Analysis of Characteristic Changes of Blended Very Low Sulfur Fuel Oil on Ultrasonic Frequency for Marine Fuel
Abstract
1. Introduction
2. Experiments
2.1. Materials and Analysis Methods
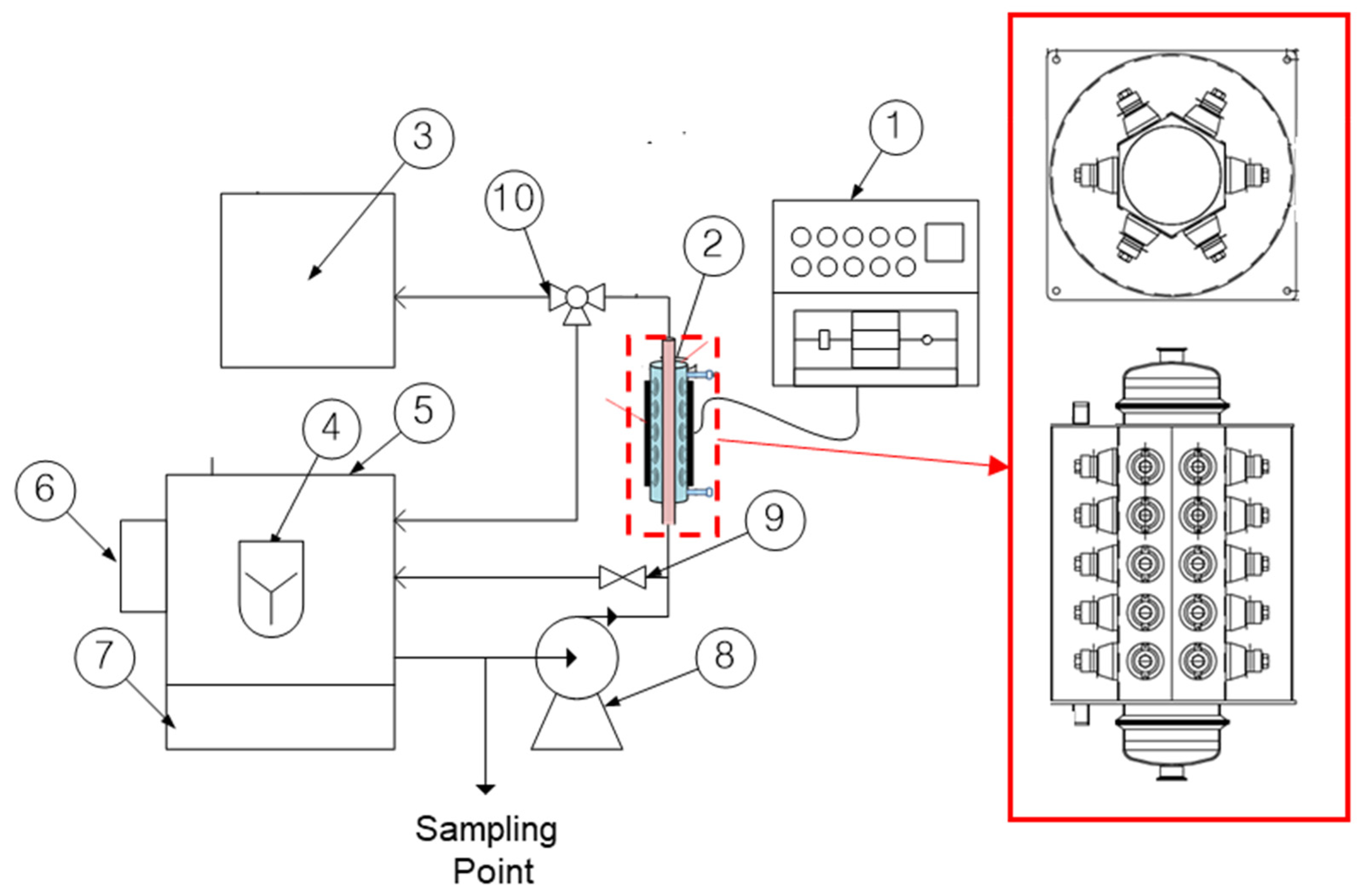
2.2. Flowcell-Type Ultrasound Equipment
2.3. Ultrasonic Irradiation Test
