Geochemical Analysis of Cretaceous Shales from the Hazara Basin, Pakistan: Provenance Signatures and Paleo-Weathering Conditions
Abstract
1. Introduction
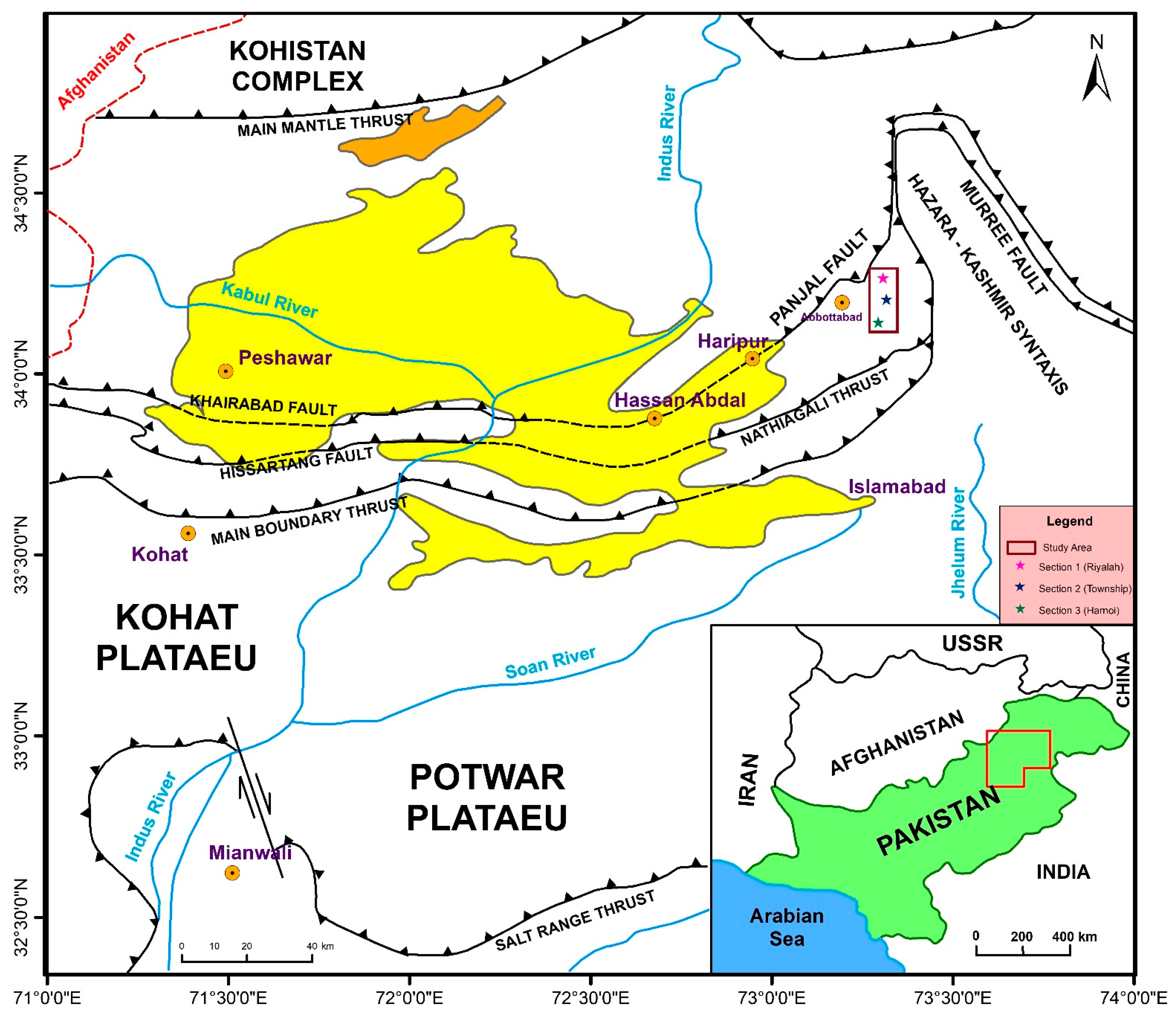
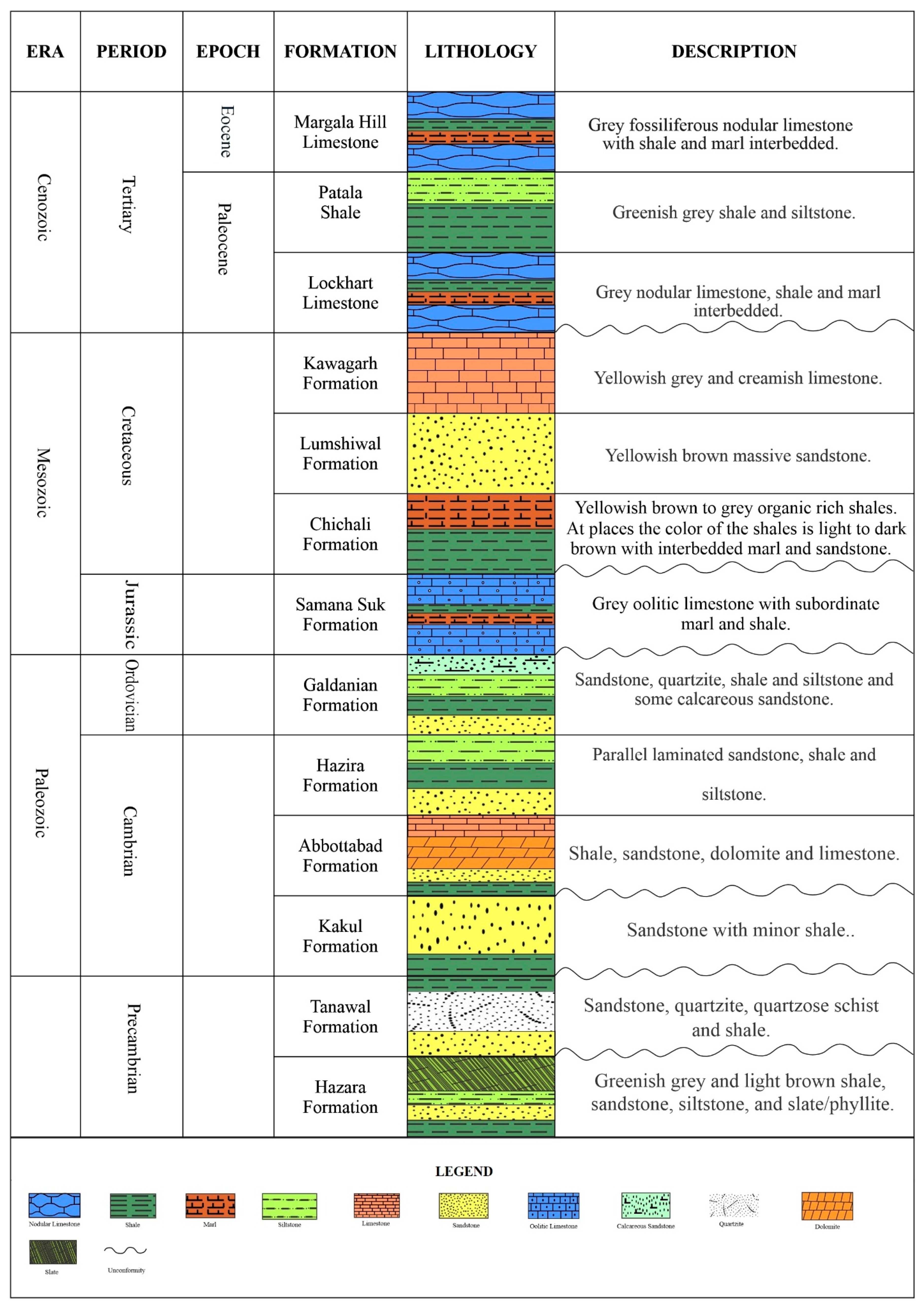
2. Geological Setting and Stratigraphy
3. Material and Methods
4. Results
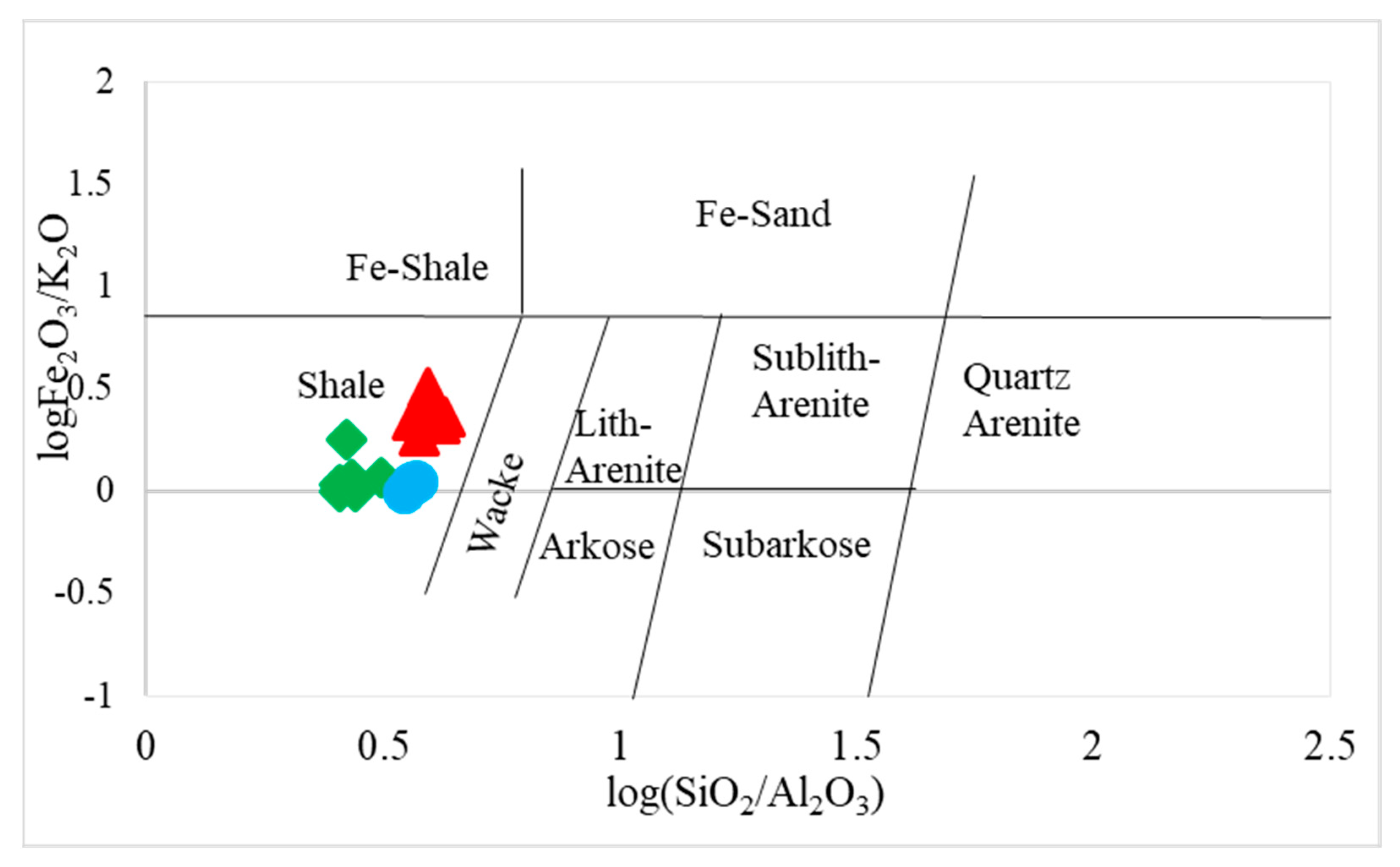
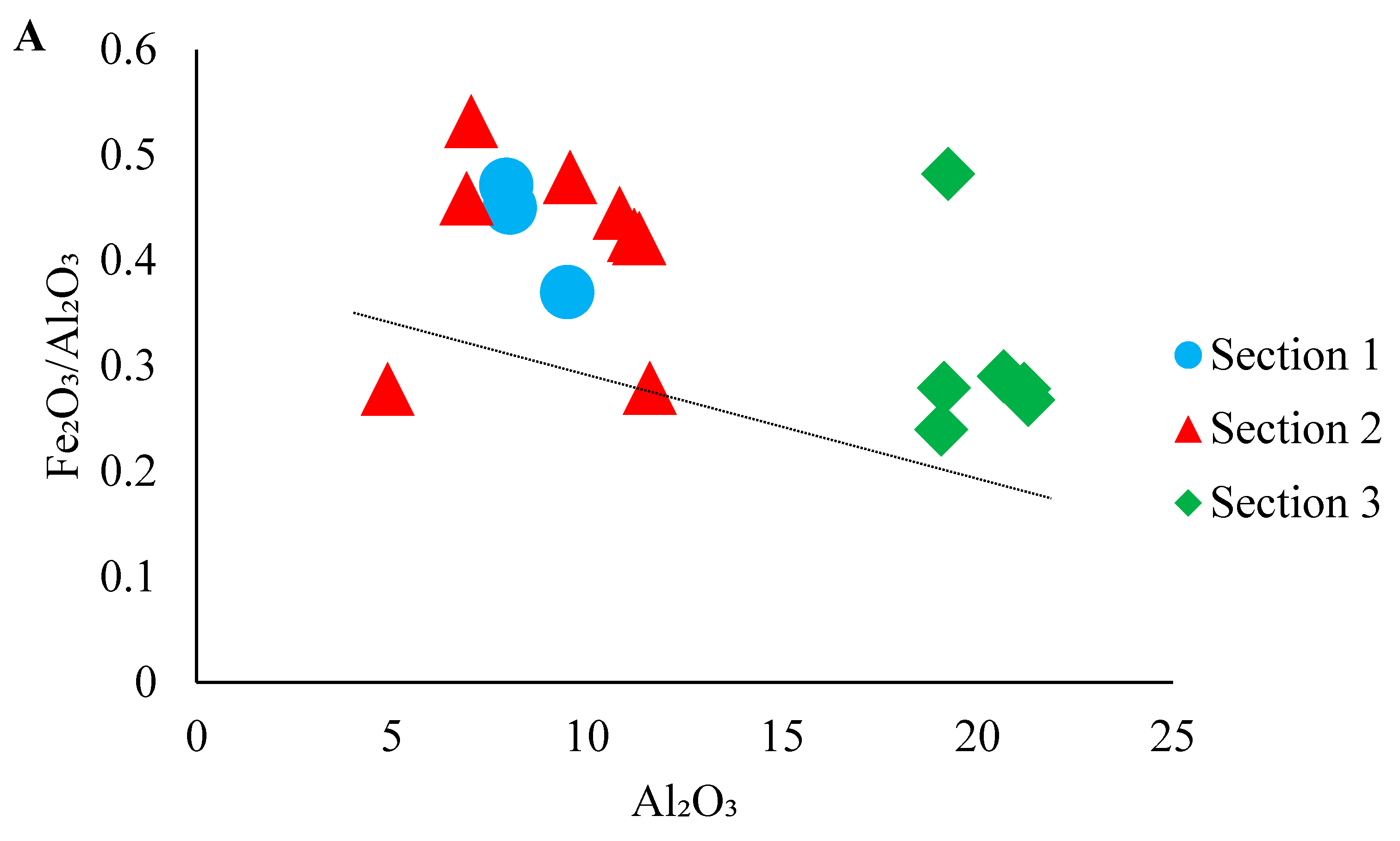
4.1. Major and Trace Elements
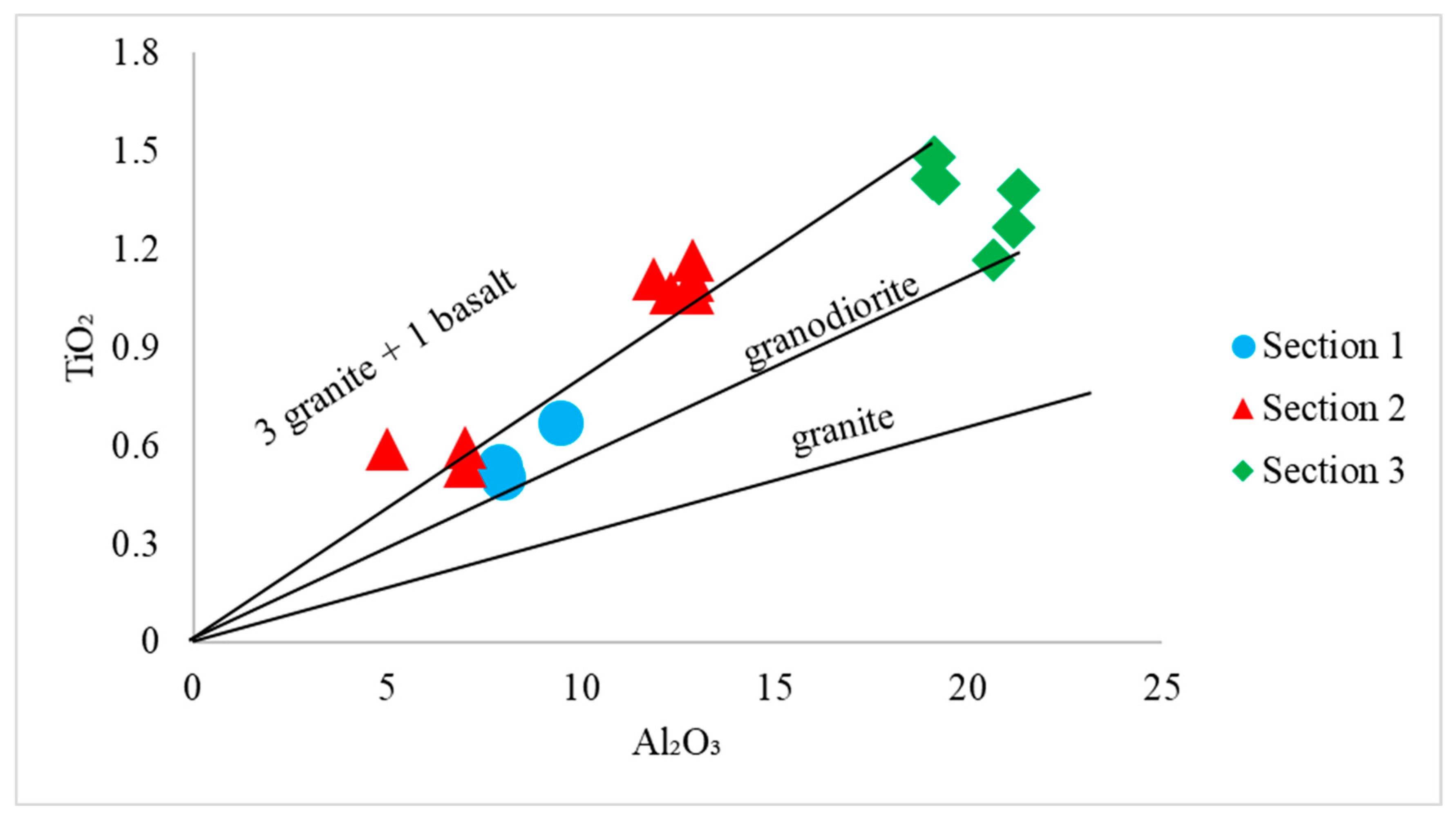
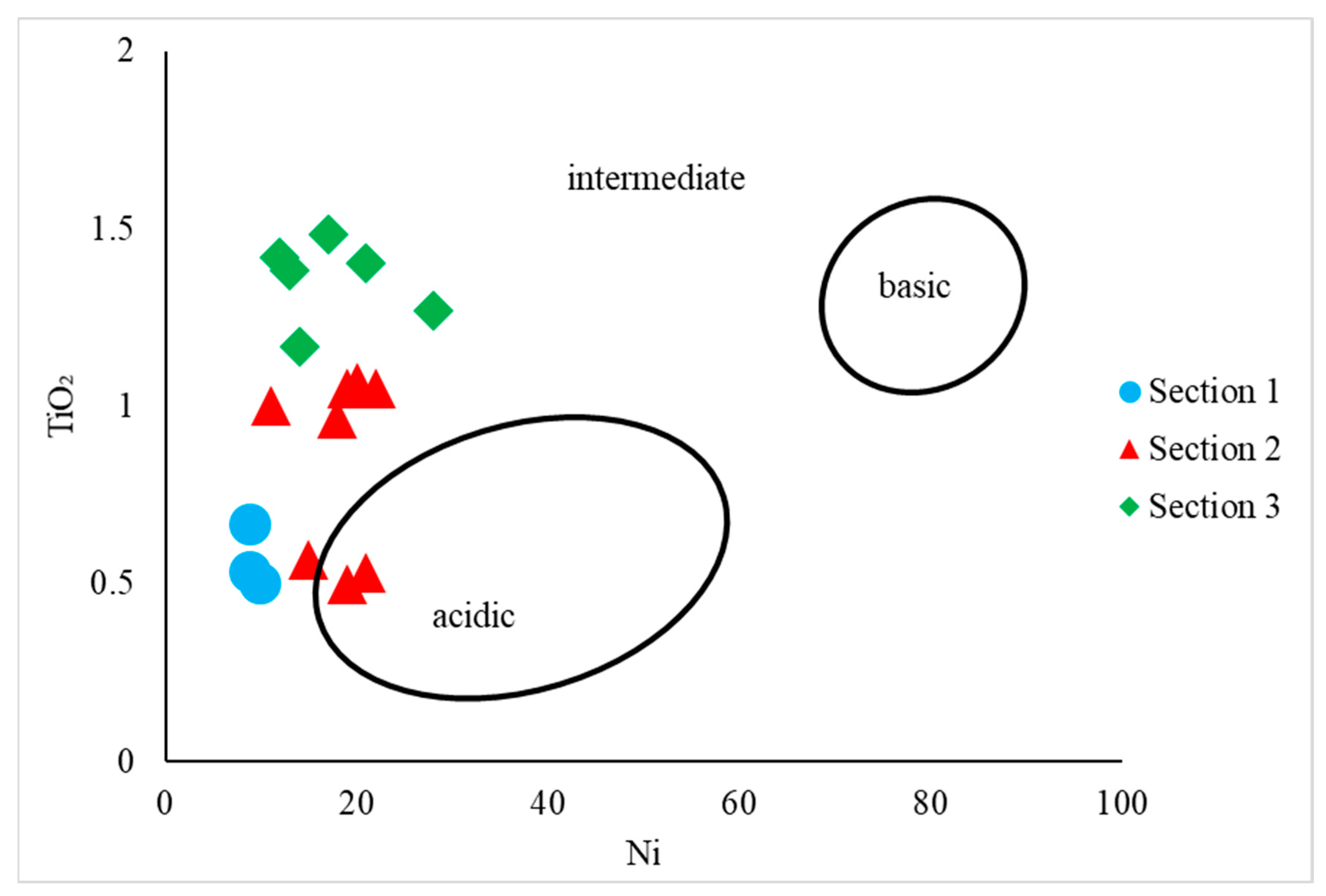
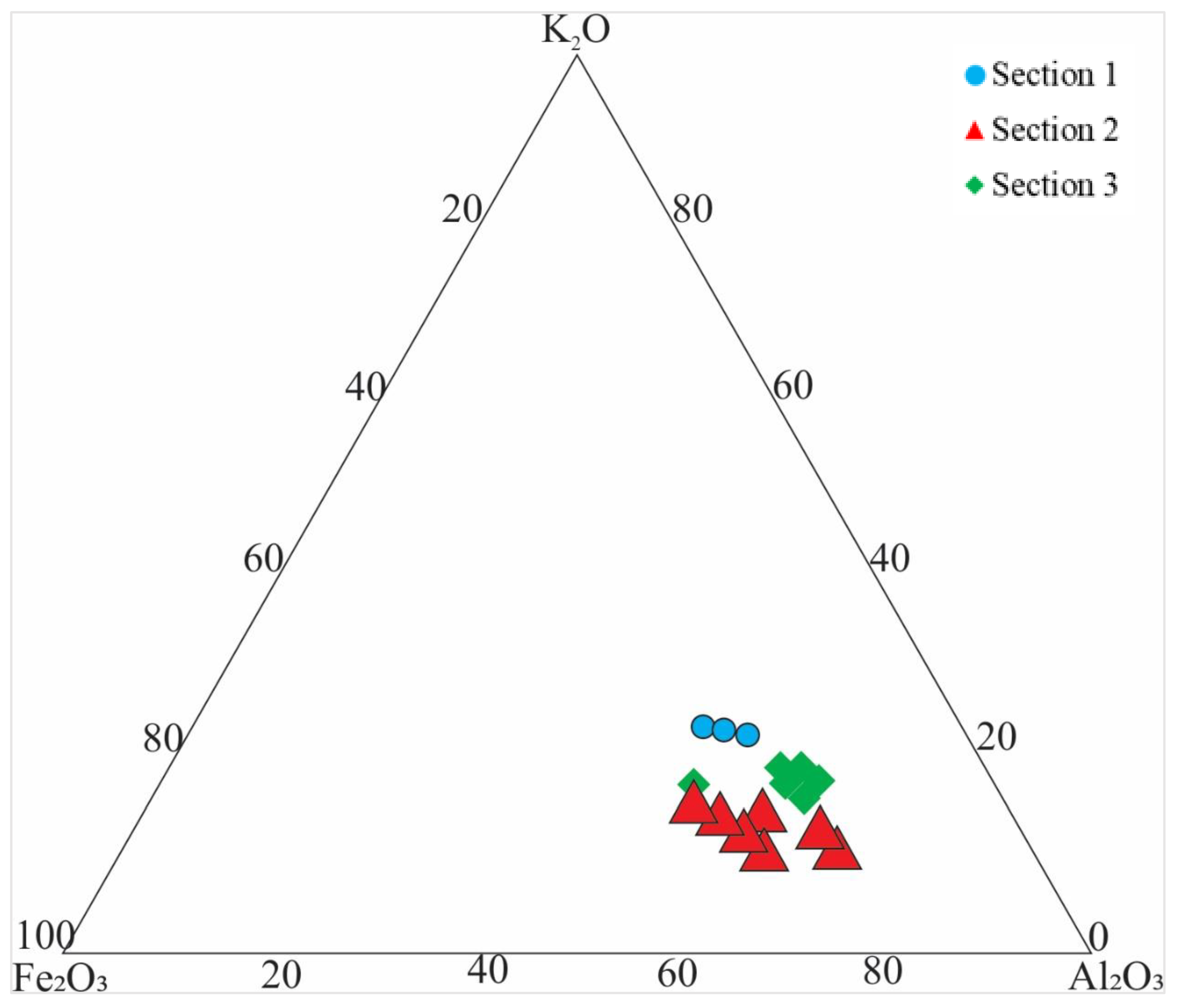
