Thermophilic Hydrocarbon-Utilizing Bacilli from Marine Shallow Hydrothermal Vents as Producers of Biosurfactants
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Screening for Lipases
2.3. Petroleum Hydrocarbons Utilization
2.4. Screening for Biosurfactant/Bioemulsifier Production
2.5. BSs Optimization and Activity
2.5.1. BSs Optimization
2.5.2. CFSs Surface-Active Properties
2.5.3. CFSs Emulsifying Properties
2.6. BSs Extraction and Characterization by ATR-FTIR
2.7. Oil Removal Test
2.8. Statistical Analysis
3. Results
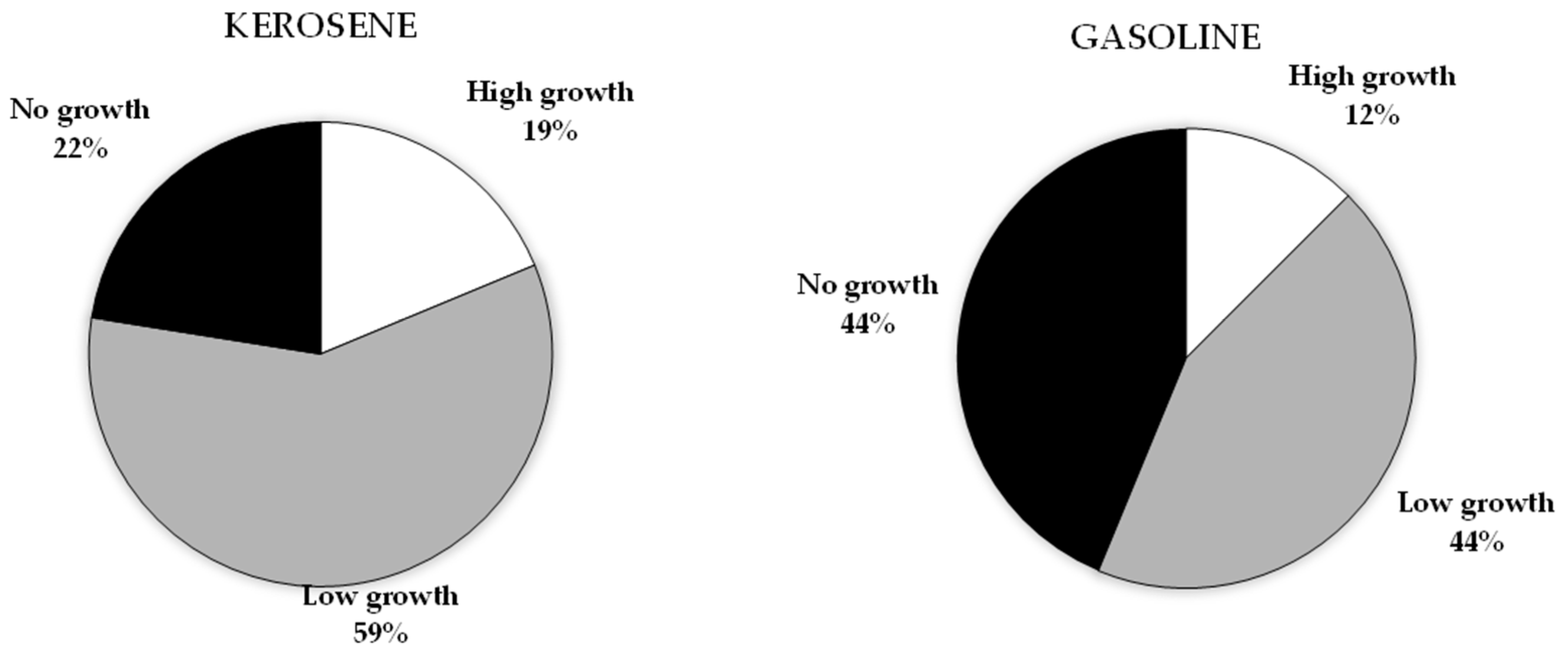
3.1. Petroleum Hydrocarbons Utilization
3.2. Screening for Biosurfactants/Bioemulsifiers Production
3.3. Biosurfactant Production and Activity
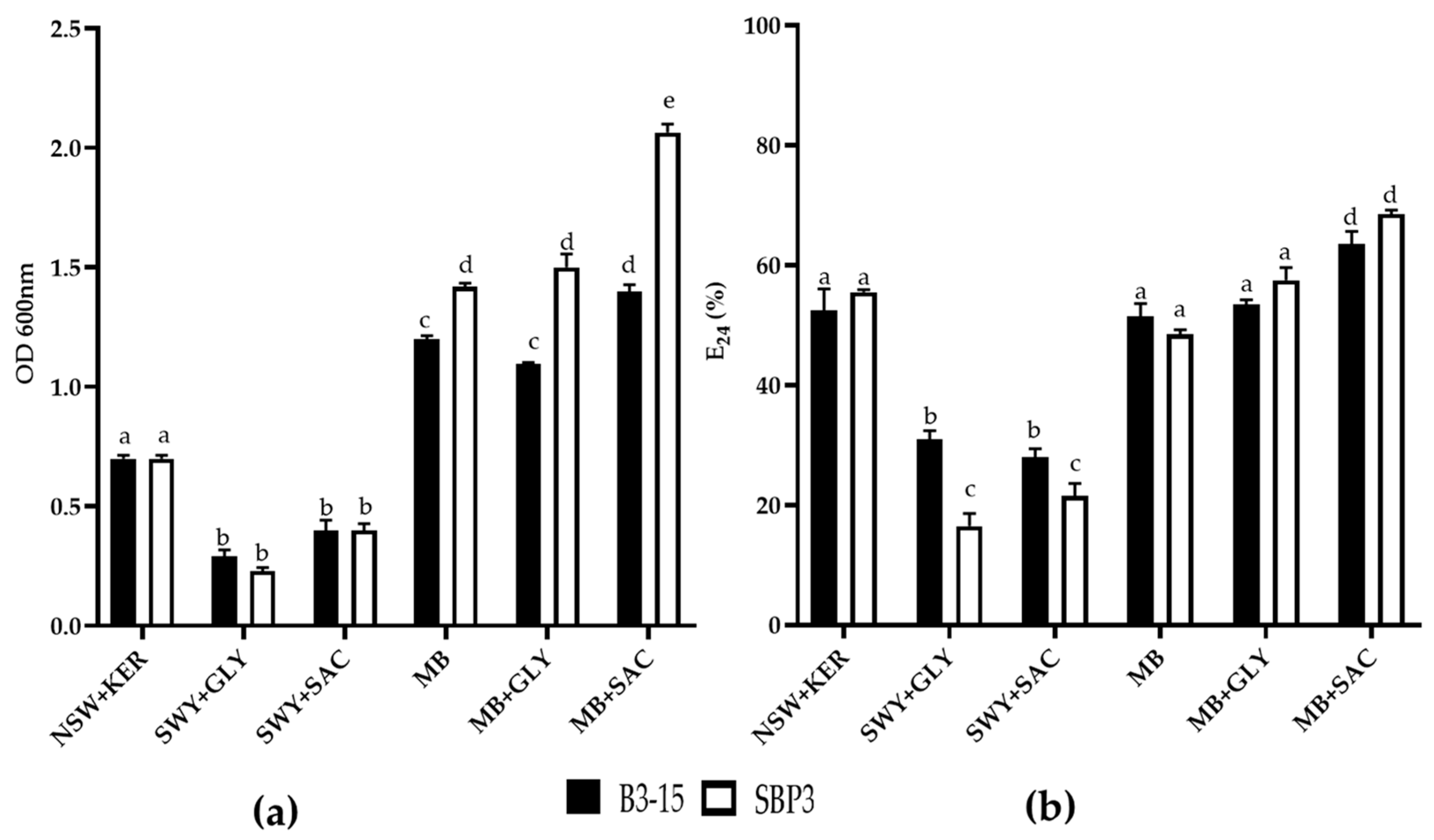
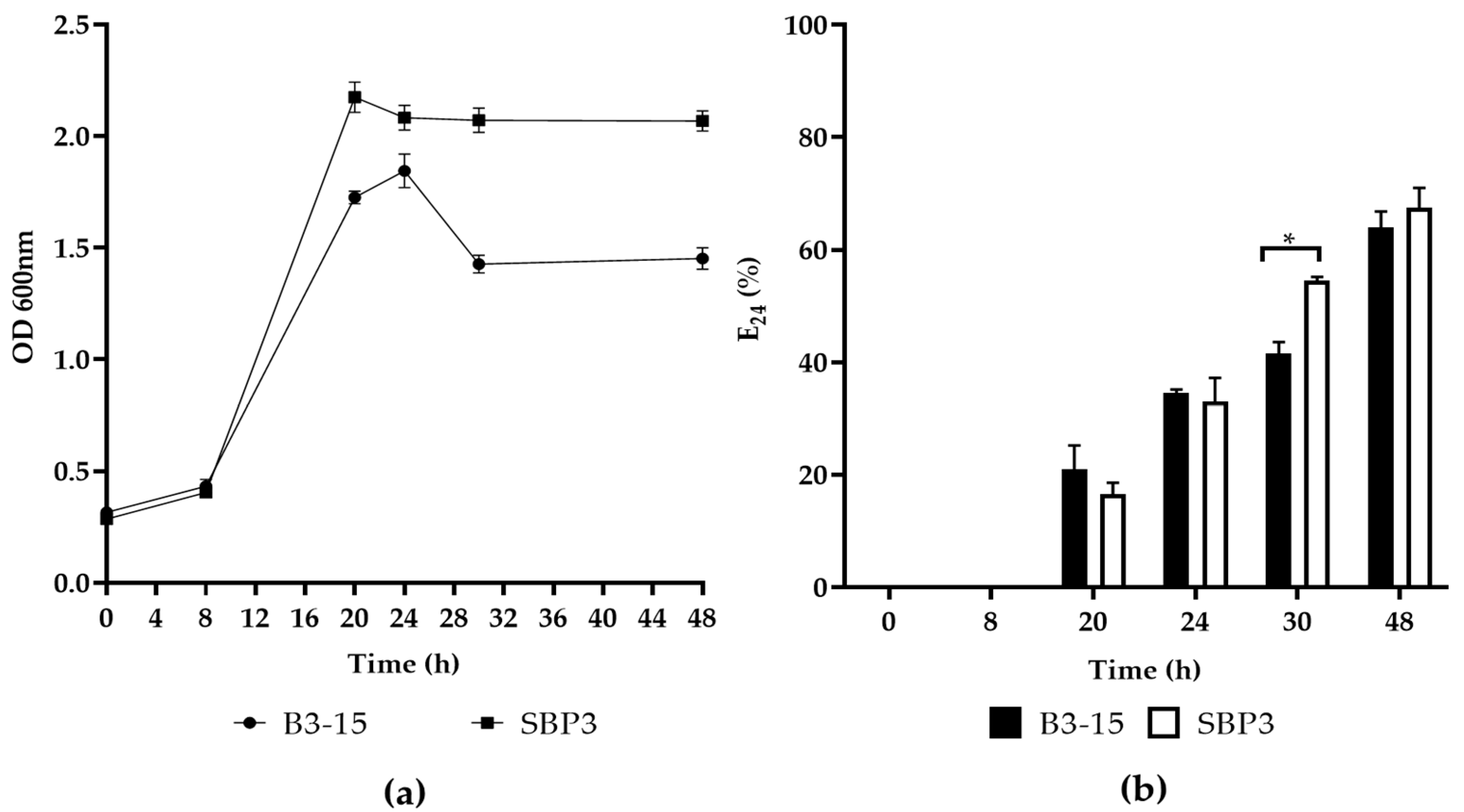
3.3.1. Biosurfactant Production
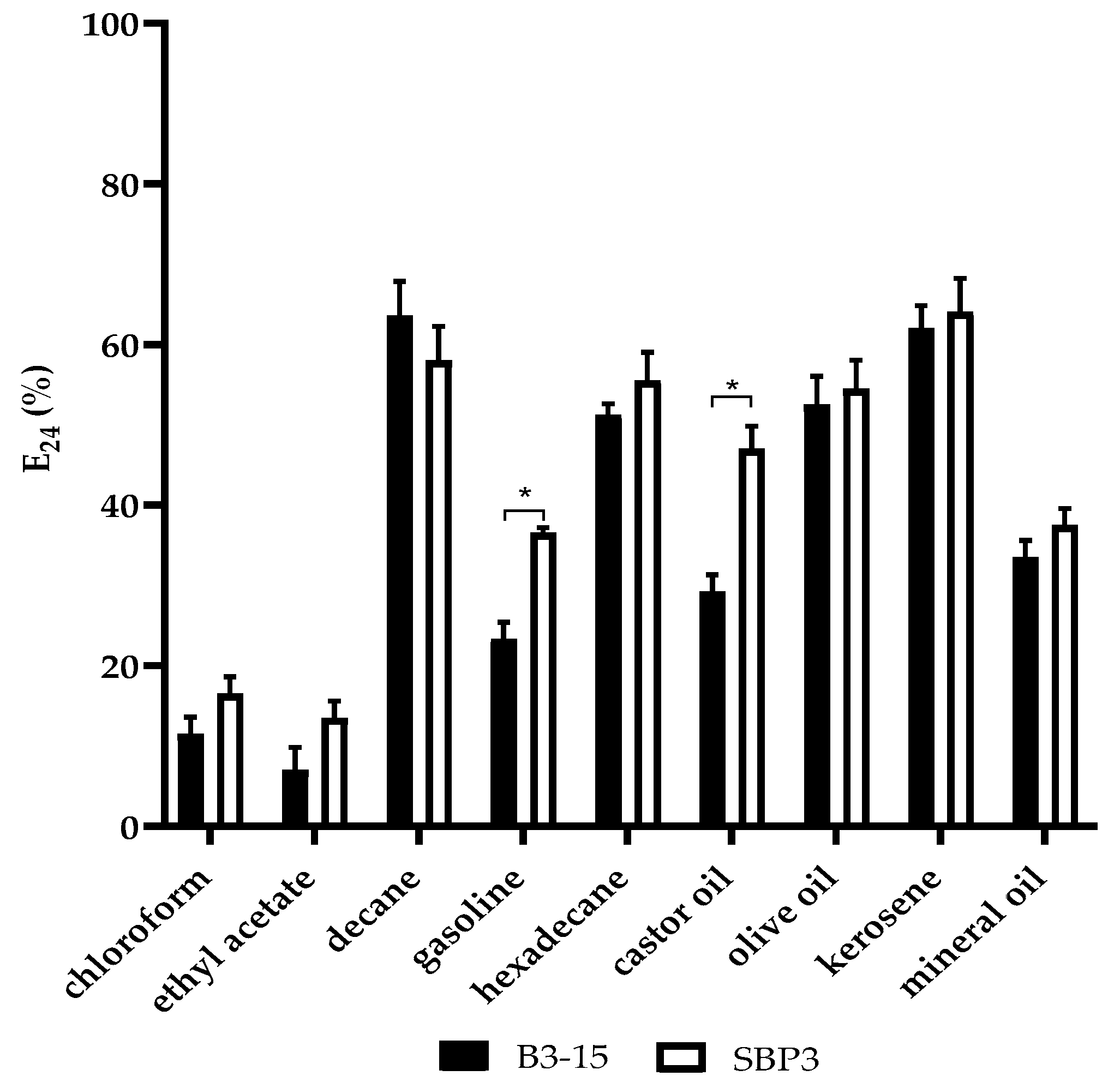
3.3.2. CFSs Biosurfactant Activities
3.4. BSs Extraction and Characterization by ATR-FTIR
3.5. Oil Removal Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kaczorek, E.; Pachholak, A.; Zdarta, A.; Smulek, W. The impact of biosurfactant on microbial properties leading to hydrocarbon bioavailability. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Rosenberg, E. Surface active and drag-reducing bacterial polymers. In Biotechnology: Bridging Research and Applications; Kamely, D., Chakrabarty, A.M., Kornguth, S.E., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 1991; pp. 231–248. [Google Scholar]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rodrigues, A.I.; Alves, E.; Domingues, M.R.; Teixeira, J.A.; Rodrigues, L.R. Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresour. Technol. 2015, 177, 87–93. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Bestetti, G.; Smyth, T.J.; Banat, I.M. Production and applications of trehalose lipid biosurfactants. Eur. J. Lipid. Sci. Technol. 2010, 112, 617–627. [Google Scholar] [CrossRef]
- De Almeida, D.G.; Soares da Silva, R.D.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef]
- Janek, T.; Łukaszewicz, M.; Krasowska, A. Identification and characterization of biosurfactants produced by the Arctic bacterium Pseudomonas putida BD2. Colloids Surf. B Biointerfaces 2013, 110, 379–386. [Google Scholar] [CrossRef]
- De França, Í.W.L.; Lima, A.P.; Lemos, J.A.M.; Lemos, C.G.F.; Melo, V.M.M.; de Sant’ana, H.B.; Gonçalves, L.R.B. Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal. Today 2015, 255, 10–15. [Google Scholar] [CrossRef]
- Perfumo, A.; Rancich, I.; Banat, I.M. Possibilities and challenges for biosurfactants use in petroleum industry. In Biosurfactants. Advances in Experimental Medicine and Biology; Sen, R., Ed.; Springer: New York, NY, USA, 2010; pp. 135–145. [Google Scholar]
- Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M. Isolation and analysis of low molecular weight microbial glycolipids. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin, Germany, 2010; pp. 3705–3723. [Google Scholar]
