Emericellopsis maritima and Purpureocillium lilacinum Marine Fungi as a Source of Functional Fractions with Antioxidant and Antitumor Potential in Colorectal Cancer: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. General Experimental Procedures
2.3. Media and Culture Conditions
2.4. Extraction and Isolation of Fractions and Compounds
2.5. Cell Culture
2.6. Cytotoxicity Assay
2.7. Cell Cycle Assay
2.8. Cancer Stem-Like Cell (CSC) Isolation and Characterization
2.9. Antioxidant Capacity Using H2O2
2.10. Antioxidant Capacity Using ABTS
2.11. Statistical Analysis
3. Results
3.1. Isolated Fractions and Compounds from Marine Fungi
3.2. In Vitro Cytotoxicity of Fractions and Compounds from Marine Fungi
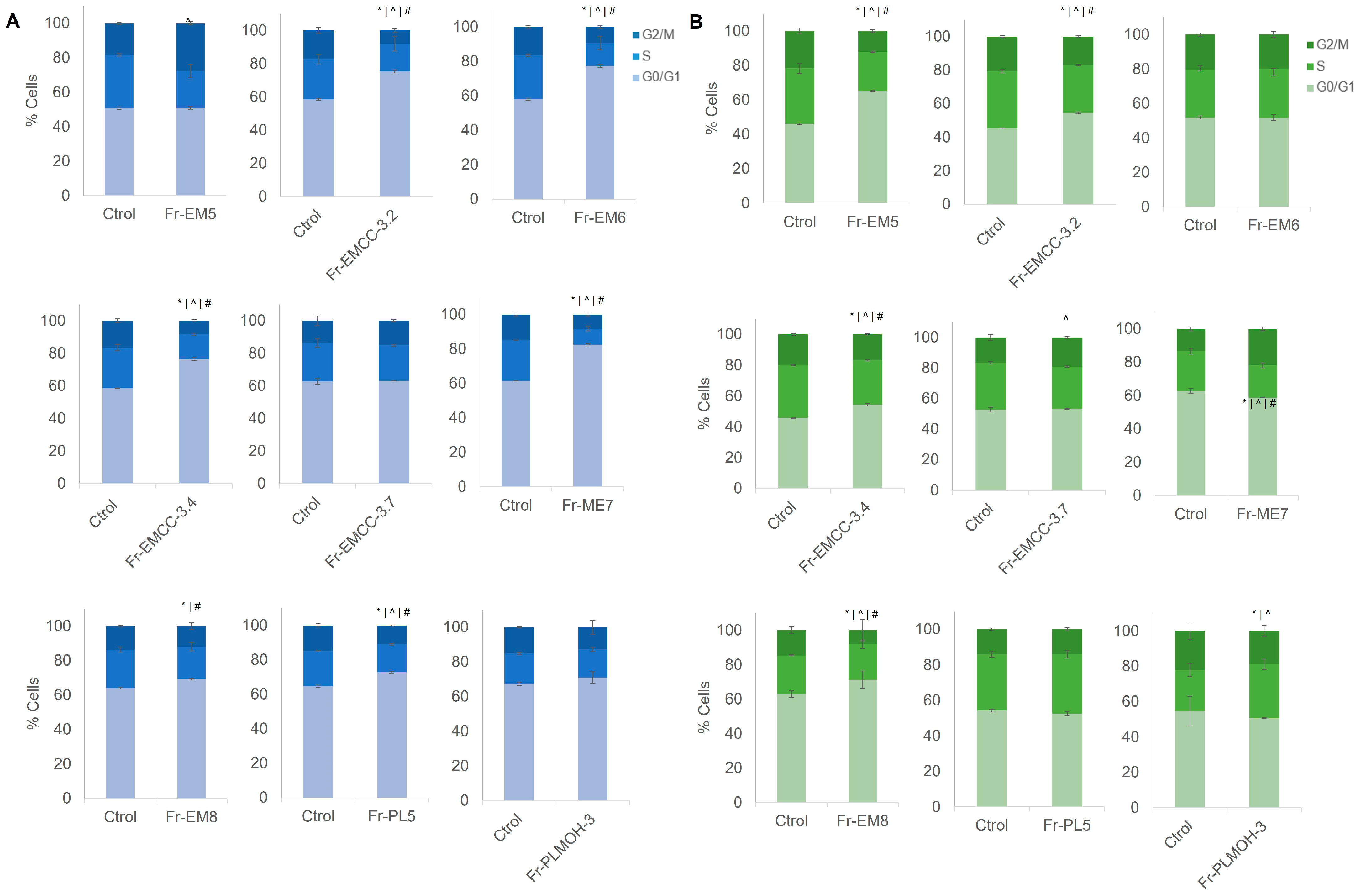
3.3. Cell Cycle Modulation by Marine-Derived Fungus Fractions
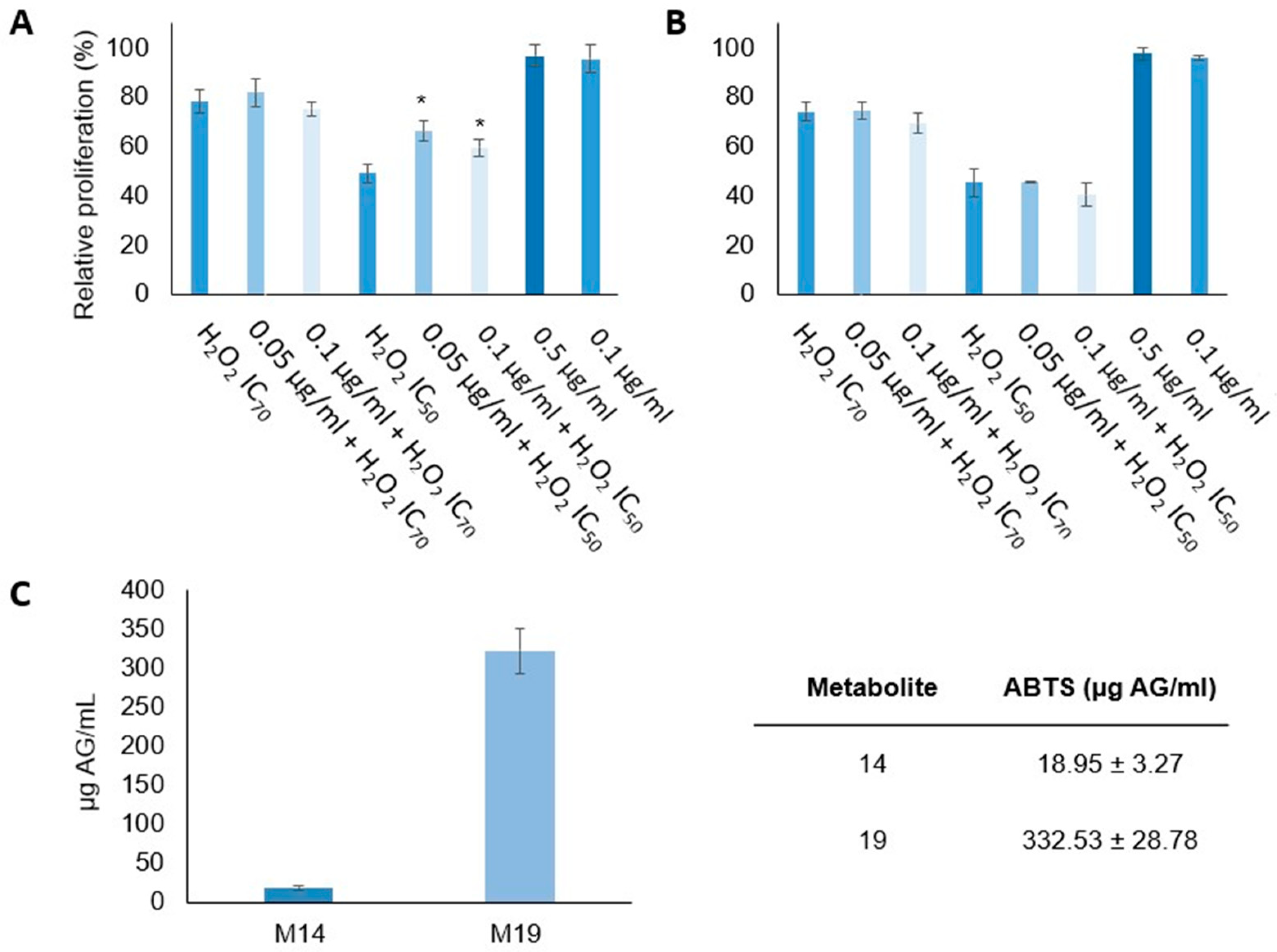
3.4. Antiproliferative Activity of Marine-Derived Fungus Fractions against Cancer Stem Cells
3.5. Antioxidant Activity of Marine-Derived Fungus Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- Hasan, S.; Ansari, M.I.; Ahmad, A.; Mishra, M. Major Bioactive Metabolites from Marine Fungi: A Review. Bioinformation 2015, 11, 176–181. [Google Scholar] [CrossRef]
- Rai, M.; Gade, A.; Zimowska, B.; Ingle, A.P.; Ingle, P. Marine-Derived Phoma-the Gold Mine of Bioactive Compounds. Appl. Microbiol. Biotechnol. 2018, 102, 9053–9066. [Google Scholar] [CrossRef]
- Ameen, F.; AlNAdhari, S.; Al-Homaidan, A.A. Marine Fungi Showing Multifunctional Activity against Human Pathogenic Microbes and Cancer. PLoS ONE 2022, 17, e0276926. [Google Scholar] [CrossRef]
- Julianti, E.; Abrian, I.A.; Wibowo, M.S.; Azhari, M.; Tsurayya, N.; Izzati, F.; Juanssilfero, A.B.; Bayu, A.; Rahmawati, S.I.; Putra, M.Y. Secondary Metabolites from Marine-Derived Fungi and Actinobacteria as Potential Sources of Novel Colorectal Cancer Drugs. Mar. Drugs 2022, 20, 67. [Google Scholar] [CrossRef]
