Effect of Hydrate Microscopic Distribution on Acoustic Characteristics during Hydrate Dissociation: An Insight from Combined Acoustic-CT Detection Study
Abstract
:1. Introduction
2. Materials and Methods
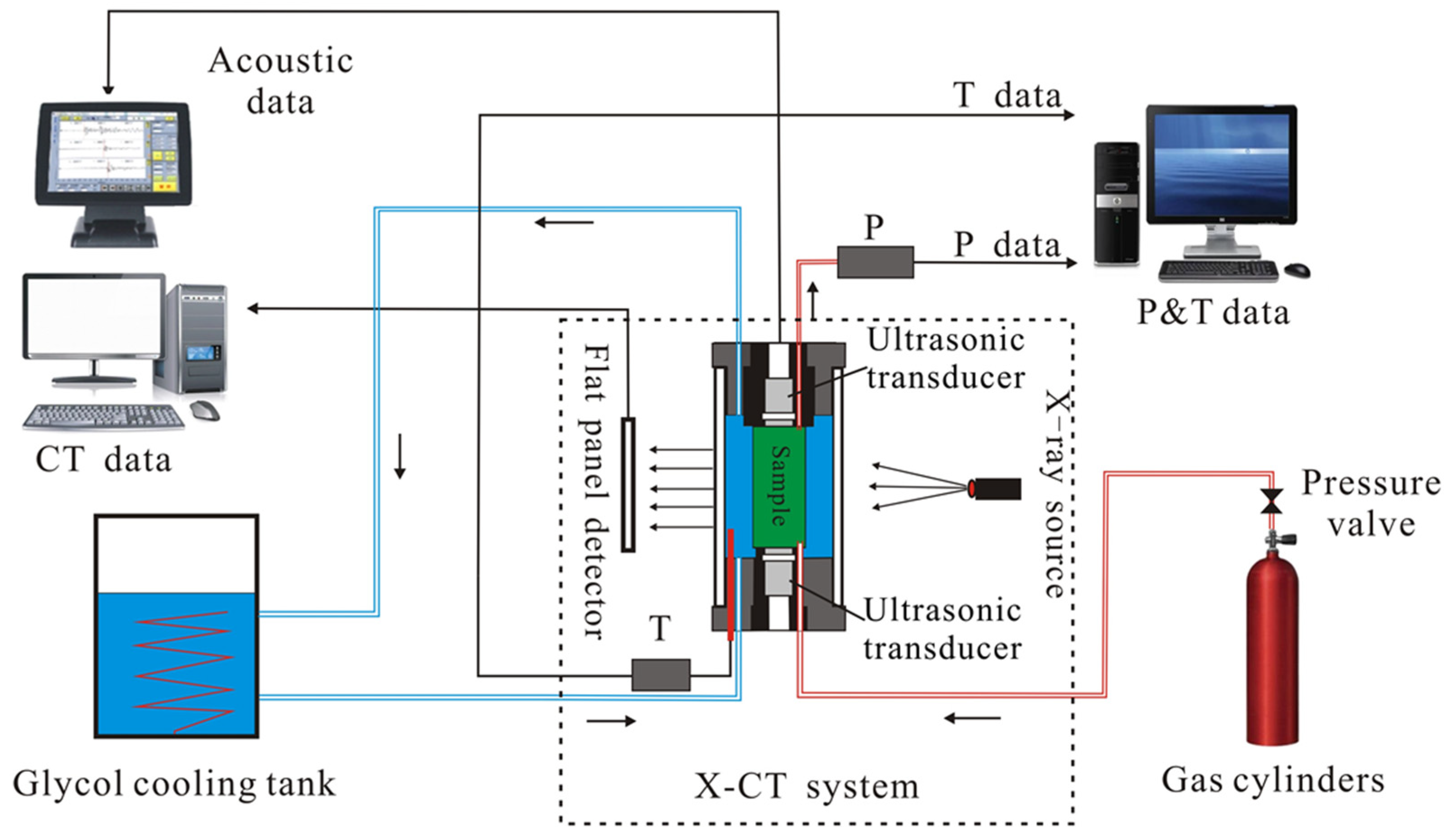
2.1. Experimental Device and Materials
2.2. Experimental Methods and Procedures
- Measure 25 mL (sample volume) of the experiment sand with a graduated cylinder, and mix 3.5% NaCl solution (80% saturated water content) with the sand;
- Install the bottom ultrasonic transducer and encapsulation end cap on the experiment sample cavity, place the mixture of sand and NaCl solution into the reaction cavity, and then install the top ultrasonic transducer and encapsulation end cap into the experiment sample cavity on the body;
- Evacuate the reaction chamber to remove the air inside;
- Open the temperature and pressure data acquisition software, and flow methane gas into the reaction chamber to reach the specified pressure;
- Lower the temperature of the refrigeration system and begin to form hydrates;
- Begin the hydrate decomposition process, and use a step-wise heating method to decompose the hydrate;
- After each temperature increase, perform two X-CT scans after the experimental system becomes stable;
- Ultrasonic data acquisition is carried out during the hydrate decomposition experiment, and ultrasonic data acquisition must be carried out synchronously during X-CT scanning until hydrate decomposition is complete;
- Hydrate decomposition is considered complete when the pressure no longer changes after the experimental system is raised to a higher temperature.
