Spatiotemporal and Environmental Dynamics of Abundances and Diversity of Larval Fish in Artificial Reef Edge Habitats of Kitros, Pieria (Northern Aegean Sea, Eastern Mediterranean)
Abstract
1. Introduction
2. Materials and Methods
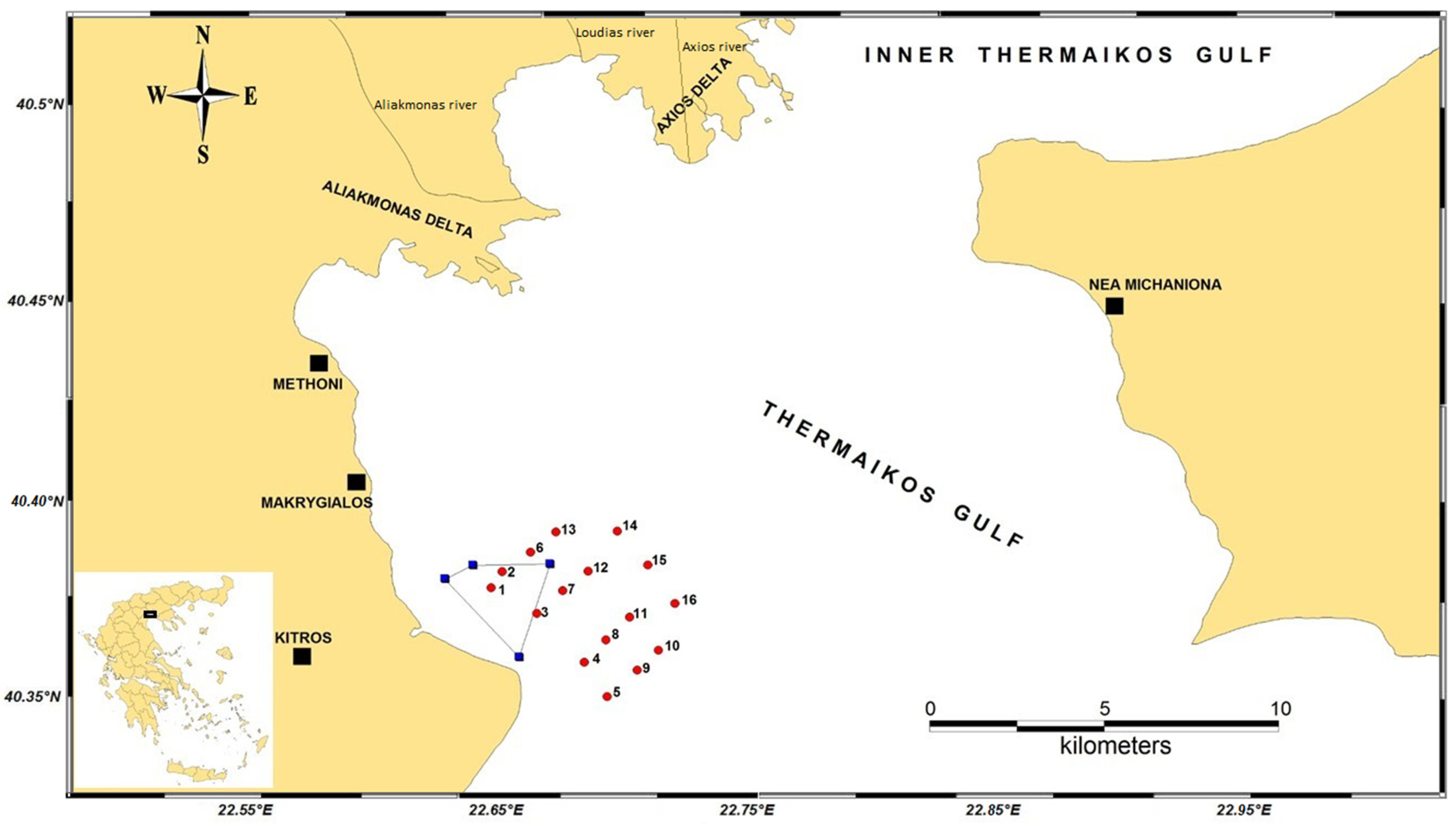
2.1. Sample Sites, Hydrographic Features
2.2. Environmental Parameters
2.3. Ichthyoplankton Collection
2.4. Statistical Analyses—Biological Indices
2.5. Statistical Analyses—Generalized Linear Modeling
2.6. Statistical Analyses—Species-Based Non-Metric Multi-Dimensional Scaling
2.7. Statistical Analyses—Seasonal-Based Non-Metric Multi-Dimensional Scaling
3. Results
3.1. Environmental Parameters
3.2. Ichthyoplankton Sampling
3.3. Ichthyoplankton Diversity Indices
3.4. Generalized Linear Models
3.5. Species-Based Non-Metric Multidimensional Scaling
3.6. Season-Based Non-Metric Multidimensional Scaling
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moser, G.H.; Smith, P.E. Larval Fish Assemblages and Oceanic Boundaries. Bull. Mar. Sci. 1993, 53, 645–691. [Google Scholar]
- Koslow, J.A.; Wright, M. Ichthyoplankton sampling design to monitor marine fish populations and communities. Mar. Policy 2016, 68, 55–64. [Google Scholar] [CrossRef]
- Cushing, D.H. The regularity of the spawning season of some fishes. ICES J. Mar. Sci. 1969, 33, 81–92. [Google Scholar] [CrossRef]
- Oozeki, Y. Biological Monitoring: Fish Eggs, Fish Larvae, and Zooplankton. In Fish Population Dynamics, Monitoring, and Management; Springer: Tokyo, Japan, 2018. [Google Scholar]
- Heath, M.R. Field investigations of the early life stages of marine fish. Adv. Mar. Biol. 1992, 28, 1–174. [Google Scholar]
- Biermann, C.H. Ecology of Marine Invertebrate Larvae; McEdward, L., Ed.; CRC Press: Boca Raton, FL, USA, 1996; Volume 71, pp. 137–138. [Google Scholar]
- Franco-Gordo, C.; Domínguez, E.; Suárez-Morales, E.; Vásquez-Yeomans, L. Diversity of ichthyoplankton in the central Mexican Pacific: A seasonal survey. Estuar. Coast. Shelf Sci. 2003, 57, 111–121. [Google Scholar] [CrossRef]
- Smith, D. The Larvae of Indo-Pacific Coastal Fishes. Copeia 2001, 2001, 1172–1174. [Google Scholar] [CrossRef]
- Loeb, V.; Kellermann, A.; Koubbi, P.; North, A.; White, M. Antarctic Larval Fish Assemblages: A Review. Bull. Mar. Sci. 1993, 53, 416–449. [Google Scholar]
- Olivar, M.; Shelton, P. Larval Fish Assemblages of the Benguela Current. Bull. Mar. Sci. 1993, 53, 450–474. [Google Scholar]
- Bolshakova, Y.; Evseenko, S. Ichthyoplankton of the Southern Waters of the North Atlantic. 2. Species Composition and Distribution Features. J. Ichthyol. 2019, 59, 657–671. [Google Scholar] [CrossRef]
- Richardson, S.L.; Laroche, J.L.; Richardson, M.D. Larval fish assemblages and associations in the north-east Pacific Ocean along the Oregon coast, winter-spring 1972–1975. Estuar. Coast. Mar. Sci. 1980, 11, 671–699. [Google Scholar] [CrossRef]
- Sabatés, A. Changes in the heterogeneity of mesoscale distribution patterns of larval fish associated with a shallow coastal haline front. Estuar. Coast. Shelf Sci. 1990, 30, 131–140. [Google Scholar] [CrossRef]
- Sabates, A.; Olivar, M.P. Variation of larval fish distributions associated with vari- ability in the location of a shelf-slope front. Mar. Ecol. Prog. Ser. 1996, 135, 11–20. [Google Scholar] [CrossRef]
- Olivar, M.P.; Sabates, A. Vertical distribution of fish larvae in the north-west Mediterranean Sea in spring. Mar. Biol. 1997, 129, 289–300. [Google Scholar] [CrossRef]