- Tang, P.; Liu, D.; Wu, Z.; Cui, H.; Zhang, R.; Kuang, Z. Inhibitory Effects and Mechanism of the Natural Compound Diaporthein B Extracted from Marine-Derived Fungi on Colon Cancer Cells. Molecules 2022, 27, 2944. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.-B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a New Genus for the Medically Important Paecilomyces Lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Liu, R.; Khan, R.A.A.; Yue, Q.; Jiao, Y.; Yang, Y.; Li, Y.; Xie, B. Discovery of a New Antifungal Lipopeptaibol from Purpureocillium Lilacinum Using MALDI-TOF-IMS. Biochem. Biophys. Res. Commun. 2020, 527, 689–695. [Google Scholar] [CrossRef]
- Kuvarina, A.E.; Gavryushina, I.A.; Kulko, A.B.; Ivanov, I.A.; Rogozhin, E.A.; Georgieva, M.L.; Sadykova, V.S. The Emericellipsins A-E from an Alkalophilic Fungus Emericellopsis Alkalina Show Potent Activity against Multidrug-Resistant Pathogenic Fungi. J. Fungi 2021, 7, 153. [Google Scholar] [CrossRef]
- Luque, C.; Cepero, A.; Perazzoli, G.; Mesas, C.; Quiñonero, F.; Cabeza, L.; Prados, J.; Melguizo, C. In Vitro Efficacy of Extracts and Isolated Bioactive Compounds from Ascomycota Fungi in the Treatment of Colorectal Cancer: A Systematic Review. Pharmaceuticals 2023, 16, 22. [Google Scholar] [CrossRef]
- Porras-Alcalá, C.; Moya-Utrera, F.; García-Castro, M.; Sánchez-Ruiz, A.; López-Romero, J.M.; Pino-González, M.S.; Díaz-Morilla, A.; Kitamura, S.; Wolan, D.W.; Prados, J.; et al. The Development of the Bengamides as New Antibiotics against Drug-Resistant Bacteria. Mar. Drugs 2022, 20, 373. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nozawa, H.; Takiyama, H.; Hasegawa, K.; Kawai, K.; Hata, K.; Tanaka, T.; Nishikawa, T.; Sasaki, K.; Kaneko, M.; Murono, K.; et al. Adjuvant Chemotherapy Improves Prognosis of Resectable Stage IV Colorectal Cancer: A Comparative Study Using Inverse Probability of Treatment Weighting. Ther. Adv. Med. Oncol. 2019, 11, 1758835919838960. [Google Scholar] [CrossRef]
- Taieb, J.; Gallois, C. Adjuvant Chemotherapy for Stage III Colon Cancer. Cancers 2020, 12, 2679. [Google Scholar] [CrossRef]
