Abstract
Harmful Algal Blooms (HAB) are phenomena that result from alterations to ecosystems. Due to their potential toxicity, the level of danger depends on the species concerned, their frequency and intensity. They can cause impacts on biodiversity and on the anthropic activities that take place in maritime and coastal areas. Primary industries such as shellfish fisheries are mainly affected. To deal with this issue, the French administration has built a governance system based on two pillars. The first relies on a water quality monitoring system that assesses the risks of HAB contamination of coastal waters. The second is a regulatory system of production and commercial bans of seafood products from the impacted areas. This public action has two objectives. The first is human health-related and aims to protect consumers of seafood. The second is economic-based and aims to minimize the economic impacts associated with the commercial bans suffered by the businesses concerned. These two objectives may appear to be antagonistic. Using the case study of the French scallop fishery in the eastern Channel and based on an analysis of the commercial bans associated with HAB and associated potential economic impacts, this paper analyses the governance scheme dealing with HAB events in France. The authors highlight that this governance is not only a matter of applying administrative closures when toxicity thresholds are exceeded, but is a dynamic decision-making process involving experts and the Administration that attempts to balance acceptable health risks and economic impacts.
1. Introduction
Harmful algal blooms (HAB) are phenomena that result from imbalances in ecosystems due to natural processes or anthropogenic effects [1]. They are highly complex processes that are difficult to predict. Under certain conditions, these microalgae release toxins that can have negative impacts on aquatic biodiversity [2]. Beyond the ecological considerations, these events can also generate economic and social impacts of a different nature. HAB can thus lead to human health issues through the ingestion of contaminated seafood leading to nausea and vomiting to more severe symptoms such as paralysis and amnesia, or by direct exposure to contaminated waters causing respiratory disturbances due to inhalation of aerosols or skin irritations [3,4]. In order to preserve human health and reduce the associated health costs, access bans to affected areas and commercial bans on shellfish production are usually decreed by the Administration. These bans can also lead to economic impacts on marine and coastal regions, and fishing and aquaculture are among the most exposed sectors. These impacts are related to deferred sales, loss of biomass and the additional costs associated with adaptation strategies to limit the consequences of HAB [5]. Other activities may be affected such as recreational fishing [6], beach walking or bathing. In addition to the loss of amenity for recreational activities, HAB can also cause economic impacts on local and regional economies [7,8]. Moreover, management and monitoring expenses to prevent or cope with HAB also represent a social cost [9,10].
Assessing the impacts associated with HAB is complex due to the heterogeneity, availability and quality of information related to the impacted activities. The economic impacts of HAB are difficult to compare due to the diversity of these events, their frequency, intensity, spatial distribution, the diversity of activities affected and the socio-cultural and institutional contexts [10]. According to Bernard et al. [11], the annual costs associated with HAB events solely in marine areas that are related to research, monitoring and mitigation activities are in the order of USD 55 million in the USA, USD 850 million in Europe and nearly USD one billion in Japan. Although it is interesting for accounting purposes to highlight the importance of these events, the single and aggregated monetary valuation of the impacts associated with HAB is of little interest regarding the decision-making process. How impacts are distributed among stakeholders and within activities tells much more in terms of coping and mitigation strategies.
The mechanisms that are implemented to respond to these phenomena are part of the management or governance of HAB [2]. The complexity this governance is related to the lack of accurate models to predict their trends and occurrences and, therefore, to anticipate their effects. In this context, Zingone and Enevoldsen [1] question the practical modes of action to be implemented in terms of marine resource management, protection of dependent economic activities, health protection, and anthropogenic actions likely to engage efficient responses against HAB impacts.
In terms of practical governance actions, there are three main types of possible responses to HAB events [2]. The first is mitigation which refers to using all available means to reduce the negative effects associated with HAB. This includes establishing monitoring systems to safeguard human health, avoiding or reducing the impacts, avoiding biomass kills and improving knowledge to enhance the ability to predict these events [12]. In the case of aquaculture, individual mitigation responses may rely on towing of fish cages [1], safeguarding in storage tanks or detoxifying contaminated products [13,14]. The adoption of these different options depends on the costs associated with their implementation and the capacity of stakeholders to fund them. In the case of fisheries, responses from companies to avoid or to reduce economic impacts rely on moving to other uncontaminated fishing areas, diversifying fishing activity to other species that are not affected by HAB or transferring activity to other businesses. These different responses depend on the capacity of companies to adapt according to the economic gain associated with each option.
The second type of action concerns anticipatory prevention rather than post-crisis adaptation. This type of action involves implementing measures capable of reducing problems at the source in order to reduce the intensity and frequency of HAB. Although modelling of these phenomena is not yet reliable [15], some works have progressed on identifying factors that can exacerbate the occurrence of HAB [16]. Prevention via integrated coastal zone management processes to improve water quality is, therefore, a means of combating HAB by reducing anthropogenic pressures on ecosystems from the agricultural sector, coastal economic activities or urban pollution rather than engaging in extensive restoration efforts [17]. These measures have results only in the long term.
Finally, the third type of measure concerns control. These are curative measures implemented after the impact. They aim to compensate HAB effects by various means, mechanical, biological, chemical, genetic and environmental [2,18]. However, they are controversial due to the consequences they may have for biodiversity in the water column and on the seabed [2].
