Photophysiological Characterization of Phytoplankton by Measuring Pigment Production Rates: A Description of Detail Method and a Case Study
Abstract
1. Introduction
2. Materials and Methods
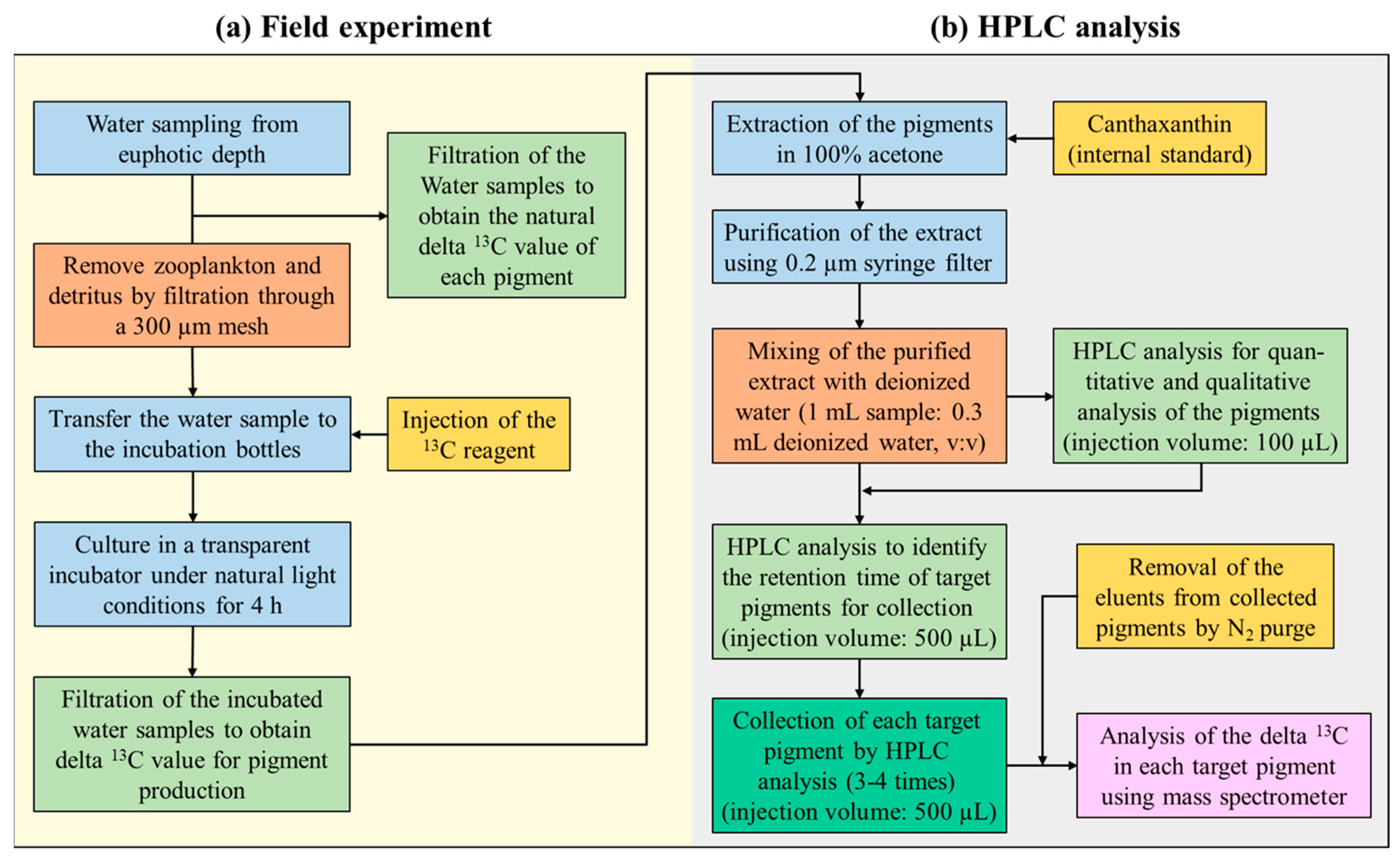
2.1. Carbon Stable Isotope Fractionation Experiment for Pigment Production
2.2. In Situ Culture Experiment Procedure for Pigment Production Using a 13C Tracer
2.3. Extraction and Analysis of Phytoplankton Pigments for Production Assessment
2.4. Calculation of the Pigment Concentration and Production Rate
2.5. Chemotaxonomic Analysis
2.6. Statical Analyses
3. Results
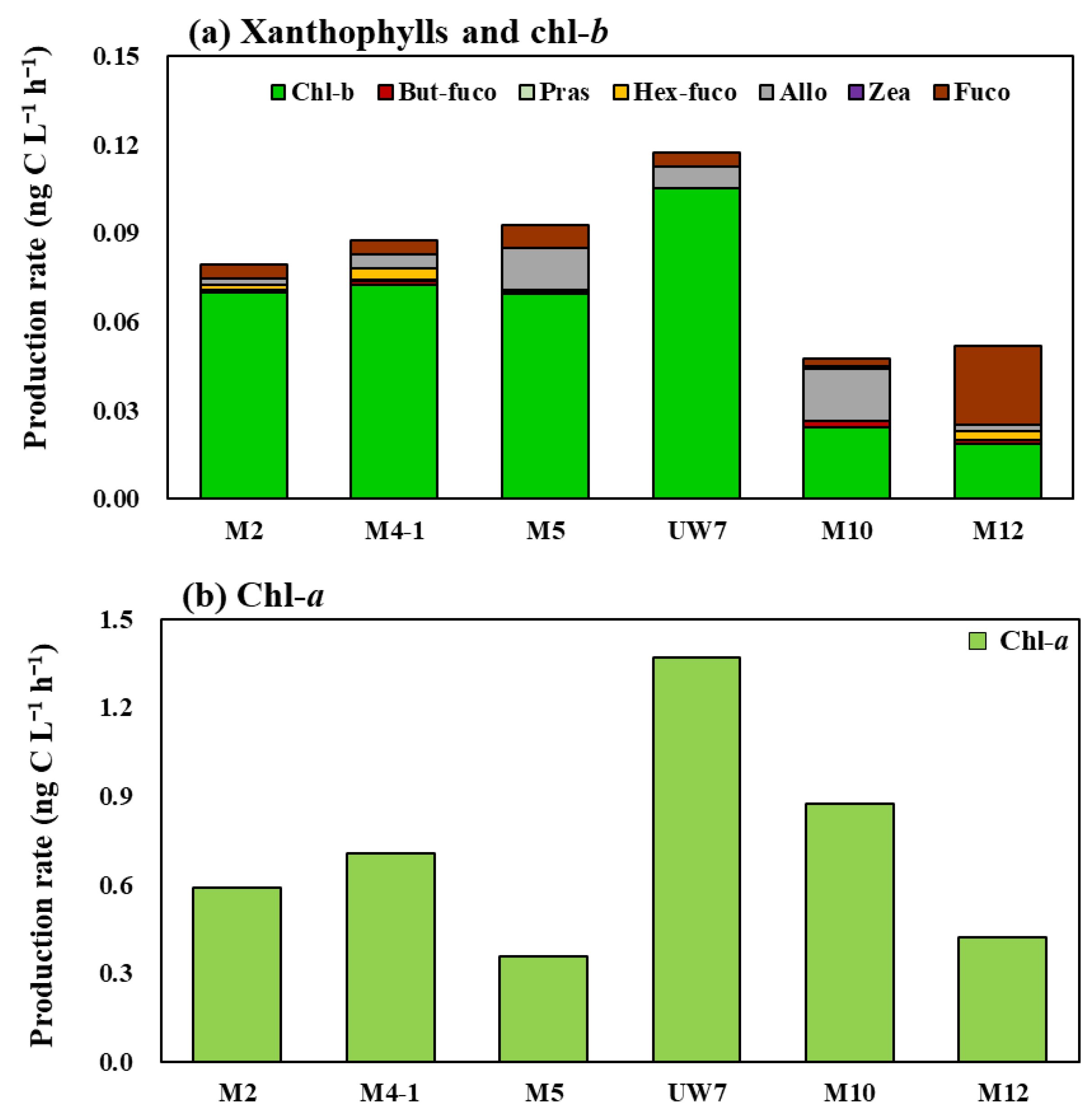
3.1. Pigment Production Analysis Using HPLC
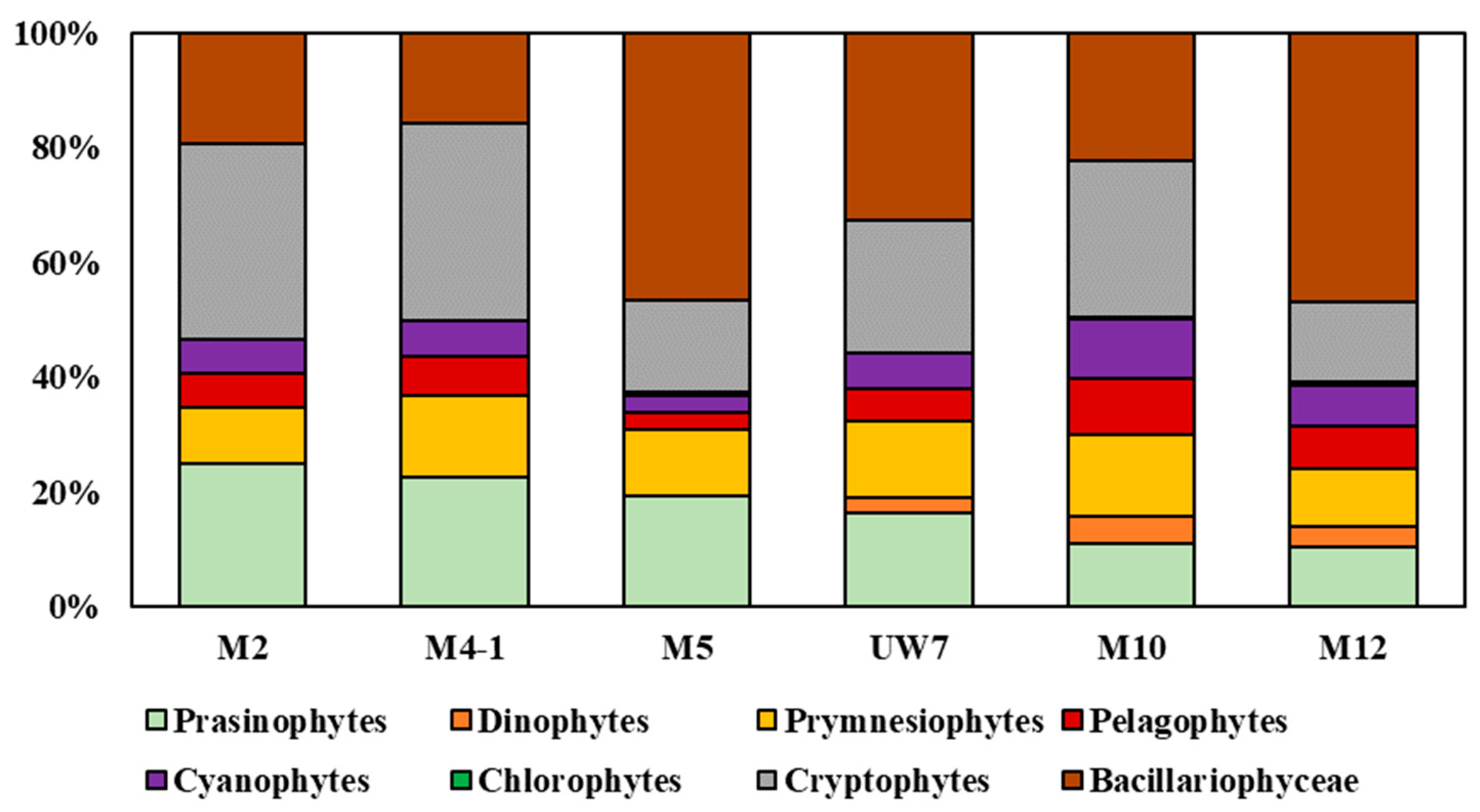
3.2. Phytoplankton Community Structure and Pigment Production Rates
4. Discussion
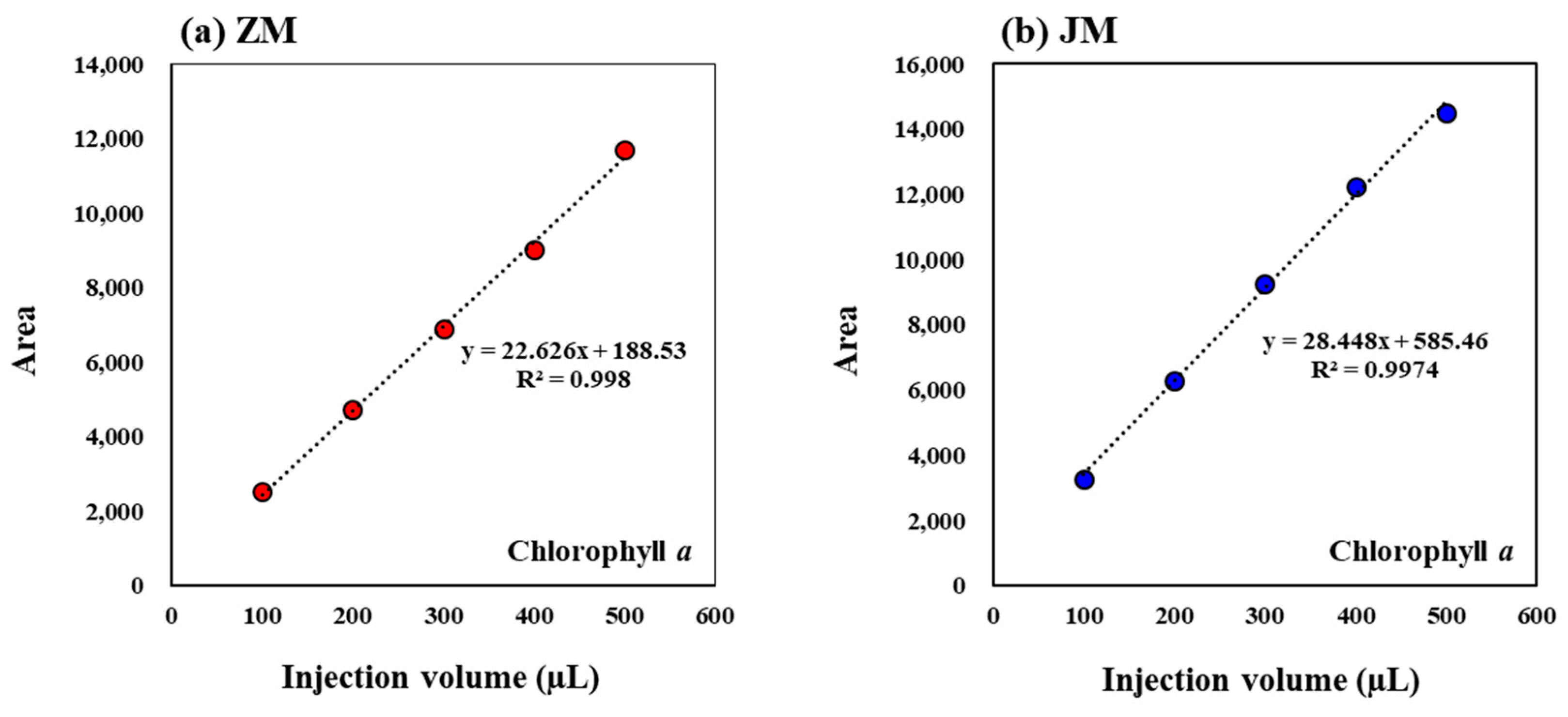
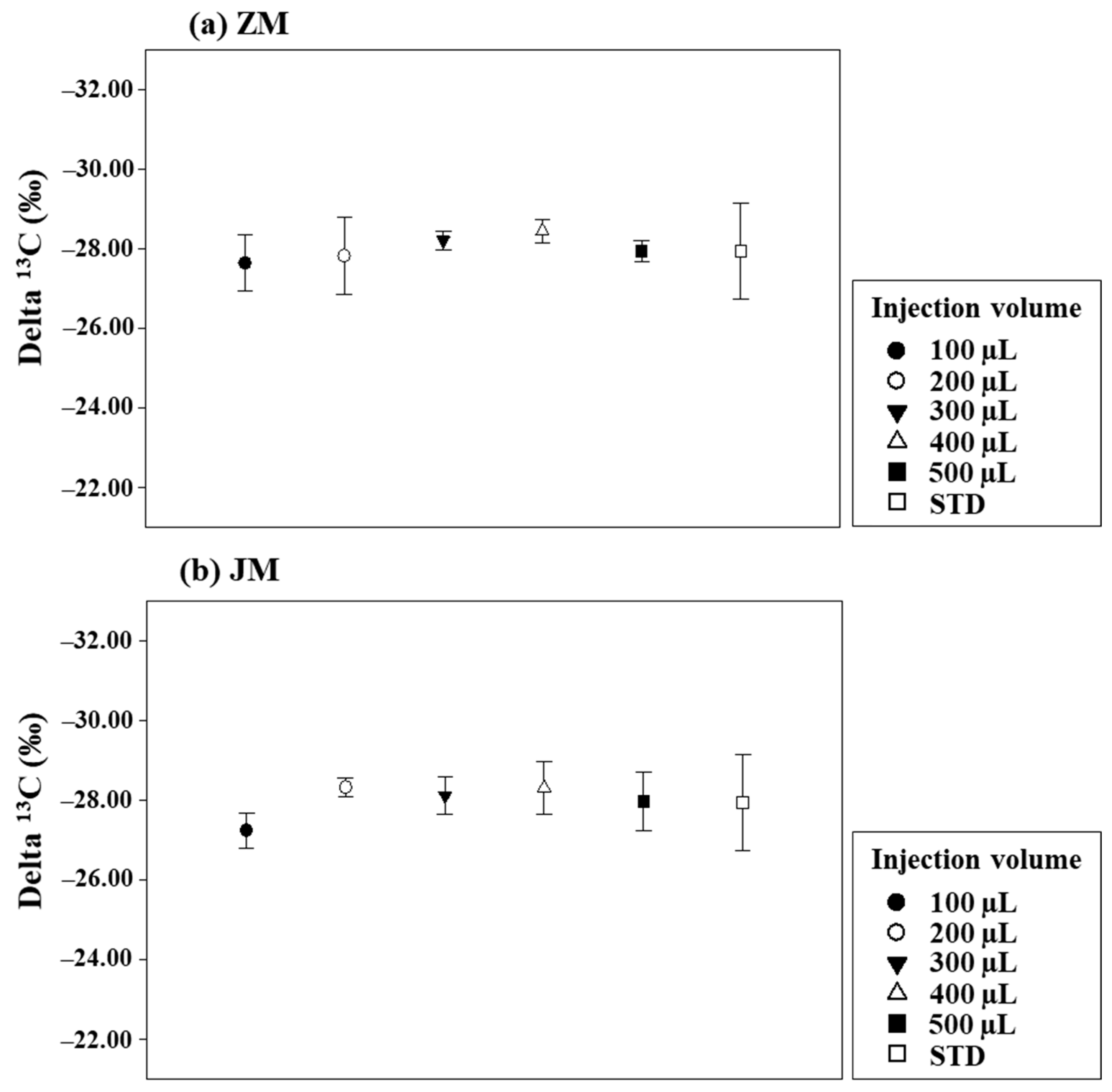
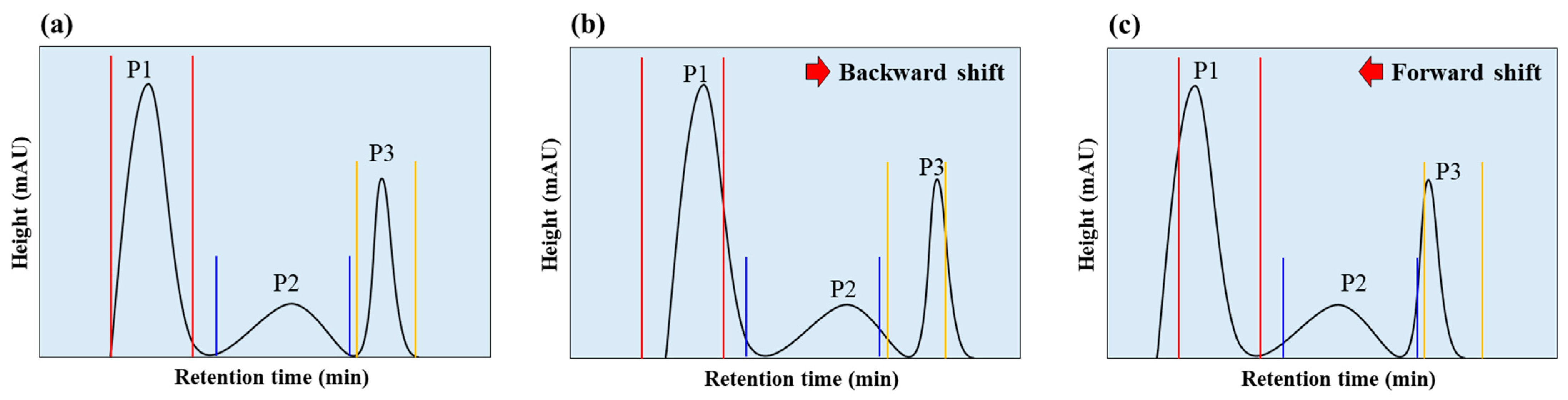
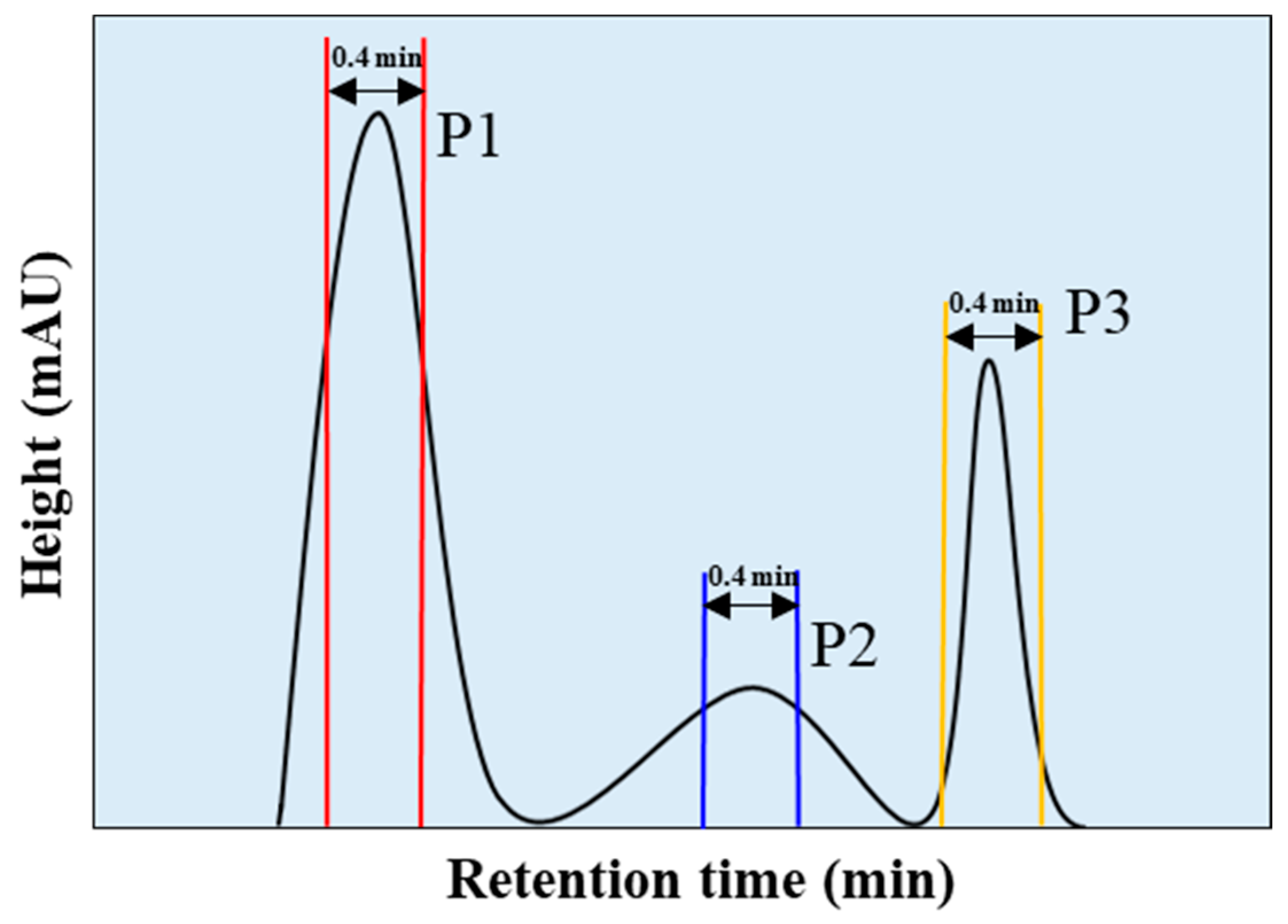
4.1. Enhancing Sensitivity in Pigment Production Rate Measurement
4.2. Recommendations for Pigment Production in Field Experiment
4.3. Recommendations for Sample Analysis in Home Laboratory
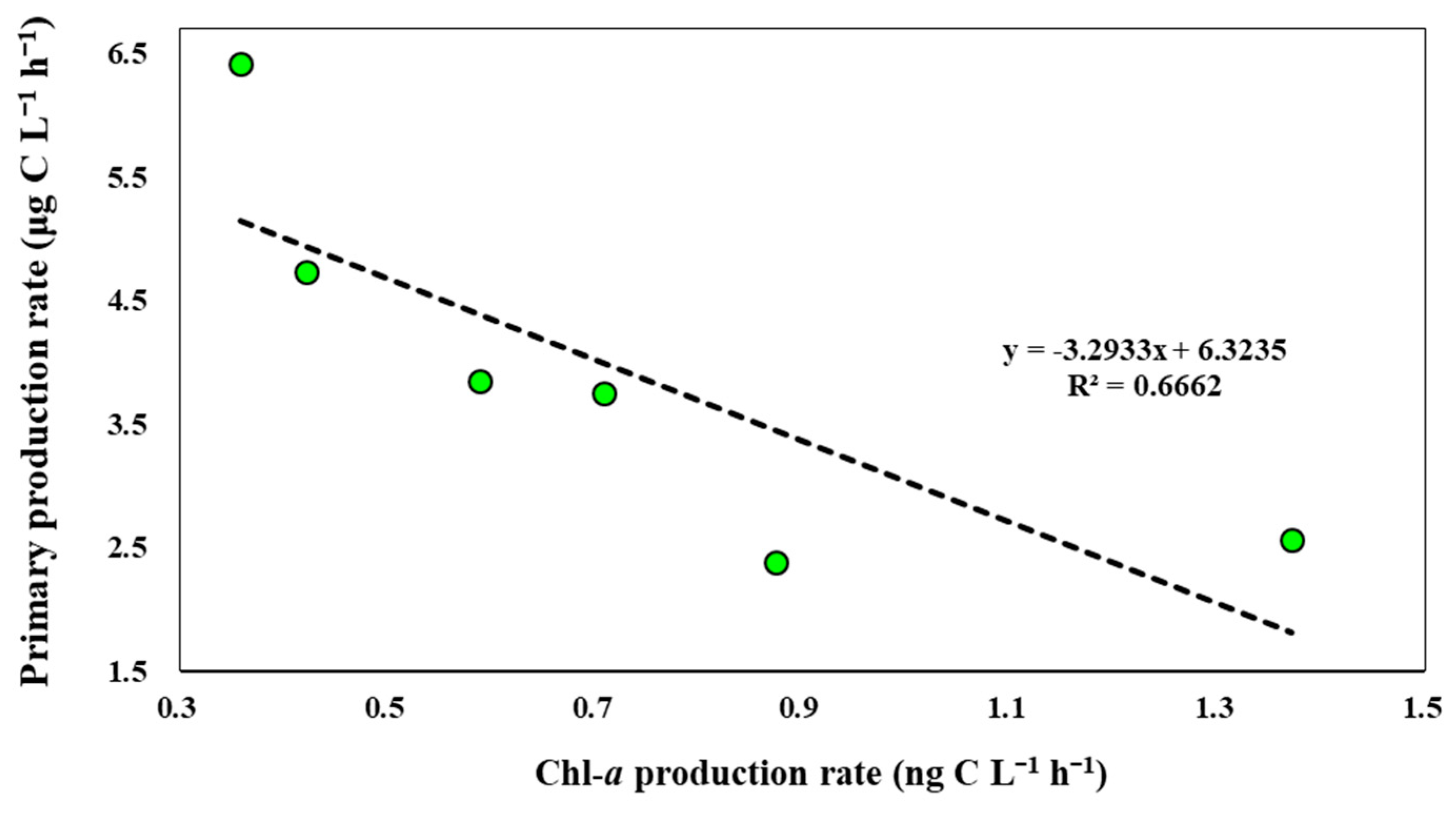
4.4. Photophysiological Status of Natural Phytoplankton Community
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buma, A.G.J.; Treguer, P.; Kraay, G.W.; Morvan, J. Algal pigment patterns in different watermasses of the Atlantic sector of the Southern Ocean during fall 1987. Polar Biol. 1990, 11, 55–62. [Google Scholar] [CrossRef]
- Gieskes, W.W.; Kraay, G.W. Floristic and physiological differences between the shallow and the deep nanophytoplankton community in the euphotic zone of the open tropical Atlantic revealed by HPLC analysis of pigments. Mar. Biol. 1986, 91, 567–576. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Hallegraeff, G.M. Phytoplankton pigments, species and light climate in a complex warm-core eddy of the East Australian Current. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 1987, 34, 649–673. [Google Scholar] [CrossRef]