3. Results
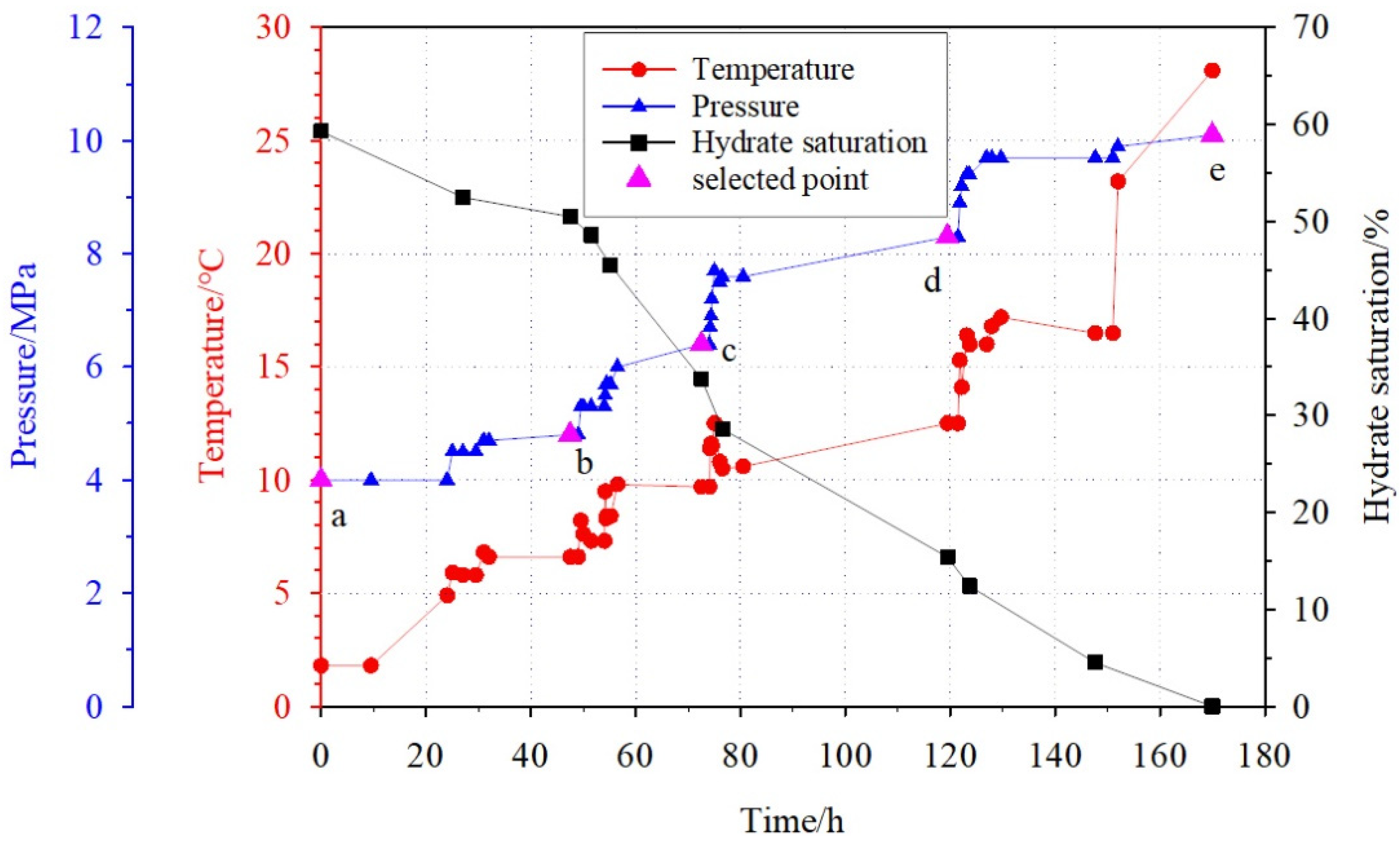
3.1. Hydrate Dissociation Process
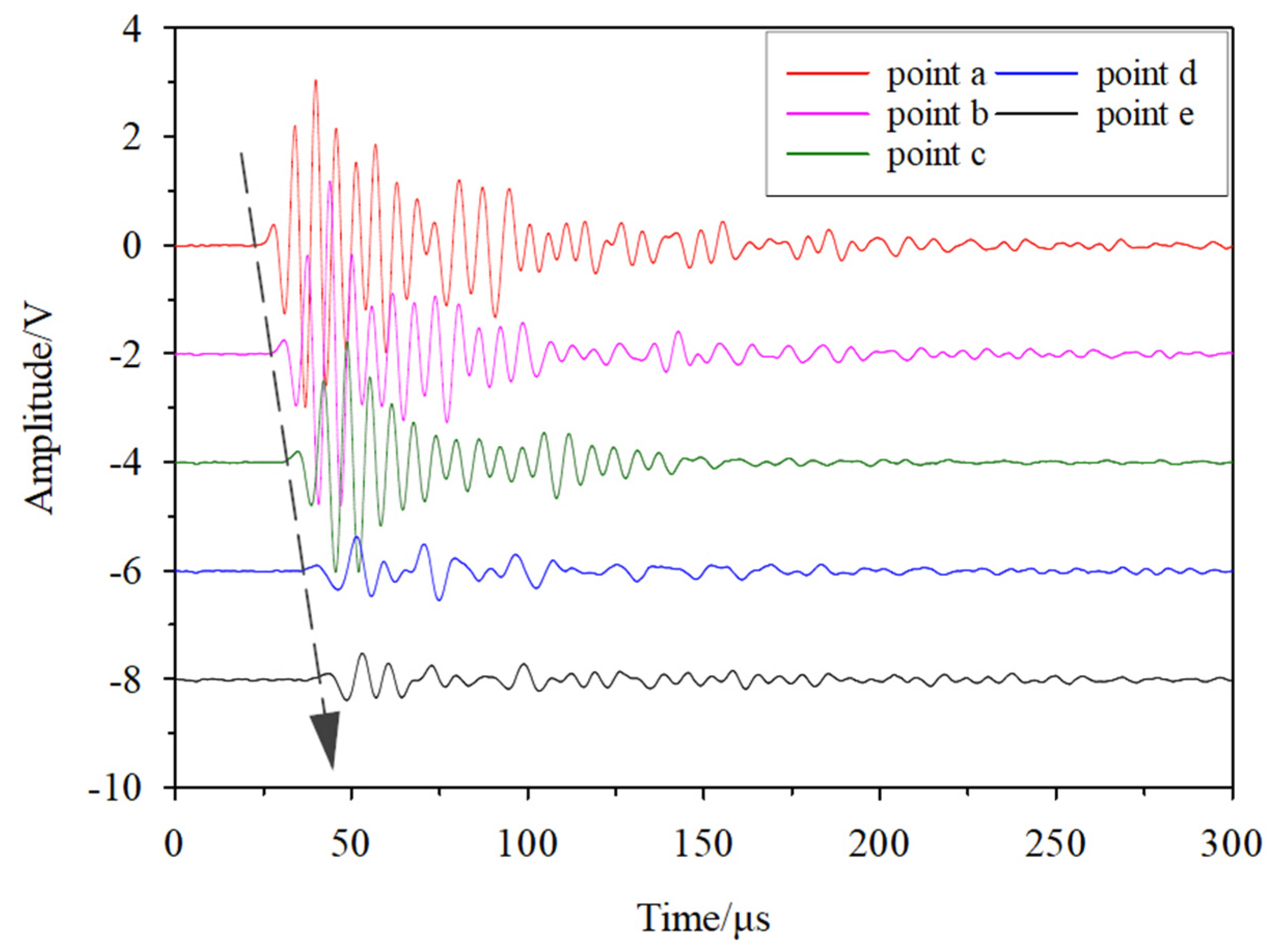
3.2. Variations in Wave Velocity during the Hydrate Dissociation Process
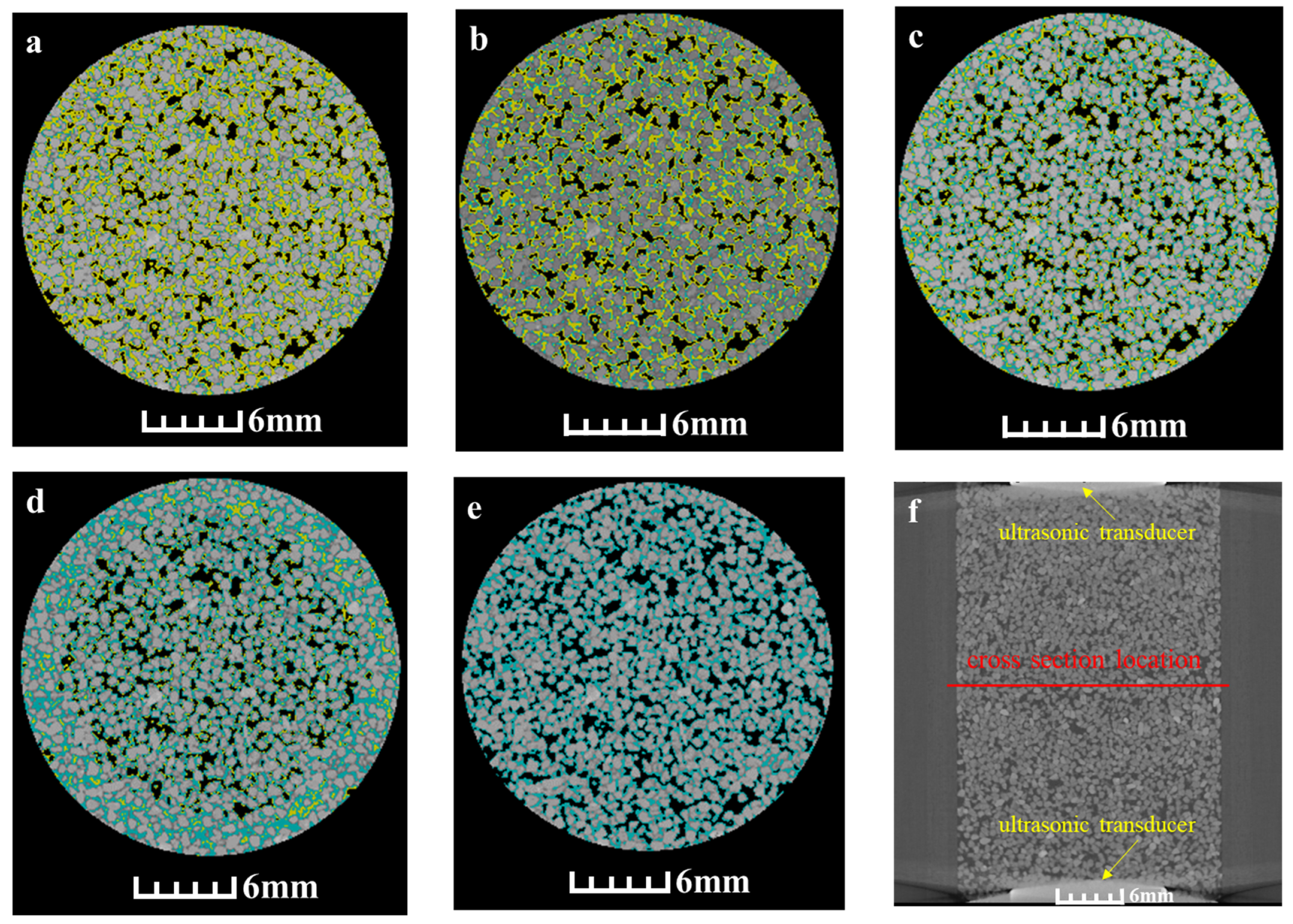
3.3. Microscopic Distribution of Hydrate during the Dissociation Process
4. Discussion
4.1. Effect of Hydrate Saturation on Acoustic Velocity
4.2. Influence of the Microscopic Hydrate Distribution on Acoustic Velocity during Hydrate Dissociation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Linga, P. Methane hydrates: A future clean energy resource. Chin. J. Chem. Eng. 2019, 27, 36–46. [Google Scholar] [CrossRef]
- Dong, L.; Wan, Y.Z.; Li, Y.L.; Liao, H.L.; Liu, C.L.; Wu, N.Y.; Leonenko, Y. 3D numerical simulation on drilling fluid invasion into natural gas hydrate reservoirs. Energy 2022, 241, 122932. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Wu, N.Y.; Chen, Q.; Li, W.T.; Hu, G.W.; Huang, L.; Ouyang, W.P. Coupled thermal-hydrodynamic-mechanical-chemical numerical simulation for gas production from hydrate-bearing sediments based on hybrid finite volume and finite element method. Comput. Geotech. 2022, 145, 104692. [Google Scholar] [CrossRef]
- Li, Y.L.; Wu, N.Y.; Gao, D.L.; Chen, Q.; Liu, C.L.; Yang, D.Y.; Jin, Y.R.; Ning, F.L.; Tan, M.J.; Hu, G.W. Optimization and Analysis of Gravel Packing Parameters in Horizontal Wells for Natural Gas Hydrate Production. Energy 2020, 219, 119585. [Google Scholar] [CrossRef]
- Li, Y.L.; Wu, N.Y.; Ning, F.L.; Gao, D.L.; Hao, X.L.; Chen, Q.; Liu, C.L.; Sun, J.Y. Hydrate induced clogging of sand-control screen and its implication on hydrate production operation. Energy 2020, 206, 118030. [Google Scholar] [CrossRef]
- He, T.; Lu, H.L.; Lin, J.Q.; Dong, Y.F.; He, J. Geophysical techniques of reservoir monitoring for marine gas hydrate exploitation. Earth Sci. Front. 2017, 24, 368–382. [Google Scholar]