In the case of France, the governance of HAB impacts aims to protect consumers of seafood and refers to the European regulatory framework. The main regulations concern (1) the specific hygiene rules applicable to food products of animal origin [19], with a specific chapter applicable to live bivalve molluscs which details the toxicity thresholds of marine biotoxins that must not be exceeded and (2) the organization of controls on food products of animal origin intended for human consumption [20], including the construction of monitoring systems, the description of sampling to be implemented for sensitive areas and the modalities of the closure of areas contaminated by HAB. Three specific groups of toxic microalgae are monitored through these directives: Dinophysis (which produces diarrethic toxins), Pseudo-nitzschia (amnesia toxins), and Alexandrium (paralytic toxins). While regulations dealing with HAB are built at the national level, the structure of this governance differs according to the species at local or regional levels. The organization of public governance is led by the territorial Administration (Direction Départementale des Territoires et de la Mer—DDTM) with the support of scientific expertise provided by IFREMER (the French Institute for Ocean Science).
More specifically, this paper focus on the French scallop fishery operating in the eastern English Channel area due to the socio-economic importance of this activity and its high potential for exposure to HAB. This maritime area has experienced changing HAB dynamics to which monitoring strategies, management measures and governance structure have had to adapt [21]. Before 2012, management focused on local scales and measures were applied to small areas. In 2012, the scallop production zones (Figure 1), that rely on particular located stocks of scallop were created following the major ASP (Amnesic Shellfish Poisoning) crisis that affected the entire eastern Channel area. In order to reduce the economic impact on the fishing sector, this zoning aimed at dividing the maritime space in order to control the production areas more finely so that only the areas affected by HAB were closed. A balance must, therefore, be found between the effectiveness and the cost of monitoring since applying controls and closures over smaller areas reduces the number of vessels concerned but increases the monitoring costs. After 2012, the monitoring locations and sampling have also been modified to determine the level of toxicity by production area. Since 2016, the measures for closing production areas have evolved, with a particular focus on the expected evolution of blooms and not only on the toxicity levels measured by in situ analyses. This has led to the implementation of precautionary closures in the case of detection of phycotoxins and depending on the phytoplankton biomass observed. Some areas may be thus closed for some concentrations under the regulatory thresholds, and this is known as the half-threshold (80 µg/kg instead of 160 µg/kg for Diarrhetic Shellfish Poisoning toxins (DSP) and 10 mg/kg instead of 20 mg/kg for Amnesic Shellfish Poisoning toxins (ASP)). This measure is implemented after consultation with experts from IFREMER and depending on the particular conditions of each event. The objective of such a measure is to avoid ex post closures when future toxicity is expected, which may result in the sale of batches of shellfish that have been contaminated but whose control result has been obtained late. This is of particular concern to producers because, in the event of a batch being recalled, the company has to take responsibility for the whole operation, i.e., finding the batch already sold, ensuring its destruction and cancelling the sale or even reimbursing the buyer. This operation is costlier for companies, which would prefer to solely suffer a closure than be forced to manage batch recall operations.
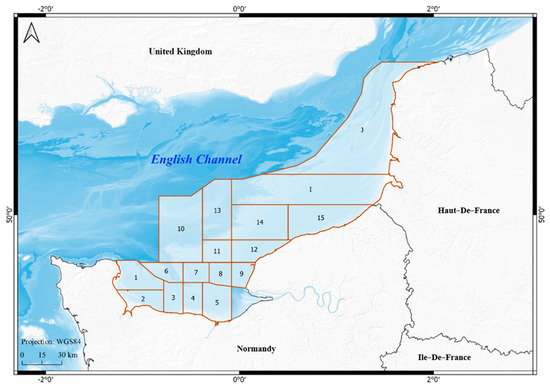
Figure 1.
Location map of the study area (zones from 1 to J represent the scallop management areas or production areas).
Since 2018, each administrative department must therefore organize and manage the phytoplankton and phycotoxins monitoring programs. For the scallop fishery, the Interregional Directorate for the Sea—East Channel, North Sea (DIRM-MEMN) is in charge of the entire monitoring and management procedures in this area, from observation to decision-making. This Administration, therefore, (1) manages the sampling protocols with approved laboratories to carry out the sampling and analysis of phycotoxins, and then (2) determines the appropriate management measures. For other professional activities, DDTMs ensure the monitoring and management of production areas in close collaboration with the regional health agencies (ARS), which are in charge of the monitoring for recreational activities.
This paper discusses the governance of HAB impacts in France as a decision-making process that engages stakeholders in a framework with two potentially contradictory objectives: minimizing health risks and minimizing economic impacts of the affected activities. The analysis conducted is focused on the example of HAB management in the scallop fishery on the eastern French coasts of the English Channel. To analyze this governance, this work focuses on the dynamics of fishing area closures based on the analysis of historical data from the REPHY (“REseau d’observation et de surveillance du PHYtoplancton”) and REPHYTOX (“REseaux de surveillance des PHYcoTOXines dans les organismes marins”) networks, the two French national monitoring networks for phytoplankton and phycotoxins [22]. The analysis of the structure and organization of the monitoring and management of HAB allowed the identification of the stakes and the strategies that guide the Administration’s decision-making processes in the event of a HAB. This analysis of how decisions are made will lead to a better understanding of the governance gaps in achieving public policy goals related to the sustainability of social ecological systems affected by HAB.
