Analysis of the Sabellaria spinulosa Bioconstruction Growth in a Laboratory
Abstract
1. Introduction
- reproduce reliable sedimentation mechanism and worm reef growth in the laboratory;
- directly observe and video-record long-term tests (1 month);
- overcome the experimental limitations of previous laboratory procedures;
- evaluate the reef growth rates;
- establish the relative role of mineralogy, grain size, and particle sand shape for growth/stasis stages of the worm reefs;
- understand how different types of sand can influence the choices of polychaetes.
2. Materials and Methods
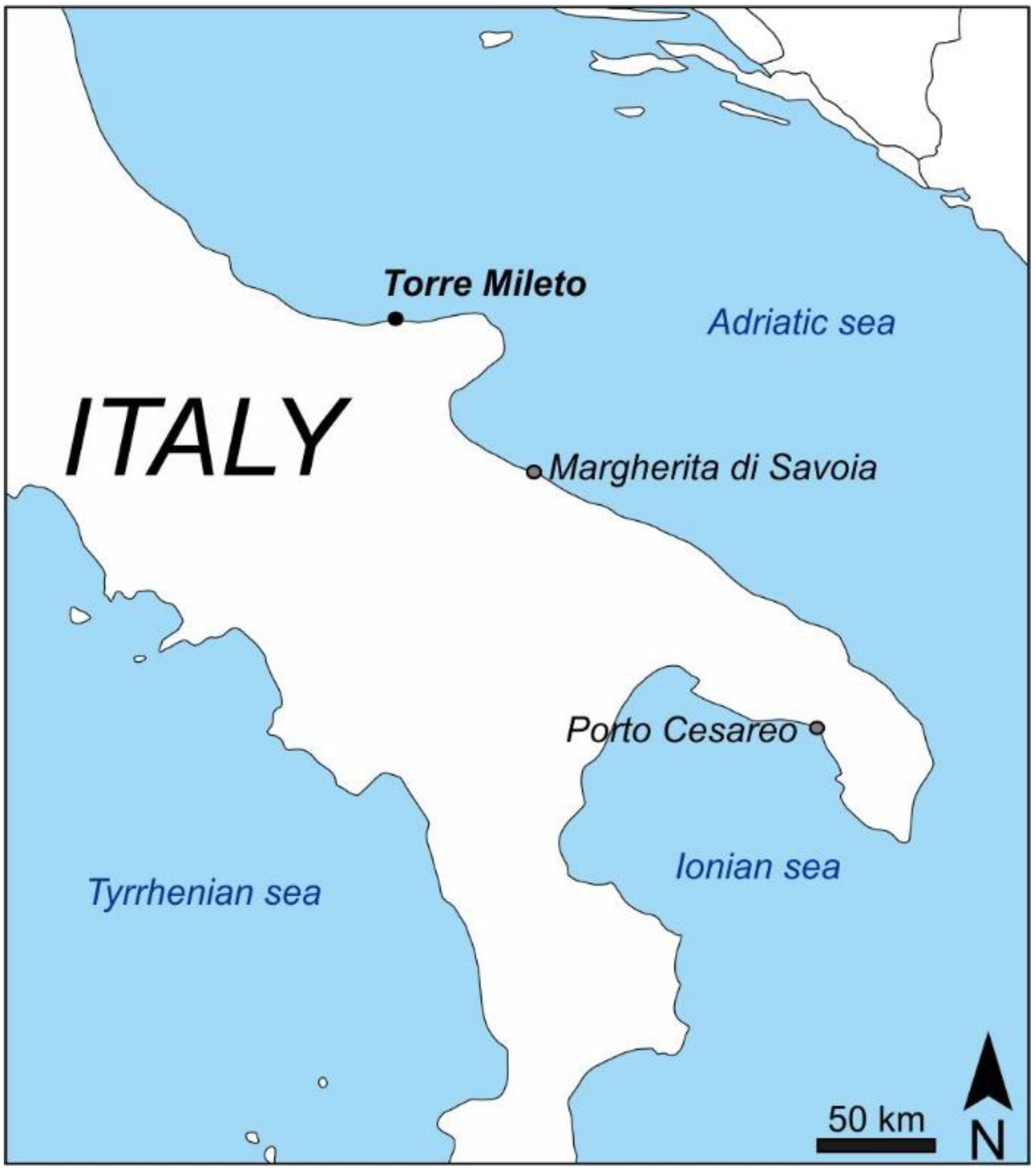
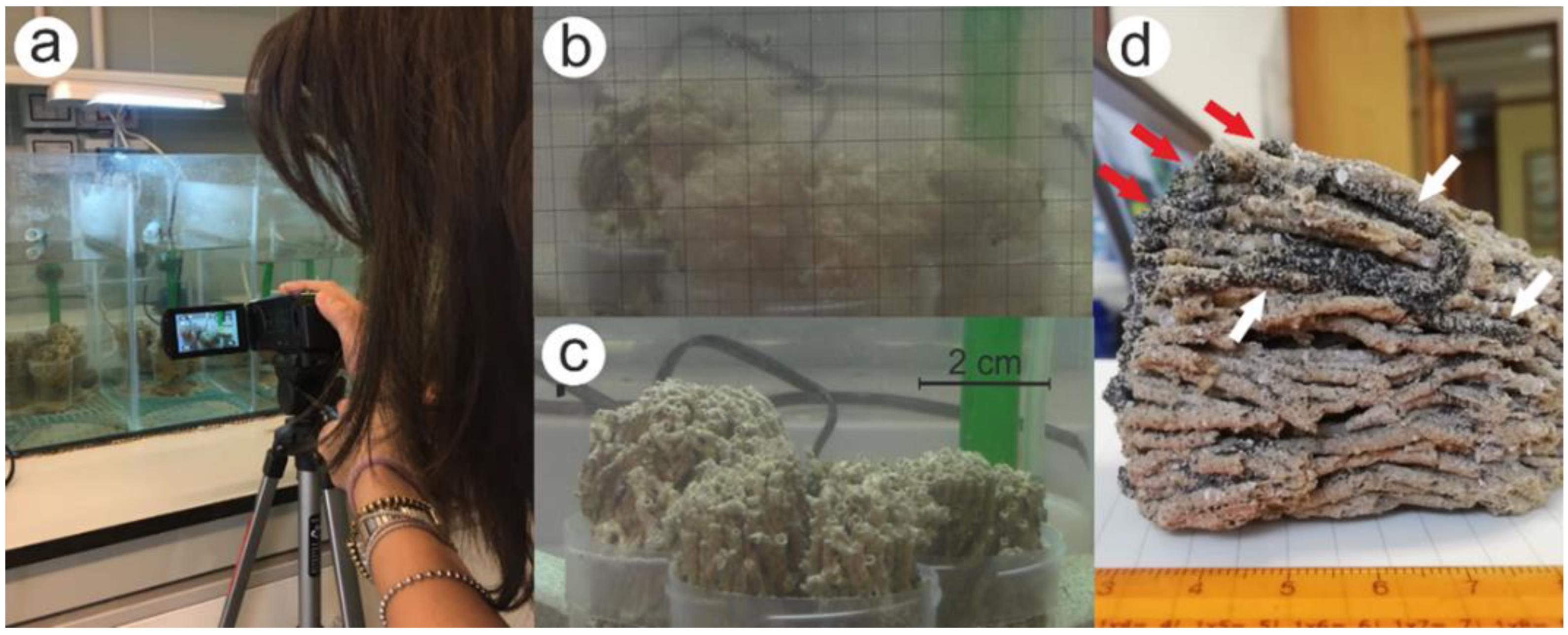
2.1. Sampling Procedures
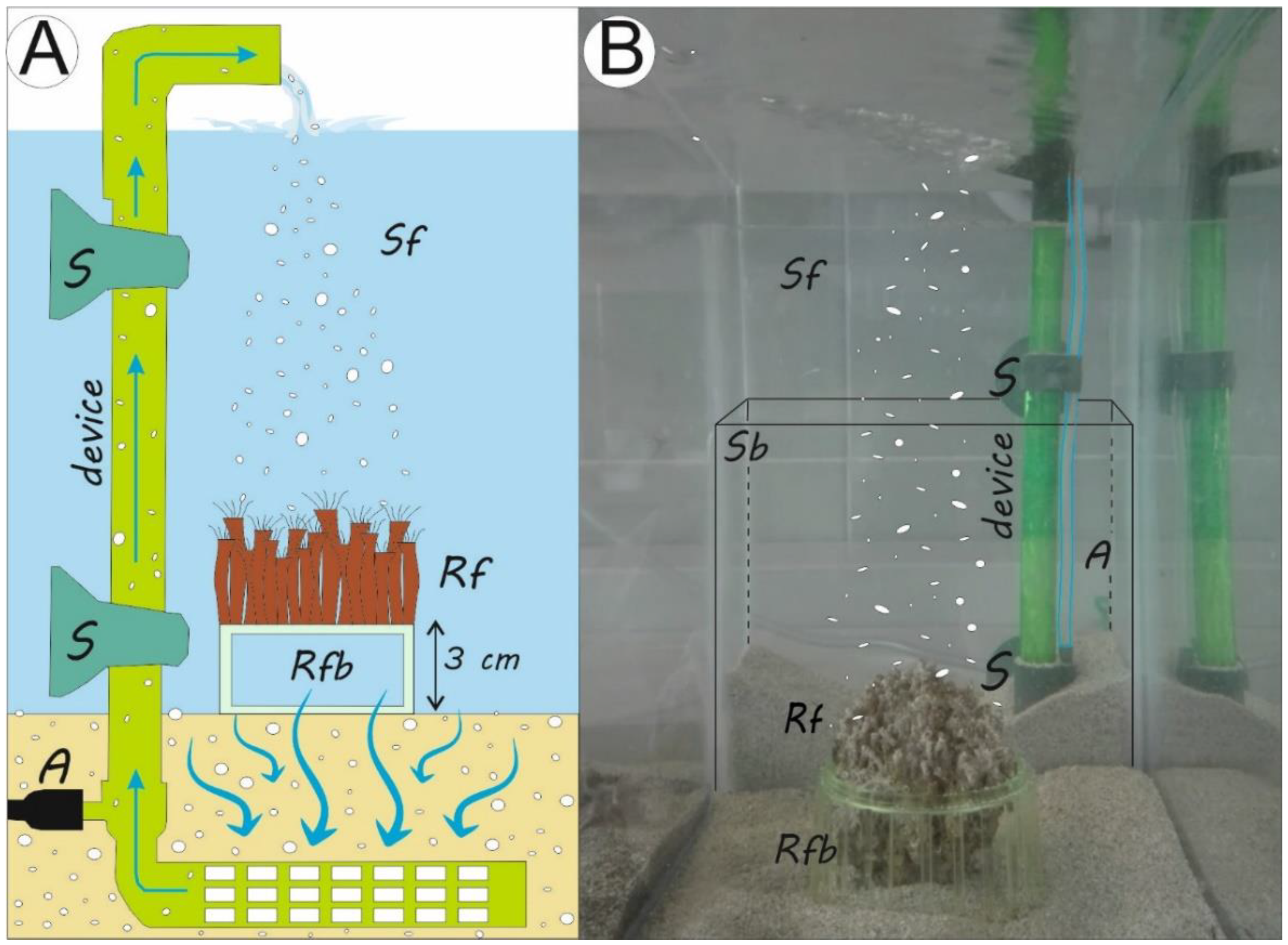
2.2. Laboratory Procedures
2.2.1. The Simulation of Sedimentation during Storm Wave Events
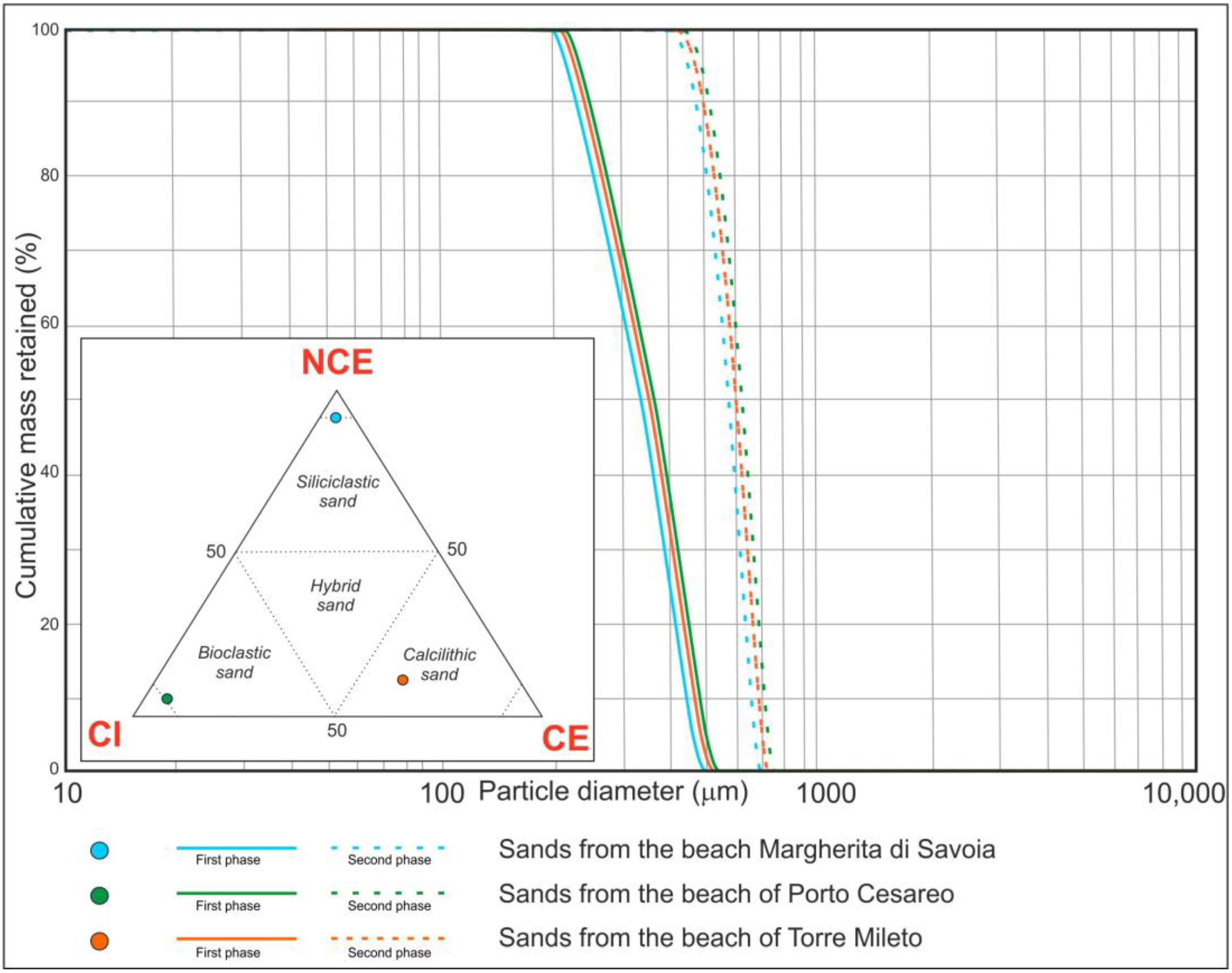
2.2.2. Sand Grain Size and Composition
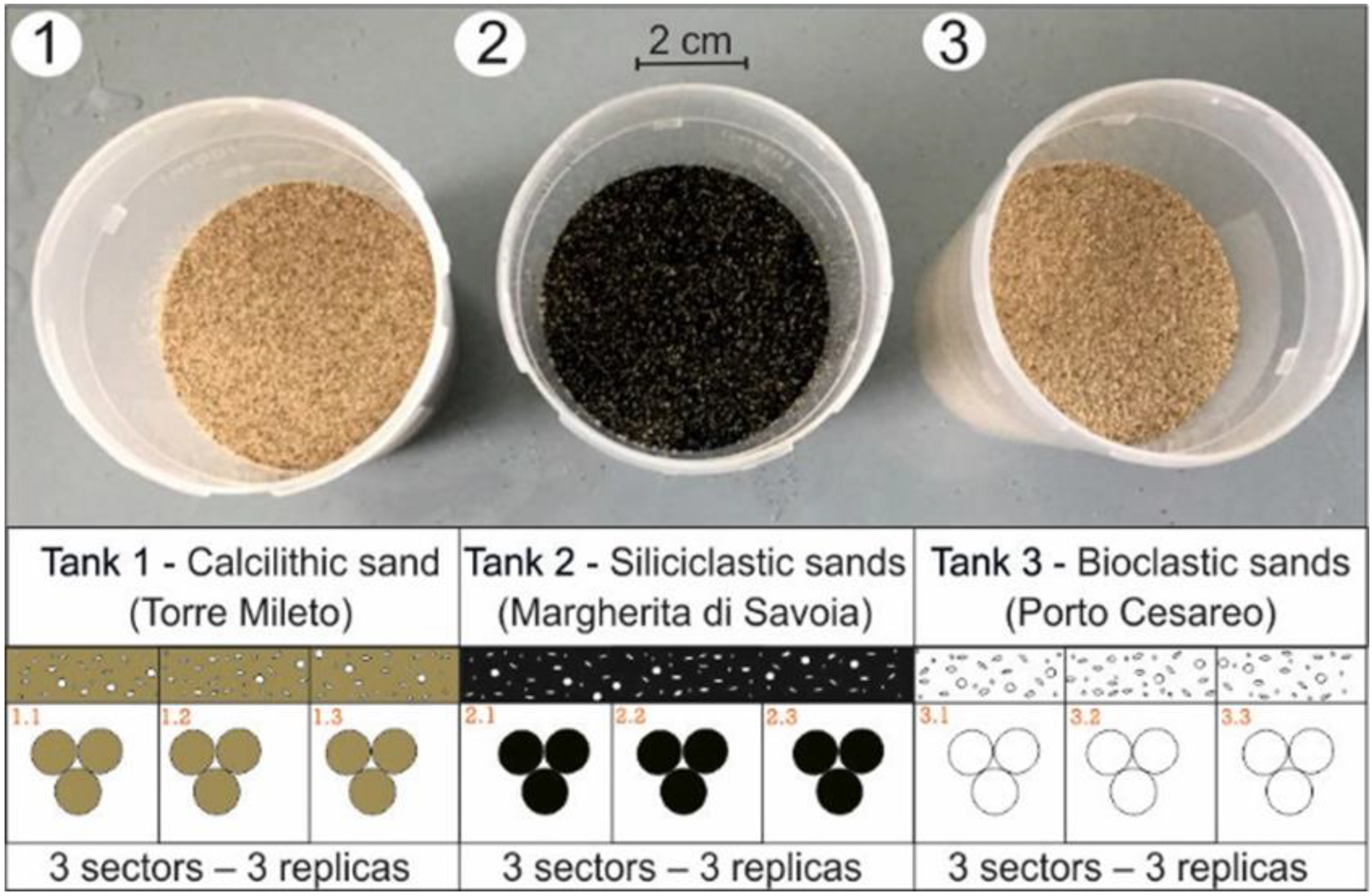
2.2.3. A Two-Phase Modelling Experiment
2.3. Procedures for Measuring the Growth of Tubes
3. Results
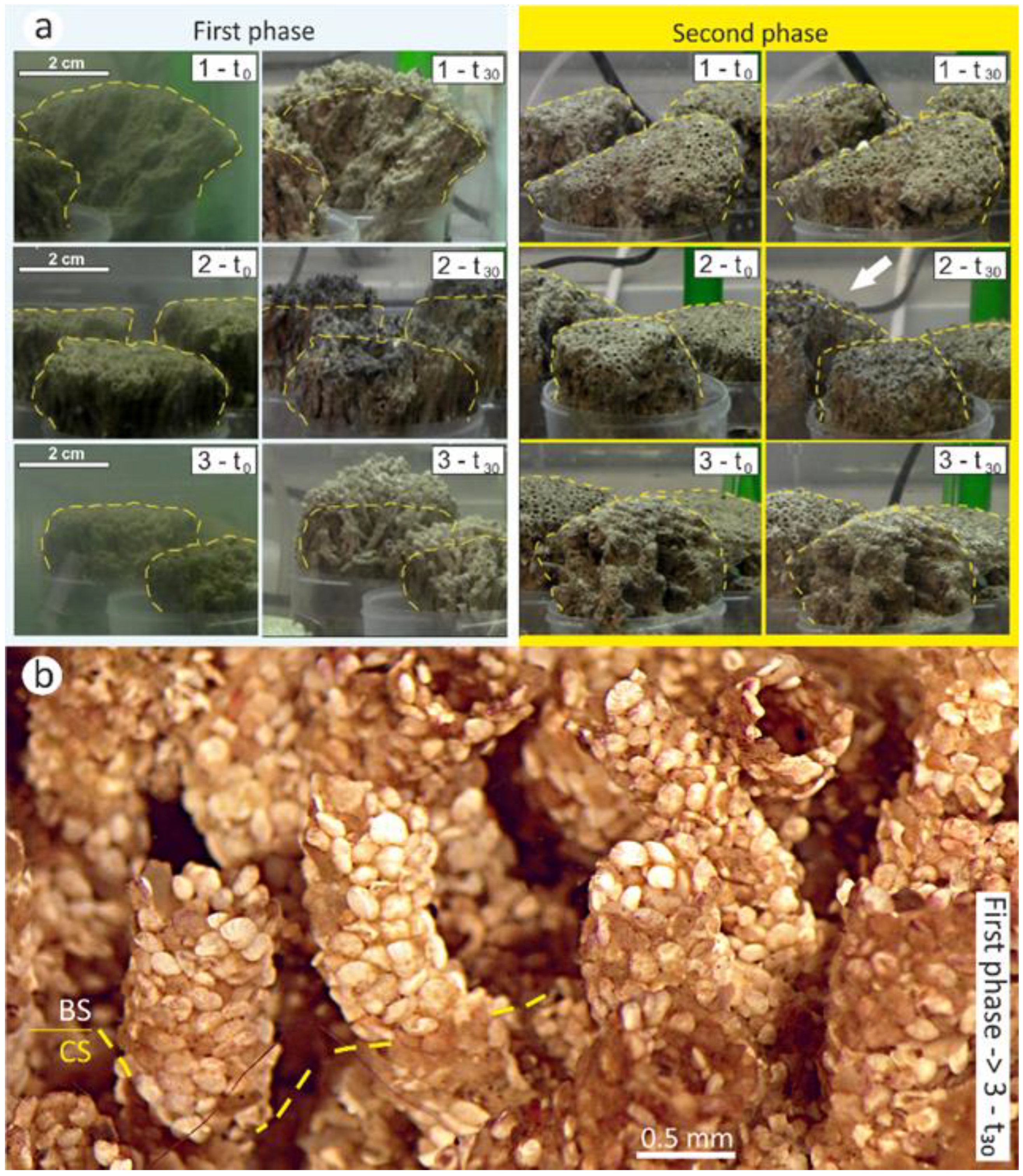