- Cuttitta, A.; Quinci, E.; Patti, B.; Bonomo, S.; Bonanno, A.; Musco, M.; Torri, M.; Placenti, F.; Basilone, G.; Genovese, S.; et al. Different key roles of mesoscale oceanographic structures and ocean bathymetry in shaping larval fish distribution pattern: A case study in Sicilian waters in summer 2009. J. Sea Res. 2016, 115, 6–17. [Google Scholar] [CrossRef]
- Olivar, M.P.; Emelianov, M.; Villate, F.; Uriarte, I.; Maynou, F.; Alvarez, I.; Morote, E. The role of oceanographic conditions and plankton availability in larval fish assemblages off the Catalan coast (NW Mediterranean). Fish. Oceanogr. 2010, 19, 209–229. [Google Scholar] [CrossRef]
- Sabatés, A.; Masó, M. Unusual larval fish distribution pattern in coastal zone of the western Mediterranean. Limnol. Oceanogr. 1992, 37, 1252–1260. [Google Scholar] [CrossRef]
- Palomera, I.; Olivar, M.P. Nearshore icthyoplankton off the Costa Brava (north-west Mediterranean Sea). Publ. Espec. Inst. Esp. Oceanogr. 1996, 22, 71–75. [Google Scholar]
- Friligos, N.; Kondylakis, I.; Psillidou-Giouranovits, R.; Georgakopoulou-Gregoriadou, E.; Voutsinou-Taliadouri, F. Distribution of nutrients and phytoplankton biomass in the water masses of the Thermaikos Gulf, Hellas. Fresenius Environ. Bull. 1998, 7, 368–373. [Google Scholar]
- Apostolopoulos, G.; Fotiadis, N.; Kotsiri, Z.; Providakis, N.; Siapatis, A.; Theodorou, I.; Verriopoulos, G. Size and abundance relations between mesozooplankton and ichthyoplankton in the Thermaikos gulf in 2006. In Proceedings of the 51st European Marine Biology Symposium (EMBS), Rhodes, Greece, 26–30 September 2016. [Google Scholar]
- Krestenitis, Y.; Kombiadou, K.; Androulidakis, Y.; Makris, C.; Baltikas, V.; Skoulikaris, C.; Kontos, Y.; Kalantzi, G. Operational Oceanographic Platform in Thermaikos Gulf (Greece): Forecasting and Emergency Alert System for Public Use. In Proceedings of the 36th International Association of Hydraulic Research (IAHR) World Congress, Hague, The Netherlands, 28 June–3 July 2015. [Google Scholar]
- Androulidakis, Y.S.; Krestenitis, Y.N. Sea Surface Temperature Variability and Marine Heat Waves over the Aegean, Ionian, and Cretan Seas from 2008–2021. J. Mar. Sci. Eng. 2022, 10, 42. [Google Scholar] [CrossRef]
- Skoulikidis, N.T. Significance evaluation of factors controlling river water composition. Environ. Geol. 1993, 22, 178–185. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Karageorgis, A.P.; Kapsimalis, V.; Drakopoulou, P.; Skoulikidis, N.; Behrendt, H.; Levkov, Z. Management of nutrient emissions of Axios River catchment: Their effect in the coastal zone of Thermaikos Gulf, Greece. Ecol. Model. 2009, 220, 383–396. [Google Scholar] [CrossRef]
- Poulos, S.E.; Chronis, G.T.; Collins, M.B.; Lykousis, V. Thermaikos Gulf coastal system, NW Aegean Sea: An overview of water/sediment fluxes in relation to air–land–ocean interactions and human activities. J. Mar. Syst. 2000, 25, 47–76. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Tsagarakis, K.; Sylaios, G.; Tsikliras, A.C. Ecosystem trophic structure and fishing effort simulations of a major fishing ground in the northeastern Mediterranean Sea (Thermaikos Gulf). Estuar. Coast. Shelf Sci. 2022, 264, 107667. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Russell, F.S. The Eggs and Planktonic Stages of British Marine Fishes; Academic Press: London, UK, 1976; p. 524. [Google Scholar]