- Wright, S.W. Phytoplankton pigment data: Prydz Bay region. Aust. Antarct. Res. Exped. Res. Notes 1987, 58, 1–106. [Google Scholar]
- Ondrusek, M.E.; Bidigare, R.R.; Sweet, S.T.; Defreitas, D.A.; Brooks, J.M. Distribution of phytoplankton pigments in the North Pacific Ocean in relation to physical and optical variability. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 1991, 38, 243–266. [Google Scholar] [CrossRef]
- Jeffrey, S.; Wright, S.; Zapata, M. Microalgal classes and their signature pigments. In Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge Environmental Chemistry Series; Roy, S., Llewellyn, C., Egeland, E., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 3–77. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2023, 850, 2691–2706. [Google Scholar] [CrossRef]
- Kyewalyanga, M. Phytoplankton primary production. In The Regional State of the Coast Report: Western Indian Ocean; UNEP-Nairobi Convention: Nairobi, Kenya, 2016; pp. 213–230. [Google Scholar]
- Mendes, C.R.B.; Tavano, V.M.; Dotto, T.S.; Kerr, R.; De Souza, M.S.; Garcia, C.A.E.; Secchi, E.R. New insights on the dominance of cryptophytes in Antarctic coastal waters: A case study in Gerlache Strait. Deep-Sea Res. Part II-Top. Stud. Oceanogr. 2018, 149, 161–170. [Google Scholar] [CrossRef]
- Neeley, A.R.; Lomas, M.W.; Mannino, A.; Thomas, C.; Vandermeulen, R. Impact of Growth Phase, Pigment Adaptation, and Climate Change Conditions on the Cellular Pigment and Carbon Content of Fifty-One Phytoplankton Isolates. J. Phycol. 2022, 58, 669–690. [Google Scholar] [CrossRef]
- Li, Z.; Sun, D.; Wang, S.; Huan, Y.; Zhang, H.; Liu, J.; He, Y. A global satellite observation of phytoplankton taxonomic groups over the past two decades. Glob. Chang. Biol. 2023, 19, 4511–4529. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. (Eds.) Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Barton, A.D.; Pershing, A.J.; Litchman, E.; Record, N.R.; Edwards, K.F.; Finkel, Z.V.; Kiørboe, T.; Ward, B.A. The biogeography of marine plankton traits. Ecol. Lett. 2013, 16, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Z.V.; Irwin, A.J.; Schofield, O. Resource limitation alters the 3/4 size scaling of metabolic rates in phytoplankton. Mar. Ecol. Prog. Ser. 2004, 273, 269–279. [Google Scholar] [CrossRef]
