Characterization of Fine-Scale Turbulence Generated in a Laboratory Orbital Shaker and Its Influence on Skeletonema costatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Turbulence Simulation
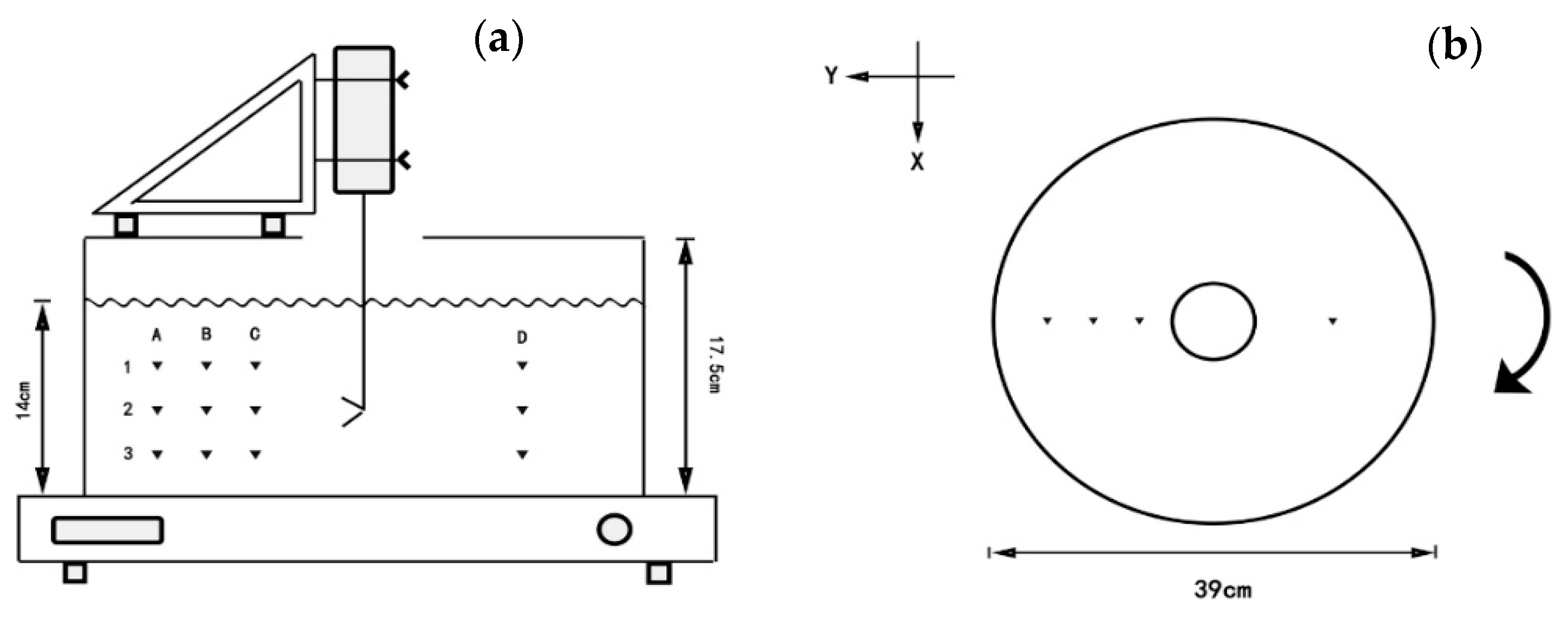
2.1.1. Setup of Orbital Shaker
2.1.2. Velocity Measurement
2.1.3. Estimation of the Turbulent Kinetic Energy Dissipation Rate
2.2. Application of Orbital Shaker in Biological Culture
2.2.1. Organism and Culture Conditions
2.2.2. Physiological Parameters of Diatom
2.2.3. Calculation of the Batchelor Length Scale
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. The Exploration of Orbital Oscillator
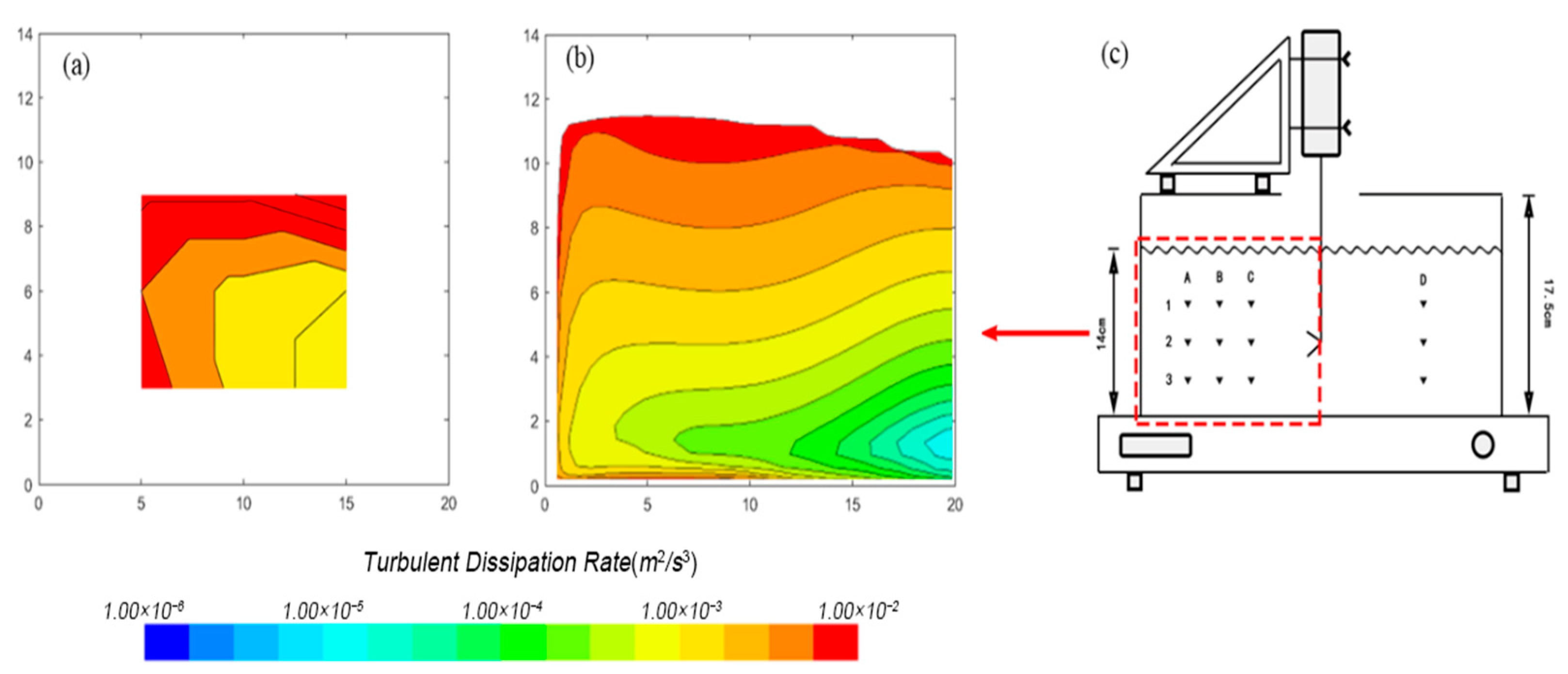
3.1.1. Turbulent Dissipation Rate Distribution
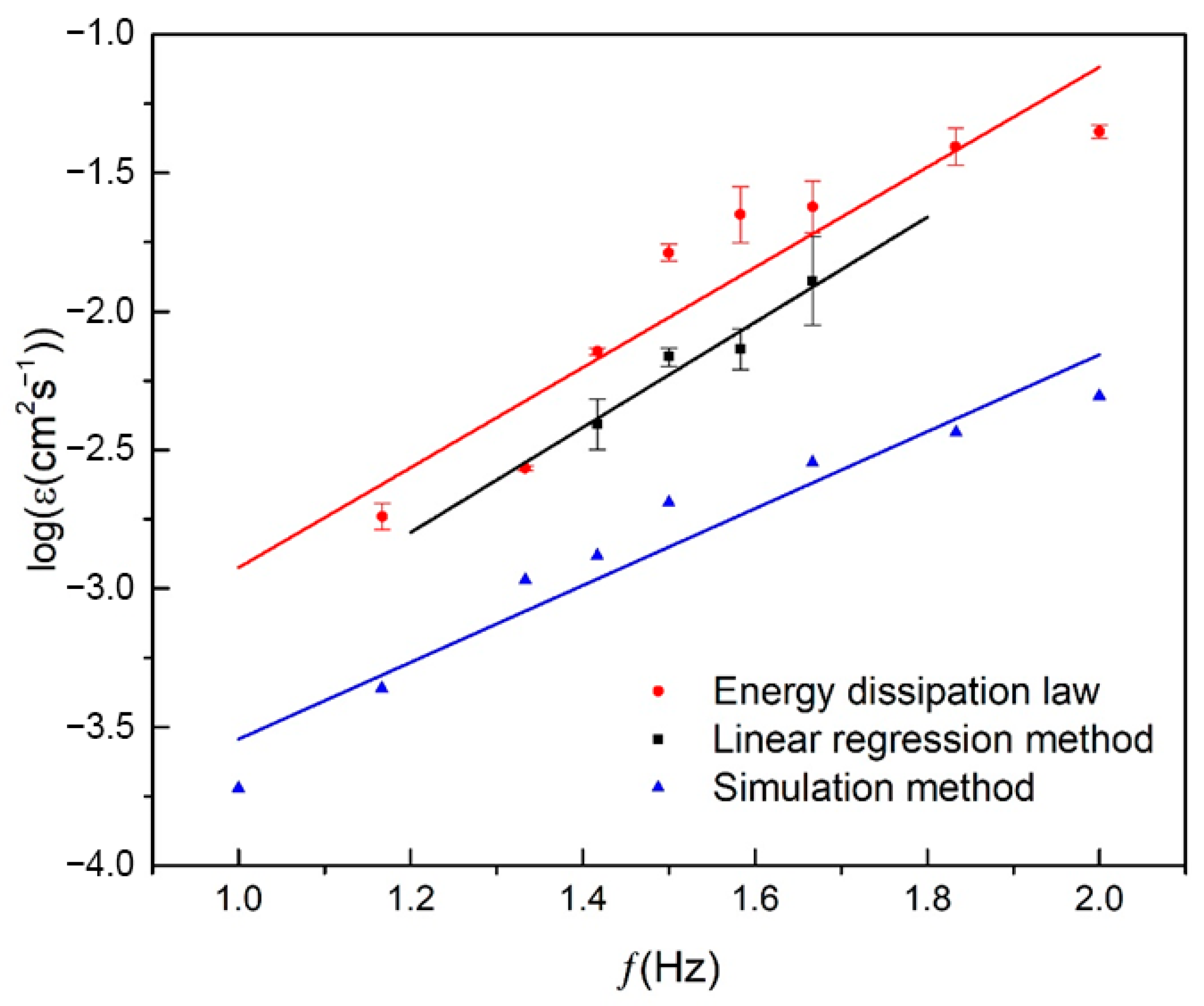
3.1.2. Comparative Analysis of Three Turbulence Simulation Methods
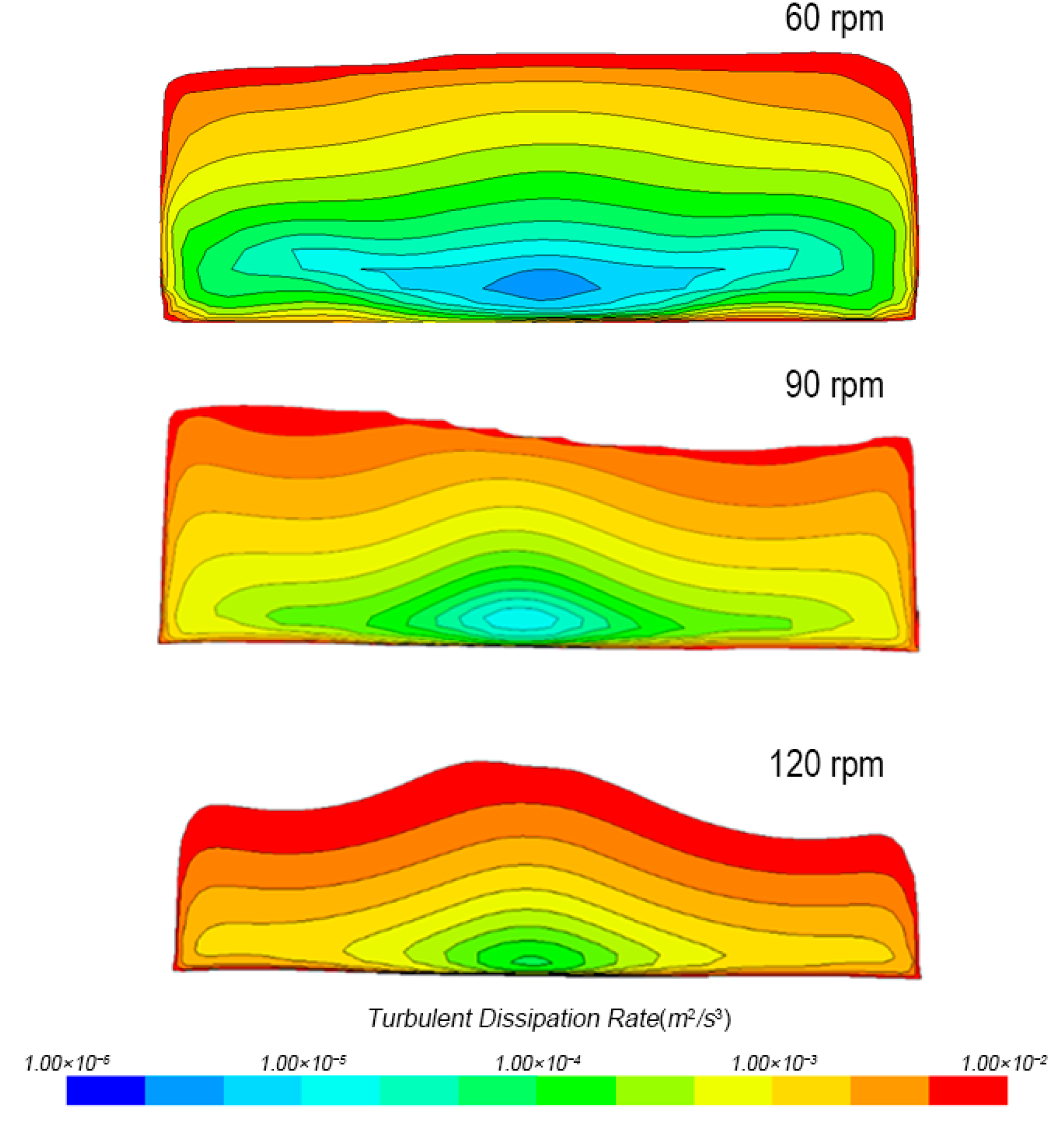
3.1.3. Turbulence Distribution in Tank
3.2. Cultivation of Skeletonema Costatum with Turbulence
3.2.1. Turbulence Simulation
3.2.2. The Response of Skeletonema Costatum under Different Turbulence Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Thorpe, S.A. The Turbulent Ocean; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; p. 439. [Google Scholar]
- Margalef, R. Life-Forms of Phytoplankton as Survival Alternatives in an Unstable Environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Berdalet, E.; Peters, F.; Koumandou, V.L.; Roldan, C.; Guadayol, O.; Estrada, M. Species-specific physiological response of dinoflagellates to quantified small-scale turbulence. J. Phycol. 2007, 43, 965–977. [Google Scholar] [CrossRef]
- Karp-Boss, L.; Boss, E.; Jumars, P.A. Motion of dinoflagellates in a simple shear flow. Limnol. Oceanogr. 2000, 45, 1594–1602. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Swift, E. Effects of small-scale turbulence on net growth rate and size of ten species of marine dinoflagellates. J. Phycol. 2003, 39, 83–94. [Google Scholar] [CrossRef]
- Du Puits, R.; Resagk, C.; Thess, A. Thickness of the diffusive sublayer in turbulent convection. Phys. Rev. E 2010, 81, 16307. [Google Scholar] [CrossRef]