- Olivar, M.P.; Fortuño, J.M. Guide to ichthyoplankton of the Southeast Atlantic (Benguela Current Region). Sci. Mar. 1991, 55, 1–383. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Natural Environment Research Council: Swindon, UK; Plymouth Marine Laboratory: Plymouth, UK, 1994. [Google Scholar]
- Zuur, A.; Ieno, E.; Elphick, C. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Robertson, R. An Introduction to Statistical Modelling. Technometrics 2012, 42, 435–436. [Google Scholar] [CrossRef]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Mather, P.M. Computational Methods of Multivariate Analysis in Physical Geography; John Wiley and Sons: London, UK, 1976. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data, Version 4.34; MjM Software: Glenden Beach, OR, USA, 1999. [Google Scholar]
- Estrada, M. Primary production in the northwestern Mediterranean. Sci. Mar. 1996, 60, 55–64. [Google Scholar]
- Skoulikidis, N.T.; Bertahas, I.; Koussouris, T. The environmental state of fresh- water resources in Greece (rivers and lakes). Environ. Geol. 1998, 36, 1–17. [Google Scholar] [CrossRef]
- Giannoulaki, M.; Iglesias, M.; Tugores, M.; Bonnano, A.; Patti, B.; Felice, A.; Leonori, I.; Bigot, J.L.; Tičina, V.; Pyrounaki, M.; et al. Characterizing the potential habitat of European anchovy Engraulis encrasicolus in the Mediterranean Sea, at different life stages. Fish. Oceanogr. 2013, 22, 69–89. [Google Scholar] [CrossRef]
- Lloret, J.; Rätz, H.J. Condition of cod (Gadus morhua) off Greenland during 1982–98. Fish. Res. 2000, 48, 79–86. [Google Scholar] [CrossRef]
- Jørgensen, T. Long-term changes in growth of North-east Arctic cod (Gadus morhua) and some environmental influences. ICES J. Mar. Sci. 1992, 49, 263–277. [Google Scholar] [CrossRef]
- Nilssen, E.M.; Pedersen, T.; Hopkins CC, E.; Thyholt, K.; Pope, J.G. Recruitment variability and growth of Northeast Arctic cod: Influence of physical environment, demography, and predator-prey energetics. ICES Mar. Sci. Symp. 1994, 198, 449–470. [Google Scholar]
- Salat, J.; Pascual, J. The oceanographic and meteorological station at l’Estartit (NW Mediterranean). Tracking long-term hydrological change in the Mediterranean Sea. CIESM Workshop Ser. 2002, 16, 29–32. [Google Scholar]
- Sabatés, A.; Martín, P.; Lloret, J.; Raya, V. Sea warming and fish distribution: The case of the small pelagic fish, Sardinella aurita, in the western Mediterranean. Glob. Change Biol. 2006, 12, 2209–2219. [Google Scholar] [CrossRef]
- Golani, D.; Orsi-Relini, L.; Massuti, E.; Quignard, J.P. Fishes. In CIESM Atlas of Exotic Species in the Mediterranean; Briand, F., Ed.; CIESM: Monaco, Monaco, 2002. [Google Scholar]
- Galil, B.S. Alien species in the Mediterranean Sea—Which, when, where, why? Hydrobiologia 2008, 606, 105–116. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Ben Tuvia, A. Red Sea fishes recently found in the Mediterranean. Copeia 1966, 2, 254–275. [Google Scholar] [CrossRef]
- Ben, M.; Glaser, T. The invasion of Saurida undosquamis (Richardson) into the Levant Basin. Fish. Bull. 1974, 72, 359–373. [Google Scholar]
- Klimova, T.; Podrezova, P. Seasonal distribution of the Black Sea ichthyoplankton near the Crimean Peninsula. Reg. Stud. Mar. Sci. 2018, 24, 260–269. [Google Scholar] [CrossRef]
- Palomera, I.; Sabatés, A. Co-occurrence of Engraulis encrasicolus and Sardinella aurita eggs and larvae in the Northwestern Mediterranean. Sci. Mar. 1990, 54, 63–69. [Google Scholar]