- Luo, C.X. Japan’s Methane Hydrate Development Today and Advances. Sino-Glob. Energy 2013, 18, 30–37. [Google Scholar]
- Li, J.F.; Ye, J.L.; Qin, X.W.; Qiu, H.J.; Wu, N.Y.; Lu, H.L.; Xie, W.W.; Lu, J.A.; Peng, F.; Xu, Z.Q.; et al. The first offshore natural gas hydrate production test in South China Sea. China Geol. 2018, 1, 5–16. [Google Scholar] [CrossRef]
- Liu, J.W.; Li, X.S. Recent Advances on Natural Gas Hydrate Exploration and Development in the South China Sea. Energy Fuels 2021, 35, 7528–7552. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, L.L.; Jin, Y.R.; Wu, N.Y. Characterization and development of natural gas hydrate in marine clayey-silt reservoirs: A review and discussion. Adv. Geo-Energy Res. 2021, 5, 75–86. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, H.L.; Meng, Q.G.; Liu, C.L.; Chen, Q.; Xing, L.C. 2-D electrical resistivity tomography assessment of hydrate formation in sandy sediments. Nat. Gas Ind. B 2020, 7, 278–284. [Google Scholar] [CrossRef]
- Cai, J.C.; Xia, Y.X.; Xu, S.; Tian, H.T. Advances in multiphase seepage characteristics of natural gas hydrate sediments. Chin. J. Theor. Appl. Mech. 2019, 52, 208–223. [Google Scholar]
- Cai, J.C.; Wei, W.; Hu, X.Y.; Wood, D.A. Electrical conductivity models in saturated porous media: A review. Earth-Sci. Rev. 2017, 171, 419–433. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, 465–484. [Google Scholar] [CrossRef]
- Winters, W.J.; Pecher, I.A.; Waite, W.F.; Mason, D.H. Physical properties and rock physics models of sediment containing natural and laboratory-formed methane gas hydrate. Am. Mineral. 2004, 89, 1221–1227. [Google Scholar] [CrossRef]