- Lewis, M.R.; Horne, E.P.W.; Cullen, J.J.; Oakey, N.S.; Platt, T. Turbulent Motions May Control Phytoplankton Photosynthesis in the Upper Ocean. Nature 1984, 311, 49–50. [Google Scholar] [CrossRef]
- Huisman, J.; Sharples, J.; Stroom, J.M.; Visser, P.M.; Kardinaal, W.E.A.; Verspagen, J.M.H.; Sommeijer, B. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 2004, 85, 2960–2970. [Google Scholar] [CrossRef]
- Peters, F.; Arin, L.; Marrase, C.; Berdalet, E.; Sala, M.M. Effects of small-scale turbulence on the growth of two diatoms of different size in a phosphorus-limited medium. J. Mar. Syst. 2006, 61, 134–148. [Google Scholar] [CrossRef]
- Barton, A.D.; Ward, B.A.; Williams, R.G.; Follows, M.J. The impact of fine-scale turbulence on phytoplankton community structure. Limnol. Oceanogr. Fluids Environ. 2014, 4, 34–49. [Google Scholar] [CrossRef]
- Mackenzie, B.R.; Miller, T.J.; Cyr, S.; Leggett, W.C. Evidence for a Dome-Shaped Relationship between Turbulence and Larval Fish Ingestion Rates. Limnol. Oceanogr. 1994, 39, 1790–1799. [Google Scholar] [CrossRef]
- Harkonen, L.; Pekcan-Hekim, Z.; Hellen, N.; Ojala, A.; Horppila, J. Combined Effects of Turbulence and Different Predation Regimes on Zooplankton in Highly Colored Water-Implications for Environmental Change in Lakes. PLoS ONE 2014, 9, e111942. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.F.; Litchman, E.; Klausmeier, C.A. Functional traits explain phytoplankton community structure and seasonal dynamics in a marine ecosystem. Ecol. Lett. 2013, 16, 56–63. [Google Scholar] [CrossRef]
- Sengupta, A.; Carrara, F.; Stocker, R. Phytoplankton can actively diversify their migration strategy in response to turbulent cues. Nature 2017, 543, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Fraisse, S.; Bormans, M.; Lagadeuc, Y. Turbulence effects on phytoplankton morphofunctional traits selection. Limnol. Oceanogr. 2015, 60, 872–884. [Google Scholar] [CrossRef]