2. Materials and Methods
This work focuses on the French eastern coasts bordering the English Channel, from the Pas-de-Calais Strait in the east to the Anglo-Norman Gulf in the West, bordering Brittany. This area covers the coasts of two administrative regions: Hauts-de-France and Normandy (See Figure 1). The geographical location of the English Channel and its morphological, hydrological and biological characteristics create the conditions for an area rich in biodiversity and economic activities [23,24]. This area is significantly affected by HAB events with potentially important economic and social impacts [25]. It has experienced several toxic bloom events over the last thirty years, with toxicity and contamination crises by ASP and DSP affecting several shellfish species, mainly scallops (Pecten maximus). In addition, this area has long been affected by other harmful non-toxic algal blooms, such as Phaeocystis and Karenia mikimotoi, which produce scum that can cause mortality of marine animals by anoxia [26,27].
The analysis of governance of HAB impacts in this paper is based on a quantitative analysis aiming at understanding the basis of administrative decisions regarding commercial bans associated with HAB events in the French eastern English Channel. The management area is divided into 17 zones based on scallop beds, and resource management, monitoring and administrative decisions are based on this zoning.
The analysis is based firstly on a study of the evolution of scallop toxicities in the area in order to translate them into theoretical closures. The theoretical closure of a production area is the administrative decision that should be taken when toxicity concentrations of the water or shellfish flesh analyzed at a control point exceed the regulatory thresholds defined by the European regulation [28]. These theoretical closures are then compared with the actual observed closures that occurred by administrative decree. When the concentration of toxins does not exceed the regulatory thresholds, the area should theoretically remain open.
The comparison between theoretical and observed closures was made on cumulative annual durations of closures per area and per fishing season from 2012 to 2019. More recent years were not considered due to the lack of data about localized closures as there is no centralized management nor data collection of observed closures. A more detailed analysis of closures data (theoretical and observed) was also conducted for weekly data between October 2012 and May 2015 to better understand the observed discrepancies between theoretical and effective closures in production areas 9 and 12. These two production areas were chosen as they are important and frequently used fishing areas, and also because they appear to have a higher risk of blooms and phycotoxin contamination. As a consequence, they support the analysis of the decision-making process regarding closures and illustrate the global functioning of governance. To that purpose, the interpretation of the discrepancy between theoretical and observed closures is used to explain the different governance strategies.
The data used in this analysis are of a varying nature and come from various sources. Data on the actual administrative closures of scallop production areas were collected from the DIRM-MEMN. This database provides details about the date and duration of closures in number of days for each production area and by fishing season from 2012 to 2019.
This period was characterized by numerous HAB events. Table 1 shows the heterogeneity of HAB risk in this marine area, both in space and time. Some fishing areas are not subject to HAB. On the other hand, others such as Areas 9 and 12, had numerous closures during the period observed. While toxic blooms resulting in closures are observed every year, there is no clear trend. A longer data set would be needed to verify a potential trend, but the closures do not appear to follow any particular cycle.

Table 1.
Closures of scallop fishing areas (duration in days). Source: DIRM-MEMN.
Theoretical closures were simulated using (1) the REPHY-REPHYTOX national monitoring network using data about scallop phycotoxin contamination, (2) the calendar of scallop fishing operations in the eastern Channel, and (3) regulations that define the toxicity thresholds not to be exceeded and the conditions for closing and opening the production areas. Utilizing these information sources made it possible to build a new database on theoretical closures based on the toxicity levels revealed by the in situ analyses (Chenouf et al., 2020). Data processing enabled the creation of a calendar of closures/openings using a binary coding (0 and 1) that compares the results of shellfish toxicity tests to the regulatory thresholds. A “0” represented data below or equal to the thresholds and openings, and “1” represented non-compliant analysis results (results above the thresholds) that led to access bans on fishing areas. This calendar was then used to simulate the number and duration of closures of production areas according to the following rule: if the results of the phycotoxicity tests are non-compliant, the areas concerned are closed. According to the regulations in force, these areas are only reopened after two successive compliant test results, i.e., results below or equal to the regulatory thresholds. The algorithm programmed under the R software analyses the REPHY-REPHYTOX database, line by line [29]. This analysis is complemented by indirect qualitative information on HAB management strategies obtained from stakeholders via face-to-face interviews.
3. Results
3.1. Analysis of Scallop Toxicity by Phycotoxins and the Rational of Commercial Bans
Figure 2 provides an overview of phycotoxin concentrations in scallops over the last ten years. This historical view highlights trends over two different time periods. The first is characterized by ASP toxin contamination due to the development of the Pseudo-nitzschia group between January 2012 and May 2014, which resulted in long closures of production areas due to slow natural detoxification of the shellfish. The second period from the beginning of the 2014–2015 fishing season was dominated by contamination by DSP toxins released by the genus Dinophysis. This period is more characterized by shorter periods of toxicity with higher frequencies in contrast to the ASP contaminations.
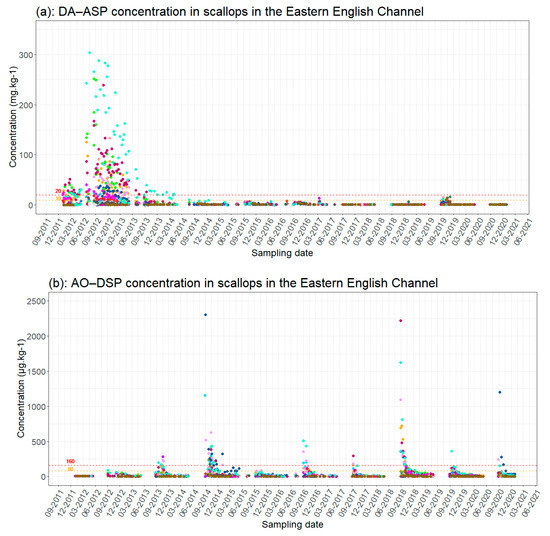
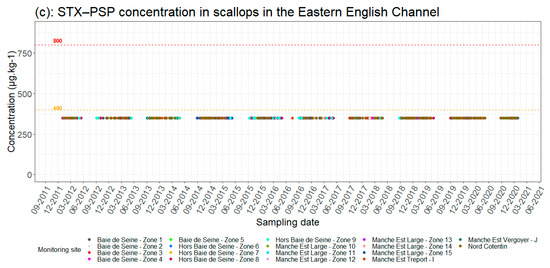
Figure 2.