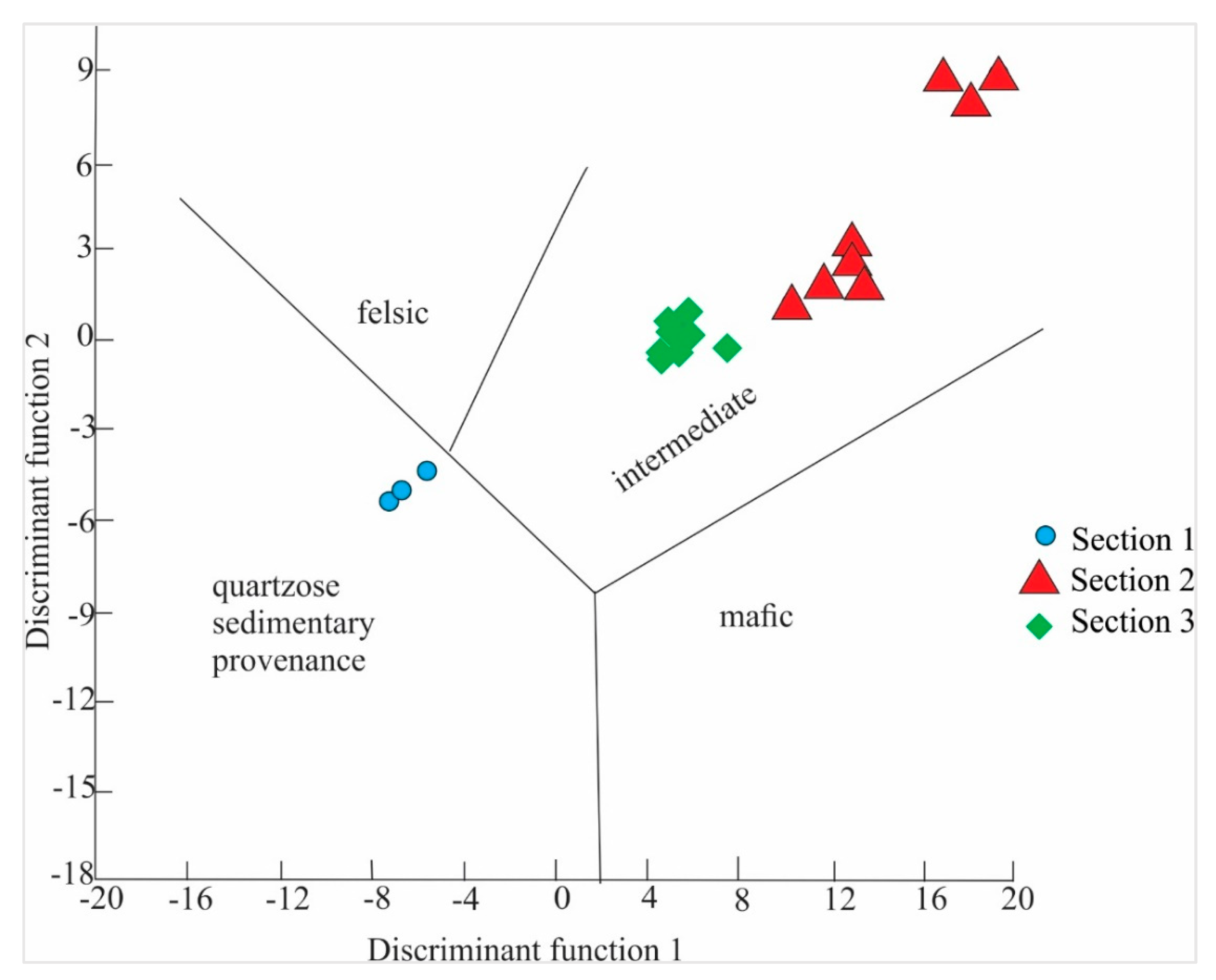
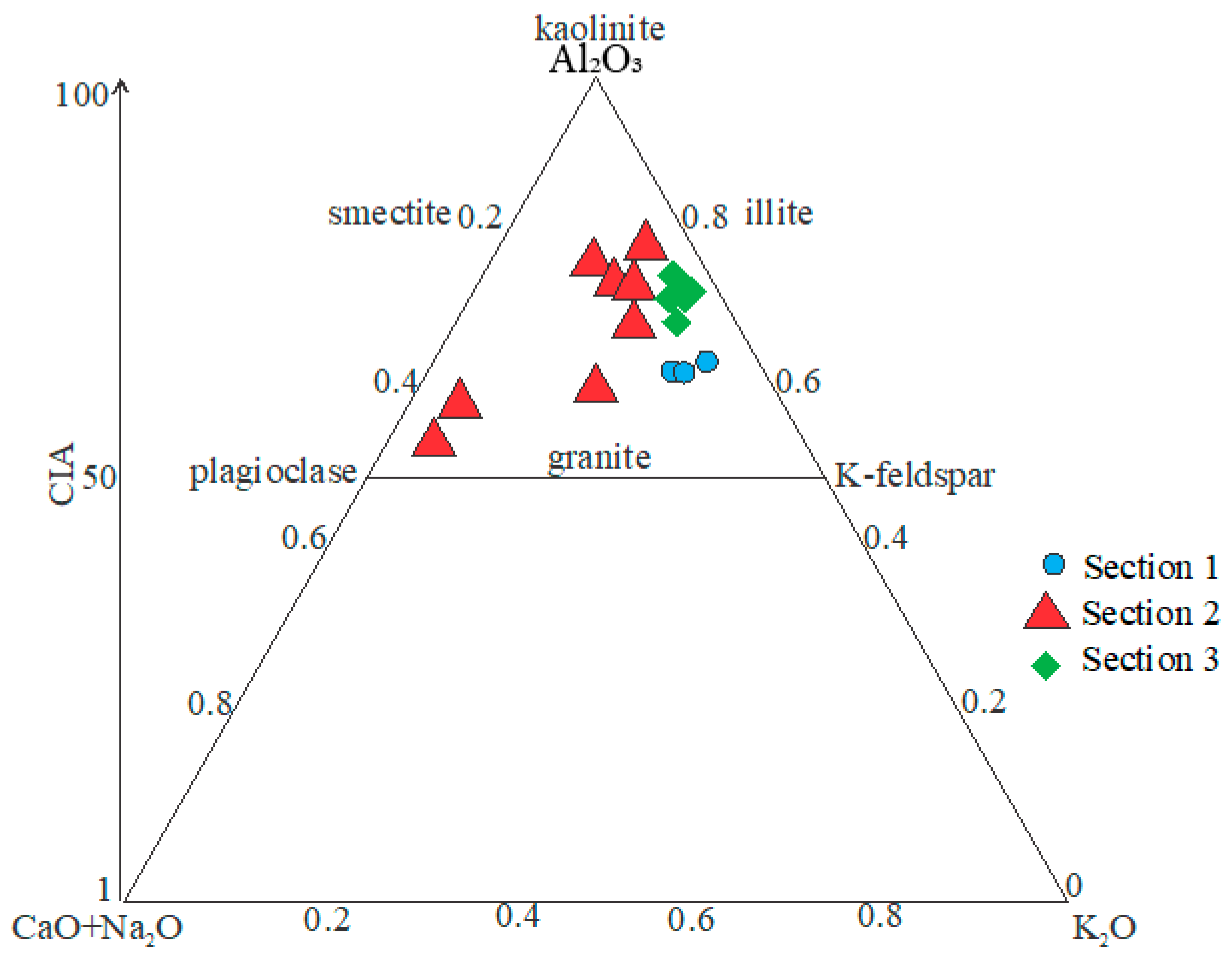
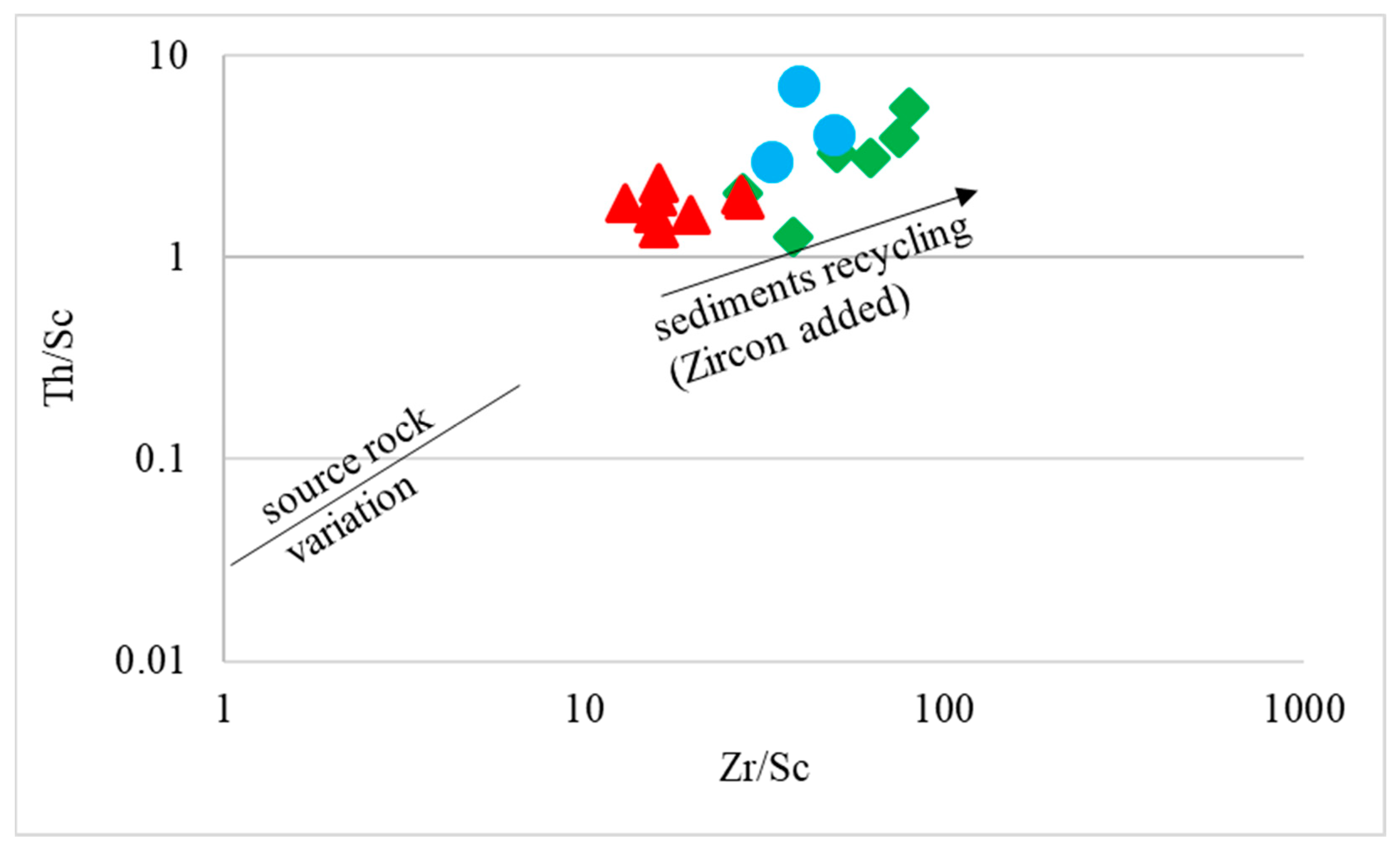
4.2. Provenance and Source Rock Potential
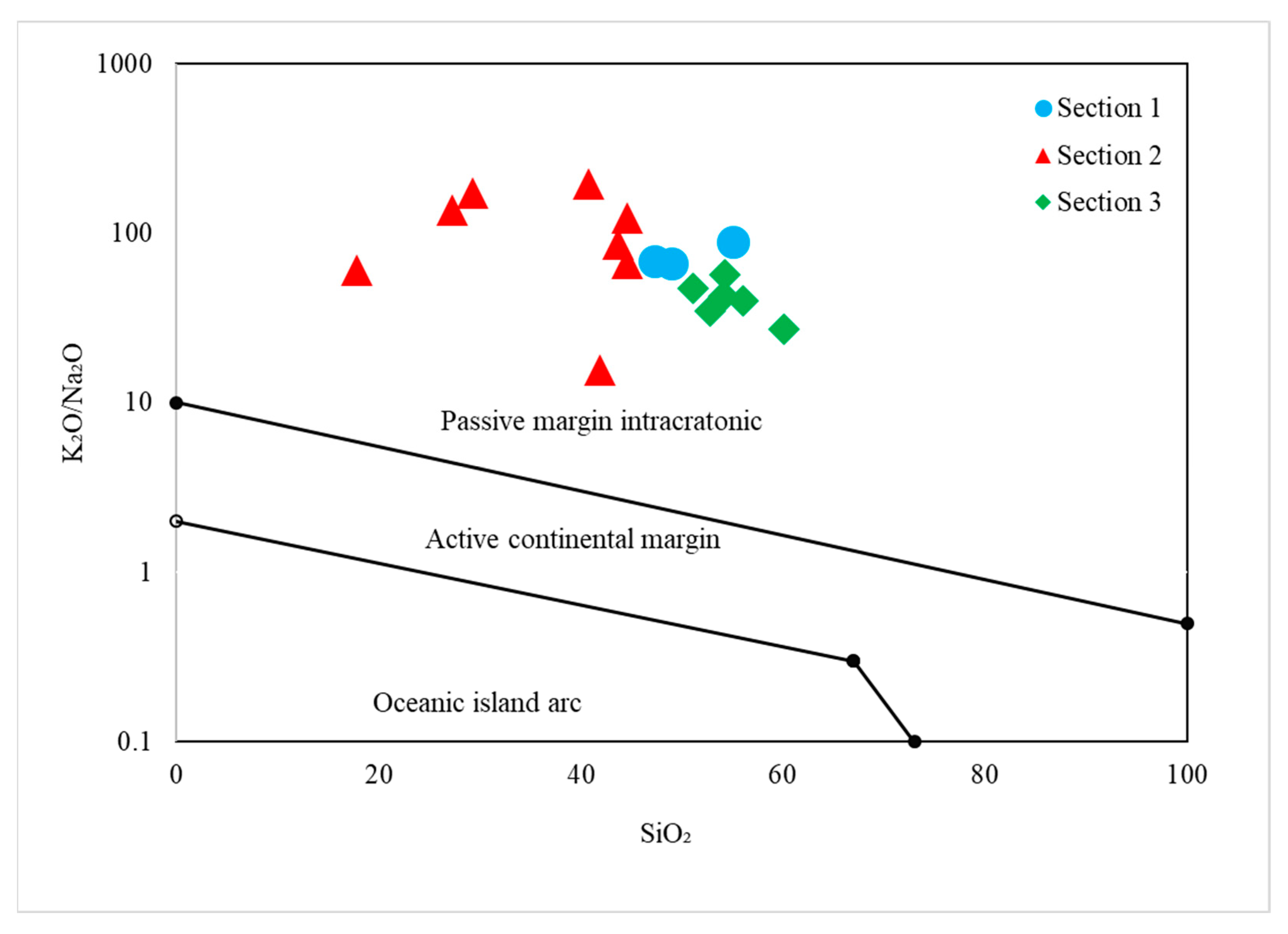
4.3. Tectonic Setting
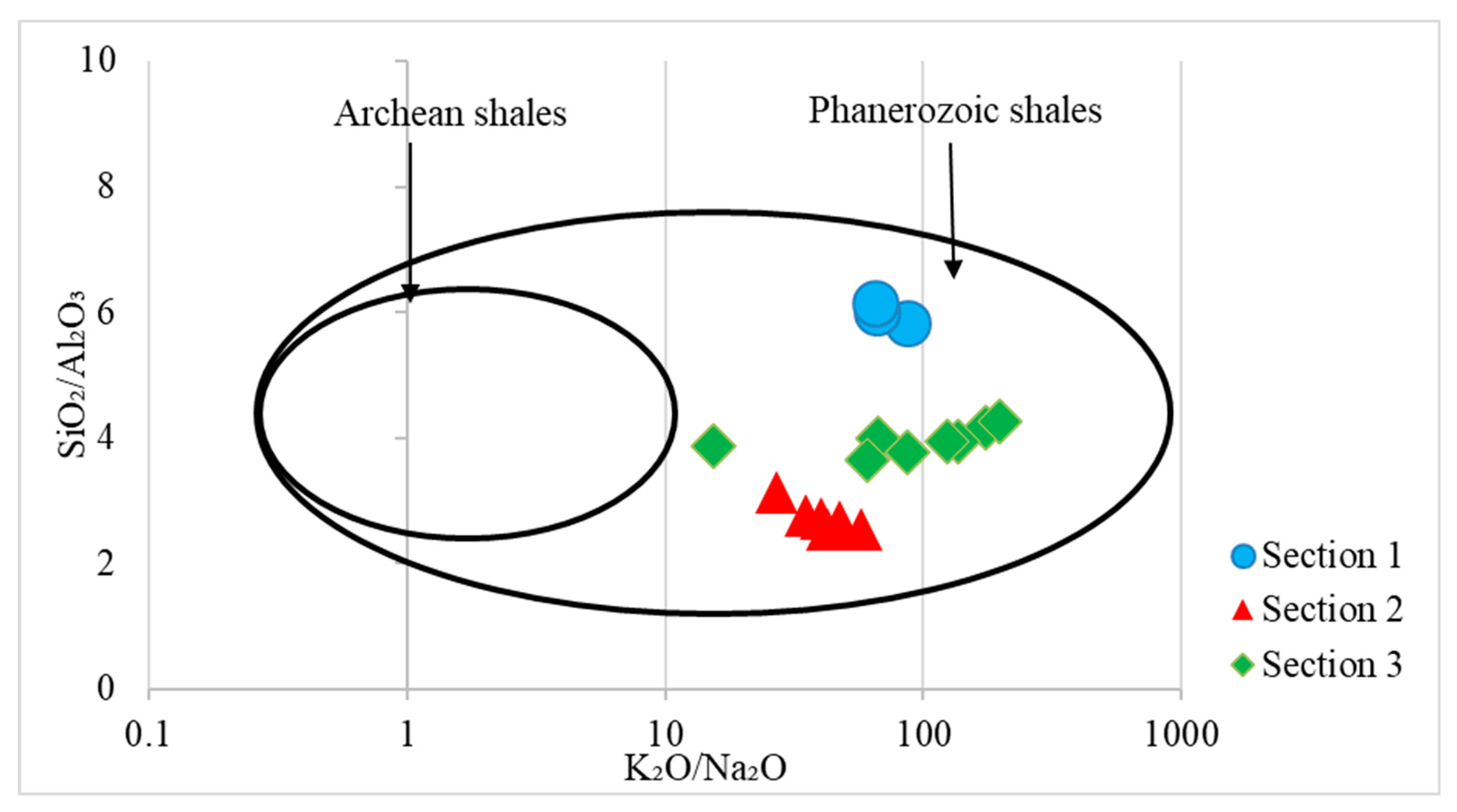
4.4. Paleo-Weathering in the Source Area
4.5. Geochemical Analysis of Chichali Formation
4.6. Statistical Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umar, M.; Sabir, M.A.; Farooq, M.; Khan, M.M.S.S.; Faridullah, F.; Jadoon, U.K.; Khan, A.S. Stratigraphic and sedimentological