- Winters, W.J.; Waite, W.F.; Mason, D.H.; Gilbert, L.Y.; Pecher, I.A. Methane gas hydrate effect on sediment acoustic and strength properties. J. Pet. Sci. Eng. 2007, 56, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Priest, J.A.; Best, A.I.; Clayton, C.R.I. Attenuation of seismic waves in methane gas hydrate-bearing sand. Geophys. J. Int. 2006, 164, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Priest, J.A.; Best, A.; Clayton, C. A laboratory investigation into the seismic velocities of methane gas hydrate-bearing sand. J. Geophys. Res. 2005, 110, B04102. [Google Scholar] [CrossRef]
- Priest, J.A.; Rees, E.V.L.; Clayton, C.R.I. Influence of gas hydrate morphology on the seismic velocities of sands. J. Geophys. Res. Solid Earth 2009, 114, B11205. [Google Scholar] [CrossRef] [Green Version]
- Sultaniya, A.K.; Priest, J.A.; Clayton, C.R.I. Measurements of the changing wave velocities of sand during the formation and dissociation of disseminated methane hydrate. J. Geophys. Res. Solid Earth 2015, 120, 778–789. [Google Scholar] [CrossRef]
- Yun, T.S.; Narsilio, G.A.; Santamarina, J.C.; Ruppel, C. Instrumented pressure testing chamber for characterizing sediment cores recovered at in situ hydrostatic pressure. Mar. Geol. 2006, 229, 285–293. [Google Scholar] [CrossRef]
- Hu, G.W.; Ye, Y.G.; Zhang, J.; Liu, C.L.; Diao, S.B.; Wang, J.S. Acoustic properties of gas hydrate-bearing consolidated sediments and experimental testing of elastic velocity models. J. Geophys. Res. 2010, 115, B02102. [Google Scholar] [CrossRef]
- Bu, Q.T.; Hu, G.W.; Ye, Y.G.; Liu, C.L.; Li, C.F.; Best, A.I.; Wang, J.S. The elastic wave velocity response of methane gas hydrate formation in vertical gas migration systems. J. Geophys. Eng. 2017, 14, 555–569. [Google Scholar] [CrossRef] [Green Version]
- Bu, Q.T.; Hu, G.W.; Ye, Y.G.; Liu, C.L.; Li, C.F.; Wang, J.S. Experimental study on 2-d acoustic characteristics and hydrate distribution in sand. Geophys. J. Int. 2017, 211, 990–1004. [Google Scholar] [CrossRef]
- Bu, Q.T.; Hu, G.W.; Liu, C.L.; Xing, T.J.; Li, C.F.; Meng, Q.G. Acoustic characteristics and microscopic distribution prediction during hydrate dissociation in sediments from the South China Sea. J. Nat. Gas Sci. Eng. 2019, 65, 135–144. [Google Scholar] [CrossRef]