Changes in phycotoxin concentrations in scallops (a) [DA(Domoic Acid) − ASP, (b) OA(Okadaic Acid) − DSP and (c) SXT (Saxitoxins) − PSP] in scallops in the eastern Channel by production area (see Figure 1). Red dotted line = regulatory toxicity threshold; orange dotted line = half toxicity threshold.
This shift is likely related to the diversity of phytoplanktonic species, and thus toxin production, which varies annually, and the level of cellular toxicity of each species for which environmental conditions, particularly nutrient ratio, play an important role [16]. The composition of the Pseudo-nitzschia phytoplanktonic group in 2012–2013, which is characterized by highly toxic species (the broad-celled species such as P. australis), is different from the composition observed since 2014, which is probably characterized by non-toxic species (in general, the group of fine-celled species). There is a succession of species, both toxic and non-toxic, within the Pseudo-nitzschia group but the dynamics of this diversity remain difficult to explain due to the complex nature of algal blooms and the lack of sufficient data sets [16]. This shift could also be explained by the changes in environmental conditions that seem, on the one hand, not favorable to the development of toxic species of Pseudo-nitzschia or to the production of toxins. On the other hand, these conditions boost the development of the Dinophysis group and the resulting increase in DSP toxicities. Concerning PSP toxins, their concentrations in scallops have never reached the regulatory threshold in the eastern Channel.
The diversity of HAB represents a considerable challenge for the management to adapt over time to the dynamics of environmental conditions and blooms. Figure 3 underlines the dependence of the fishery management system on the changes and dynamics of HAB and their toxicities illustrated by the two examples of production areas 9 and 12. The results show some inconsistencies between the degree of toxicity and administrative decisions. Closures (represented by a pink background color) were observed when some toxicity results were below the regulatory thresholds (red line on Figure 3). Conversely, some toxicity results were not compliant with regulatory thresholds but the zones remained open.
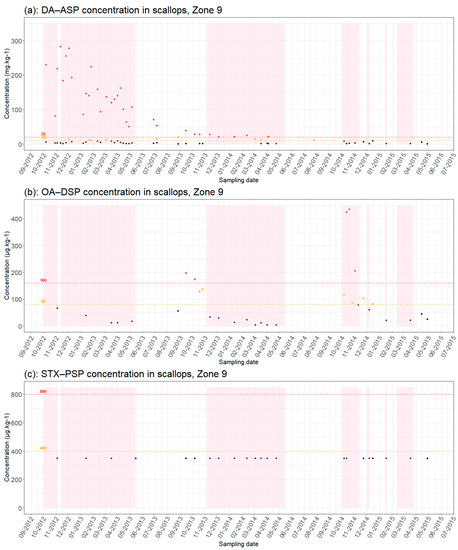
Figure 3.
Results of phycotoxin analyses in scallops (a) [DA(Domoic Acid) − ASP, (b) OA(Okadaic Acid) − DSP and (c) SXT (Saxitoxins) − PSP] from production areas 9 from September 2012 to July 2015: Red dots = results > regulatory thresholds; orange dots = results > half regulatory threshold; black dots = results below regulatory threshold; pink background color = closure periods; red dotted line = regulatory toxicity threshold; orange dotted line = half toxicity threshold.
These results show two scenarios in the management of closures. The first is a situation of “normality” when there is a match between the estimated theoretical closure and the administrative decision. This is often the case when toxin concentrations are not subject to interpretation. Thus, if toxin concentrations are far above regulatory thresholds or persist over time, an area must be closed. This was the case in 2012 and 2013 when episodes of ASP were observed and led to numerous closures in Zone 9 and 12. While toxin concentrations were far below regulatory thresholds, these areas remained open to fishermen.
The second scenario concerns more questionable situations, where the toxicity levels of the analyses are between the regulatory thresholds and the half-thresholds. As mentioned earlier, these half-sets were established with the agreement of the fishery sector to avoid costly batch recalls. The interpretation of each situation leads to a decision in favor of opening or closing the area. This is the case, for example, of these two areas 9 and 12 for the period between June and November 2013. In Figure 3 and Figure 4, red dots are plotted corresponding to analyses of the three toxins that describe a situation of theoretical closure, where concentrations exceed regulatory half-thresholds, but these fishing areas remained open. Conversely, in Area 12, between November and December 2013, the observed toxins concentrations were below regulatory thresholds but the area remained closed. This was also the case for the closures observed in early 2015 in these two areas while concentrations were close to the half threshold but still below the regulatory threshold. The discrepancy between the decision that should have been applied following the toxicity analyses and the final decision to open or close a production zone underlines that decision is, therefore, not always the result of a simple protocol.
