, L.; Andersson, E.; Taranger, G.L.; Schulz, R.W.; Edvardsen, R.B. Dnd Knockout Ablates Germ Cells and Demonstrates Germ Cell Independent Sex Differentiation in Atlantic Salmon. Sci. Rep. 2016, 6, 21284. [Google Scholar] [CrossRef]
- Wong, T.-T.; Zohar, Y. Production of Reproductively Sterile Fish by a Non-Transgenic Gene Silencing Technology. Sci. Rep. 2015, 5, 15822. [Google Scholar] [CrossRef]
- Du, S.J.; Devlin, R.H.; Hew, C.L. Genomic Structure of Growth Hormone Genes in Chinook Salmon (Oncorhynchus tshawytscha): Presence of Two Functional Genes, GH-I and GH-II, and a Male-Specific Pseudogene, GH-ψ. DNA Cell Biol. 1993, 12, 739–751. [Google Scholar] [CrossRef]
- Yano, A.; Nicol, B.; Jouanno, E.; Quillet, E.; Fostier, A.; Guyomard, R.; Guiguen, Y. The Sexually Dimorphic on the Y-chromosome Gene (SdY) Is a Conserved Male-specific Y-chromosome Sequence in Many Salmonids. Evol. Appl. 2013, 6, 486–496. [Google Scholar] [CrossRef]
- Moore, L.J.; Somamoto, T.; Lie, K.K.; Dijkstra, J.M.; Hordvik, I. Characterisation of Salmon and Trout CD8α and CD8β. Mol. Immunol. 2005, 42, 1225–1234. [Google Scholar] [CrossRef]
- Trummel, D.E.; Fulcher, K.D.; Beck, J.C.; Cloud, J.G. Fertility of Rainbow Trout Males Relative to Differences in the Proportion of Sperm That Binds to a Specific Antibody. Aquaculture 1992, 104, 175–182. [Google Scholar] [CrossRef]
- Krise, W.F.; Hendrix, M.A.; Bonney, W.A.; Baker-Gordon, S.E. Evaluation of Sperm-Activating Solutions in Atlantic Salmon Salmo salar Fertilization Tests. J. World Aquac. Soc. 1995, 26, 384–389. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nishimura, T.; Goto-Kazeto, R.; Kawakami, Y.; Yamaha, E.; Arai, K. Sexual Dimorphism of Gonadal Structure and Gene Expression in Germ Cell-Deficient Loach, a Teleost Fish. Proc. Natl. Acad. Sci. USA 2010, 107, 17211–17216. [Google Scholar] [CrossRef]
- Goto, R.; Saito, T.; Takeda, T.; Fujimoto, T.; Takagi, M.; Arai, K.; Yamaha, E. Germ Cells Are Not the Primary Factor for Sexual Fate Determination in Goldfish. Dev. Biol. 2012, 370, 98–109. [Google Scholar] [CrossRef]
- Linhartová, Z.; Saito, T.; Kašpar, V.; Rodina, M.; Prášková, E.; Hagihara, S.; Pšenička, M. Sterilization of Sterlet Acipenser ruthenus by Using Knockdown Agent, Antisense Morpholino Oligonucleotide, against Dead End Gene. Theriogenology 2015, 84, 1246–1255.e1. [Google Scholar] [CrossRef]