- Ha, S.Y.; La, H.S.; Min, J.O.; Chung, K.H.; Kang, S.H.; Shin, K.H. Photoprotective function of mycosporine-like amino acids in a bipolar diatom (Porosira glacialis): Evidence from ultraviolet radiation and stable isotope probing. Diatom Res. 2014, 29, 399–409. [Google Scholar] [CrossRef]
- Grumbach, K.H.; Lichtenthaler, H.K.; Erismann, K.H. Incorporation of 14CO2 in photosynthetic pigments of Chlorella pyrenoidosa. Planta 1978, 140, 37–43. [Google Scholar] [CrossRef]
- Riper, D.M.; Owens, T.G.; Falkowski, P.G. Chlorophyll turnover in Skeletonema costatum, a marine plankton diatom. Plant Physiol. 1979, 64, 49–54. [Google Scholar] [CrossRef]
- Goericke, R.; Welschmeyer, N.A. Pigment turnover in the marine diatom Thalassiosira weissflogii. I. The 14CO2-labeling kinetics of chlorophyll a1. J. Phycol. 1992, 28, 498–507. [Google Scholar] [CrossRef]
- Hama, T.; Miyazaki, T.; Ogawa, Y.; Iwakuma, T.; Takahashi, M.; Otsuki, A.; Ichimura, S. Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar. Biol. 1983, 73, 31–36. [Google Scholar] [CrossRef]
- Mantoura, R.F.C.; Llewellyn, C.A. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reversephase high-performance liquid chromatography. Anal. Chim. Acta 1983, 151, 297–314. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffrey, S.W.; Mantoura, R.F.C.; Llewellyn, C.A.; Bjornland, T.; Repeta, D.; Welschmeyer, N. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 77, 183–196. [Google Scholar] [CrossRef]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A. 2001, 910, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.W. Qualitative and quantitative HPLC analysis of SCOR reference algal cultures. In Phytoplankton Pigments in Oceanography; UNESCO: Paris, France, 1997; pp. 343–360. [Google Scholar]
- Lee, S.H.; Joo, H.M.; Liu, Z.; Chen, J.; He, J. Phytoplankton productivity in newly opened waters of the Western Arctic Ocean. Deep-Sea Res. Part II-Top. Stud. Oceanogr. 2012, 81, 18–27. [Google Scholar] [CrossRef]