- Schapira, M.; Seuront, L.; Gentilhomme, V. Effects of small-scale turbulence on Phaeocystis globosa (Prynmesiophyceae) growth and life cycle. J. Exp. Mar. Biol. Ecol. 2006, 335, 27–38. [Google Scholar] [CrossRef]
- Beardall, J.; Raven, J.A. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 2004, 43, 26–40. [Google Scholar] [CrossRef]
- Beardall, J.; Stojkovic, S.J.S. Microalgae under Global Environmental Change: Implications for Growth and Productivity, Populations and Trophic Flow. ScienceAsia 2006, 32, 1–10. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, B.Q.; Han, X.X. Effects of the magnitude and persistence of turbulence on phytoplankton in Lake Taihu during a summer cyanobacterial bloom. Aquat. Ecol. 2016, 50, 197–208. [Google Scholar] [CrossRef]
- Peters, F.; Marrase, C. Effects of turbulence on plankton: An overview of experimental evidence and some theoretical considerations. Mar. Ecol. Prog. Ser. 2000, 205, 291–306. [Google Scholar] [CrossRef]
- Guadayol, O.; Peters, F.; Stiansen, J.E.; Marrase, C.; Lohrmann, A. Evaluation of oscillating grids and orbital shakers as means to generate isotropic and homogeneous small-scale turbulence in laboratory enclosures commonly used in plankton studies. Limnol. Oceanogr. Methods 2009, 7, 287–303. [Google Scholar] [CrossRef]
- Bluteau, C.E.; Jones, N.L.; Ivey, G.N. Estimating turbulent kinetic energy dissipation using the inertial subrange method in environmental flows. Limnol. Oceanogr. Methods 2011, 9, 302–321. [Google Scholar] [CrossRef]
- Peters, F.; Redondo, J.M. Turbulence generation and measurement: Application to studies on plankton. Sci. Mar. 1997, 61, 205–228. [Google Scholar]
- Li, C.; Xia, J.Y.; Chu, J.; Wang, Y.H.; Zhuang, Y.P.; Zhang, S.L. CFD analysis of the turbulent flow in baffled shake flasks. Biochem. Eng. J. 2013, 70, 140–150. [Google Scholar] [CrossRef]
- Striebel, M.; Kirchmaier, L.; Hingsamer, P. Different mixing techniques in experimental mesocosms-does mixing affect plankton biomass and community composition? Limnol. Oceanogr. Methods 2013, 11, 176–186. [Google Scholar] [CrossRef]