- Ecker, C. Seismic Characterization of Methane Hydrate Structures; Stanford University: Santa Clara County, CA, USA, 2001. [Google Scholar]
- Jiang, M.J.; Zhu, F.Y.; Liu, F.; Utili, S. A bond contact model for methane hydrate-bearing sediments with interparticle cementation. Int. J. Numer. Anal. Methods Geomech. 2014, 38, 1823–1854. [Google Scholar] [CrossRef]
- Yang, L.; Falenty, A.; Chaouachi, M.; Haberthür, D.; Kuhs, W.F. Synchrotron X-ray computed microtomography study on gas hydrate decomposition in a sedimentary matrix. Geochem. Geophys. Geosystems 2016, 17, 3717–3732. [Google Scholar] [CrossRef] [Green Version]
- Chaouachi, M.; Falenty, A.; Sell, K.; Enzmann, F.; Kersten, M.; Haberth€ur, D.; Kuhs, W.F. Microstructural evolution of gas hydrates in sedimentary matrices observed with synchrotron X-ray computed tomographic microscopy. Geochem. Geophys. Geosystems 2015, 16, 1711–1722. [Google Scholar] [CrossRef] [Green Version]
- Le, T.X.; Bornert, M.; Aimedieu, P.; Chabot, B.; King, A.; Tang, A.M. An experimental investigation on methane hydrate morphologies and pore habits in sandy sediment using synchrotron X-ray computed tomography. Mar. Pet. Geol. 2020, 122, 104646. [Google Scholar] [CrossRef]
- Han, G.; Kwon, T.H.; Lee, J.Y.; Kneafsey, T.J. Depressurization-induced fines migration in sediments containing methane hydrate: X-ray computed tomography imaging experiments. J. Geophys. Res. Solid Earth 2018, 123, 2539–2558. [Google Scholar] [CrossRef]
- Li, Y.H.; Song, X.G.; Wu, P.; Sun, X.; Song, Y.C. Consolidation deformation of hydrate-bearing sediments: A pore-scale computed tomography investigation. J. Nat. Gas Sci. Eng. 2021, 95, 104184. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Zhao, J.Z.; Shi, D.X.; Feng, Z.C.; Liang, W.G.; Yang, D. Micro-ct analysis of structural characteristics of natural gas hydrate in porous media during decomposition. J. Nat. Gas Sci. Eng. 2016, 31, 139–148. [Google Scholar] [CrossRef]