- Quaatey, S.N.K.; Maravelias, C.D. Maturity and spawning pattern of Sardinella aurita in relation to water temperature and zooplankton abundance off Ghana, West Africa. J. Appl. Ichthyol. 1999, 15, 63–69. [Google Scholar] [CrossRef]
- Koutrakis, E.; Kallianiotis, A.; Tsikliras, A. Temporal patterns of larval fish distribution and abundance in a coastal area of Northern Greece. Sci. Mar. 2004, 68, 585–595. [Google Scholar] [CrossRef]
- Caragitsou, E.; Siapatis, A.; Anastasopoulou, A. Seasonal structure of fish larvae assemblages in the Pagasitikos Gulf (Greece). Rapp. Comm. Int. Mer. Médit. 2001, 36, 250. [Google Scholar]
- Caragitsou, E.; Siapatis, A.; Anastasopoulou, A.; Papaconstantinou, C. Seasonal Distribution of Ichthyoplankton in the Aegean Sea. In Proceedings of the 5th Congress on Oceanography & Fisheries, Kavala, Greece, 15–18 April 1997; pp. 143–145. [Google Scholar]
- Jennings, S.; Kaiser, M.J.; Reynolds, J.D. Marine Fisheries Ecology; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Longhurst, A.R.; Sathyendranath, S.; Platt, T.; Cavehill, C. An estimate of global primary production in the ocean from satellite radiometer data. J. Plankton Res. 1995, 17, 1245–1271. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Pertierra, J.P. Growth rates of the anchovy Engraulis encrasicolus and the sardine Sardina pilchardus in the north-western Mediterranean Sea. Mar. Biol. 1990, 107, 349–356. [Google Scholar] [CrossRef]
- Palomera, I. Spawning of anchovy Engraulis encrasicolus, in the north-western Mediterranean relative to hydrographic features in the region. Mar. Ecol. Prog. Ser. 1992, 79, 215–223. [Google Scholar] [CrossRef]
- Shelton, P.A.; Hutchings, L. Transport of anchovy, Engraulis capensis Gilchrist, eggs and early larvae by a frontal jet current. ICES J. Mar. Sci. 1982, 40, 185–198. [Google Scholar] [CrossRef]
- Salat, J.; Olivar, M.; Sabatés, A. Advection of continental water as an ex- port mechanism for anchovy, Engraulis encrasicolus, larvae. Sci. Mar. 2001, 65, 77–88. [Google Scholar]
- Tsikliras, A.C.; Koutrakis, E.T.; Stergiou, K.I. Age and growth of round sardinella (Sardinella aurita) in the north-eastern Mediterranean. Sci. Mar. 2005, 69, 231–240. [Google Scholar] [CrossRef][Green Version]



| Year | Julian Day of the Year | Statistic | Surface chl-a | chl-a | Surface Salinity | Salinity | Salinity Gradient | Surface Temperature | Temp. | Temperature Gradient |
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 176 | Mean | 5.375 | 1.557 | 33.134 | 36.102 | −4.561 | 23.527 | 19.03 | 8.092 |
| Std. Dev. | 2.491 | 0.352 | 1.239 | 0.489 | 1.058 | 0.203 | 0.562 | 0.48 | ||
| 195 | Mean | 4.452 | 1.888 | 33.53 | 36.002 | −4.178 | 25.947 | 20.68 | 10.146 | |
| Std. Dev. | 2.281 | 0.667 | 0.89 | 0.605 | 0.664 | 0.48 | 1.419 | 1.054 | ||
| 303 | Mean | 3.492 | 2.157 | 32.546 | 35.913 | −5.117 | 17.938 | 19.75 | −3.041 | |
| Std. Dev. | 0.521 | 1.013 | 1.308 | 0.641 | 1.066 | 0.573 | 0.343 | 0.495 | ||
| Yearly | Mean | 4.354 | 1.896 | 33.064 | 35.997 | −4.624 | 22.375 | 19.89 | 4.79 | |
| Std. Dev. | 2.017 | 0.776 | 1.203 | 0.581 | 1.001 | 3.556 | 1.127 | 6.088 | ||
| 2016 | 98 | Mean | 6.623 | 2.5 | 29.66 | 37.222 | −7.993 | 17.543 | 14.69 | 3.286 |
| Std. Dev. | 3.462 | 1.013 | 1.44 | 0.36 | 2.406 | 0.222 | 0.155 | 0.228 | ||
| 206 | Mean | 6.51 | 2.491 | 30.963 | 36.33 | −6.941 | 20.235 | 18.88 | 2.816 | |
| Std. Dev. | 1.104 | 0.855 | 1.935 | 0.948 | 1.864 | 0.257 | 0.292 | 0.452 | ||
| 210 | Mean | 4.962 | 2.797 | 34.19 | 36.2 | −3.686 | 22.137 | 22.7 | 0.174 | |
| Std. Dev. | 1.976 | 1.192 | 1.261 | 0.648 | 1.008 | 0.235 | 0.215 | 0.919 | ||