3.1. First Phase (125–350 μm)
3.2. Second Phase (350–500 μm)
3.3. Statistical Significance of Tube Growth Data Measured in the Laboratory
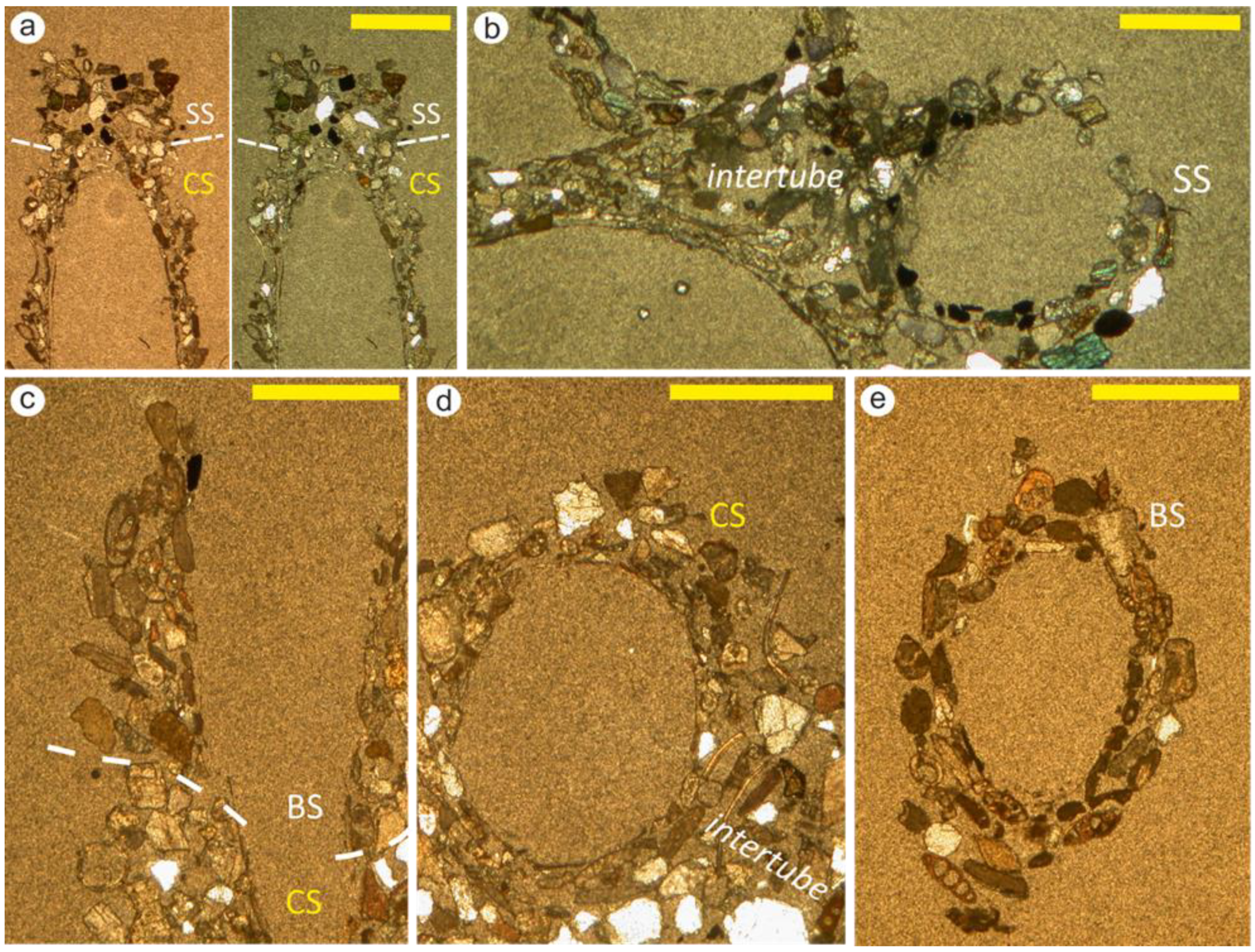
3.4. Thin Section Analysis
3.5. μX-CT Analysis
4. Discussion
4.1. The Growth of Sabellaria spinulosa Bioconstructions in the Laboratory
4.2. Microstructure of Sabellaria spinulosa Tubes Grown in the Laboratory
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wust, R.A.J. Binding organisms. In Encyclopedia of Modern Coral Reefs: Structure, Form and Process. Encyclopedia of Earth Science; Hopley, D., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 136–139. [Google Scholar]
- Allen, J.H.; Billings, I.; Cutts, N.; Elliott, M. Mapping, Condition and Conservation assessment of Honeycomb Worm Sabellaria alveolata Reefs on the Eastern Irish Sea Coast. Report to English Nature, 2002, Institute of Estuarine and Coastal Studies University of Hull, Reference No: Z122-F-2002.
- Gruet, Y. Spatio-temporal Changes of Sabellarian Reefs Built by the Sedentary Polychaete Sabellaria alveolata (Linné). Mar. Ecol. 1986, 7, 303–319. [Google Scholar] [CrossRef]