- Amato, A.; Fortini, S.; Watteaux, R.; Diano, M.; Espa, S.; Esposito, S.; Ferrante, M.I.; Peters, F.; Iudicone, D.; Ribera d’Alcalà, M. TURBOGEN: Computer-controlled vertically oscillating grid system for small-scale turbulence studies on plankton. Rev. Sci. Instrum. 2016, 87, 035119. [Google Scholar] [CrossRef]
- Kysela, B.; Konfrst, J.; Chara, Z.; Sulc, R.; Jasikova, D. Evaluation of the turbulent kinetic dissipation rate in an agitated vessel. Epj. Web. Conf. 2017, 143, 2062. [Google Scholar] [CrossRef]
- Sanford, L.P. Turbulent mixing in experimental ecosystem studies. Mar. Ecol. Prog. Ser. 1997, 161, 265–293. [Google Scholar] [CrossRef]
- Kaku, V.J.; Boufadel, M.C.; Venosa, A.D. Evaluation of mixing energy in laboratory flasks used for dispersant effectiveness testing. J. Environ. Eng. 2006, 132, 93–101. [Google Scholar] [CrossRef]
- Buchs, J. Introduction to advantages and problems of shaken cultures. Biochem. Eng. J. 2001, 7, 91–98. [Google Scholar] [CrossRef]
- Pujara, N.; Du Clos, K.T.; Ayres, S.; Variano, E.A.; Karp-Boss, L. Measurements of trajectories and spatial distributions of diatoms (Coscinodiscus spp.) at dissipation scales of turbulence. Exp. Fluids 2021, 62, 149. [Google Scholar] [CrossRef]
- Li, M.; Xiao, M.; Zhang, P.; Hamilton, D.P. Morphospecies-dependent disaggregation of colonies of the cyanobacterium Microcystis under high turbulent mixing. Water Res. 2018, 141, 340–348. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Z.; Li, C.; Zhang, Z.; Guo, J.S. Effect of Small-Scale Turbulence on the Physiology and Morphology of Two Bloom-Forming Cyanobacteria. PLoS ONE 2016, 11, e0168925. [Google Scholar] [CrossRef] [PubMed]
- Burns, W.G.; Marchetti, A.; Ziervogel, K. Enhanced formation of transparent exopolymer particles (TEP) under turbulence during phytoplankton growth. J. Plankton Res. 2019, 41, 349–361. [Google Scholar] [CrossRef]
- Liu, M.Z.; Ma, J.R.; Kang, L.; Wei, Y.Y.; He, Q.; Hu, X.B.; Li, H. Strong turbulence benefits toxic and colonial cyanobacteria in water: A potential way of climate change impact on the expansion of Harmful Algal Blooms. Sci. Total Environ. 2019, 670, 613–622. [Google Scholar] [CrossRef]