- Dariya, B.; Aliya, S.; Merchant, N.; Alam, A.; Nagaraju, G.P. Colorectal Cancer Biology, Diagnosis, and Therapeutic Approaches. Crit. Rev. Oncog. 2020, 25, 71–94. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.-C.; Li, Y.-J.; Lu, X.-B.; Ji, G. Drug Resistance and New Therapies in Colorectal Cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Kokhdan, E.P.; Sadeghi, H.; Ghafoori, H.; Sadeghi, H.; Danaei, N.; Javadian, H.; Aghamaali, M.R. Cytotoxic Effect of Methanolic Extract, Alkaloid and Terpenoid Fractions of Stachys Pilifera against HT-29 Cell Line. Res. Pharm. Sci. 2018, 13, 404–412. [Google Scholar] [CrossRef]
- Lin, Y.; Li, B.; Zhao, J.; Wei, L.; Wang, Y.; Wang, M.; Dia, V.P.; Meng, X. Combinatorial Effect of Blueberry Extracts and Oxaliplatin in Human Colon Cancer Cells. J. Cell Physiol. 2019, 234, 17242–17253. [Google Scholar] [CrossRef]
- GAO, S.-S.; SHANG, Z.; LI, X.-M.; LI, C.-S.; CUI, C.-M.; WANG, B.-G. Secondary Metabolites Produced by Solid Fermentation of the Marine-Derived Fungus Penicillium Commune QSD-17. Biosci. Biotechnol. Biochem. 2012, 76, 358–360. [Google Scholar] [CrossRef]
- Oliver, J.A.; Ortiz, R.; Melguizo, C.; Álvarez, P.J.; Gómez-Millán, J.; Prados, J. Prognostic Impact of MGMT Promoter Methylation and MGMT and CD133 Expression in Colorectal Adenocarcinoma. BMC Cancer 2014, 14, 511. [Google Scholar] [CrossRef][Green Version]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Izuta, I.; Oka, H.; Sakaguchi, R.; Kobayashi, M.; Satoh, T. Studies on Terpenoids and Related Alicyclic Compounds. XVII. Total Syntheses of Sesquiterpenoids; (±)-Isopetasol, (±)-3-Epiisopetasol, (±)-Warburgiadione, and (±)-Petasitol. Chem. Pharm. Bull. 1979, 27, 331–340. [Google Scholar] [CrossRef][Green Version]
- Yamakawa, K.; Izuta, I.; Oka, H.; Sakaguchi, R. Total Synthesis of (±)-Isopetasol, (±)-3-Epiisopetasol, and (±)-Warburgiadion. Tetrahedron Lett. 1974, 15, 2187–2190. [Google Scholar] [CrossRef]
- Bohlmann, F.; Knoll, K.-H. Natürlich vorkommende Terpen-Derivate, 200 Zwei neue Eremophilan-Derivate aus Senecio suaveolens. Liebigs Ann. Der. Chem. 1979, 1979, 470–472. [Google Scholar] [CrossRef]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and Antifungal Compounds from Aspergillus, Penicillium and Other Filamentous Fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed]