- Sekhon-Randhawa, K.K.; Rahman, P.K. Rhamnolipid biosurfactants—past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Iwabuchi, T.; Matsui, Y.; Toki, H.; Hamada, E.; Tanaka, H. Distribution and biodegradation potential of oil-degrading bacteria in North Eastern Japanese coastal waters. FEMS Microbiol. Ecol. 1991, 86, 113–122. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Panico, A.; Lama, L.; Gambacorta, A.; Nicolaus, B. A halophilic thermotolerant Bacillus isolated from a marine hot spring able to produce a new exopolysaccharide. Biotechnol. Lett. 2002, 24, 515–519. [Google Scholar] [CrossRef]
- Zarilla, K.A.; Perry, J.J. Bacillus thermoleovorans, sp. nov., a species of obligately thermophilic hydrocarbon utilizing endospore-forming bacterial. Syst. Appl. Microbial. 1987, 9, 258–264. [Google Scholar] [CrossRef]
- Sorkhoh, N.A.; Ibrahim, A.S.; Ghannoum, M.A.; Radwan, S.S. High-temperature hydrocarbon degradation by Bacillus stearothermophilus from oil-polluted Kuwaiti desert. Appl. Microbial. Biotechnol. 1993, 39, 123–126. [Google Scholar] [CrossRef]
- Nazina, T.N.; Tourova, T.P.; Poltaraus, A.B.; Novikova, E.V.; Grigoryan, A.A.; Ivanova, A.E.; Lysenko, A.M.; Petrunyaka, V.V.; Osipov, G.A.; Belyaev, S.S.; et al. Taxonomic study of aerobic thermophilic bacilli: Descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int. J. Syst. Evol. Microbiol. 2001, 51, 433–446. [Google Scholar] [PubMed]
- Gugliandolo, C.; Lentini, V.; Spanò, A.; Maugeri, T.L. New bacilli from shallow hydrothermal vents of Panarea Island (Italy) and their biotechnological potential. J. Appl. Microbiol. 2012, 112, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Calanchi, N.; Capaccioni, B.; Martini, M.; Tassi, F.; Valentini, L. Submarine gas-emission from Panarea Island Aeolian Archipelago: Distribution of inorganic and organic compounds and inferences about source conditions. Acta Vulcanol. 1995, 7, 43–48. [Google Scholar]
- Wang, W.; Li, Z.; Zeng, L.; Dong, C.; Shao, Z. The oxidation of hydrocarbons by diverse heterotrophic and mixotrophic bacteria that inhabit deep-sea hydrothermal ecosystems. ISME J. 2020, 14, 1994–2006. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. A polyphasic taxonomic study of thermophilic bacilli from shallow, marine vents. Syst. Appl. Microbiol. 2001, 24, 451–468. [Google Scholar] [CrossRef]
- Lentini, V.; Gugliandolo, C.; Maugeri, T.L. Identification of enzyme-producing thermophilic bacilli isolated from marine vents of Aeolian Islands (Italy). Ann. Microbiol. 2007, 57, 355–361. [Google Scholar] [CrossRef]
- Spanò, A.; Gugliandolo, C.; Lentini, V.; Maugeri, T.L.; Anzelmo, G.; Poli, A.; Nicolaus, B. A novel EPS-producing strain of Bacillus licheniformis isolated from a shallow vent of Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [Google Scholar] [CrossRef]