| Yearly | Mean | 6.032 | 2.596 | 31.604 | 36.584 | −6.207 | 19.972 | 18.76 | 2.092 | |
| Std. Dev. | 2.459 | 1.018 | 2.463 | 0.82 | 2.591 | 1.919 | 3.316 | 1.506 | ||
| 2017 | 116 | Mean | 1.521 | 1.227 | 36.17 | 37.706 | −2.166 | 15.685 | 14.69 | 1.73 |
| Std. Dev. | 0.85 | 0.347 | 1.505 | 0.731 | 1.368 | 0.395 | 0.222 | 0.385 | ||
| 144 | Mean | 0.944 | 0.451 | 35.304 | 36.389 | −1.872 | 20.542 | 19.28 | 2.2 | |
| Std. Dev. | 0.468 | 0.191 | 0.77 | 0.264 | 0.724 | 0.325 | 0.21 | 0.503 | ||
| 272 | Mean | 0.558 | 0.623 | 36.721 | 37.29 | −1.523 | 22.804 | 22.89 | 1.462 | |
| Std. Dev. | 0.326 | 0.428 | 0.335 | 0.221 | 0.386 | 0.457 | 0.191 | 0.709 | ||
| Yearly | Mean | 1.008 | 0.767 | 36.065 | 37.128 | −1.854 | 19.677 | 18.95 | 1.797 | |
| Std. Dev. | 0.703 | 0.471 | 1.138 | 0.719 | 0.94 | 3.026 | 3.398 | 0.619 |
| Family | Species | 15-Jun | 15-Jul | 15-Oct | 16-Apr | 16-May | 16-Sep | 17-Apr | 17-May | 17-Sep |
|---|---|---|---|---|---|---|---|---|---|---|
| Atherinidae | Atherina boyeri | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 136 | 0 |
| Blenniidae | Blennius gattorugine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 23 | 0 |
| Blennius ocellaris | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Blennius pilicornis | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Parablennius gattorugine | 0 | 0 | 0 | 0 | 4 | 11 | 15 | 10 | 10 | |
| Parablennius pilicornis | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | |
| Bothidae | Arnoglossus spp. | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Arnoglossus thori | 0 | 15 | 7 | 11 | 0 | 10 | 22 | 0 | 8 | |
| Callanthiidae | Callanthias ruber | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 |
| Callyonimidae | Callionymus lyra | 15 | 18 | 0 | 12 | 1 | 0 | 20 | 20 | 11 |
| Callionymus maculatus | 34 | 28 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | |
| Callionymus spp. | 15 | 21 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | |
| Carangidae | Trachurus mediterraneus | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachurus trachurus | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Centracanthidae | Spicara maena | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Spicara smaris | 0 | 0 | 0 | 0 | 6 | 0 | 59 | 24 | 6 | |
| Spicara spp. | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | |
| Centracanthus cirrus | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 6 | |
| Spicara flexuosa | 0 | 0 | 0 | 14 | 1 | 0 | 51 | 0 | 0 | |
| Cepolidae | Cepola macrophthalma | 0 | 21 | 6 | 0 | 0 | 7 | 0 | 0 | 9 |
| Clupeidae | Sardina pilchardus | 0 | 0 | 6 | 16 | 0 | 0 | 0 | 0 | 0 |
| Sardinella aurita | 0 | 50 | 0 | 12 | 1 | 0 | 0 | 0 | 0 | |
| Cynoglossidae | Symphurus nigrescens | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 7 |
| Echeneidae | Remora remora | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Engraulidae | Engraulis encrasicholus | 152 | 248 | 52 | 57 | 16 | 261 | 48 | 104 | 324 |
| Gadidae | 62 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Micromesistius poutassou | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Trisopterus minutus capelanus | 0 | 8 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | |
| Gobiesocidae | Lepadogaster candolii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Lepadogaster lepadogaster | 0 | 0 | 0 | 19 | 0 | 5 | 0 | 0 | 0 | |
| Lepadogaster purpurea | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | |
| Gobiidae | Aphia minuta | 0 | 0 | 0 | 9 | 16 | 9 | 0 | 0 | 0 |
| Deltentosteus quadrimaculatus | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | |
| Gobius niger | 0 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | |
| Gobius spp. II | 32 | 42 | 10 | 13 | 0 | 18 | 145 | 96 | 31 | |
| Labridae | Crenilabrus melops | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 |
| Crenilabrus spp. | 0 | 18 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |
| Symphodus cinereus | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 16 | 0 | |
| Mugilidae | Chelon labrosus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Liza aurata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | |
| Liza saliens | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 6 | |
| Liza spp. | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mugil cephalus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | |
| Mugilidae | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | |
| Mullidae | Mullus surmuletus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Myctophidae | Ceratoscopelus maderensis | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hygophum benoiti | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Notoscopelus spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | |
| Ophidiidae | Parophidion vassali | 0 | 0 | 0 | 0 | 0 | 106 | 0 | 0 | 0 |
| Pomacentridae | Chromis chromis | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scombridae | Scomber japonicus | 0 | 16 | 0 | 0 | 0 | 6 | 0 | 0 | 13 |
| Scorpaena porcus | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 12 | |
| Scorpaena spp. | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 7 | |
| Serranidae | Anthias anthias | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 9 |
| Serranus cabrilla | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 18 | |
| Serranus hepatus | 63 | 7 | 0 | 0 | 2 | 10 | 0 | 7 | 5 | |
| Serranus scriba | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | |
| Soleidae | Buglossidium luteum | 0 | 0 | 0 | 8 | 0 | 6 | 0 | 0 | 0 |
| Solea lascaris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | |
| Solea spp. | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | |
| Sparidae | Boops boops | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diplodus annularis | 0 | 0 | 0 | 10 | 0 | 0 | 7 | 0 | 6 | |
| Diplodus vulgaris | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | |
| Pagellus acarne | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 17 | |
| Pagellus bogaraveo | 0 | 0 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | |
| Pagrus pagrus | 30 | 12 | 0 | 0 | 0 | 9 | 0 | 0 | 9 | |
| Sparus aurata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | |
| Sparidae | 39 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | |
| Sygnathidae | Syngnathus acus | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 7 | 0 |
| Trachinidae | Trachinus draco | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 6 |
| Total abundance | 442 | 619 | 155 | 227 | 60 | 615 | 419 | 447 | 601 |
| Sample Month, Year | S | d | J’ | Brillouin | Fisher | H’(Loge) | 1-Lambda’ |
|---|---|---|---|---|---|---|---|
| June 2015 | 9 | 1.31 | 0.88 | 1.89 | 1.6 | 1.93 | 0.82 |
| July 2015 | 21 | 3.11 | 0.78 | 2.30 | 4.2 | 2.38 | 0.82 |
| October 2015 | 11 | 1.98 | 0.88 | 2.01 | 2.7 | 2.12 | 0.84 |
| April 2016 | 15 | 2.57 | 0.92 | 2.37 | 3.57 | 2.50 | 0.90 |
| May 2016 | 15 | 3.42 | 0.79 | 1.84 | 6.42 | 2.13 | 0.84 |