- Weidinger, G.; Stebler, J.; Slanchev, K.; Dumstrei, K.; Wise, C.; Lovell-Badge, R.; Thisse, C.; Thisse, B.; Raz, E. Dead End, a Novel Vertebrate Germ Plasm Component, Is Required for Zebrafish Primordial Germ Cell Migration and Survival. Curr. Biol. 2003, 13, 1429–1434. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Takashiba, K.; Shimamori, S.; Fujinuma, K.; Shikina, S.; Okutsu, T.; Kume, S.; Hayashi, M. Production of Germ Cell-Deficient Salmonids by Dead End Gene Knockdown, and Their Use as Recipients for Germ Cell Transplantation. Mol. Reprod. Dev. 2016, 83, 298–311. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, M.; Ryu, J.H.; Hayman, E.S.; Fairgrieve, W.T.; Zohar, Y.; Luckenbach, J.A.; Wong, T.-T. Reproductive Sterility in Aquaculture: A Review of Induction Methods and an Emerging Approach with Application to Pacific Northwest Finfish Species. Rev. Aquac. 2023, 15, 220–241. [Google Scholar] [CrossRef]
- Bongers, A.B.J.; in’t Veld, E.P.C.; Abo-Hashema, K.; Bremmer, I.M.; Eding, E.H.; Komen, J.; Richter, C.J.J. Androgenesis in Common Carp (Cyprinus carpio L.) Using UV Irradiation in a Synthetic Ovarian Fluid and Heat Shocks. Aquaculture 1994, 122, 119–132. [Google Scholar] [CrossRef]
- Corley-Smith, G.E.; Lim, C.J.; Brandhorst, B.P. Production of Androgenetic Zebrafish (Danio rerio). Genetics 1996, 142, 1265–1276. [Google Scholar] [CrossRef]
- Sakai, N.; Burgess, S.; Hopkins, N. Delayed in Vitro Fertilization of Zebrafish Eggs in Hank’s Saline Containing Bovine Serum Albumin. Mol. Mar. Biol. Biotechnol. 1997, 6, 84–87. [Google Scholar]
- Rosengrave, P.; Taylor, H.; Montgomerie, R.; Metcalf, V.; McBride, K.; Gemmell, N.J. Chemical Composition of Seminal and Ovarian Fluids of Chinook Salmon (Oncorhynchus tshawytscha) and Their Effects on Sperm Motility Traits. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 152, 123–129. [Google Scholar] [CrossRef]
- Ernst, V.V.; Neff, J.M.; Anderson, J.W. The Effects of the Water-Soluble Fractions of No. 2 Fuel Oil on the Early Development of the Estuarine Fish, Fundulus Grandis Baird and Girard. Environ. Pollut. (1970) 1977, 14, 25–35. [Google Scholar] [CrossRef]
- Manner, H.W.; Vancura, M.; Muehleman, C. The Ultrastructure of the Chorion of the Fathead Minnow, Pimephales promelas. Trans. Am. Fish. Soc. 1977, 106, 110–114. [Google Scholar] [CrossRef]
- Gellert, G.; Heinrichsdorff, J. Effect of Age on the Susceptibility of Zebrafish Eggs to Industrial Wastewater. Water Res. 2001, 35, 3754–3757. [Google Scholar] [CrossRef]
- González-Doncel, M.; Larrea, M.; Sánchez-Fortún, S.; Hinton, D.E. Influence of Water Hardening of the Chorion on Cadmium Accumulation in Medaka (Oryzias latipes) Eggs. Chemosphere 2003, 52, 75–83. [Google Scholar] [CrossRef]
- Harvey, B.; Chamberlain, J.B. Water Permeability in the Developing Embryo of the Zebrafish, Brachydanio rerio. Can. J. Zool. 1982, 60, 268–270. [Google Scholar] [CrossRef]
- Mangor-Jensen, A. Water Balance in Developing Eggs of the Cod Gadus morhua L. Fish. Physiol. Biochem. 1987, 3, 17–24. [Google Scholar] [CrossRef]