- Noman, E.; Al-Shaibani, M.M.; Bakhrebah, M.A.; Almoheer, R.; Al-Sahari, M.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Almulaiky, Y.Q.; Abdulaal, W.H. Potential of Anti-Cancer Activity of Secondary Metabolic Products from Marine Fungi. J. Fungi 2021, 7, 436. [Google Scholar] [CrossRef]
- Kawada, M.; Inoue, H.; Ohba, S.-I.; Masuda, T.; Momose, I.; Ikeda, D. Leucinostatin A Inhibits Prostate Cancer Growth through Reduction of Insulin-like Growth Factor-I Expression in Prostate Stromal Cells. Int. J. Cancer 2010, 126, 810–818. [Google Scholar] [CrossRef]
- Kil, Y.-S.; Risinger, A.L.; Petersen, C.L.; Mooberry, S.L.; Cichewicz, R.H. Leucinostatins from Ophiocordyceps Spp. and Purpureocillium Spp. Demonstrate Selective Antiproliferative Effects in Cells Representing the Luminal Androgen Receptor Subtype of Triple Negative Breast Cancer. J. Nat. Prod. 2020, 83, 2010–2024. [Google Scholar] [CrossRef]
- Mfengwana, P.H.; Mashele, S.S.; Manduna, I.T. Cytotoxicity and Cell Cycle Analysis of Asparagus Laricinus Burch. and Senecio Asperulus DC. on Breast and Prostate Cancer Cell Lines. Heliyon 2019, 5, e01666. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Cabeza, L.; Ortiz, R.; Prados, J.; Melguizo, C.; Cheng-Sánchez, I.; López-Romero, J.M.; Sarabia, F. Bengamide Analogues Show A Potent Antitumor Activity against Colon Cancer Cells: A Preliminary Study. Mar. Drugs 2020, 18, 240. [Google Scholar] [CrossRef]
- Phi, T.D.; Doan Thi Mai, H.; Tran, V.H.; Truong, B.N.; Tran, T.A.; Vu, V.L.; Chau, V.M.; Pham, V.C. Design, Synthesis and Cytotoxicity of Bengamide Analogues and Their Epimers. Medchemcomm 2017, 8, 445–451. [Google Scholar] [CrossRef]
- Wang, C.C.C.; Chiang, Y.M.; Kuo, P.L.; Chang, J.K.; Hsu, Y.L. Norsolorinic Acid Inhibits Proliferation of T24 Human Bladder Cancer Cells by Arresting the Cell Cycle at the G0/G1 Phase and Inducing a FAS/Membrane-Bound FAS Ligand-Mediated Apoptotic Pathway. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1301–1308. [Google Scholar] [CrossRef]