| September 2016 | 24 | 3.60 | 0.68 | 2.08 | 5.02 | 2.16 | 0.77 |
| April 2017 | 15 | 1.37 | 0.88 | 1.89 | 1.6 | 1.93 | 0.82 |
| May 2017 | 12 | 1.17 | 0.78 | 1.50 | 2.2 | 1.38 | 0.82 |
| September 2017 | 30 | 2.98 | 0.88 | 1.71 | 3.7 | 2.12 | 0.84 |
| Intercept | Year | Site | Surface Salinity (PSU) | Surface Temp (°C) | chl-a ug/L | Surface chl-a ug/L | Julian Day of the Year | Salinity Gradient (PSU) | Salinity (PSU) | Temp Gradient (°C) | Temp (°C) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deg. freedom | 1 | 2 | 15 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||
| W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | W C S | S I G | |
| Anthias anthias | 0.00 | 0.957 | 0.65 | 0.721 | 17.98 | 0.264 | 7.05 | 0.008 | 6.28 | 0.012 | 2.34 | 0.126 | 0.32 | 0.570 | 1.46 | 0.227 | 6.69 | 0.010 | 8.46 | 0.004 | 4.09 | 0.043 | 6.35 | 0.012 |
| Aphia minuta | 1.24 | 0.265 | 16.27 | 0.000 | 19.95 | 0.174 | 1.03 | 0.309 | 1.45 | 0.228 | 0.70 | 0.402 | 0.21 | 0.650 | 7.17 | 0.007 | 0.00 | 0.960 | 5.42 | 0.020 | 0.53 | 0.467 | 4.86 | 0.027 |
| Arnoglossus thori | 0.75 | 0.385 | 0.42 | 0.809 | 28.39 | 0.019 | 1.78 | 0.182 | 1.74 | 0.187 | 0.23 | 0.632 | 0.04 | 0.833 | 1.47 | 0.225 | 0.08 | 0.776 | 1.16 | 0.282 | 0.00 | 0.986 | 1.29 | 0.256 |
| Atherina boyeri | 1.63 | 0.201 | 3.48 | 0.176 | 20.19 | 0.165 | 0.05 | 0.815 | 0.00 | 0.987 | 1.42 | 0.233 | 0.02 | 0.897 | 2.80 | 0.094 | 0.16 | 0.690 | 1.01 | 0.314 | 0.80 | 0.372 | 1.50 | 0.221 |
| Callionymus lyra | 1.40 | 0.237 | 1.52 | 0.468 | 16.85 | 0.328 | 0.34 | 0.558 | 0.24 | 0.626 | 0.01 | 0.937 | 2.25 | 0.134 | 2.41 | 0.121 | 0.18 | 0.671 | 1.03 | 0.309 | 0.03 | 0.861 | 1.02 | 0.312 |
| Callionymus maculatus | 6.98 | 0.008 | 0.64 | 0.726 | 22.47 | 0.096 | 14.55 | 0.000 | 12.78 | 0.000 | 0.16 | 0.691 | 10.23 | 0.001 | 0.43 | 0.511 | 3.83 | 0.050 | 18.24 | 0.000 | 0.91 | 0.341 | 16.45 | 0.000 |
| Callionymus spp. | 0.32 | 0.571 | 2.85 | 0.241 | 13.23 | 0.585 | 13.80 | 0.000 | 13.01 | 0.000 | 0.84 | 0.359 | 0.00 | 0.949 | 0.23 | 0.632 | 2.33 | 0.127 | 6.05 | 0.014 | 1.15 | 0.283 | 0.32 | 0.571 |
| Engraulis encrasicholus | 0.11 | 0.739 | 9.66 | 0.008 | 47.48 | 0.000 | 0.32 | 0.572 | 0.88 | 0.349 | 0.07 | 0.789 | 6.35 | 0.012 | 1.16 | 0.281 | 1.49 | 0.223 | 1.62 | 0.203 | 2.76 | 0.096 | 2.04 | 0.153 |
| Gobius niger | 1.53 | 0.216 | 26.42 | 0.000 | 21.66 | 0.117 | 2.96 | 0.085 | 0.94 | 0.333 | 0.56 | 0.453 | 0.13 | 0.715 | 4.86 | 0.027 | 1.84 | 0.175 | 0.00 | 0.972 | 0.46 | 0.500 | 0.23 | 0.632 |
| Gobius spp. II | 1.97 | 0.160 | 7.62 | 0.022 | 17.47 | 0.291 | 0.89 | 0.345 | 0.55 | 0.457 | 1.01 | 0.314 | 1.21 | 0.271 | 4.01 | 0.045 | 0.26 | 0.610 | 5.07 | 0.024 | 0.00 | 0.985 | 4.20 | 0.040 |
| Lepadogaster lepadogaster | 0.24 | 0.622 | 2.22 | 0.330 | 16.75 | 0.334 | 0.98 | 0.322 | 1.23 | 0.267 | 0.07 | 0.792 | 0.05 | 0.818 | 0.07 | 0.794 | 0.53 | 0.468 | 2.47 | 0.116 | 0.59 | 0.444 | 2.36 | 0.124 |
| Liza saliens | 2.13 | 0.144 | 0.66 | 0.718 | 15.30 | 0.430 | 1.48 | 0.223 | 2.09 | 0.148 | 1.34 | 0.248 | 0.13 | 0.713 | 0.10 | 0.750 | 66.68 | 0.000 | 0.36 | 0.546 | 72.65 | 0.000 | 0.08 | 0.783 |
| Mugilidae | 0.00 | 0.969 | 3.47 | 0.177 | 21.01 | 0.136 | 17.90 | 0.000 | 15.87 | 0.000 | 0.01 | 0.924 | 2.73 | 0.098 | 0.07 | 0.797 | 2.47 | 0.116 | 9.71 | 0.002 | 0.68 | 0.410 | 7.77 | 0.005 |
| Pagellus acarne | 0.46 | 0.500 | 1.24 | 0.539 | 18.26 | 0.249 | 7.92 | 0.005 | 8.76 | 0.003 | 0.04 | 0.839 | 0.99 | 0.319 | 1.86 | 0.172 | 2.75 | 0.098 | 14.08 | 0.000 | 2.73 | 0.098 | 12.43 | 0.000 |