3. Results and Discussion
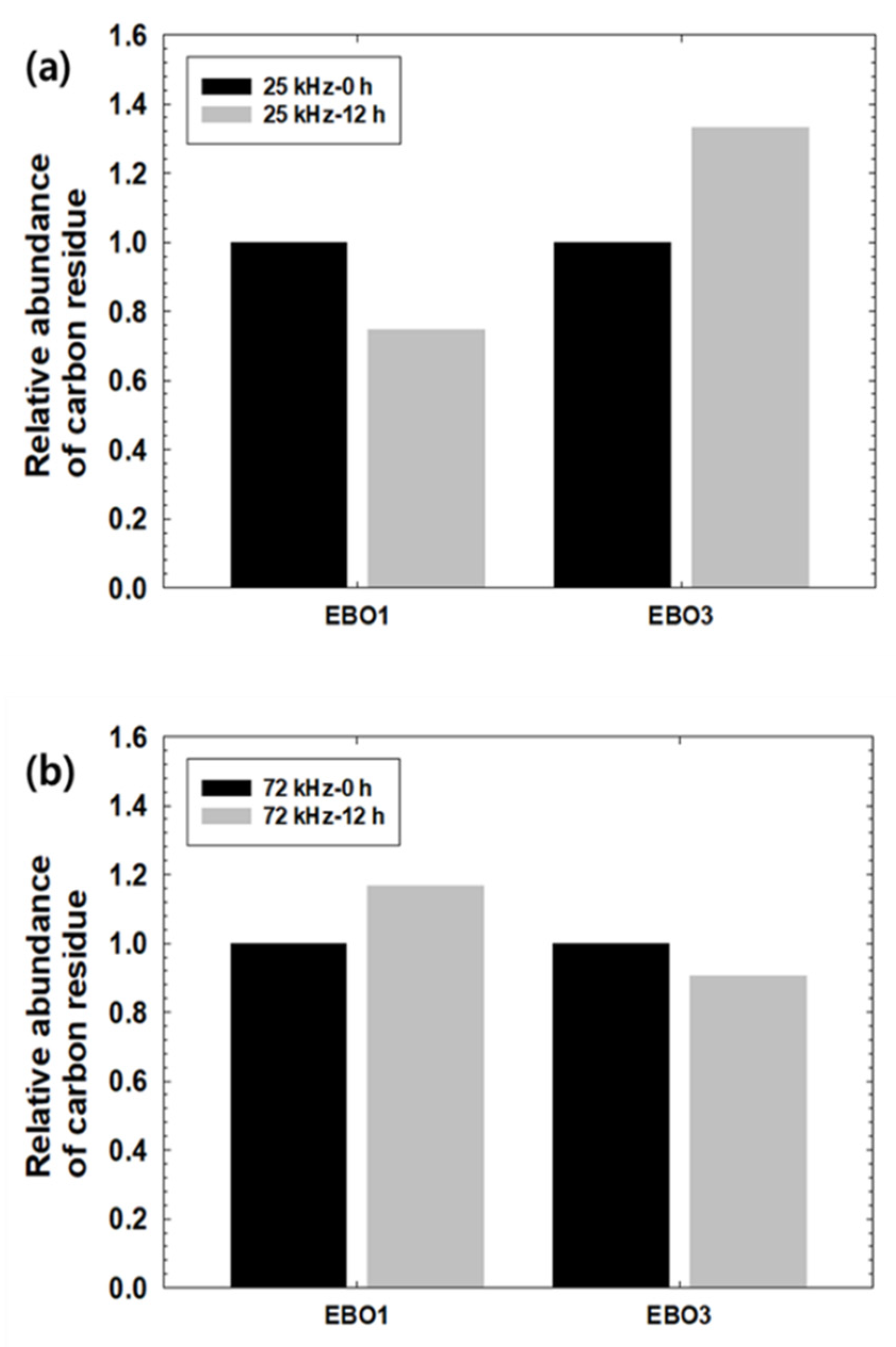
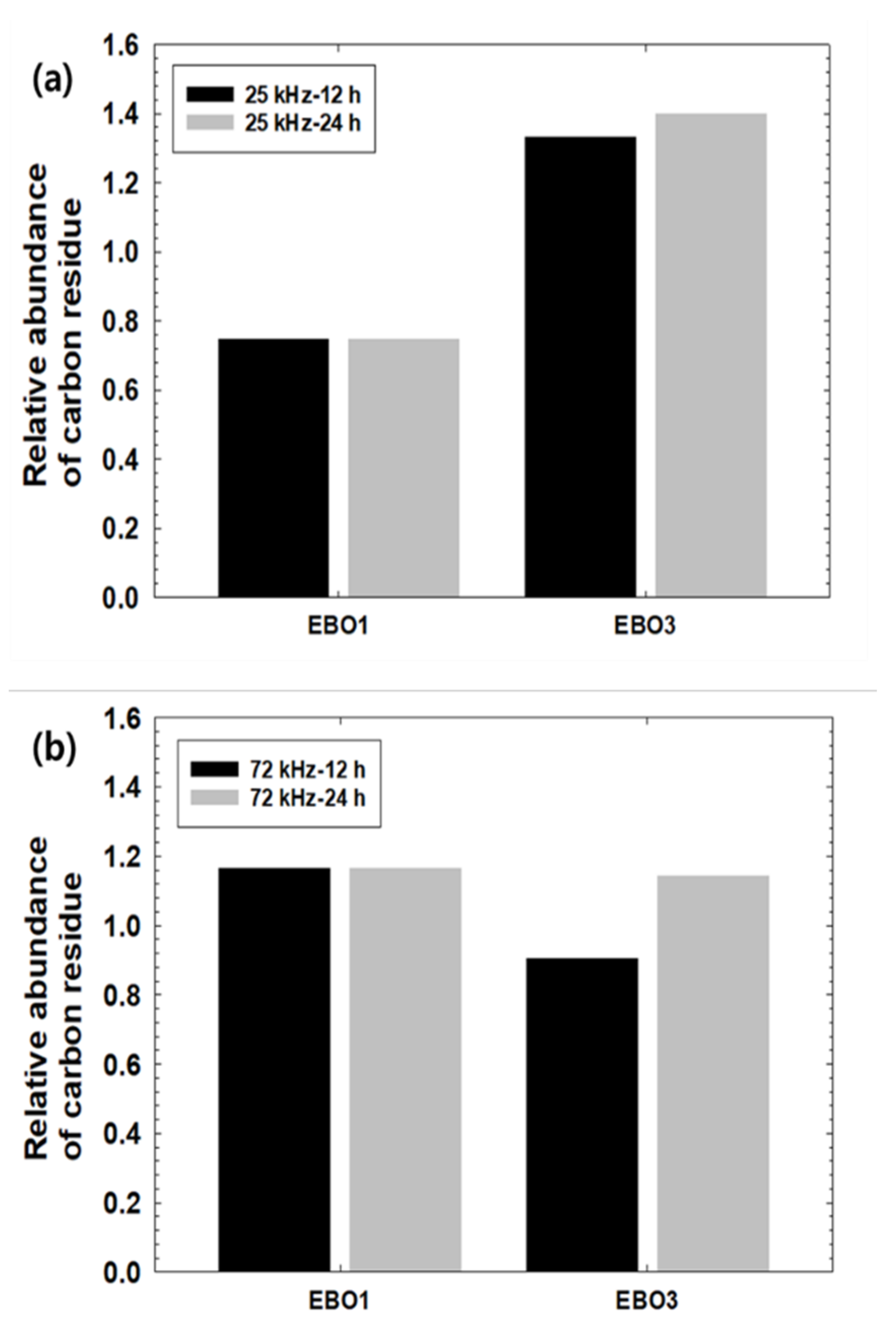
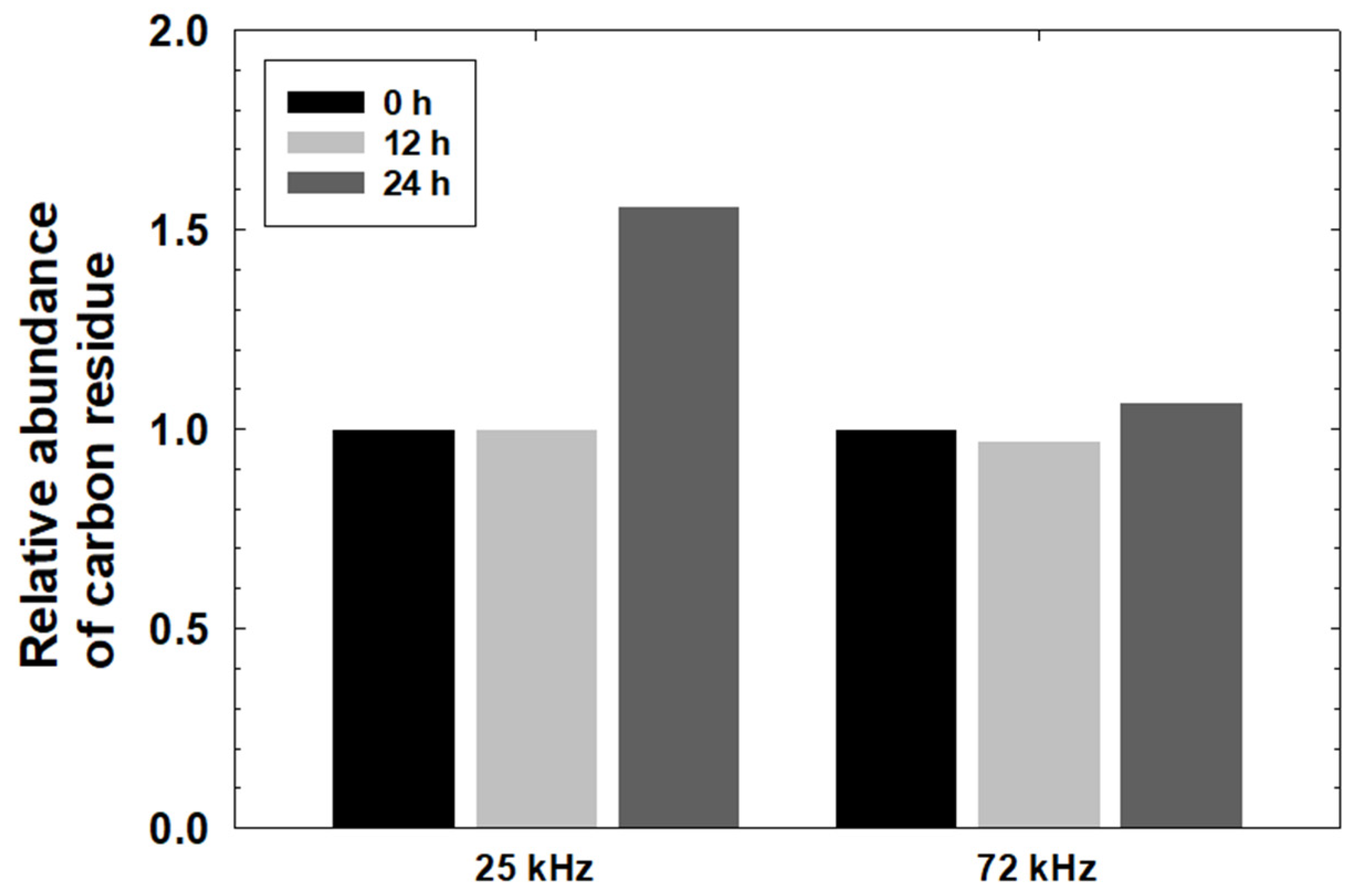
3.1. Carbon Residue
3.2. Kinematic Viscosity and Density
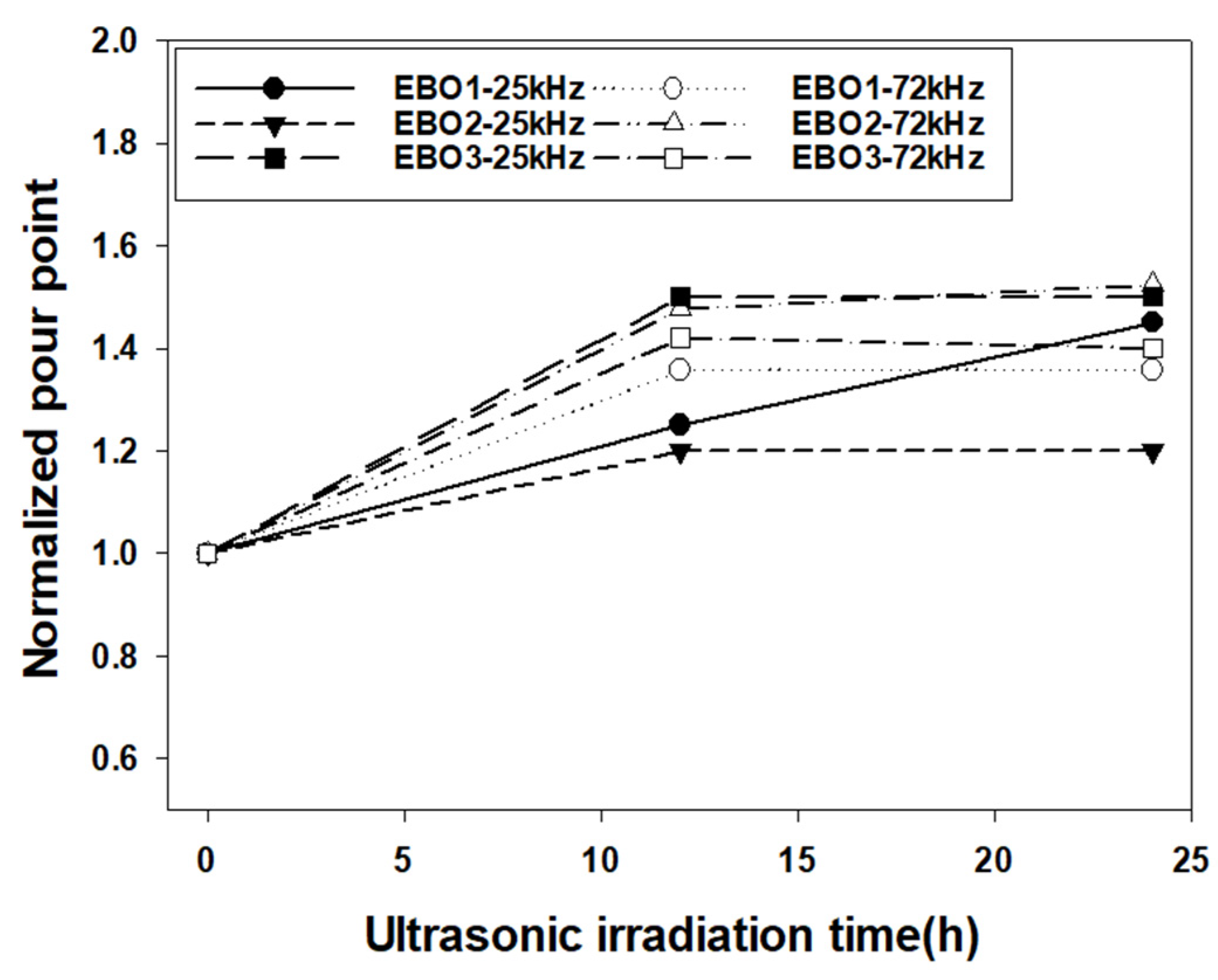
3.3. Pour Point
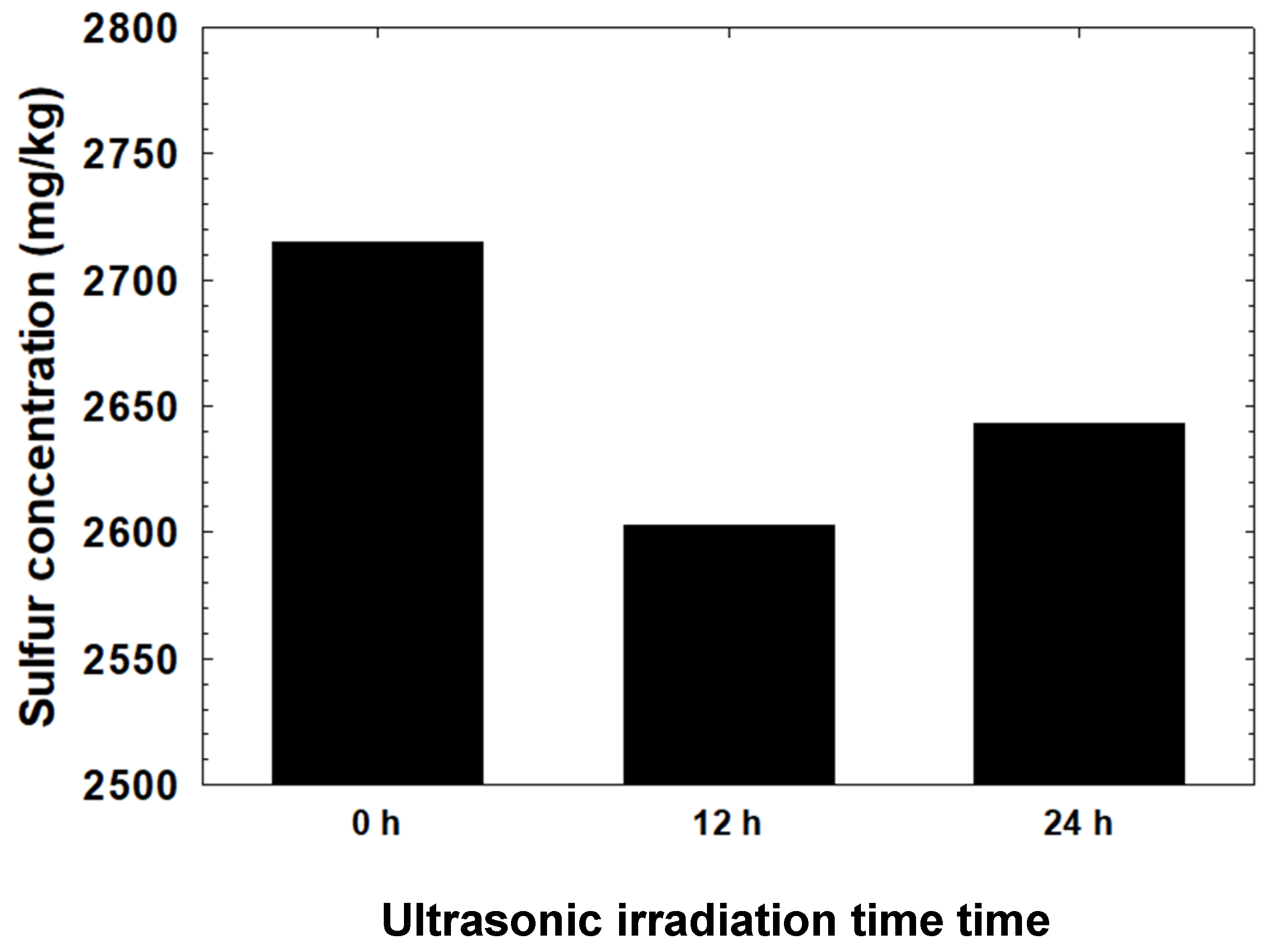
3.4. Sulfur
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| B-A | Bunker-A |
| EBO | Experimental blended oil |
| EBO1 | Experimental blended oil 1 (mixing ratio by weight of 25:75 for marine gas oil and bunker-A) |
| EBO2 | Experimental blended oil 2 (mixing ratio by weight of 50:50 for marine gas oil and bunker-A) |
| EBO3 | Experimental blended oil 3 (mixing ratio by weight of 75:25 for marine gas oil and bunker-A) |
| HSFO | High sulfur fuel oil |
| MGO | Marine gas oil |
| ODS | Oxidative desulfurization |
| UAOD | Ultrasonic-assisted oxidation desulfurization |
| VLSFO | Very low sulfur fuel oil |
References
- International Maritime Organization. Sulphur Oxides (SOX) and Particulate Matter (PM)–Regulation 14; IMO: London, UK, 2015; Available online: https://www.imo.org/en/OurWork/Environment/Pages/Sulphur-oxides-(SOX)—Regulation-14.aspx (accessed on 1 June 2022).