- Gruet, Y. Granulometric evolution of the sand tube in relation to growth of the polychaete Annelid Sabellaria alveolate (Linné) (Sabellariidae). Ophelia 1984, 23, 181–193. [Google Scholar] [CrossRef]
- Naylor, L.A.; Viles, H.A. A temperate reef builder: An evaluation of the growth, morphology and composition of Sabellaria alveolata (L.) colonies on carbonate platforms in South Wales. In Carbonate Platform Systems: Components and Interactions; Insalaco, E., Skelton, P.W., Palmer, T.J., Eds.; Geological Society, Special Publications: London, UK, 2000; Volume 178, pp. 9–19. [Google Scholar]
- Delbono, I.; Bianchi, C.N.; Morri, C. Le biocostruzioni di Sabellaria alveolata come indicatori ambientali: Area costiera fra Chiavari e Sestri Levante. In Studi per la Creazione di Strumenti di Gestione Costiera. Golfo del Tigullio; Ferretti, O., Ed.; Golfo del Tigullio: Genoa, Italy, 2003; pp. 130–140. [Google Scholar]
- La Porta, B.; La Valle, P.; Gusso, C. Sabellaria alveolata (Linnaeus, 1776): La selezione dei granuli di sedimento per la costruzione dei tubi. Biol. Mar. Mediterr. 2006, 13, 593–596. [Google Scholar]