- Ha, S.Y.; Lee, Y.; Kim, M.S.; Kumar, K.S.; Shin, K.H. Seasonal changes in mycosporine-like amino acid production rate with respect to natural phytoplankton species composition. Mar. Drugs 2015, 13, 6740–6758. [Google Scholar] [CrossRef]
- Lee, S.H.; Joo, H.; Lee, J.H.; Lee, J.H.; Kang, J.J.; Lee, H.W.; Lee, D.; Kang, C.K. Seasonal carbon uptake rates of phytoplankton in the northern East/Japan Sea. Deep-Sea Res. Part II-Top. Stud. Oceanogr. 2017, 143, 45–53. [Google Scholar] [CrossRef]
- Kang, J.J.; Jang, H.K.; Lim, J.H.; Lee, D.; Lee, J.H.; Bae, H.; Lee, C.H.; Kang, C.-K.; Lee, S.H. Characteristics of Different Size Phytoplankton for Primary Production and Biochemical Compositions in the Western East/Japan Sea. Front. Microbiol. 2020, 11, 3306. [Google Scholar] [CrossRef]
- Jang, S.J.; Park, M.O. Evaluation of Grinding Effects on the Extraction of Photosynthetic Pigments for HPLC Analysis. Sea 2015, 20, 71–77. [Google Scholar] [CrossRef][Green Version]
- Park, M.O. Composition and distribution of phytoplankton with size fraction results at southwestern East/Japan Sea. Ocean. Sci. J. 2006, 41, 301–313. [Google Scholar] [CrossRef]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX—A program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 256–283. [Google Scholar] [CrossRef]
- Wright, S.W.; Thomas, D.P.; Marchant, H.J.; Higgins, H.W.; Mackey, M.D.; Mackey, M.D. Analysis of phytoplankton of the Australian sector of the Southern Ocean: Comparisons of microscopy and size frequency data with interpretations of pigment HPLC data using the ‘CHEMTAX’ matrix factorisation program. Mar. Ecol. Prog. Ser. 1996, 144, 285–298. [Google Scholar] [CrossRef]
- Wright, S.W.; van den Enden, R.L. Phytoplankton community structure and stocks in the Eastern Antarctic marginal ice zone (BROKE survey, January e March 1996) determined by CHEMTAX analysis of HPLC pigment signatures. Deep-Sea Res. Part II-Top. Stud. Oceanogr. 2000, 47, 2363–2400. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B.Q.; Huang, Q.; Wang, L.; Ni, X.B.; Tang, Q.S.; Sun, S.; Wei, H.; Liu, S.M.; Li, C.L.; et al. Seasonal phytoplankton response to physical processes in the southern Yellow Sea. J. Sea Res. 2015, 95, 45–55. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Landry, M.R.; Chiang, K.-P.; Wang, L.; Huang, B. Responses of phytoplankton communities to environmental variability in the East China Sea. Ecosystems 2016, 19, 832–849. [Google Scholar] [CrossRef]
- Latasa, M. A simple method to increase sensitivity for RP-HPLC phytoplankton pigment analysis. Limnol. Oceanogr. Meth. 2014, 12, 46–53. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, T.W.; Lee, S.; Lee, D.; Park, J.; Kim, B.K.; Kim, K.; Jang, H.K.; Bhavya, P.S.; Lee, S.H. Seasonal variations in the small phytoplankton contribution to the total primary production in the Amundsen Sea, Antarctica. J. Geophys. Res.-Oceans 2019, 124, 8324–8341. [Google Scholar] [CrossRef]
- Kim, K.; Ha, S.Y.; Kim, B.K.; Mundy, C.J.; Gough, K.M.; Pogorzelec, N.M.; Lee, S.H. Carbon and nitrogen uptake rates and macromolecular compositions of bottom-ice algae and phytoplankton at Cambridge Bay in Dease Strait, Canada. Ann. Glaciol. 2020, 61, 106–116. [Google Scholar] [CrossRef]
- Yun, M.S.; Kim, Y.; Jeong, Y.; Joo, H.T.; Jo, Y.H.; Lee, C.H.; Bae, H.; Lee, D.; Bhavya, P.S.; Kim, D.; et al. Weak response of biological productivity and community structure of phytoplankton to mesoscale eddies in the oligotrophic Philippine Sea. J. Geophys. Res.-Oceans 2020, 125, e2020JC016436. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, J.J.; Jang, H.K.; Jo, N.; Lee, D.; Yun, M.S.; Lee, S.H. Major controlling factors for spatio-temporal variations in the macromolecular composition and primary production by phytoplankton in Garolim and Asan bays in the Yellow Sea. Reg. Stud. Mar. Sci. 2020, 36, 101269. [Google Scholar] [CrossRef]
- Johnsen, G.; Prézelin, B.B.; Jovine, R.V.M. Fluorescence excitation spectra and light utilization in two red tide dinoflagellates. Limnol. Oceanogr. 1997, 42, 1166–1177. [Google Scholar] [CrossRef]