- Arena, A.; Gugliandolo, C.; Stassi, G.; Pavone, B.; Iannello, D.; Bisignano, G.; Maugeri, T.L. An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: Antiviral activity on immunocompetent cells. Immunol. Lett. 2009, 123, 132–137. [Google Scholar] [CrossRef]
- Nicolaus, B.; Panico, A.; Manca, A.C.; Lama, L.; Gambacorta, A.; Maugeri, T.; Gugliandolo, C.; Caccamo, D. A thermophilic Bacillus isolated from an Eolian shallow hydrothermal vent, able to produce exopolysaccharides. Syst. Appl. Microbiol. 2000, 23, 426–432. [Google Scholar] [CrossRef]
- Spanò, A.; Laganà, P.; Visalli, G.; Maugeri, T.L.; Gugliandolo, C. In vitro antibiofilm activity of an exopolysaccharide from the marine thermophilic Bacillus licheniformis T14. Curr. Microbiol. 2016, 72, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, M.T.; Zammuto, V.; Gugliandolo, C.; Madeleine-Perdrillat, C.; Spanò, A.; Magazù, S. Thermal restraint of a bacterial exopolysaccharide of shallow vent origin. Int. J. Biol. Macromol. 2018, 114, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. Three novel halotolerant and thermophilic Geobacillus strains from shallow marine vents. Syst. Appl. Microbiol. 2002, 25, 450–455. [Google Scholar] [CrossRef]
- Sierra, G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek 1957, 23, 15–22. [Google Scholar] [CrossRef]
- Siegmund, I.; Wagner, F. New method for detecting rhamnolipids excreted by Pseudomonas species during growth on mineral agar. Biotechnol. Technol. 1991, 5, 265–268. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacilllus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Tuleva, B.; Christova, N.; Jordanov, B.; Nikolova-Damyanova, B.; Petrov, P. Naphthalene degradation and biosurfactant activity by Bacillus cereus 28BN. Z. Naturforsch. C. J. Biosci. 2005, 60, 577–582. [Google Scholar] [CrossRef]
- Sharma, N.; Lavania, M.; Kukreti, V.; Rana, D.P.; Lal, B. Laboratory investigation of indigenous consortia TERIJ-188 for incremental oil recovery. Front. Microbiol. 2018, 9, 2357. [Google Scholar] [CrossRef]
- Tripathi, V.; Gaur, V.K.; Dhiman, N.; Gautam, K.; Manickam, N. Characterization and properties of the biosurfactant produced by PAH-degrading bacteria isolated from contaminated oily sludge environment. Environ. Sci. Pollut. Res. Int. 2020, 27, 27268–27278. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, N.; Li, D.; Long, S.; Tang, X.; Xiao, G.; Wang, L. Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. Int. 2018, 25, 14934–14943. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Fuchs, F.M.; Fiebrandt, M.; Stapelmann, K.; Ulrich, N.J.; Maugeri, T.L.; Pukall, R.; Gugliandolo, C.; Moeller, R. Comparing spore resistance of Bacillus strains isolated from hydrothermal vents and spacecraft assembly facilities to environmental stressors and decontamination treatments. Astrobiology 2018, 18, 1425–1434. [Google Scholar] [CrossRef]
- Arrondo, J.L.; Goñi, F.M. Special Issue: Infrared spectroscopy of membrane lipids. Chem. Phys. Lipids 1998, 96, 1–164. [Google Scholar]