- Taramelli Rivosecchi, E. Osservazioni sulle biocenosi del banco a Sabellaria di Lavinio. Rend. Accad. Naz. 1961, 40–41, 1–2. [Google Scholar]
- Multer, H.G.; Milliman, J.D. Geological aspects of Sabellarian reefs, southeastern Florida. Bull. Mar. Sci. 1967, 17, 257.e267. [Google Scholar]
- Lisco, S.; Moretti, M.; Cardone, F.; Corriero, G.; Longo, C. Sedimentological features of Sabellaria spinulosa biocontructions. Mar. Pet. Geol. 2017, 87, 203–212. [Google Scholar] [CrossRef]
- Desroy, N.; Dubois, S.; Fournier, J.; Ricquiers, L.; Le Mao, P.; Guérin, L.; Gerla, D.; Rougerie, M.; Legendre, A. Conservation status of Sabellaria alveolata (L.) (Polychaeta: Sabellariidae) reefs in the Mont-Saint-Michel bay. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2011, 21, 462–471. [Google Scholar] [CrossRef]
- Firth, L.B.; Mieszkowska, N.; Grant, L.M.; Bush, L.E.; Davies, A.J.; Frost, M.T.; Moschella, P.S.; Burrows, M.T.; Cunningham, P.N.; Dye, S.R.; et al. Historical comparisons reveal multiple drivers of decadal change of an ecosystem engineer at the range edge. Ecol. Evol. 2015, 5, 3210–3222. [Google Scholar] [CrossRef]