- Dell’Aquila, G.; Ferrante, M.I.; Gherardi, M.; Lagomarsino, M.C.; d’Alcala, M.R.; Iudicone, D.; Amato, A. Nutrient consumption and chain tuning in diatoms exposed to storm-like turbulence. Sci. Rep. 2017, 7, 1828. [Google Scholar] [CrossRef]
- Ravizza, M.; Hallegraeff, G. Environmental conditions influencing growth rate and stalk formation in the estuarine diatom Licmophora flabellata (Carmichael ex Greville) C.Agardh. Diatom Res. 2015, 30, 197–208. [Google Scholar] [CrossRef]
- Bolli, L.; Llaveria, G.; Garces, E.; Guadayol, O.; van Lenning, K.; Peters, F.; Berdalet, E. Modulation of ecdysal cyst and toxin dynamics of two Alexandrium (Dinophyceae) species under small-scale turbulence. Biogeosciences 2007, 4, 559–567. [Google Scholar] [CrossRef]
- Yeung, P.; Wong, J. Inhibition of cell proliferation by mechanical agitation involves transient cell cycle arrest at G1 phase in dinoflagellates. Protoplasma 2003, 220, 173–178. [Google Scholar] [CrossRef]
- Zirbel, M.J.; Veron, F.; Latz, M.I. The reversible effect of flow on the morphology of Ceratocorys horrida (Peridiniales, Dinophyta). J. Phycol. 2000, 36, 46–58. [Google Scholar] [CrossRef]
- McLelland, S.J.; Nicholas, A.P. A new method for evaluating errors in high-frequency ADV measurements. Hydrol. Process 2000, 14, 351–366. [Google Scholar] [CrossRef]
- Taylor, G.I. Statistical Theory of Turbulence. Proc. R. Soc. Lond. 1935, 151, 421–444. [Google Scholar] [CrossRef]
- Chisita, C.T.; Enakrire, R.T.; Olumide, D.O.; Tsabedze, V.W. Handbook of Research on Records and Information Management Strategies for Enhanced Knowledge Coordination; Information Science Reference: Hershey, PA, USA, 2021; p. 1, online resource. [Google Scholar]
- Stiansen, J.E.; Sundby, S. Improved methods for generating and estimating turbulence in tanks suitable for fish larvae experiments. Sci. Mar. 2001, 65, 151–167. [Google Scholar] [CrossRef]
- Tennekes, H. Eulerian and Lagrangian Time Microscales in Isotropic Turbulence. J. Fluid Mech. 1975, 67, 561–567. [Google Scholar] [CrossRef]