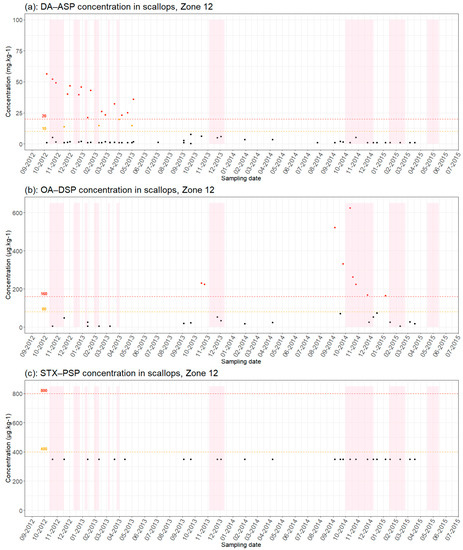
Figure 4.
Results of phycotoxin analyses in scallops (a) [DA(Domoic Acid)−ASP, (b) OA(Okadaic Acid)−DSP and (c) SXT (Saxitoxins)−PSP] from production areas 12 from September 2012 to July 2015: Red dots = results > regulatory thresholds; orange dots = results > half regulatory threshold; black dots = results below regulatory threshold; pink background color = closure periods; red dotted line = regulatory toxicity threshold; orange dotted line = half toxicity threshold.
3.2. Comparison between Theoretical and Observed Closures for the Whole Period and Production Areas
The comparison between the theoretical closures and observed administrative closures was carried out for all the production areas between 2012 and 2019. This comparison again highlighted discrepancies between the durations of the two closures as shown in Figure 5. The results of these analyses provided a complementary vision of management measures in order to better handle economic and social issues associated with HAB.
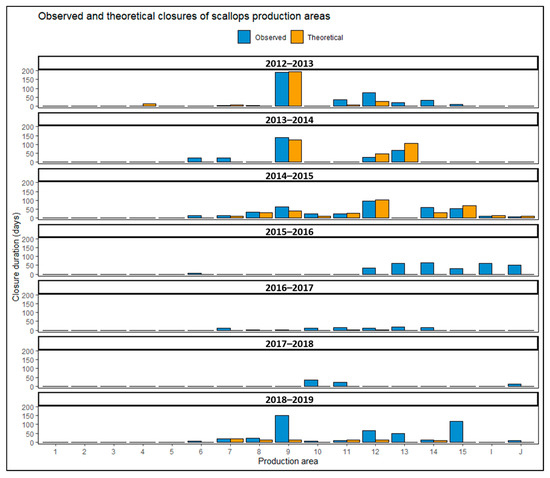
Figure 5.
Comparison of theoretical and observed closures duration in scallop production areas in the eastern Channel.
The retrospective analysis of REPHY-REPHYTOX data and administrative decisions on closures during the period analyzed highlights two cases. The first concerns the underestimation of the duration of theoretical closures compared with observed closures (7% of cases). The production areas can be closed if phycotoxin levels in shellfish are below the regulatory thresholds. As previously explained, these are precautionary closures before the results of the analyses are obtained, or closures at the half-threshold (80 µg/kg for lipophilic toxins (DSP), and between 10 and 16 mg/kg for ASP). Other reasons for the discrepancies between theoretical and observed closures are related to the absence of shellfish samples or the absence of phycotoxin analysis results. These absences are due to difficulties in accessing the sampling areas (e.g., bad weather conditions), problems related to the analysis themselves (transport issues, loss of samples) or disinterest in sampling an area of lower economic importance at some point in the season. A lack of sampling represents 72.34% of the cases of underestimation of theoretical closures compared with the observed closures. Other management strategies including the implementation of a rest periods to protect the scallop stock can also explain the underestimations of theoretical closures. This measure in support of the stock management of scallop was implemented in 2015 based on the results of the IFREMER programs and it consists of identifying an area each year to be closed for the entire season. Therefore, commercial bans due to HAB proved the effectiveness of biological rest and thus facilitated its implementation for resource management objectives. According to the REPHYTOX data, sampling and toxicity analyses are not conducted within these areas, showing that the choice of rest areas is not related to scallop toxicity events.
The second case, which accounts for 93% of the data, involves overestimations of closures by the algorithm, mainly between the 2012–2013 and 2014–2015 seasons. This is due to the fact that access to production areas may be open during toxicity periods despite regulatory thresholds being exceeded. This case only concerns ASP contaminations and this measure is implemented under several conditions. When the concentrations of amnesic toxins are between 20 and 250 mg/kg (total drained flesh), a second analysis is made on the edible parts (muscle and gonad). If the result is less than 4.6 mg/kg, the fishery is authorized for shucking industries, otherwise fishing activities are prohibited. Toxicity tests on the edible parts are only implemented if the fishing companies have an economic interest in the shucking industry; they are, therefore, not carried out systematically. Figure 6 and Figure 7 illustrate the concentrations of ASP in the edible parts of scallops and the number of tests conducted per month from January 2012, respectively. They show that related tests for shucking occurred in the first seasons of the analyzed time series (2011–2012, 2012–2013) and in a few months of the 2013–2014 season, with a significant number of tests in 2012–2013 (between 14 and 32 tests per month). After these periods, the number of tests for shucking dropped and no tests were performed after the 2013–2014 season (two tests for ASP concentration in edible parts of scallops were conducted in 2016 (July and September) but this was outside the fishing season).
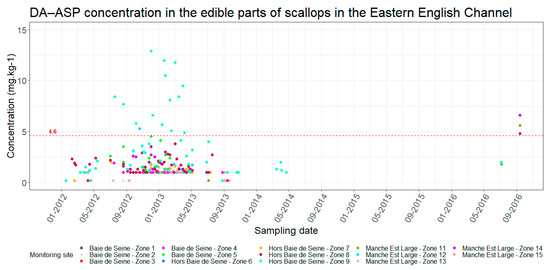
Figure 6.