- Naumann, D.; Fabian, H.; Lasch, P. FTIR spectroscopy of cells, tissues and body fluids. In Advances in Biomedical Spectroscopy; IOSPress BV: Amsterdam, The Netherlands, 2009; Volume 2, pp. 312–354. [Google Scholar]
- Yoshida, S.; Miyazaki, M.; Sakai, K.; Takeshita, M.; Yuasa, S.; Sato, A.; Kobayashi, T.; Watanabe, S.; Okuyama, H. Fourier Transform Infrared spectroscopic analysis of rat brain microsomal membranes modified by dietary fatty acids: Possible correlation with altered learning behavior. Biospectroscopy 1997, 3, 281–290. [Google Scholar] [CrossRef]
- Ramani, K.; Jain, S.C.; Mandal, A.B.; Sekaran, G. Microbial induced lipoprotein biosurfactant from slaughterhouse lipid waste and its application to the removal of metal ions from aqueous solution. Colloids Surf. B 2012, 97, 254–263. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Production and applications of lipopeptide biosurfactant for bioremediation and oil recovery by Bacillus subtilis CN2. Biochem. Eng. J. 2015, 101, 168–178. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.C. Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res. Int. 2010, 43, 886–892. [Google Scholar] [CrossRef]
- Joy, S.; Rahman, K.S.M.P.; Sharma, S. Biosurfactant production and concomitant hydrocarbon degradation potentials of bacteria isolated from extreme and hydrocarbon contaminated environments. Biochem. Eng. J. 2017, 317, 232–241. [Google Scholar] [CrossRef]
- Lin, S.C.; Minton, M.A.; Sharma, M.M.; Georgiou, G. Structural and immunological characterization of a biosurfactant produced by Bacillus licheniformis JF-2. Appl. Environ. Microbiol. 1994, 60, 31–38. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Ijah, U.J.J.; Manga, S.B.; Bilbis, L.S.; Umar, S. Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int. Biodeterior. Biodegrad. 2013, 81, 28–34. [Google Scholar] [CrossRef]
- Paula, A.V.; Barboza, J.C.S.; Castro, H.F. Study of the influence of solvent, carbohydrate and fatty acid in the enzymatic synthesis of sugar esters by lipases. Quím. Nova 2005, 28, 792–796. [Google Scholar] [CrossRef][Green Version]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Yusoff, D.F.; Raja Abd Rahman, R.N.Z.; Masomian, M.; Ali, M.S.M.; Leow, T.C. Newly isolated alkane hydroxylase and lipase producing Geobacillus and Anoxybacillus species involved in crude oil degradation. Catalysts 2020, 10, 851. [Google Scholar] [CrossRef]
- Mulani, N.; Fulke, A.B.; D’souza, E.; Ram, A.; Maloo, A.; Sayed, F.; Gajbhiye, S.N. Biodegradation of crude oil using marine Bacillus species from Vadinar coast, Gujarat, India. Curr. Sci. 2017, 112, 569–576. [Google Scholar] [CrossRef]
- Margesin, R.; Zimmerbauer, A.; Schinner, F. Soil lipase activity—A useful indicator of oil biodegradation. Biotechnol. Technol. 1999, 13, 859–863. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Bao, M.; Sun, P. Metabolic pathway for a new strain Pseudomonas synxantha LSH-70: From chemotaxis to uptake of n-hexadecane. Sci. Rep. 2017, 7, 39068. [Google Scholar] [CrossRef] [PubMed]