- Taylor, J.R.; Stocker, R. Trade-Offs of Chemotactic Foraging in Turbulent Water. Science 2012, 338, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Kaku, V.J.; Boufadel, M.C.; Venosa, A.D.; Weaver, J. Flow dynamics in eccentrically rotating flasks used for dispersant effectiveness testing. Environ. Fluid Mech. 2006, 6, 385–406. [Google Scholar] [CrossRef]
- Mackenzie, B.R.; Leggett, W.C. Wind-based models for estimating the dissipation rates of turbulent energy in aquatic environments—Empirical comparisons. Mar. Ecol. Prog. Ser. 1993, 94, 207–216. [Google Scholar] [CrossRef]
- Skyllingstad, E.D.; Smyth, W.D.; Moum, J.N.; Wijesekera, H. Upper-ocean turbulence during a westerly wind burst: A comparison of large-eddy simulation results and microstructure measurements. J. Phys. Oceanogr. 1999, 29, 5–28. [Google Scholar] [CrossRef]
- Lozovatsky, I.; Roget, E.; Planella, J.; Fernando, H.J.S.; Liu, Z.Y. Intermittency of near-bottom turbulence in tidal flow on a shallow shelf. J. Geophys. Res. Ocean. 2010, 115, c05006. [Google Scholar] [CrossRef]
- Tanaka, M. Changes in Vertical Distribution of Zooplankton under Wind-Induced Turbulence: A 36-Year Record. Fluids 2019, 4, 195. [Google Scholar] [CrossRef]
- Katija, K. Biogenic inputs to ocean mixing. J. Exp. Biol. 2012, 215, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Arnott, R.N.; Cherif, M.; Bryant, L.D.; Wain, D.J. Artificially generated turbulence: A review of phycological nanocosm, microcosm, and mesocosm experiments. Hydrobiologia 2021, 848, 961–991. [Google Scholar] [CrossRef]
- Yamazaki, H.; Osborn, T.R. In Review of Oceanic Turbulence: Implications for Biodynamics. In Toward a Theory on Biological-Physical Interactions in the World Ocean; Springer: Dordrecht, The Netherlands, 1988; pp. 215–234. [Google Scholar]
- Sukhodolov, A.; Thiele, M.; Bungartz, H. Turbulence structure in a river reach with sand bed. Water Resour. Res. 1998, 34, 1317–1334. [Google Scholar] [CrossRef]
- Guadayol, O.; Peters, F. Analysis of wind events in a coastal area: A tool for assessing turbulence variability for studies on plankton. Sci. Mar. 2006, 70, 9–20. [Google Scholar] [CrossRef][Green Version]