Evolution of ASP concentrations in edible parts of scallops in the eastern Channel. Red dotted line = regulatory toxicity threshold.
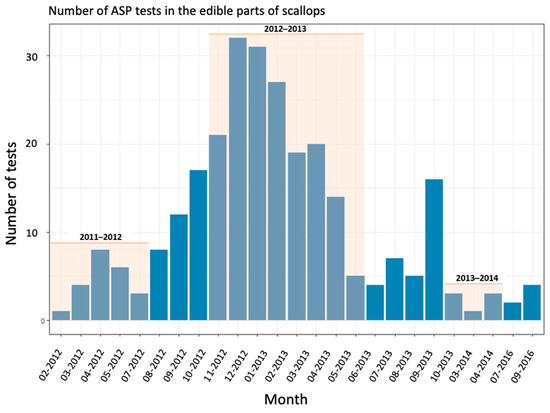
Figure 7.
Evolution of the number of ASP tests per month on edible parts of scallops in the eastern Channel. Pink areas correspond to the scallop fishing seasons.
The absolute error calculated between the theoretical closure values and the observed closures showed that of the total data, 49.58% had results of theoretical closure estimates consistent with the recorded and observed closures. For the rest of the data, 42.86% show discrepancies that were explained by management strategies adapting to the evolution of HAB in order to minimize the associated socio-economic impacts. Some of the differences between the theoretical and observed closures (7.56% of the data) were not explained due to lack of data or errors in the databases used. This comparison approach allowed verification of the quality of the estimates of the theoretical closures by the algorithm and showed that these discrepancies are not related to programming errors but rather to the noise generated by human factors that were not considered in the programming.
4. Discussion
The societal issues resulting from HAB are complex to address due to the difficulties in predicting these events. Much of the literature that deals with HAB mainly focuses on biological and ecological dimensions in order to better understand their dynamics [30,31]. This work often aims at identifying the factors that contribute to these blooms and to quantify the way in which they operate [32]. Moreover, most of the works on socio-economic issues related to HAB are devoted to assessing the financial impacts on the affected sectors [9,10]. Although each of the ecological, economic and social dimensions of these phenomena is in itself highly complex, the uncertainty and non-linear nature of the processes involved in HAB increase the level of difficulty in understanding these blooms and managing their impacts. While HAB issues are mainly addressed by the natural sciences and, to a lesser extent, by economists who seek to assess their economic impacts, few works deal with HAB governance at an operational level.
The results of the analysis in this work show that decision processes regarding HAB are not automatic and are based on the comparisons of observed and allowed toxicity levels. Decisions taken by public authorities are also complex due to their responsibility for economic and health issues. An excessively strict implementation of the rules could lead to increased economic impacts on fisheries. As observed in other fishing areas in Brittany, such as the Bay of Brest, long closure periods have led a majority of the concerned fishing fleet stopping their activity and abandoning the fishery [33]. Similarly, the Administration has a responsibility to protect seafood consumers from health risks. Although the main priority is to protect human health, as explained in the previous chapter, under certain conditions the Administration can implement the regulation in a more flexible way. Fishing areas can be closed or opened to minimize economic impacts when toxicity is between the regulatory threshold and the half-threshold. This flexibility does not increase the risk for consumers. The results of monitoring carried out by the Administration did not show any correlation to specific food poisoning events that could be linked to HAB events. For this reason, the flexibility in decision-making to deal with HAB allows for better consideration of the socio-economic impacts associated with these events, which are not considered by the European health-related regulations.
In a context where control systems remain efficient in protecting consumers, decision-making processes with the involvement of experts allow interpretation of the conditions of each event to anticipate a closure or postpone it if the risk is considered low. In the first case, the anticipation of a closure makes it possible to prevent the sale of contaminated batches just before the realization of an analysis revealing a HAB contamination.
While decision-makers are doing the best they can with the tools at their disposal, there is still room for progress in achieving the objectives of governance. This is because there are several gaps that need to be addressed in future work. The departments of the Administration in charge of managing fisheries and those in charge of managing HAB impacts are not the same. Those in charge of HAB issues have only a global vision of the sector. Without the integration of these two institutional frameworks, fisheries and health, the initiatives undertaken by decision-makers to minimize the economic impacts are only based on the optimization of the monitoring networks. They do not take into account the complexity and heterogeneity of the fishing sector, i.e., the diversity of spatial and temporal production strategies of the companies, nor their vulnerability to risk, sensitivity or coping capacities. Agreements for self-regulation, carried out at the initiative of the fishermen, to accelerate the reopening of a fishing area if the analyses demonstrate the absence of toxicity before the official tests, also reinforces the measures in favor of reducing economic impacts. Moreover, considering the absence of reliable HAB prediction models, governance of HAB impacts occurs within a framework of action in crisis. Consequently, management procedures are based on reacting to impacts rather than avoiding and anticipating them. There are no active collective plans about how to adapt to HAB; instead, all decisions are adapted to each event.
The discrepancies between the theoretical and observed closures reveal the usefulness of this work. Moreover, this approach also demonstrates the dynamics of the HAB risk management strategies in adapting to the evolution of the blooms and the fishery social ecological systems. The subjectivity inherent in the decision-making processes with respect to the “objective” application of the rules results from the analysis of the conditions of each event by the Administration and experts and leads to a specific decision that is not simple on all occasions. As a result, socio-economic risk analysis based on observed closures (e.g. [34]) may be less suitable for these specific contexts such as scallop fisheries. The use of theoretical closures, as proposed in this work, refers to the consideration of a notion of risk based on toxicity, which is a pertinent proxy to assess ecological risks and socio-economic impacts associated with HAB.