- Adlan, N.A.; Sabri, S.; Masomian, M.; Ali, M.S.M.; Rahman, R.N.Z.R.A. Microbial biodegradation of paraffin wax in malaysian crude oil mediated by degradative enzymes. Front. Microbiol. 2020, 11, 565608. [Google Scholar] [CrossRef]
- Hamdan, S.H.; Maiangwa, J.; Ali, M.S.M.; Normi, Y.M.; Sabri, S.; Leow, T.C. Thermostable lipases and their dynamics of improved enzymatic properties. Appl. Microbiol. Biotechnol. 2021, 105, 7069–7094. [Google Scholar] [CrossRef]
- Abouseouda, M.; Maachi, R.; Amranec, A.; Boudergua, S.; Nabia, A. Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 2008, 223, 143–151. [Google Scholar] [CrossRef]
- Fooladi, T.; Hamid, A.; Yusoff, W.; Moazami, N.; Shafiee, Z. Production of biosurfactant by indigenous isolated bacteria in fermentation system. AIP Conf. Proc. 2013, 1571, 197–201. [Google Scholar]
- Reis, F.A.; Sérvulo, E.F.; De França, F.P. Lipopeptide surfactant production by Bacillus subtilis grown on low-cost raw materials. Appl. Biochem. Biotechnol. 2004, 115, 899–912. [Google Scholar] [CrossRef]
- Pemmaraju, S.C.; Sharma, D.; Singh, N.; Panwar, R.; Cameotra, S.S.; Pruthi, V. Production of microbial surfactants from oily sludge-contaminated soil by Bacillus subtilis DSVP23. Appl. Biochem. Biotechnol. 2012, 167, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Pastore, G.M. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Biores. Technol. 2006, 97, 336–341. [Google Scholar] [CrossRef]
- Anburajan, L.; Meena, B.; Toms Cheriath, J.; Dheenan Palaiya, S.; Vinithkumar Nambali, V.; Dharani, G.; Kirubagaran, R. Functional and molecular characterization of a lipopeptide surfactant from the marine sponge-associated eubacteria Bacillus licheniformis NIOT-AMKV06 of Andaman and Nicobar Islands, India. Mar. Pollut. Bull. 2014, 82, 76–85. [Google Scholar]
- Vaz, D.A.; Gudiña, E.J.; Alameda, E.J.; Teixeira, J.A.; Rodrigues, L.R. Performance of a biosurfactant produced by a Bacillus subtilis strain isolated from crude oil samples as compared to commercial chemical surfactants. Colloids Surf. B Biointerfaces 2012, 89, 167–174. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef]
- Campos, J.M.; Stamford, T.L.M.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Formulation of mayonnaise with the addition of a bioemulsifier isolated from Candida utilis. Toxicol. Rep. 2015, 2, 1164–1170. [Google Scholar] [CrossRef]
- Rendueles, O.; Kaplan, J.B.; Ghigo, J.M. Antibiofilm polysaccharides. Environ. Microbiol. 2012, 15, 334–346. [Google Scholar] [CrossRef]
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Wray, V.; Fredrickson, H.L. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol. 1995, 61, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Lee, S.K.; Cha, B.J.; Kim, Y.H.; Shin, K.S. Detection and characterization of the Gloeosporium gloeosporioides growth inhibitory compound iturin A from Bacillus subtilis strain KS03. FEMS Microbiol. Lett. 2003, 223, 47–51. [Google Scholar] [CrossRef]