- Stolte, W.; Kraay, G.W.; Noordeloos, A.A.M.; Riegman, R. Genetic and physiological variation in pigment composition of Emiliania huxleyi (Prymnesiophyceae) and the potential use of its pigment rations as a quantitative physiological marker. J. Phycol. 2000, 36, 529–539. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Chen, Y.-B. Photoacclimation of light harvesting systems in eukaryotic algae. In Light-Harvesting Antennas in Photosynthesis; Springer: Dordrecht, The Netherlands, 2003; pp. 423–447. [Google Scholar]
- Rodríguez, F.; Chauton, M.; Johnsen, G.; Andresen, K.; Olsen, L.M.; Zapata, M. Photoacclimation in phytoplankton: Implications for biomass estimates, pigment functionality and chemotaxonomy. Mar. Biol. 2006, 148, 963–971. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Lewitus, A.J.; Kana, T.M.; Craft, N.E. Improved separations of phytoplankton pigments using temperature-controlled highperformance liquid chromatography. Mar. Ecol. Prog. Ser. 1994, 114, 303–313. [Google Scholar] [CrossRef]
- Garrido, J.L.; Zapata, M. Reversed phase high performance liquid chromatographic separation of mono- and divinyl chlorophyll forms using pyridine-containing mobile phases and a polymeric octadecyl silica column. Chromatographia 1997, 44, 43–49. [Google Scholar] [CrossRef]
- Inbaraj, S.B.; Chien, J.T.; Chen, B.H. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Canuti, E. Phytoplankton pigment in situ measurements uncertainty evaluation: An HPLC interlaboratory comparison with a European-scale dataset. Front. Mar. Sci. 2023, 10, 1197311. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Zhang, Z.; Ding, C.; Sun, J. The impact of environmental factors on the phytoplankton communities in the Western Pacific Ocean: HPLC-CHEMTAX approach. Front. Mar. Sci. 2023, 10, 1185939. [Google Scholar] [CrossRef]
- Goericke, R.; Welschmeyer, N.A. Pigment turnover in the marine diatom Thalassiosira weissflogii. II. the 14CO2-labeling kinetics of Carotenoids1. J. Phycol. 1992, 28, 507–517. [Google Scholar] [CrossRef]