| Pagellus bogaraveo | 0.31 | 0.579 | 18.43 | 0.000 | 16.20 | 0.369 | 0.02 | 0.893 | 0.01 | 0.904 | 2.60 | 0.107 | 0.35 | 0.556 | 10.95 | 0.001 | 0.02 | 0.895 | 1.09 | 0.298 | 0.00 | 0.958 | 0.39 | 0.532 |
| Pagrus pagrus | 0.00 | 0.990 | 8.32 | 0.016 | 19.54 | 0.190 | 3.84 | 0.050 | 3.82 | 0.051 | 3.20 | 0.074 | 1.15 | 0.283 | 0.67 | 0.414 | 2.42 | 0.120 | 4.87 | 0.027 | 2.06 | 0.152 | 3.85 | 0.050 |
| Parablennius gattorugine | 8.68 | 0.003 | 4.86 | 0.088 | 22.82 | 0.088 | 0.02 | 0.880 | 0.04 | 0.840 | 0.41 | 0.521 | 2.36 | 0.125 | 11.43 | 0.001 | 0.30 | 0.582 | 4.41 | 0.036 | 0.21 | 0.649 | 5.16 | 0.023 |
| Parophidion vassali | 0.31 | 0.579 | 3.28 | 0.194 | 18.97 | 0.215 | 1.72 | 0.190 | 0.45 | 0.501 | 1.70 | 0.192 | 0.82 | 0.364 | 0.11 | 0.735 | 1.02 | 0.312 | 0.53 | 0.467 | 0.10 | 0.754 | 0.02 | 0.891 |
| Sardinella aurita | 0.00 | 0.949 | 0.55 | 0.761 | 22.06 | 0.106 | 0.66 | 0.417 | 0.24 | 0.621 | 4.33 | 0.037 | 5.44 | 0.020 | 0.43 | 0.513 | 1.09 | 0.297 | 0.27 | 0.606 | 2.43 | 0.119 | 0.05 | 0.817 |
| Scomber japonicus | 0.11 | 0.739 | 4.18 | 0.124 | 28.51 | 0.019 | 0.59 | 0.441 | 1.66 | 0.198 | 0.35 | 0.557 | 6.88 | 0.009 | 8.33 | 0.004 | 3.14 | 0.077 | 1.89 | 0.169 | 5.72 | 0.017 | 3.03 | 0.082 |
| Serranus hepatus | 1.61 | 0.204 | 2.95 | 0.229 | 19.31 | 0.200 | 3.77 | 0.052 | 4.08 | 0.043 | 2.36 | 0.125 | 0.74 | 0.391 | 0.03 | 0.858 | 0.18 | 0.674 | 0.99 | 0.319 | 0.05 | 0.818 | 0.84 | 0.360 |
| Sparidae | 2.18 | 0.140 | 11.25 | 0.004 | 26.25 | 0.035 | 0.95 | 0.330 | 1.34 | 0.246 | 0.66 | 0.416 | 4.87 | 0.027 | 0.02 | 0.880 | 1.05 | 0.306 | 0.02 | 0.902 | 0.86 | 0.354 | 0.03 | 0.866 |
| Spicara smaris | 2.63 | 0.105 | 6.97 | 0.031 | 25.29 | 0.046 | 0.06 | 0.812 | 0.22 | 0.638 | 3.80 | 0.051 | 0.20 | 0.651 | 7.73 | 0.005 | 0.17 | 0.678 | 3.54 | 0.060 | 0.60 | 0.439 | 4.05 | 0.044 |
| Spicara spp. | 0.00 | 0.969 | 7.37 | 0.025 | 20.13 | 0.167 | 0.46 | 0.499 | 0.68 | 0.411 | 1.60 | 0.207 | 0.20 | 0.654 | 3.00 | 0.083 | 0.46 | 0.500 | 1.20 | 0.273 | 0.06 | 0.812 | 1.04 | 0.308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallianiotis, A.A.; Kamidis, N.; Tselepides, A.; Batjakas, I.E. Spatiotemporal and Environmental Dynamics of Abundances and Diversity of Larval Fish in Artificial Reef Edge Habitats of Kitros, Pieria (Northern Aegean Sea, Eastern Mediterranean). J. Mar. Sci. Eng. 2023, 11, 40. https://doi.org/10.3390/jmse11010040
Kallianiotis AA, Kamidis N, Tselepides A, Batjakas IE. Spatiotemporal and Environmental Dynamics of Abundances and Diversity of Larval Fish in Artificial Reef Edge Habitats of Kitros, Pieria (Northern Aegean Sea, Eastern Mediterranean). Journal of Marine Science and Engineering. 2023; 11(1):40. https://doi.org/10.3390/jmse11010040
Chicago/Turabian StyleKallianiotis, Athanasios A., Nikolaos Kamidis, Anastasios Tselepides, and Ioannis E. Batjakas. 2023. "Spatiotemporal and Environmental Dynamics of Abundances and Diversity of Larval Fish in Artificial Reef Edge Habitats of Kitros, Pieria (Northern Aegean Sea, Eastern Mediterranean)" Journal of Marine Science and Engineering 11, no. 1: 40. https://doi.org/10.3390/jmse11010040
APA StyleKallianiotis, A. A., Kamidis, N., Tselepides, A., & Batjakas, I. E. (2023). Spatiotemporal and Environmental Dynamics of Abundances and Diversity of Larval Fish in Artificial Reef Edge Habitats of Kitros, Pieria (Northern Aegean Sea, Eastern Mediterranean). Journal of Marine Science and Engineering, 11(1), 40. https://doi.org/10.3390/jmse11010040