- Prescott, D.M. Effect of Activation on the Water Permeability of Salmon Eggs. J. Cell. Comp. Physiol. 1955, 45, 1–12. [Google Scholar] [CrossRef]
- Rudy, P.P.; Potts, W.T. Sodium Balance in the Eggs of the Atlantic Salmon, Salmo salar. J. Exp. Biol. 1969, 50, 239–246. [Google Scholar] [CrossRef]
- Terner, C. Studies of Metabolism in Embryonic Development-I. The Oxidative Metabolic of Unfertilized and Embryonated Eggs of the Rainbow Trout. Comp. Biochem. Physiol. 1968, 24, 933–940. [Google Scholar] [CrossRef]
- Onozato, H.; Usui, K.; Hamada, K. A Method for Dechorionation in Goldfish Carassius auratus. Nippon Suisan Gakkaishi 1986, 52, 1929–1934. [Google Scholar] [CrossRef]
- Sakai, Y.T. Method for Removal of Chorion and Fertilization of the Naked Egg in Oryzias latipes. Embryologia 1961, 5, 357–368. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Oshiro, T.; Takashima, F. Prevention of Hardening of Chorion and Dechorionation for Microinjection into Fish Eggs. Nippon Suisan Gakkaishi 1989, 55, 369. [Google Scholar] [CrossRef][Green Version]
- Bendriem, N.; Roman, R.; Sumaila, U.R. Enhancing Land-Based Culture of Coho Salmon through Genomic Technologies: An Economic Analysis. Aquac. Econ. Manag. 2021, 25, 27–52. [Google Scholar] [CrossRef]
- Gaffney, L.P.; Franks, B.; Weary, D.M.; Von Keyserlingk, M.A.G. Coho Salmon (Oncorhynchus kisutch) Prefer and Are Less Aggressive in Darker Environments. PLoS ONE 2016, 11, e0151325. [Google Scholar] [CrossRef]
- Crouse, C.; Davidson, J.; May, T.; Summerfelt, S.; Good, C. Production of Market-Size European Strain Atlantic Salmon (Salmo salar) in Land-Based Freshwater Closed Containment Aquaculture Systems. Aquac. Eng. 2021, 92, 102138. [Google Scholar] [CrossRef]
- Good, C.; Davidson, J. A Review of Factors Influencing Maturation of Atlantic Salmon, Salmo salar, with Focus on Water Recirculation Aquaculture System Environments. J. World Aquac. Soc. 2016, 47, 605–632. [Google Scholar] [CrossRef]


| Target Gene | Accession Number | Primer Name | Direction | Sequences (5′ to 3′) | Tm (°C) | Anticipated Amplicon Size (bp) |
|---|---|---|---|---|---|---|
| Start codon verification | ||||||
| dnd | XM_020452699.1 | WBCS1 | Forward | CACCTAGAACTACCTGTCGAAAC | 55 | 566 |
| WBCS2 | Reverse | GCTGTCGTACTTGGCGTAG | ||||
| Sex genotyping | ||||||
| GH1; GH2; GHΨ 1 | Gene IDs: 109893213; 109898300 | WBCS11 | Forward | CCTGGATGACAATGACTCTCA | 60 | 779; 404; 273 |
| WBCS12 | Reverse | CTACAGAGTGCAGTTGGCCTC | ||||
| sdY 2 | - | WBCS17 | Forward | ATGGCTGACAGAGAGGCCAGAATCCAA | 55 | ~700 |
| WBCS18 | Reverse | TGCTCTCTGTTGAAGAGCATCAC | ||||
| Gonadal tissue gene expression | ||||||
| vasa | XM_020457444.2 | qrCS5 | Forward | TTTGGGAGACCGACTGATAAAG | 60 | 124 |
| qrCS6 | Reverse | CACCAGCACCTGAAGAGAAA | ||||