- Kang, J.J.; Min, J.O.; Kim, Y.; Lee, C.H.; Yoo, H.; Jang, H.K.; Kim, M.-J.; Oh, H.-J.; Lee, S.H. Vertical distribution of phytoplankton community and pigment production in the Yellow Sea and the East China Sea during the late summer season. Water 2021, 13, 3321. [Google Scholar] [CrossRef]
- Lee, C.H.; Kang, J.J.; Min, J.O.; Bae, H.; Kim, Y.; Park, S.; Kim, J.; Kim, D.; Lee, S.H. Physiological characteristics of phytoplankton in response to different light environments in the Philippine Sea, Northwestern Pacific Ocean. Front. Mar. Sci. 2022, 9, 930690. [Google Scholar] [CrossRef]
| Equipment | Conditions | ||
|---|---|---|---|
| Pump |
| ||
| Time | Ratio | ||
| A | B | ||
| 0 | 100 | 0 | |
| 21 | 60 | 40 | |
| 27 | 5 | 95 | |
| 37 | 5 | 95 | |
| 45 | 100 | 0 | |
| |||
| Sampler |
| ||
| Column |
| ||
| Detector |
| ||
| Collector |
| ||
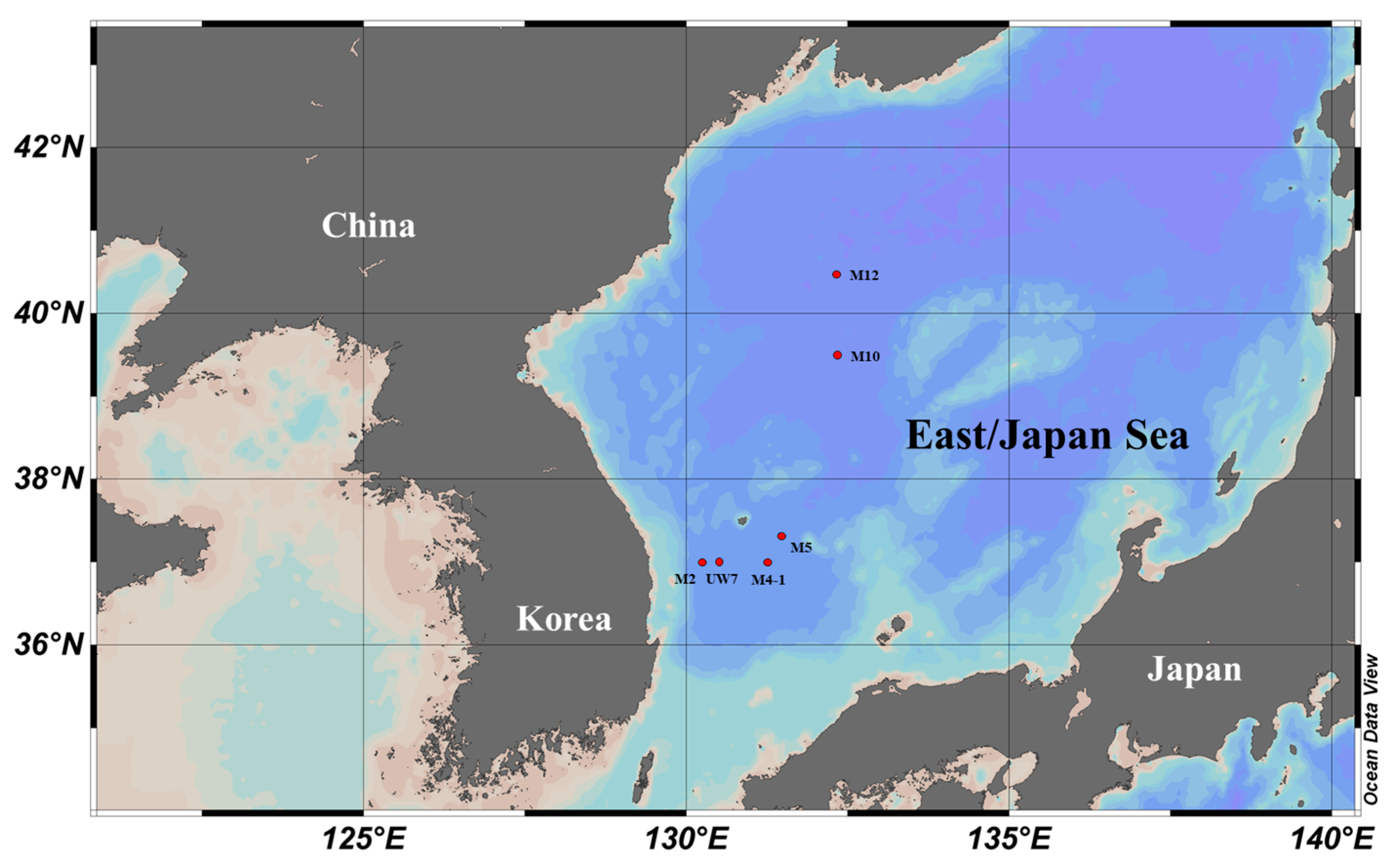
| Station | Date | Latitude (°N) | Longitude (°E) | * PP (mg C m–3 h–1) |
|---|---|---|---|---|
| M2 | 07-Apr | 37.010 | 130.250 | 3.85 |
| M4-1 | 08-Apr | 36.995 | 131.260 | 3.75 |
| M5 | 09-Apr | 37.328 | 131.457 | 6.42 |
| UW7 | 13-Apr | 37.017 | 130.502 | 2.57 |
| M10 | 11-Apr | 39.493 | 132.340 | 2.38 |
| M12 | 10-Apr | 40.473 | 132.317 | 4.74 |
| ZM | JM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection Volume (µL) | 100 | 200 | 300 | 400 | 500 | Injection Volume (µL) | 100 | 200 | 300 | 400 | 500 |
| Area | 2525.5 | 4732.3 | 6940.1 | 8952.1 | 11,686.6 | Area | 3282.1 | 6302.6 | 9251.6 | 12,246.7 | 14,546.3 |
| 2526.1 | 4736.3 | 6896.5 | 9010.4 | 11,658.2 | 3276.7 | 6284.2 | 9277.7 | 12,227.9 | 14,498.6 | ||
| 2532.8 | 4727.8 | 6889.3 | 9071.9 | 11,759.9 | 3277.7 | 6273.9 | 9250.8 | 12,288.5 | 14,512.3 | ||
| 1 S.D. | 4.1 | 4.3 | 27.5 | 59.9 | 52.5 | S.D. | 2.9 | 14.5 | 15.3 | 31.0 | 24.6 |
| 2 R.S.D. (%) | 0.16 | 0.09 | 0.40 | 0.66 | 0.45 | R.S.D. (%) | 0.09 | 0.23 | 0.17 | 0.25 | 0.17 |
| Eluents | A | B | C | A + B | A + C | B + C | A + B + C |
|---|---|---|---|---|---|---|---|
| ZM | −26.58 | −30.30 | - | −30.12 | - | - | - |
| JM | −40.43 | −33.69 | −30.39 | −40.51 | −39.19 | −30.96 | −39.39 |
| Method | Application | Reference No. |
|---|---|---|
| Pigment analysis | Analysis of chlorophylls and carotenoids from marine phytoplankton | [23] |