- Vedachalam, S.; Baquerizo, N.; Dalai, A.K. Review on impacts of low sulfur regulations on marine fuels and compliance options. Fuel 2022, 310, 122243. [Google Scholar] [CrossRef]
- Lee, H.C.; Rue, H.Y. Development Strategy of Shipping Industry in Korea to prepare for IMO Emission Regulations; Korea Maritime Institute: Busan, Korea, 2019; Available online: https://www.kmi.re.kr/synap/skin/doc.html?fn=201902011317546431.pdf&rs=/synap/efiles/result/&page=null&type=null (accessed on 1 June 2022).
- Korea Ocean Business Corporation. IMO 2020 and Green Transition; KOBC: Busan, Korea, 2020; Available online: https://www.meic.kr/REPORT/rboard/1?type=SP (accessed on 1 June 2022).
- Odey, F.; Lacey, M. IMO 2020–Short Term Implications for the Oil Market; Schroder Investment Management North America Inc.: New York, NY, USA, 2018; Available online: https://prod.schroders.com/en/sysglobalassets/digital/insights/2018/pdf/imo-report2.pdf (accessed on 1 June 2022).
- Javadli, R.; De Klerk, A. Desulfurization of heavy oil. Appl. Petrochem. Res. 2012, 1, 3–19. [Google Scholar] [CrossRef]
- De Filippis, P.; Scarsella, M. Oxidative desulfurization: Oxidation reactivity of sulfur compounds in different organic matrixes. Energy Fuels 2003, 17, 1452–1455. [Google Scholar] [CrossRef]
- Ja’fari, M.; Ebrahimi, S.L.; Khosravi-Nikou, M.R. Ultrasound-assisted oxidative desulfurization and denitrogenation of liquid hydrocarbon fuels: A critical review. Ultrason. Sonochem. 2018, 40, 955–968. [Google Scholar] [CrossRef]
- Bimco; ICS; Intercargo; Intertanko. 2020 Fuel Oil Quality and Safety Survey; BIMCO: Copenhagen, Denmark, 2020; Available online: https://www.bimco.org/-/media/bimco/news-and-trends/news/priority-news/2020/2020-fuel-oil-quality-and-safety-survey---report.ashx (accessed on 1 June 2022).
- Smyshlyaeva, K.I.; Rudko, V.A.; Kuzmin, K.A.; Povarov, V.G. Asphaltene genesis influence on the low-sulfur residual marine fuel sedimentation stability. Fuel 2022, 328, 125291. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Islamov, S.; Mardashov, D.; Beloglazov, I.; Hemmingsen, T. Research of the Influence of Marine Residual Fuel Composition on Sedimentation Due to Incompatibility. J. Mar. Sci. Eng. 2021, 9, 1067. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanova, N.; Veli, A.; Nikolova, R.; Mitkova, M.; Stanulov, K.; Argirov, G.; Yordanov, D.; Nikolaychuk, E. Colloidal stability and hot filtration test of residual fuel oils based on visbreaking and ebullated bed residue H-Oil hydrocracking. Int. J. Oil Gas Coal Technol. 2019, 20, 169–188. [Google Scholar] [CrossRef]
- Povarov, V.G.; Efimov, I.; Smyshlyaeva, K.I.; Rudko, V.A. Application of the UNIFAC Model for the Low-Sulfur Residue Marine Fuel Asphaltenes Solubility Calculation. J. Mar. Sci. Eng. 2022, 10, 1017. [Google Scholar] [CrossRef]
- Vrablik, A.; Schlehofer, D.; Dlasková Jaklová, K.; Hidalgo Herrador, J.M.; Cerny, R. Comparative Study of Light Cycle Oil and Naphthalene as an Adequate Additive to Improve the Stability of Marine Fuels. ACS Omega 2022, 7, 2127–2136. [Google Scholar] [CrossRef]
- Lee, B.; Kim, M. Identify the Quality Characteristic of Low Sulfur Fuel Oil to Implement IMO Regulation on Sox. Kor. Ind. Chem. News 2019, 22, 41–55. [Google Scholar]
- Intertanko. Contaminated Bunkers Damage Hundreds of Ships do Authorities Really Care; Intertanko: London, UK, 2018; 20p, Available online: http://www.gard.no/Content/26096787/Intertanko%20paper.pdf (accessed on 1 June 2022).