attributes in Hazara Basin Lesser Himalaya, North Pakistan: Their role in deciphering minerals potential. Arab. J. Geosci. 2015, 8, 1653–1667. [Google Scholar] [CrossRef]
- Ali, S.K.; Janjuhah, H.T.; Shahzad, S.M.; Kontakiotis, G.; Saleem, M.H.; Khan, U.; Zarkogiannis, S.D.; Makri, P.; Antonarakou, A. Depositional Sedimentary Facies, Stratigraphic Control, Paleoecological Constraints, and Paleogeographic Reconstruction of Late Permian Chhidru Formation (Western Salt Range, Pakistan). J. Mar. Sci. Eng. 2021, 9, 1372. [Google Scholar] [CrossRef]
- Abbasi, I.A.; Haneef, M.; Obaid, S.; Daud, F.; Qureshi, A.W. Mesozoic deltaic system along the western margin of the Indian plate: Lithofacies and depositional setting of Datta Formation, North Pakistan. Arab. J. Geosci. 2012, 5, 471–480. [Google Scholar] [CrossRef]
- Janjuhah, H.T.; Ishfaque, M.; Mehmood, M.I.; Kontakiotis, G.; Shahzad, S.M.; Zarkogiannis, S.D. Integrated Underground Mining Hazard Assessment, Management, Environmental Monitoring, and Policy Control in Pakistan. Sustainability 2021, 13, 13505. [Google Scholar] [CrossRef]