- Li, C.F.; Liu, L.L.; Sun, J.Y.; Zhang, Y.C.; Hu, G.W.; Liu, C.L. Finite element analysis of micro-seepage in hydrate-bearing quartz sands based on digital cores. Mar. Geol. Front. 2020, 36, 68–72. [Google Scholar]
- Zhang, Y.C.; Liu, C.L.; Wu, N.Y.; Liu, L.L.; Hao, X.L.; Meng, Q.G.; Li, C.F.; Sun, J.Y.; Wang, D.G. Advances in the pore-structure characteristics and microscopic seepage numerical simulation of the hydrate-bearing sediments. Mar. Geol. Front. 2020, 36, 23–33. [Google Scholar]
- Li, C.F.; Hu, G.W.; Zhang, W.; Ye, Y.G.; Liu, C.L.; Li, Q.; Sun, J.Y. Influence of foraminifera on formation and occurrence characteristics of natural gas hydrates in fine-grained sediments from shen hu area, South China Sea. Sci. China Earth Sci. 2016, 59, 2223–2230. [Google Scholar] [CrossRef]
- Li, C.F.; Liu, C.L.; Hu, G.W.; Sun, J.Y.; Hao, X.L.; Liu, L.L.; Meng, Q.G. Investigation on the multiparameter of hydrate—Bearing sands using nano—Focus X-ray computed tomography. J. Geophys. Res. Solid Earth 2019, 124, 2286–2296. [Google Scholar] [CrossRef]
- Lei, L.; Seol, Y.; Myshakin, E.M. Methane hydrate film thickening in porous media. Geophys. Res. Lett. 2019, 46, 11091–11099. [Google Scholar] [CrossRef]
- Lei, L.; Seol, Y.; Choi, J.H.; Kneafsey, T.J. Pore habit of methane hydrate and its evolution in sediment matrix—Laboratory visualization with phase-contrast micro-ct. Mar. Pet. Geol. 2019, 104, 451–467. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Seol, Y. High-saturation gas hydrate reservoirs—A pore scale investigation of their formation from free gas and dissociation in sediments. J. Geophys. Res. Solid Earth 2019, 124, 12430–12444. [Google Scholar] [CrossRef]
- Dvorkin, J.; Prasad, M.; Sakai, A.; Lavoie, D. Elasticity of marine sediments: Rock physics modeling. Geophys. Res. Lett. 1999, 26, 1781–1784. [Google Scholar] [CrossRef]