| nanos3 | XM_031810988.1 | WBTP51 | Forward | TCATGACTCCGGGATATGCT | 60 | 115 |
| WBTP52 | Reverse | GGGTTCCATTTCGTGCCATA | ||||
| ef1a 3 | XM_031793750.1 | qrCS13 | Forward | CCCCTCCAGGATGTTTACAAA | 60 | 57 |
| qrCS14 | Reverse | CACACGGCCCACAGGTACA | ||||
| Group | Immersion Medium | csdnd-MO-Vivo (μM) | Tm (°C) | Time (Hours) | Eyed Rates 1 | Fish Dissected | Sterile Fish | Sterility Rates 2 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Retarded Female | |||||||
| Batch B | |||||||||||
| CSB1 | - | - | - | - | 146/261 = 55.9% | 11 | 13 | 0 | |||
| CSB16 | B | 17.5 | 4 | 48 | 5/557 = 0.9% | 2 | 3 | 1 | - | ||
| CSB27 | B | 20 | 4 | 24 | 69/514 = 13.4% | 24 | 26 | 1 | 1 | 2/24 = 8.3% | |
| CSB39 | B | 12.5 | 8 | 36 | 53/258 = 20.5% | 16 | 19 | 1 | 2 | 1/16 = 6.3% | |
| Batch C | |||||||||||
| CSC13 | C1 | 0 | 8 | 12 | 198/237 = 83.5% | - | - | - | |||
| CSC14-2 | C1 | 20 | 8 | 12 | 120/375 = 32.0% | 25 | 33 | 1 | - | ||
| CSC17 | C1 | 0 | 8 | 24 | 198/230 = 86.1% | - | - | ||||
| CSC18-1 | C1 | 20 | 8 | 24 | 71/368 = 19.3% | 20 | 25 | 2 | 2/20 = 10.0% | ||
| CSC19 | C2 | 0 | 8 | 24 | 109/227 = 48.0% | - | - | - | |||
| CSC20-1 | C2 | 20 | 8 | 24 | 145/389 = 37.3% | 28 | 22 | 1 | 1/28 = 3.6% | ||
| CSC22-2 | C1 | 0 | 8 | 12 | 124/272 = 45.6% | - | - | - | |||
| CSC27-1 | C1 | 20 | 8 | 12 | 69/337 = 20.5% | 34 | 17 | 1 | 1/34 = 2.9% | ||
| CSC28 | C2 | 0 | 8 | 12 | 121/240 = 50.4% | - | - | - | |||
| CSC29-2 | C2 | 20 | 8 | 12 | 81/326 = 24.58% | 19 | 22 | 1 | 1/19 = 5.3% | ||
| CSC30-1 | C1 | 15 μM control-MO-Vivo 3 | 8 | 12 | 113/330 = 34.2% | 58 | 43 | 0 | |||
| CSC36-1 | C1 | 15 | 8 | 24 | 202/318 = 63.5% | 43 | 37 | 1 | 1/43 = 2.3% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhao, M.; Zohar, Y.; Wong, T.-T. Induction of Reproductive Sterility in Coho Salmon (Oncorhynchus kisutch) by an Immersion-Based Gene Silencing Technology. J. Mar. Sci. Eng. 2023, 11, 2208. https://doi.org/10.3390/jmse11122208
Xu L, Zhao M, Zohar Y, Wong T-T. Induction of Reproductive Sterility in Coho Salmon (Oncorhynchus kisutch) by an Immersion-Based Gene Silencing Technology. Journal of Marine Science and Engineering. 2023; 11(12):2208. https://doi.org/10.3390/jmse11122208
Chicago/Turabian StyleXu, Lan, Mingli Zhao, Yonathan Zohar, and Ten-Tsao Wong. 2023. "Induction of Reproductive Sterility in Coho Salmon (Oncorhynchus kisutch) by an Immersion-Based Gene Silencing Technology" Journal of Marine Science and Engineering 11, no. 12: 2208. https://doi.org/10.3390/jmse11122208
APA StyleXu, L., Zhao, M., Zohar, Y., & Wong, T.-T. (2023). Induction of Reproductive Sterility in Coho Salmon (Oncorhynchus kisutch) by an Immersion-Based Gene Silencing Technology. Journal of Marine Science and Engineering, 11(12), 2208. https://doi.org/10.3390/jmse11122208