| Improved separations of phytoplankton pigments | [47] | |
| Qualitative and quantitative HPLC analysis | [26] | |
| HPLC separation of mono- and divinyl chlorophyll forms | [48] | |
| Separation of chlorophylls and carotenoids from marine phytoplankton | [24] | |
| Improving HPLC method for determination of carotenoids | [49] | |
| Increasing sensitivity for RP-HPLC phytoplankton pigment analysis | [38] | |
| Evaluation of phytoplankton pigment in situ measurements uncertainty | [50] | |
| Phytoplankton communities using HPLC-CHEMTAX approach | [51] | |
| Pigment production | Chlorophyll a pigment turnover in the marine diatom | [20] |
| Carotenoid pigment turnover in the marine diatom | [52] | |
| Measuring various pigment production rates | [53] | |
| Physiological characteristics of phytoplankton | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-J.; Min, J.-O.; Joo, H.; Youn, S.-H.; Lee, S.-H. Photophysiological Characterization of Phytoplankton by Measuring Pigment Production Rates: A Description of Detail Method and a Case Study. J. Mar. Sci. Eng. 2023, 11, 1859. https://doi.org/10.3390/jmse11101859
Kang J-J, Min J-O, Joo H, Youn S-H, Lee S-H. Photophysiological Characterization of Phytoplankton by Measuring Pigment Production Rates: A Description of Detail Method and a Case Study. Journal of Marine Science and Engineering. 2023; 11(10):1859. https://doi.org/10.3390/jmse11101859
Chicago/Turabian StyleKang, Jae-Joong, Jun-Oh Min, Huitae Joo, Seok-Hyun Youn, and Sang-Heon Lee. 2023. "Photophysiological Characterization of Phytoplankton by Measuring Pigment Production Rates: A Description of Detail Method and a Case Study" Journal of Marine Science and Engineering 11, no. 10: 1859. https://doi.org/10.3390/jmse11101859
APA StyleKang, J.-J., Min, J.-O., Joo, H., Youn, S.-H., & Lee, S.-H. (2023). Photophysiological Characterization of Phytoplankton by Measuring Pigment Production Rates: A Description of Detail Method and a Case Study. Journal of Marine Science and Engineering, 11(10), 1859. https://doi.org/10.3390/jmse11101859