5. Conclusions
This paper analyzed the dynamic of decision-making processes to address the challenges faced by social-ecological systems affected by HAB. In particular, the analyses conducted, using the example of the scallop fishery in the eastern English Channel, highlighted the ways administrations in charge of managing HAB deal with the occurrences, the toxicities and the socio-economic impacts associated with these events.
The methodology developed in this work, based on the analysis of the management processes and the mechanics of the implementation of measures, has made it possible to better understand the issues and constraints of this governance, which are poorly addressed in the literature. Such a methodology is useful to assess more precisely the impacts associated with this phenomenon, but also to explore the ways to improve management systems through the identification of issues and, therefore, societal needs. The authors also highlight the need for decision makers to build adaptive governance approaches. The current European regulations are based on simple protocols built on automatic actions to be undertaken. This regulation addresses only health issues. However, the growing socio-economic stakes in coastal and marine areas lead the authorities in charge of HAB management to build adaptive mechanisms in response to the dynamics of these events in order to minimize their impacts.
In a way, this functioning of HAB management in France is relatively efficient since the combined objectives of protecting seafood consumers and the reduction of socio-economic impacts are achieved overall. However, as HAB are dynamic, the Administration and governance processes must also constantly adapt to maintain their effectiveness. The assessment of ecological risks and their translation into socio-economic risks is one of the main challenges on which the scientific community should work in order to provide the necessary knowledge to tackle the future challenges of HAB, and particularly to build anticipatory responses such as emergency plans rather than adaptive action after a crisis.
Author Contributions
Conceptualization J.A.P.A.; Methodology J.A.P.A.; Formal Analysis J.A.P.A.; Writing. J.A.P.A.; Data Curation S.C.; Methodology S.C.; Formal Analysis S.C.; Writing S.C. and P.R.; Conceptualization P.R.; Methodology P.R.; Editing P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been carried out through the S3-EUROHAB project (Sentinel-3 products for detecting EUtROphication and Harmful Algal Bloom events) and funded by the European Regional Development Fund through the INTERREG France-Channel-England (Project Number 106).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data on theoretical closures are available in https://doi.org/10.5281/zenodo.6320765 (accessed on 6 July 2022) and data on observed closures were collected from the DIRM-MEMN Administration.
Acknowledgments
The authors express their gratitude firstly to the S3-EUROHAB project for the financial support in this work and secondly to the people who were consulted to better understand the socio-economic stakes associated with HABs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zingone, A.; Enevoldsen, H.O. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HAB). Ocean. Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masó, M.; Garcés, E. Harmful microalgae blooms (HAB); problematic and conditions that induce them. Mar. Pollut. Bull. 2006, 53, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.; Lewandowska, A.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment—Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef]
- Moore, K.M.; Allison, E.H.; Dreyer, S.J.; Ekstrom, J.A.; Jardine, S.L.; Klinger, T.; Moore, S.K.; Norman, K.C. Harmful Algal Blooms: Identifying Effective Adaptive Actions Used in Fishery-Dependent Communities in Response to a Protracted Event. Front. Mar. Sci. 2020, 6, 803. [Google Scholar] [CrossRef]
- Anderson, L.E.; Plummer, M.L. Recreational Demand for Shellfish Harvesting Under Environmental Closures. Mar. Resour. Econ. 2017, 32, 43–57. [Google Scholar] [CrossRef]
- Larkin, S.L.; Adams, C.M. Harmful Algal Blooms and Coastal Business: Economic Consequences in Florida. Soc. Nat. Resour. 2007, 20, 849–859. [Google Scholar] [CrossRef]
- Dyson, K.; Huppert, D.D. Regional economic impacts of razor clam beach closures due to harmful algal blooms (HABs) on the Pacific coast of Washington. Harmful Algae 2010, 9, 264–271. [Google Scholar] [CrossRef]
- Hoagland, P.; Anderson, D.M.; Kaoru, Y.; White, A.W. The economic effects of harmful algal blooms in the United States: Estimates, assessment issues, and information needs. Estuaries 2002, 25, 819–837. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal Bloom and Its Economic Impact. EUR 27905 EN. 2016. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiBwKvQrOn4AhWpx4UKHaTmB-EQFnoECAQQAQ&url=https%3A%2F%2Fpublications.jrc.ec.europa.eu%2Frepository%2Fbitstream%2FJRC101253%2Flbna27905enn.pdf&usg=AOvVaw1-xJu5q68VR-LLV1Mb2Wai (accessed on 6 July 2022).