- Brennen, C.E. Cavitation and Bubble Dynamics; Oxford University Press: Oxford, UK, 1995; pp. 59–88. [Google Scholar]
- Hosseini, H.; Hamidi, A. Sulfur Removal of Crude Oil by Ultrasound-Assisted Oxidative Method. In Proceedings of the International Conference on Biological, Civil and Environmental Engineering, Dubai, United Arab Emirates, 17–18 March 2014. [Google Scholar] [CrossRef]
- Margeta, D.; Sertić-Bionda, K.; Foglar, L. Ultrasound assisted oxidative desulfurization of model diesel fuel. Appl. Acoust. 2016, 103, 202–206. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Huang, X.; Wu, Y.; Chen, J. Optimization of ultrasonic-assisted oxidative desulfurization of gasoline and crude oil. Chem. Eng. Process. Process Intensif. 2020, 147, 107789. [Google Scholar] [CrossRef]
- Bolla, M.K.; Choudhury, H.A.; Moholkar, V.S. Mechanistic features of ultrasound-assisted oxidative desulfurization of liquid fuels. Ind. Eng. Chem. Res. 2021, 51, 9705–9712. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, D.; Qi, Y. Sono-desulfurization oxidation reactivities of FCC diesel fuel in metal ion/H2O2 systems. Ultrason. Sonochem. 2011, 18, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Escobar, D.R.; Quintana-Jiménez, L.A.; González-Jiménez, E.E.; Olaya-Escobar, E.S. Ultrasound Applied in the Reduction of Viscosity of Heavy Crude Oil. Rev. Fac. Ing. 2020, 29, e11528. [Google Scholar] [CrossRef]
- Allami, H.A.; Tabasizadeh, M.; Rohani, A.; Nayebzadeh, H.; Farzad, A. Effect of ultrasonic irradiation on the properties and performance of biodiesel produced from date seed oil used in the diesel engine. Ultrason. Sonochem. 2020, 60, 104672. [Google Scholar] [CrossRef]
- Najafi, I.; Amani, M. Asphaltene flocculation inhibition with ultrasonic wave radiation: A detailed experimental study of the governing mechanisms. Adv. Petrol. Explor. Dev. 2011, 2, 32–36. [Google Scholar] [CrossRef]
- Duarte, F.A.; Mello, P.dA.; Bizzi, C.A.; Nunes, M.A.G.; Moreira, E.M.; Alencar, M.S.; Motta, H.N.; Dressler, V.L.; Flores, É.M.M. Sulfur removal from hydrotreated petroleum fractions using ultrasound-assisted oxidative desulfurization process. Fuel 2011, 90, 2158–2164. [Google Scholar] [CrossRef]
- Makarev, D.I.; Rybyanets, A.N.; Sukhorukov, V.L. Effects of different power high-intensity ultrasonic treatment on rheological properties of heavy oil products. Indian J. Sci. Technol. 2016, 9, 168. [Google Scholar] [CrossRef][Green Version]
- Mguni, L.L.; Yao, Y.; Liu, X.; Yuan, Z.; Hildebrandt, D. Ultra-deep desulphurization of both model and commercial diesel fuels by adsorption method. J. Environ. Chem. Eng. 2019, 7, 102957. [Google Scholar] [CrossRef]
- Ju, H.J.; Jeon, S.K. Effect of Ultrasound Irradiation on the Properties and Sulfur Contents of Blended Very Low-Sulfur Fuel Oil (VLSFO). J. Mar. Sci. Eng. 2022, 10, 980. [Google Scholar] [CrossRef]
- ISO8217. Petroleum Products-Fuels (Class-F)-Specification of Marine Fuels; ISO: Geneva, Switzerland, 2017; Available online: https://www.iso.org/standard/64247.html (accessed on 1 June 2022).