- Helgerud, M.B.; Dvorkin, J.; Nur, A.; Sakai, A.; Collett, T. Elastic-wave velocity in marine sediments with gas hydrate: Effective medium modeling. Geophys. Res. Lett. 1999, 26, 2021–2024. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Madhusudhan, B.N.; Marín-Moreno, H.; North, L.J.; Ahmed, S.; Falcon-Suarez, I.H.; Minshull, T.A.; Best, A.I. Laboratory insights into the effect of sediment-hosted methane hydrate morphology on elastic wave velocity from time-lapse 4-D synchrotron X-ray computed tomography. Geochem. Geophys. Geosystems 2018, 19, 4502–4521. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.G.; Liu, C.L. (Eds.) Natural Gas Hydrates: Experimental Techniques and Their Applications, Springer Geophysics; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]

| Time/h 1 | Pressure/MPa 2 | Temperature/°C 3 |
|---|---|---|
| 0 | 4.0 | 1.8 |
| 9.5 | 4.0 | 1.8 |
| 24 | 4.0 | 4.9 |
| 25 | 4.5 | 5.9 |
| 27 | 4.5 | 5.8 |
| 29.5 | 4.5 | 5.8 |
| 31 | 4.7 | 6.8 |
| 32 | 4.7 | 6.6 |
| 47.5 | 4.8 | 6.6 |
| 49 | 4.8 | 6.6 |
| 49.5 | 5.3 | 8.2 |
| 50 | 5.3 | 7.6 |
| 51.5 | 5.3 | 7.3 |
| 54 | 5.3 | 7.3 |
| 54.17 | 5.5 | 9.5 |
| 54.34 | 5.7 | 8.3 |
| 54.5 | 5.7 | 8.4 |
| 55.16 | 5.7 | 8.4 |
| 56.5 | 6.0 | 9.8 |
| 72.5 | 6.4 | 9.7 |
| 74 | 6.4 | 9.7 |
| 74.17 | 6.7 | 11.4 |
| 74.34 | 6.9 | 11.6 |
| 74.5 | 7.2 | 11.5 |
| 75 | 7.7 | 12.5 |
| 76 | 7.5 | 10.8 |
| 76.5 | 7.6 | 10.5 |
| 80.5 | 7.6 | 10.6 |
| 119.5 | 8.3 | 12.5 |
| 121.5 | 8.3 | 12.5 |
| 121.84 | 8.9 | 15.3 |
| 122.18 | 9.2 | 14.1 |
| 123.18 | 9.4 | 16.4 |
| 123.68 | 9.4 | 16.0 |
| 126.93 | 9.7 | 16.0 |
| 127.93 | 9.7 | 16.8 |
| 129.68 | 9.7 | 17.2 |
| 147.68 | 9.7 | 16.5 |
| 151 | 9.7 | 16.5 |
| 152 | 9.9 | 23.2 |
| 170 | 10.1 | 28.1 |
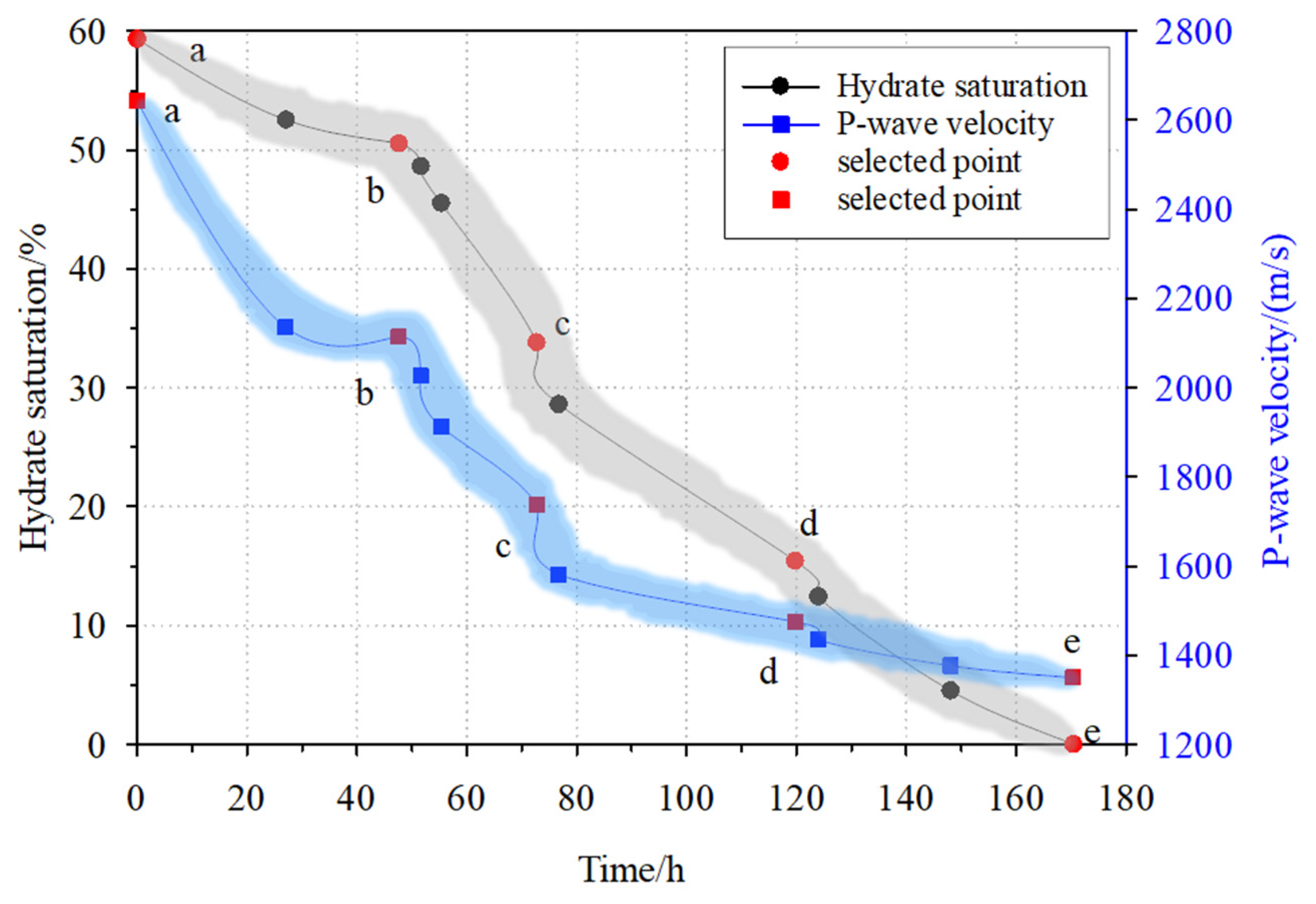
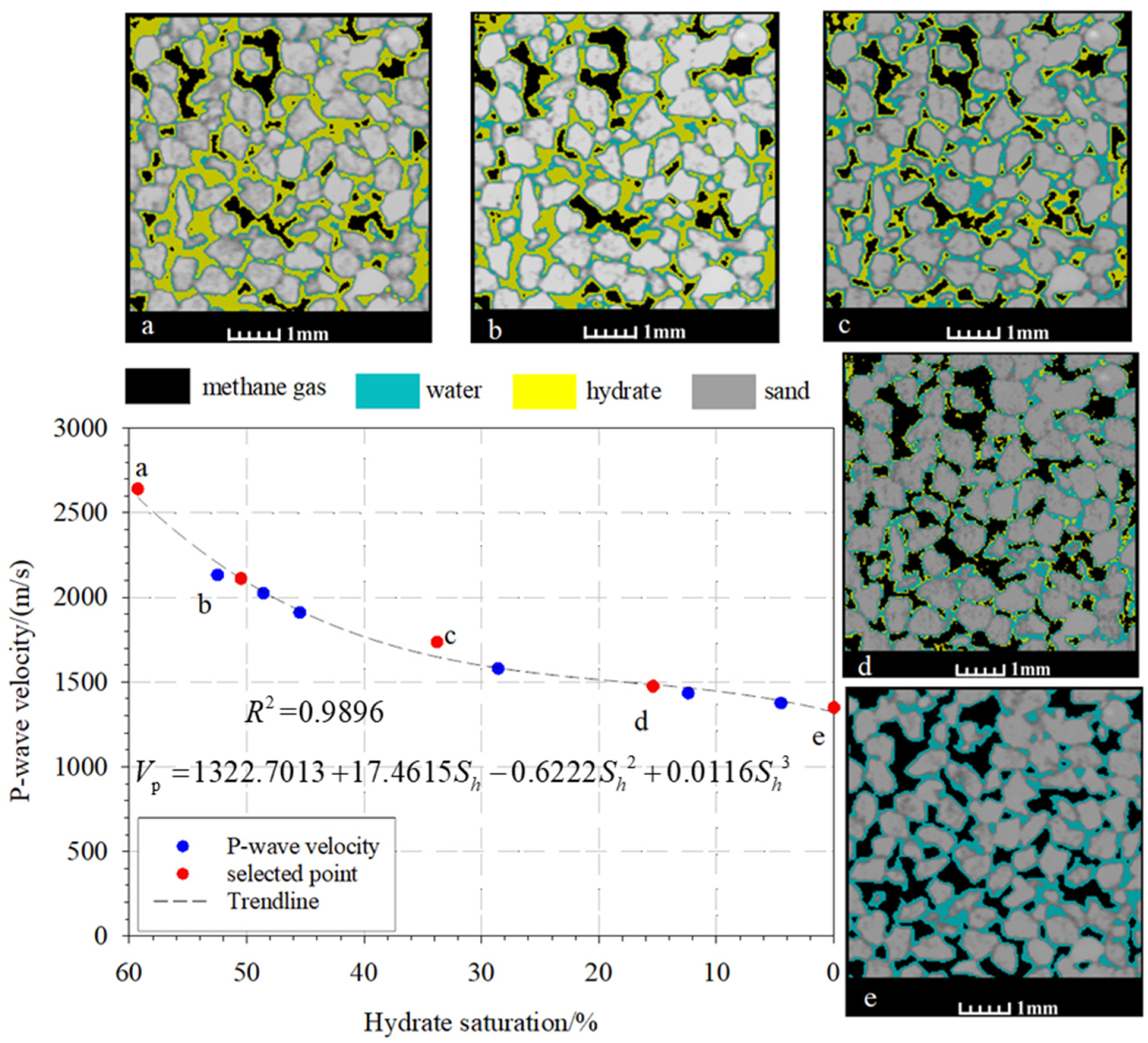
| Time/h 1 | Pressure/MPa 2 | Temperature/°C 3 | Hydrate Saturation/% 4 | Wave Velocity/(m/s) 5 |