- Nanjundan, J.; Ramasamy, R.; Uthandi, S.; Ponnusamy, M. Antimicrobial activity and spectroscopic characterization of surfactin class of lipopeptides from Bacillus amyloliquefaciens SR1. Microb. Pathog. 2019, 128, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Gandhimathi, R.; Seghal Kiran, G.; Hema, T.A.; Selvin, J.; Rajeetha Raviji, T.; Shanmughapriya, S. Production and characterization of lipopeptide biosurfactant by a sponge-associated marine actinomycetes Nocardiopsis alba MSA10. Bioprocess Biosyst. Eng. 2009, 32, 825–835. [Google Scholar] [CrossRef]
- Hentati, D.; Chebbi, A.; Hadrich, F.; Frikha, I.; Rabanal, F.; Sayadi, S.; Manresa, A.; Chamkha, M. Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol. Environ. Saf. 2019, 167, 441–449. [Google Scholar] [CrossRef]
- Anburajan, L.; Meena, B.; Raghavan, R.V.; Shridhar, D.; Joseph, T.C.; Vinithkumar, N.V.; Dharani, G.; Dheenan, P.S.; Kirubagaran, R. Heterologous expression, purification, and phylogenetic analysis of oil-degrading biosurfactant biosynthesis genes from the marine sponge-associated Bacillus licheniformis NIOT-06. Bioproc. Biosyst. Eng. 2015, 38, 1009–1018. [Google Scholar] [CrossRef]
- Sivapathasekaran, C.; Mukherjee, S.; Samanta, R.; Sen, R. High-performance liquid chromatography purification of biosurfactant isoforms produced by a marine bacterium. Anal. Bioanal. Chem. 2009, 395, 845–854. [Google Scholar] [CrossRef]
- Dey, G.; Bharti, R.; Dhanarajan, G.; Das, S.; Dey, K.K.; Kumar, B.N.; Sen, R.; Mandal, M. Marine lipopeptide Iturin A inhibits Akt mediated GSK3β and FoxO3a signaling and triggers apoptosis in breast cancer. Sci. Rep. 2015, 5, 10316. [Google Scholar] [CrossRef]
- Son, S.; Ko, S.K.; Jang, M.; Kim, J.W.; Kim, G.S.; Lee, J.K.; Jeon, E.S.; Futamura, Y.; Ryoo, I.J.; Lee, J.S.; et al. New cyclic lipopeptides of the iturin class produced by saltern-derived Bacillus sp. KCB14S006. Mar. Drugs 2016, 14, 72. [Google Scholar] [CrossRef]
- Xu, B.H.; Lu, Y.Q.; Ye, Z.W.; Zheng, Q.W.; Wei, T.; Lin, J.F.; Guo, L.Q. Genomics-guided discovery and structure identification of cyclic lipopeptides from the Bacillus siamensis JFL15. PLoS ONE 2018, 13, e0202893. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.; Amro, M.; Bock, M.; Boseker, K.; Fredrickson, H.L.; Kessel, D.G.; Timmis, K.N. The potential of Bacillus licheniformis strains for in situ enhanced oil recovery. J. Petrol. Sci. Eng. 1997, 18, 147–160. [Google Scholar] [CrossRef]
- Bouassida, M.; Fourati, N.; Ghazala, I.; Ellouze-Chaabouni, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: Compatibility study with detergent ingredients and washing performance. Eng. Life Sci. 2018, 18, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A. Potential application of cyclic lipopeptide biosurfactants produced by Bacillus subtilis strains in laundry detergent formulations. Lett. Appl. Microbiol. 2007, 45, 330–335. [Google Scholar] [CrossRef]