- Xie, C.-L.; Zhang, D.; Xia, J.-M.; Hu, C.-C.; Lin, T.; Lin, Y.-K.; Wang, G.-H.; Tian, W.-J.; Li, Z.-P.; Zhang, X.-K.; et al. Steroids from the Deep-Sea-Derived Fungus Penicillium Granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway. Mar. Drugs 2019, 17, 178. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, B.; Lin, X.; Luo, X.; Pang, X.; Tang, L.; Liu, Y.; Li, X.; Zhou, X. Nitrobenzoyl Sesquiterpenoids with Cytotoxic Activities from a Marine-Derived Aspergillus Ochraceus Fungus. J. Nat. Prod. 2018, 81, 92–97. [Google Scholar] [CrossRef]
- Pucci, B.; Kasten, M.; Giordano, A. Cell Cycle and Apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Hnit, S.S.T.; Yao, M.; Xie, C.; Ge, G.; Bi, L.; Jin, S.; Jiao, L.; Xu, L.; Long, L.; Nie, H.; et al. Transcriptional Regulation of G2/M Regulatory Proteins and Perturbation of G2/M Cell Cycle Transition by a Traditional Chinese Medicine Recipe. J. Ethnopharmacol. 2020, 251, 112526. [Google Scholar] [CrossRef]
- Astuti, P.; Erden, W.; Wahyono, N.; Wahyuono, S.; Hertiani, T. Pyrophen Produced by Endophytic Fungi Aspergillus Sp Isolated from Piper Crocatum Ruiz and Pav Exhibits Cytotoxic Activity and Induces S Phase Arrest in T47D Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2016, 17, 615–618. [Google Scholar] [CrossRef]
- Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, L.; Wang, H.; Oyang, L.; Su, M.; Liu, Q.; Lin, J.; Tan, S.; Tian, Y.; Liao, Q.; et al. Cancer Stem Cells in Progression of Colorectal Cancer. Oncotarget 2018, 9, 33403–33415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Wang, S.-Y.; Shen, H.; Yao, X.-F.; Zhang, F.-L.; Lai, D. The Marine-Derived Fungal Metabolite, Terrein, Inhibits Cell Proliferation and Induces Cell Cycle Arrest in Human Ovarian Cancer Cells. Int. J. Mol. Med. 2014, 34, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Dezaire, A.; Marchand, C.H.; Vallet, M.; Ferrand, N.; Chaouch, S.; Mouray, E.; Larsen, A.K.; Sabbah, M.; Lemaire, S.D.; Prado, S.; et al. Secondary Metabolites from the Culture of the Marine-Derived Fungus Paradendryphiella Salina PC 362H and Evaluation of the Anticancer Activity of Its Metabolite Hyalodendrin. Mar. Drugs 2020, 18, 191. [Google Scholar] [CrossRef] [PubMed]


| Sample | T84 IC50 (µg/mL) | SW480 IC50 (µg/mL) |
|---|---|---|
| Fr-EM5 | 147.1 ± 0.033 | 65.0 ± 0.039 |
| Fr-EM3.3 | 58.90 ± 0.023 | 27.94 ± 0.073 |
| Fr-EMCC-3.2 | 206.7 ± 0.022 | 125.3 ± 0.019 |