- Gondrexon, N.; Renaudin, V.; Petrier, C.; Boldo, P.; Bernis, A.; Gonthier, Y. Degradation of pentachlorophenol aqueous solutions using a continuous flow ultrasonic reactor: Experimental performance and modelling. Ultrason. Sonochem. 1999, 5, 125–131. [Google Scholar] [CrossRef]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, P.W. Sonocrystallization: The use of ultrasound for improved industrial crystallization. Org. Process Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Ghetti, P. A rapid heating TGA method for evaluating the carbon residue of fuel oil. Fuel 1994, 73, 1918–1921. [Google Scholar] [CrossRef]
- Piazza, T.; Puskas, W.L. The Ideal Ultrasonic Parameters for Delicate Parts Cleaning. In Particles on Surfaces: Detection, Adhesion and Removal; CRC Press: Boca Raton, FL, USA, 2003; Volume 8, p. 303. Available online: http://www.ultrasonic-resonators.org/misc/references/articles/Piazza__’The_Ideal_Ultrasonic_Parameters_for_Delicate_Parts_Cleaning’.pdf (accessed on 1 June 2022).
- Gollapudi, U.K.; Bang, S.S.; Islam, M.R. Ultrasonic Treatment for Removal of Asphaltene Deposits During Petroleum Production. In Proceedings of the SPE Formation Damage Control Symposium, Lafayette, LA, USA, 7–10 February 1994; p. p. SPE-27377-MS. [Google Scholar] [CrossRef]
- Diallo, M.S.; Cagin, T.; Faulon, J.L.; Goddard, W.A. Thermodynamic properties of asphaltenes: A predictive approach based on computer assisted structure elucidation and atomistic simulations. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 2000; p. 40. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on mineral oil hydrocarbons in food. EFSA J. 2012, 10, 2704. [Google Scholar] [CrossRef]
- CIMAC. WG7 Fuels Members, Cold Flow Properties of Marine Fuel Oils, International Council on Combustion Engines; CIMAC: Frankfurt, Germany, 2015; Available online: https://www.cimac.com/cms/upload/workinggroups/WG7/CIMAC_WG7_2015_01_Guideline_Cold__Flow_Properties_Marine_Fuel_Oils_final.pdf (accessed on 1 June 2022).
- Ford, M.C. A Master’s Guide to Using Fuel Oil Onboard Ships; Charles Taylor & Co, Limited: London, UK, 2012; Available online: https://www.standard-club.com/fileadmin/uploads/standardclub/Documents/Import/publications/masters-guides/24163-AMastersGuidetoUsingFuelOilOnboardships.pdf (accessed on 1 June 2022).
- Ihara, S. Feasibility of hydrogen production by direct water splitting at high temperature. Int. J. Hydrog. Energy 1978, 3, 287–296. [Google Scholar] [CrossRef]
| Case | MGO (wt.%) | B-A (wt.%) | Total (kg) |
|---|---|---|---|
| EBO1 | 75 | 25 | 40 |
| EBO2 | 50 | 50 | 40 |
| EBO3 | 25 | 75 | 40 |
| Condition | Kinematic Viscosity (mm2/s at 40 °C) | Density (kg/m3 at 15 °C) | |||||
|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 0 h | 12 h | 24 h | ||
| EBO1 | 25 kHz | 3.87 | 3.88 | 3.89 | 842.5 | 842.4 | 842.5 |
| 72 kHz | 3.84 | 3.92 | 3.93 | 842.0 | 842.5 | 842.6 | |
| EBO2 | 25 kHz | 4.17 | 4.18 | 4.19 | 847.6 | 847.6 | 847.7 |
| 72 kHz | 4.17 | 4.18 | 4.19 | 847.5 | 847.6 | 847.7 | |
| EBO3 | 25 kHz | 4.51 | 4.52 | 4.52 | 852.7 | 852.8 | 852.8 |
| 72 kHz | 4.52 | 4.53 | 4.54 | 852.8 | 852.9 | 853.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, H.-j.; Jeon, S.-k. Analysis of Characteristic Changes of Blended Very Low Sulfur Fuel Oil on Ultrasonic Frequency for Marine Fuel. J. Mar. Sci. Eng. 2022, 10, 1254. https://doi.org/10.3390/jmse10091254
Ju H-j, Jeon S-k. Analysis of Characteristic Changes of Blended Very Low Sulfur Fuel Oil on Ultrasonic Frequency for Marine Fuel. Journal of Marine Science and Engineering. 2022; 10(9):1254. https://doi.org/10.3390/jmse10091254
Chicago/Turabian StyleJu, Hae-ji, and Soo-kyung Jeon. 2022. "Analysis of Characteristic Changes of Blended Very Low Sulfur Fuel Oil on Ultrasonic Frequency for Marine Fuel" Journal of Marine Science and Engineering 10, no. 9: 1254. https://doi.org/10.3390/jmse10091254
APA StyleJu, H.-j., & Jeon, S.-k. (2022). Analysis of Characteristic Changes of Blended Very Low Sulfur Fuel Oil on Ultrasonic Frequency for Marine Fuel. Journal of Marine Science and Engineering, 10(9), 1254. https://doi.org/10.3390/jmse10091254