- Rosenberg, E.; Ron, E.Z. High- and low-molecular-mass microbial surfactants. Appl. Microbiol. Biotechnol. 1999, 52, 154–162. [Google Scholar] [CrossRef]
| Island | Site | Station and Sample Type (F,S) | Depth (m) | Temp (°C) | pH | Conductivity (mS cm−1) | Isolate |
|---|---|---|---|---|---|---|---|
| Vulcano | Levante Harbor | A (F) | 0.3 | 25 | 5.2 | A1-3, A2-4, A3, A2-3a | |
| S1 (S) | sl | 93 | S1, S1-1 | ||||
| S3 (S) | sl | 85 | S3 | ||||
| B1 (F) | 6.0 | 24 | 6.4 | B1-2 | |||
| B2, T2 (F,S) | 6.0 | 43 | 6.4 | B2-3, B2-4, B2-5, B2-70-1,T1-3, T2-3, T2-4, T2-5, T2-6 | |||
| B3, T3 (F,S) | 0.7 | 65 | 5.2 | nd | B3-3, B3-4, B3-6, B3-11, B3-12, B3-13, B3-15, B3-16, B3-18, B3-24, B3-27, B3-28, B3-S, B3-S2, B3-S3, B3-S4, B3-S5, B3-71, B3-72, B3-73, B3-74, B3-75, B3-76, T3-1, T3-2, T3-3 | ||
| 1 (F) | 6.3 | 48 | 5.6 | 47.9 | s1a-1, s1a-2, s1a-2-1, 1as-1, s1b-1a, s1b-3 | ||
| La Roya | 3 (F) | 3.0 | 49 | 6.0 | 37.2 | 3s-1, 3s-2, md3s-1, md3s-2 | |
| Pta Conigliara | 4 (F,S) | 15 | 45 | 6.1 | 40.6 | 4-1, 4s-1, md4-1-1, md4-1-2, su4-4, s4-4, s4-5, s4-6 | |
| Lipari | Inzolfata | 5 (F,S) | 3.1 | 30 | 5.9 | 5-1, 5-2, 5s-1, 5s-2, s5s-1, s5s-2 | |
| Panarea | La Calcara | 7 (S) | 19.8 | 95 | 5.1 | s7s-1, s7s-3g, s7s-5 | |
| Black point | BP (S) | 23.0 | 130 | 3.3 | 46.2 | SBP3 | |
| Campo 7 | AP (W) | 21.3 | 60 | 4.9 | 49.2 | APA, APB | |
| Stromboli | Zurro | 10 (F) | sl | 36 | 6.7 | 10-2, 10-1-65, 10-1-55, md10-1, g10 | |
| Ginostra | 11 (F) | sl | 39 | 6.7 | 11-1, g11-2 | ||
| sl: sea level |
| Strain | Opt | Opt pH | Opt NaCl (%) | Ker (2%) | Gasol (2%) | Tween 20 | Tween 80 | Esterase C4 | Esterase/Lipase C8 | Lipase C14 |
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | ||||||||||
| Bacillus sp. A1-3 | 55 | 6 | 2 | + | + | + | + | + | + | − |
| B. licheniformis B3-15 a | 45 | 7 | 2 | + | − | + | + | + | + | + |
| Bacillus sp. B3-18 | 65 | 7 | 2 | + | − | + | + | + | + | − |
| Bacillus sp. B3-24 | 65 | 7 | 2 | + | + | + | − | + | + | − |
| Bacillus sp. B3-28 | 50 | 6 | 2 | + | − | − | − | + | + | − |
| G. thermodenitrificans B3-72 b | 65 | 7 | 0 | + | + | + | − | + | + | + |
| Bacillus sp. B3-75 | 55 | 8 | 2 | + | + | + | + | + | + | − |
| Bacillus sp. B3-76 | 50 | 5.5 | 1 | + | + | + | − | + | + | + |
| B. licheniformis md4-1-1 | 50 | 7.5 | 5 | + | + | − | − | + | + | − |
| Bacillus sp. md4-1-2 | 70 | 5.5 | 1 | + | + | − | − | + | + | − |
| Bacillus sp. T1-3 | 60 | 8 | 1 | + | − | + | + | + | + | + |
| G. thermodenitrificans S1 | 65 | 8 | 2 | + | − | + | + | + | + | + |
| Bacillus sp. S1-1 | 50 | 5.5 | 2 | + | + | + | + | + | + | + |
| B. licheniformis s7s-1 | 50 | 7 | 2 | + | + | + | + | + | + | + |
| B. horneckiae SBP3 DSM 103063 c | 45 | 8 | 2 | + | + | + | + | + | + | + |
| Strain | BM | E24 (%) |
|---|---|---|
| A1-3 | + | 42.1 ± 2.3 |
| B3-15 a | + | 51.4 ± 1.4 |