|---|---|---|---|---|
| 0 | 4.0 | 1.8 | 59.3 | 2642.0 |
| 27 | 4.5 | 5.8 | 52.5 | 2134.5 |
| 47.5 | 4.8 | 6.6 | 50.5 | 2113.0 |
| 51.5 | 5.3 | 7.3 | 48.6 | 2026.3 |
| 55.16 | 5.7 | 8.4 | 45.5 | 1911.1 |
| 72.5 | 6.4 | 9.7 | 33.8 | 1736.9 |
| 76.5 | 7.6 | 10.5 | 28.6 | 1580.4 |
| 119.5 | 8.3 | 12.5 | 15.4 | 1474.4 |
| 123.68 | 9.4 | 16.0 | 12.4 | 1434.2 |
| 147.68 | 9.7 | 16.5 | 4.5 | 1375.5 |
| 170 | 10.1 | 28.1 | 0 | 1348.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Q.; Xing, T.; Li, C.; Zhao, J.; Liu, C.; Wang, Z.; Zhao, W.; Kang, J.; Meng, Q.; Hu, G. Effect of Hydrate Microscopic Distribution on Acoustic Characteristics during Hydrate Dissociation: An Insight from Combined Acoustic-CT Detection Study. J. Mar. Sci. Eng. 2022, 10, 1089. https://doi.org/10.3390/jmse10081089
Bu Q, Xing T, Li C, Zhao J, Liu C, Wang Z, Zhao W, Kang J, Meng Q, Hu G. Effect of Hydrate Microscopic Distribution on Acoustic Characteristics during Hydrate Dissociation: An Insight from Combined Acoustic-CT Detection Study. Journal of Marine Science and Engineering. 2022; 10(8):1089. https://doi.org/10.3390/jmse10081089
Chicago/Turabian StyleBu, Qingtao, Tongju Xing, Chengfeng Li, Jinhuan Zhao, Changling Liu, Zihao Wang, Wengao Zhao, Jiale Kang, Qingguo Meng, and Gaowei Hu. 2022. "Effect of Hydrate Microscopic Distribution on Acoustic Characteristics during Hydrate Dissociation: An Insight from Combined Acoustic-CT Detection Study" Journal of Marine Science and Engineering 10, no. 8: 1089. https://doi.org/10.3390/jmse10081089
APA StyleBu, Q., Xing, T., Li, C., Zhao, J., Liu, C., Wang, Z., Zhao, W., Kang, J., Meng, Q., & Hu, G. (2022). Effect of Hydrate Microscopic Distribution on Acoustic Characteristics during Hydrate Dissociation: An Insight from Combined Acoustic-CT Detection Study. Journal of Marine Science and Engineering, 10(8), 1089. https://doi.org/10.3390/jmse10081089