- Holt, T.J.; Rees, E.I.; Hawkins, S.J.; Seed, R. Biogenic Reefs (Volume IX). An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs; Scottish Association for Marine Science (UK Marine SACs Project): Obam, UK, 1998; p. 170. [Google Scholar]
- Pearce, B.; Hill, J.M.; Grubb, L.; Harper, G. Impacts of marine aggregate dredging on adjacent Sabellaria spinulosa aggregations and other benthic fauna. In Marine Aggregates Levy Sustainability Fund MEPF 08/P39 and The Crown Estate. Marine Ecological Surveys Limited; 3 Palace Yard Mews: Bath, UK, 2011; p. 35. ISBN 978-0-9506920-5-0. [Google Scholar]
- Plicanti, A.; Domínguez, R.; Dubois, S.F.; Bertocci, I. Human impacts on biogenic habitats: Effects of experimental trampling on Sabellaria alveolata (Linnaeus, 1767) reefs. J. Exp. Mar. Biol. Ecol. 2016, 478, 34–44. [Google Scholar] [CrossRef]
- Lisco, S.N.; Pierri, C.; Lazic, T.; Bonifazi, A.; Gravina, M.F.; Giangrande, A.; Acquafredda, P.; Moretti, M. Sabellaria alveolata versus Sabellaria spinulosa Reefs along the Italian Coasts: A New Methodological Proposal to Compare Different Growth Models. Geosciences 2021, 11, 426. [Google Scholar] [CrossRef]
- Lisco, S.N.; Acquafredda, P.; Gallicchio, S.; Sabato, L.; Bonifazi, A.; Cardone, F.; Corriero, G.; Gravina, M.F.; Pierri, C.; Moretti, M. The sedimentary dynamics of Sabellaria alveolata bioconstructions (Ostia, Tyrrhenian Sea, central Italy). J. Palaeogeogr. 2020, 9, 2. [Google Scholar] [CrossRef]
- Fournier, J. Bioconstructions d’annélides polychètes. In Mémoire d’Habilitation à Diriger des Recherches, Volume 2; Synthese scientifique—Université de Bretagne Occidentale, Institut Universitaire Européen de la Mer, CNRS: Brest, France, 2013; p. 171. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [CrossRef] [PubMed]
- Eckelbarger, K.J. Larval development and population aspects of the reef-building polychaete Phragmatopoma lapidosa from the east coast of Florida. Bull. Mar. Sci. 1976, 26, 117–132. [Google Scholar]