- Bernard, S.; Kudela, R.M.; Velo-Suarez, L. Developing global capabilities for the observation and prediction of harmful algal blooms. In Oceans and Society: Blue Planet; Djavidnia, S., Cheung, V., Ott, M., Seeyave, S., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2014. [Google Scholar]
- Heil, C.A.; Steidinger, K.A. Monitoring, management, and mitigation of Karenia blooms in the eastern Gulf of Mexico. Harmful Algae 2009, 8, 611–617. [Google Scholar] [CrossRef]
- Anderson, D.M.; Andersen, P.; Bricelj, V.M.; Cullen, J.J.; Rensel, J.J. Monitoring and Management Strategies for Harmful Algal Blooms in Coastal Waters; Unesco: Paris, France, 2001; p. 268. [Google Scholar]
- Pérez Agúndez, J.A.; Raux, P.; Girard, S.; Mongruel, R. Technological Adaptation to Harmful Algal Blooms: Socioeconomic Consequences for the Shellfish Farming Sector in Bourgneuf Bay (France). Aquac. Econ. Manag. 2013, 17, 341–359. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, P.; Fu, F.; Tennenbaum, N.; Tatters, A.O.; Hutchins, D.A. Understanding the blob bloom: Warming increases toxicity and abundance of the harmful bloom diatom Pseudo-nitzschia in California coastal waters. Harmful Algae 2017, 67, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Thorel, M.; Claquin, P.; Schapira, M.; Le Gendre, R.; Riou, P.; Goux, D.; Le Roy, B.; Raimbault, V.; Deton-Cabanillas, A.-F.; Bazin, P.; et al. Nutrient ratios influence variability in Pseudo-nitzschia species diversity and particulate domoic acid production in the Bay of Seine (France). Harmful Algae 2017, 68, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.-V.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Kim, H.G. Mitigation and controls of HAB. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- European Union. Regulation (EC) No 853/2004 of the European parliament and of the council of 29 April 2004 laying down specific hygiene rules for food of animal origin. J. Eur. Union 2004, 139, 55–205. [Google Scholar]
- European Union. Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organization of official controls on products of animal origin intended for human consumption. J. Eur. Union L 2004, 226, 83–127. [Google Scholar]
- Chenouf, S. Caractérisation des Impacts des Efflorescences Algales Nuisibles en Manche. Application au cas de la Pêcherie de Coquilles Saint-Jacques de la Manche Est; Sciences du Vivant [q-bio]; HAL Open Science: Lyon, France, 2018; p. 67. [Google Scholar]
- Belin, C.; Soudant, D. Trente Années D’observation des Micro-Algues et des Toxines D’algues Sur le Littoral; Quae: Versailles, France, 2018; p. 258. [Google Scholar]
- Carpentier, A.; Coppin, F.; Curet, L.; Dauvin, J.-C.; Delavenne, J.; Dewarumez, J.-M.; Dupuis, L.; Foveau, A.; Garcia, C.; Gardel, L.; et al. Atlas des Habitats des Ressources Marines de la Manche Orientale—CHARM II, Channel Habitat Atlas for marine Resource Management—CHARM II. 2009. Available online: https://archimer.ifremer.fr/doc/00000/7377/ (accessed on 2 February 2022).
- Dauvin, J.C. The English Channel: La Manche. In World Seas: An Environmental Evaluation; Academic Press: Cambridge, MA, USA, 2019; pp. 153–188. [Google Scholar]
- Husson, B.; Hernández-Fariñas, T.; Le Gendre, R.; Schapira, M.; Chapelle, A. Two decades of Pseudo-nitzschia spp. blooms and king scallop (Pecten maximus) contamination by domoic acid along the French Atlantic and English Channel coasts: Seasonal dynamics, spatial heterogeneity and interannual variability. Harmful Algae 2016, 51, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Napoléon, C.; Fiant, L.; Raimbault, V.; Riou, P.; Claquin, P. Dynamics of phytoplankton diversity structure and primary productivity in the English Channel. Mar. Ecol. Prog. Ser. 2014, 505, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, A.; Dezécache, C. Trajectories of Changes in Phytoplankton Biomass, Phaeocystis globosa and Diatom (incl. Pseudo-nitzschia sp.) Abundances Related to Nutrient Pressures in the Eastern English Channel, Southern North Sea. J. Mar. Sci. Eng. 2020, 8, 401. [Google Scholar] [CrossRef]
- Chenouf, S.; Merzereaud, M.; Pérez Agúndez, J.; Raux, P. Determination of scallop production areas closures due to phycotoxins along the Eastern English Channel using data from REPHY and REPHYTOX monitoring networks. SEANOE 2020. [Google Scholar] [CrossRef]
- Chenouf, S.; Merzereaud, M. R code to simulate closures of scallops fishing areas due to harmful algal blooms (Theoretical-closures). Zenodo 2022. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Harmful algal blooms: A global overview. In Manual on Harmful Marine Microalgae; Unesco: Paris, France, 2003; Volume 33, pp. 1–22. [Google Scholar]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms (HAB): Paradigm shifts and new technologies for research, monitoring and management. Annu. Rev. Mar. Sci. 2012, 4, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, D.; Xie, G.; Tian, J.; Tseng, K.-H.; Shum, C.K.; Lee, J.; Liang, S. Spatiotemporal variability and environmental factors of harmful algal blooms (HABs) over western Lake Erie. PLoS ONE 2017, 12, e0179622. [Google Scholar] [CrossRef] [PubMed]
- Ragueneau, O.; Ragueneau, O.; Raimonet, M.; Raimonet, M.; Mazé, C.; Mazé, C.; Coston-Guarini, J.; Coston-Guarini, J.; Chauvaud, L.; Chauvaud, L.; et al. The Impossible Sustainability of the Bay of Brest? Fifty Years of Ecosystem Changes, Interdisciplinary Knowledge Construction and Key Questions at the Science-Policy-Community Interface. Front. Mar. Sci. 2018, 5, 124. [Google Scholar] [CrossRef] [Green Version]
- Guillotreau, P.; Le Bihan, V.; Morineau, B.; Pardo, S. The vulnerability of shellfish farmers to HAB events: An optimal matching analysis of closure decrees. Harmful Algae 2021, 101, 101968. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).