- Guadayol, O.; Marrase, C.; Peters, F.; Berdalet, E.; Roldan, C.; Sabata, A. Responses of coastal osmotrophic planktonic communities to simulated events of turbulence and nutrient load throughout a year. J. Plankton Res. 2009, 31, 583–600. [Google Scholar] [CrossRef][Green Version]
- Guasto, J.S.; Rusconi, R.; Stocker, R. Fluid Mechanics of Planktonic Microorganisms. In Annual Review of Fluid Mechanics; Massachusetts Institute of Technology: Cambridge, MA, USA, 2012; Volume 44, pp. 373–400. [Google Scholar]
- Clarson, S.J.; Steinitz-Kannan, M.; Patwardhan, S.V.; Kannan, R.; Hartig, R.; Schloesser, L.; Hamilton, D.W.; Fusaro, J.K.A.; Beltz, R. Some Observations of Diatoms Under Turbulence. Silicon 2009, 1, 79–90. [Google Scholar] [CrossRef]
- Langdon, C. On the causes of interspecific differences in the growth irradiance relationship for phytoplankton. 2. A general-review. J. Plankton Res. 1988, 10, 1291–1312. [Google Scholar] [CrossRef]
- Garrison, H.S.; Tang, K.W. Effects of episodic turbulence on diatom mortality and physiology, with a protocol for the use of Evans Blue stain for live-dead determinations. Hydrobiologia 2014, 738, 155–170. [Google Scholar] [CrossRef][Green Version]

| Algae | Turbulence Generator | ε (m2·s−3) | Reference |
|---|---|---|---|
| diatom | Paddles | 10−3–10−1 | [32] |
| cyanobacterium | Paddles | 10−2–10−1 | [33] |
| cyanobacterium | Paddles | 10−3–10−2 | [34] |
| diatom haptophyte | oscillating grids | 10−6–10−4 | [35] |
| natural communities | oscillating grids | 10−3–10−2 | [36] |
| diatom | oscillating grids | 10−5 | [37] |
| Zygnematophyceae Bacillariophyceae Chlorophyceae Prasinophyceae | oscillating grids | 10−5–10−3 | [15] |
| prymnesiophyceae | oscillating grids | 10−6–10−4 | [16] |
| dinoflagellates | oscillating grids | 10−8–10−4 | [5] |
| diatom | orbital shaker | - | [38] |
| dinoflagellates | orbital shaker | 10−5–10−2 | [39] |
| dinoflagellates | orbital shaker | 10−4 | [3] |
| diatom | orbital shaker | 10−3 | [9] |
| dinoflagellates | orbital shaker | 10−5–10−4 | [40] |