| Fr-EM6 | 91.6 ± 0.048 | 48.2 ± 0.029 |
| Fr-EMCC-3.4 | 84.0 ± 0.075 | 75.2 ± 0.047 |
| Fr-EMCC-3.6 | 114.7 ± 0.086 | 106.5 ± 0.051 |
| Fr-EMCC-3.7 | 56.3 ± 0.053 | 23.2 ± 0.039 |
| Fr-EM7 | 93.8 ± 0.022 | 51.1 ± 0.054 |
| Fr-EM8 | 71.7 ± 0.036 | 38.3 ± 0.027 |
| Fr-PL5 | 88.4 ± 0.052 | 41.4 ± 0.008 |
| Fr-PLMOH-3 | 11.3 ± 0.041 | 6.4 ± 0.016 |
| 1 | 24.75 ± 0.150 | 24.15 ± 0.065 |
| 2 | 44.47 ± 0.112 | 66.35 ± 0.030 |
| 3 | 32.06 ± 0.082 | 14.46 ± 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perazzoli, G.; de los Reyes, C.; Pinedo-Rivilla, C.; Durán-Patrón, R.; Aleu, J.; Cabeza, L.; Melguizo, C.; Prados, J. Emericellopsis maritima and Purpureocillium lilacinum Marine Fungi as a Source of Functional Fractions with Antioxidant and Antitumor Potential in Colorectal Cancer: A Preliminary Study. J. Mar. Sci. Eng. 2023, 11, 2024. https://doi.org/10.3390/jmse11102024
Perazzoli G, de los Reyes C, Pinedo-Rivilla C, Durán-Patrón R, Aleu J, Cabeza L, Melguizo C, Prados J. Emericellopsis maritima and Purpureocillium lilacinum Marine Fungi as a Source of Functional Fractions with Antioxidant and Antitumor Potential in Colorectal Cancer: A Preliminary Study. Journal of Marine Science and Engineering. 2023; 11(10):2024. https://doi.org/10.3390/jmse11102024
Chicago/Turabian StylePerazzoli, Gloria, Carolina de los Reyes, Cristina Pinedo-Rivilla, Rosa Durán-Patrón, Josefina Aleu, Laura Cabeza, Consolación Melguizo, and Jose Prados. 2023. "Emericellopsis maritima and Purpureocillium lilacinum Marine Fungi as a Source of Functional Fractions with Antioxidant and Antitumor Potential in Colorectal Cancer: A Preliminary Study" Journal of Marine Science and Engineering 11, no. 10: 2024. https://doi.org/10.3390/jmse11102024
APA StylePerazzoli, G., de los Reyes, C., Pinedo-Rivilla, C., Durán-Patrón, R., Aleu, J., Cabeza, L., Melguizo, C., & Prados, J. (2023). Emericellopsis maritima and Purpureocillium lilacinum Marine Fungi as a Source of Functional Fractions with Antioxidant and Antitumor Potential in Colorectal Cancer: A Preliminary Study. Journal of Marine Science and Engineering, 11(10), 2024. https://doi.org/10.3390/jmse11102024