- Davies, A.J.; Last, K.S.; Attard, K.; Hendrick, V.J. Maintaining turbidity and current flow in laboratory aquarium studies, a case study using Sabellaria spinulosa. J. Exp. Mar. Biol. Ecol. 2009, 370, 35–40. [Google Scholar] [CrossRef]
- Jones, L.A.; Hiscock, K.; Connor, D.W. Marine Habitat Reviews: A Summary of Ecological Requirements and Sensitivity Characteristics for the Conservation and Management of Marine SACs; Joint Nature Conservation Committee: Peterborough, UK, 2000.
- Braithwaite, C.J.R.; Robinson, R.J.; Jones, G. Sabellarids: A hidden danger or an aid to subsea pipelines? Q. J. Eng. Geol. Hydrogeol. 2006, 39, 259–265. [Google Scholar] [CrossRef]
- Last, K.; Hendrick, V.J.; Beveridge, C.M.; Davies, A.J. Measuring the effects of suspended particulate matter and smothering on the behaviour, growth and survival of key species found in areas associated with aggregate dredging. Report for the Marine Aggregate Levy Sustainability Fund; Commercial Report, 2011; MEPF 08/P76. Available online: https://pure.uhi.ac.uk/en/publications/measuring-the-effects-of-suspended-particulate-matter-and-burial- (accessed on 12 December 2022).