| dinoflagellates | orbital shaker | 10−5–10−4 | [41] |
| Rotation Rate (rpm) | Frequence (Hz) | The Energy Dissipation Law Method | The Linear Regression Method | The Simulation Method |
|---|---|---|---|---|
| 60 | 1.000 | 1.90 × 10−4 | ||
| 70 | 1.167 | 1.83 × 10−3 | 4.35 × 10−4 | |
| 80 | 1.333 | 2.72 × 10−3 | 1.07 × 10−3 | |
| 85 | 1.417 | 7.16 × 10−3 | 3.97 × 10−3 | 1.31 × 10−3 |
| 90 | 1.500 | 1.63 × 10−2 | 6.86 × 10−3 | 2.04 × 10−3 |
| 95 | 1.583 | 2.28 × 10−2 | 7.38 × 10−3 | |
| 100 | 1.667 | 2.41 × 10−2 | 1.33 × 10−2 | 2.85 × 10−3 |
| 110 | 1.833 | 4.68 × 10−2 | 3.65 × 10−3 | |
| 120 | 2.000 | 4.20 × 10−2 | 4.94 × 10−3 |
| Rotation Rate | 60 rpm | 120 rpm |
|---|---|---|
| Frequency (Hz) | 1.000 | 2.000 |
| ε (m2·s−3) | 2.4 × 10−7 | 5.2 × 10−5 |
| The Kolmogorov length scale (, μm) | 1433 | 35 |
| The Batchelor length scale (, μm) | 374 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Li, Y.; Yao, Z.; You, L.; Jiang, Z.-P.; Fan, W.; Pan, Y. Characterization of Fine-Scale Turbulence Generated in a Laboratory Orbital Shaker and Its Influence on Skeletonema costatum. J. Mar. Sci. Eng. 2022, 10, 1053. https://doi.org/10.3390/jmse10081053
Yu L, Li Y, Yao Z, You L, Jiang Z-P, Fan W, Pan Y. Characterization of Fine-Scale Turbulence Generated in a Laboratory Orbital Shaker and Its Influence on Skeletonema costatum. Journal of Marine Science and Engineering. 2022; 10(8):1053. https://doi.org/10.3390/jmse10081053
Chicago/Turabian StyleYu, Lin, Yifan Li, Zhongzhi Yao, Long You, Zong-Pei Jiang, Wei Fan, and Yiwen Pan. 2022. "Characterization of Fine-Scale Turbulence Generated in a Laboratory Orbital Shaker and Its Influence on Skeletonema costatum" Journal of Marine Science and Engineering 10, no. 8: 1053. https://doi.org/10.3390/jmse10081053
APA StyleYu, L., Li, Y., Yao, Z., You, L., Jiang, Z.-P., Fan, W., & Pan, Y. (2022). Characterization of Fine-Scale Turbulence Generated in a Laboratory Orbital Shaker and Its Influence on Skeletonema costatum. Journal of Marine Science and Engineering, 10(8), 1053. https://doi.org/10.3390/jmse10081053