| B3-18 | + | 42.2 ± 2.7 |
| B3-24 | + | 40.1 ± 1.9 |
| B3-28 | + | 41.4 ± 1.8 |
| B3-72 b | + | 41.3 ± 1.8 |
| B3-75 | + | 32.3 ± 1.5 |
| B3-76 | + | 43.5 ± 1.8 |
| md4-1-1 | + | 42.3 ± 1.9 |
| md4-1-2 | − | 18.2 ± 1.5 |
| T1-3 | − | 22.3 ± 1.8 |
| S1 | + | 35.6 ± 1.4 |
| S1-1 | + | 44.3 ± 1.8 |
| s7s-1 | + | 32.2 ± 1.6 |
| SBP3 c | + | 55.2 ± 1.6 |
| Triton X-100 | nd | 74 ± 1.8 |
| Sterile NSW + KER | nd | 0 |
| Oil Drop Collapse | ST (mN m−1) | ||||
|---|---|---|---|---|---|
| CFSs (MB + SAC) | CFSs (MB + SAC) | ||||
| Time (h) | B3-15 | SBP3 | B3-15 | SBP3 | Sterile MB + SAC |
| 24 | nd | nd | 57 ± 1.3 | 53 ± 1.1 * | 68.3 ± 1.2 |
| 48 | + | + | 52 ± 1.4 | 39 ± 1.1 ** | 68.3 ± 1.6 |
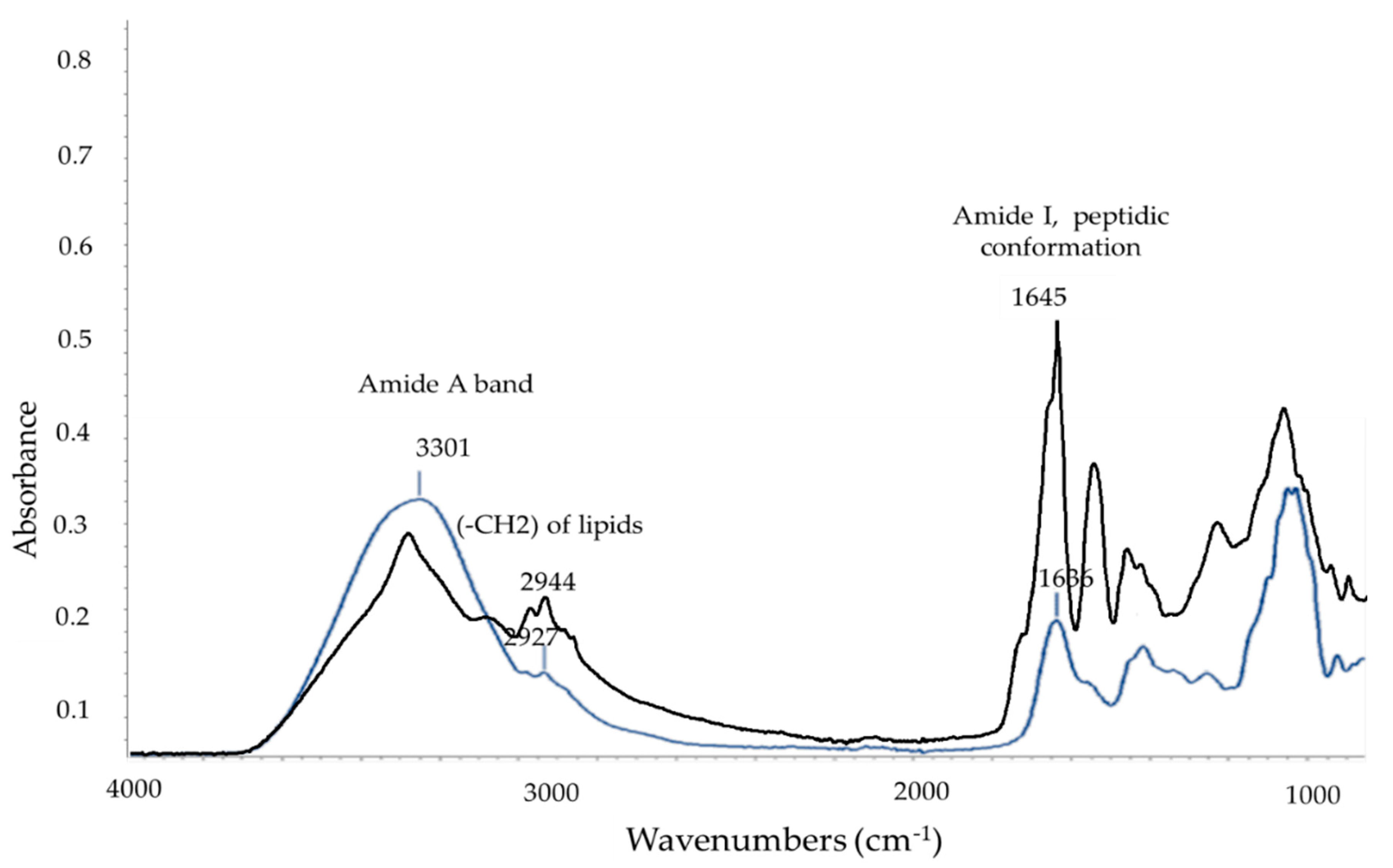
| Wavenumber Values (cm−1) | Assignment | References |
|---|---|---|
| 3300–3200 | Amide A | [37,38] |
| 3000–2800 | CH2 and CH3 of lipids | [39] |
| 1690–1618 | Amide I peptidic conformation | [37] |
| ~1548 | Amide II peptidic conformation | [38] |
| 1456–1453 | -CH2 of lipids | [38] |
| 1400–1380 | CH2 and CH3 of lipids, dipicolinic acid, amide III | [38] |
| ~1066 | (R-O-p-O-R) from ring vibrations of carbohydrates | [38] |
| ~1250 | CH-NH stretching | [40] |
| 1055–1050 | Phosphate groups | [41] |
| 1035–1030 | Stretching vibrations of the C–O group in esters | [42] |
| Washing Solution | Mineral Oil | Crude Oil | Castor Oil |
|---|---|---|---|
| Tap water | 0.52 ± 0.04 | 0.42 ± 0.01 | 0.87 ± 0.02 |
| BS B3-15 | 53.0 ± 0.04 | 45.0 ± 0.04 | 70.0 ± 2.20 |
| BS SBP3 | 48.0 ± 0.02 | 80.0 ± 2.50 | 32.0 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammuto, V.; Spanò, A.; Nicolò, M.S.; Grillo, E.; Caccamo, M.T.; Magazù, S.; Cappello, S.; Gugliandolo, C. Thermophilic Hydrocarbon-Utilizing Bacilli from Marine Shallow Hydrothermal Vents as Producers of Biosurfactants. J. Mar. Sci. Eng. 2022, 10, 1077. https://doi.org/10.3390/jmse10081077
Zammuto V, Spanò A, Nicolò MS, Grillo E, Caccamo MT, Magazù S, Cappello S, Gugliandolo C. Thermophilic Hydrocarbon-Utilizing Bacilli from Marine Shallow Hydrothermal Vents as Producers of Biosurfactants. Journal of Marine Science and Engineering. 2022; 10(8):1077. https://doi.org/10.3390/jmse10081077
Chicago/Turabian StyleZammuto, Vincenzo, Antonio Spanò, Marco Sebastiano Nicolò, Emanuela Grillo, Maria Teresa Caccamo, Salvatore Magazù, Simone Cappello, and Concetta Gugliandolo. 2022. "Thermophilic Hydrocarbon-Utilizing Bacilli from Marine Shallow Hydrothermal Vents as Producers of Biosurfactants" Journal of Marine Science and Engineering 10, no. 8: 1077. https://doi.org/10.3390/jmse10081077
APA StyleZammuto, V., Spanò, A., Nicolò, M. S., Grillo, E., Caccamo, M. T., Magazù, S., Cappello, S., & Gugliandolo, C. (2022). Thermophilic Hydrocarbon-Utilizing Bacilli from Marine Shallow Hydrothermal Vents as Producers of Biosurfactants. Journal of Marine Science and Engineering, 10(8), 1077. https://doi.org/10.3390/jmse10081077