- Giangrande, A.; Lezzi, M.; Cardone, F.; Mikac, B. Variation and ontogenetic changes of opercular paleae in a population of Sabellaria spinulosa (Polychaeta: Sabellaridae) from the South Adriatic Sea, with remarks on larval development. Sci. Mar. 2015, 79, 137–150. [Google Scholar] [CrossRef]
- Gravina, M.F.; Cardone, F.; Bonifazi, A.; Bertrandino, M.S.; Chimienti, G.; Longo, C.; Marzano, C.N.; Moretti, M.; Lisco, S.; Moretti, V.; et al. Sabellaria spinulosa (Polychaeta, Annelida) reefs in the Mediterranean Sea: Habitat mapping, dynamics and associated fauna for conservation management. Estuarine Coast. Shelf Sci. 2018, 200, 248–257. [Google Scholar] [CrossRef]
- Lowe, D.R. Water escape structures in coarse-grained sediments. Sedimentology 1975, 22, 157–204. [Google Scholar] [CrossRef]
- Van Loon, A.J.; Moretti, M.; Tropeano, M.; Acquafredda, P.; Baldacconi, R.; Festa, V.; Lisco, S.; Mastronuzzi, G.; Moretti, V.; Scotti, R. Tracing the Source of the Bio/Siliciclastic Beach Sands at Rosa Marina (Apulian Coast, SE Italy). In Sediment Provenance; Mazumder, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 25–47. [Google Scholar] [CrossRef]
- Lapietra, I.; Lisco, S.N.; Milli, S.; Moretti, M. Sediment provenance of a bioclastic carbonate pocket beach—Le Dune (Ionian Sea, South Italy). J. Palaeogeogr. 2022, 11, 1–18. [Google Scholar] [CrossRef]
- Poppe, L.J.; Eliason, A.H.; Fredericks, J.J.; Rendigs, R.R.; Blackwood, D.; Polloni, C.F. Grain-Size Analysis of Marine Sediments e Methodology and Data Processing. In Geological Survey Open File Report 00e358; U.S. Geological Survey: Woods Hole, MA, USA, 2000. Available online: https://pubs.usgs.gov/of/2000/of00-358/text/chapter1.htm (accessed on 8 January 2023).
- Sanfilippo, R.; Rosso, A.; Viola, A.; Guido, A.; Deias, C. Architecture and tube structure of Sabellaria spinulosa (Leuckart, 1849): Comparison with the Mediterranean S. alveolata congener. J. Morphol. 2022, 283, 1350–1358. [Google Scholar] [CrossRef]
- Zuffa, G.G. Hybrid arenites: Their composition and classification. J. Sediment. Petrol. 1980, 50, 21–29. [Google Scholar]
- Zuffa, G.G. Optical analysis of arenites: Influence of methodology on compositional results. In Provenance of Arenites; Zuffa, G.G., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 165–189. [Google Scholar]
- Liu, X.; Sasov, A. Cluster reconstruction strategies for microCT/nanoCTscanners. In Proceedings of the of Fully 3D Image Reconstruction Meeting in Radiology and Nuclear Medicine, Salt Lake City, UT, USA, 6–9 July 2005. [Google Scholar]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Kench, P.S.; McLean, R.F. A comparison of settling and sieve techniques for the analysis of bioclastic sediments. Sediment. Geol. 1997, 109, 111–119. [Google Scholar] [CrossRef]
- Franzitta, G.; Colletti, A.; Savinelli, B.; Martire, M.L.; Corinaldesi, C.; Musco, L. Feasibility of the Sabellarid Reef Habitat Restoration. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Pearce, B.; Fariñas-Franco, J.M.; Wilson, C.; Pitts, J.; Deburgh, A.; Somerfield, P.J. Repeated mapping of reefs constructed by Sabellaria spinulosa Leuckart 1849 at an offshore wind farm site. Cont. Shelf Res. 2014, 83, 3–13. [Google Scholar] [CrossRef]
- Bue, G.L.; Marchini, A.; Mancin, N. Selection or random picking? Foraminiferal tests in Sabellaria alveolata (Linnaeus, 1767) bioconstructions. Mar. Environ. Res. 2022, 176, 105616. [Google Scholar] [CrossRef] [PubMed]
- Choquette, P.W.; Pray, L.C. Geologic nomenclature and classification of porosity in sedimentary carbonates. Am. Assoc. Pet. Geol. Bull. 1970, 54, 207–250. [Google Scholar]
- Jackson, D.R.; Richardson, M.D.; Isakson, M.J.; Wilson, P.S. High-Frequency Seafloor Acoustics. J. Acoust. Soc. Am. 2007, 122, 2497. [Google Scholar] [CrossRef]
- Maiklem, W.R. Some hydraulic properties of bioclastic carbonate grains. Sedimentology 1968, 10, 101–109. [Google Scholar] [CrossRef]
- Fox, H.M. Functions of the Tube in Sabellid Worms. Nature 1938, 141, 163. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisco, S.; Lazic, T.; Pierri, C.; Mele, D.; de Luca, A.; Moretti, M. Analysis of the Sabellaria spinulosa Bioconstruction Growth in a Laboratory. J. Mar. Sci. Eng. 2023, 11, 204. https://doi.org/10.3390/jmse11010204
Lisco S, Lazic T, Pierri C, Mele D, de Luca A, Moretti M. Analysis of the Sabellaria spinulosa Bioconstruction Growth in a Laboratory. Journal of Marine Science and Engineering. 2023; 11(1):204. https://doi.org/10.3390/jmse11010204
Chicago/Turabian StyleLisco, Stefania, Tamara Lazic, Cataldo Pierri, Daniela Mele, Alessia de Luca, and Massimo Moretti. 2023. "Analysis of the Sabellaria spinulosa Bioconstruction Growth in a Laboratory" Journal of Marine Science and Engineering 11, no. 1: 204. https://doi.org/10.3390/jmse11010204
APA StyleLisco, S., Lazic, T., Pierri, C., Mele, D., de Luca, A., & Moretti, M. (2023). Analysis of the Sabellaria spinulosa Bioconstruction Growth in a Laboratory. Journal of Marine Science and Engineering, 11(1), 204. https://doi.org/10.3390/jmse11010204