- Qasim, M.; Khan, M.A.; Haneef, M. Stratigraphic characterization of the Early Cambrian Abbottabad Formation in the Sherwan area, Hazara region, N. Pakistan: Implications for Early Paleozoic stratigraphic correlation in NW Himalayas, Pakistan. J. Himal. Earth Sci. 2014, 47, 25. [Google Scholar]
- Ahsan, N. Facies modeling, depositional and diagenetic environments of Kawagarh Formation, Hazara Basin, Pakistan. Doctoral Dissertation, College of Earth and Environmental Sciences, Punjab University, Lahore, Pakistan, 2007. [Google Scholar]
- Hasterok, D.; Gard, M.; Webb, J. On the radiogenic heat production of metamorphic, igneous, and sedimentary rocks. Geosci. Front. 2018, 9, 1777–1794. [Google Scholar] [CrossRef]
- Armstrong-Altrin, J.S.; Lee, Y.I.; Verma, S.P.; Ramasamy, S. Geochemistry of Sandstones from the Upper Miocene Kudankulam Formation, Southern India: Implications for Provenance, Weathering, and Tectonic Setting. J. Sediment. Res. 2004, 74, 285–297. [Google Scholar] [CrossRef]
- Armstrong-Altrin, J.S.; Lee, Y.I.; Kasper-Zubillaga, J.J.; Carranza-Edwards, A.; Garcia, D.; Eby, G.N.; Balaram, V.; Cruz-Ortiz, N.L. Geochemistry of beach sands along the western Gulf of Mexico, Mexico: Implication for provenance. Geochemistry 2012, 72, 345–362. [Google Scholar] [CrossRef]
- Zaid, S.M. Integrated petrographic, mineralogical, and geochemical study of the Late Cretaceous–Early Tertiary Dakhla Shales, Quseir–Nile Valley Province, central Egypt: Implications for source area weathering, provenance, and tectonic setting. Arab. J. Geosci. 2015, 8, 9237–9259. [Google Scholar] [CrossRef]
- Martini, A.M.; Walter, L.M.; Ku, T.C.W.; Budai, J.M.; McIntosh, J.C.; Schoell, M. Microbial production and modification of gases in sedimentary basins: A geochemical case study from a Devonian shale gas play, Michigan basin. AAPG Bull. 2003, 87, 1355–1375. [Google Scholar] [CrossRef]
- Lin, N.H.; Guo, Y.; Wai, S.N.; Tamehe, L.S.; Wu, Z.; Naing, N.M.; Zhang, J. Sedimentology and geochemistry of Middle Eocene-Lower Oligocene sandstones from the western Salin Sub-Basin, the Central Myanmar Basin: Implications for provenance, source area weathering, paleo-oxidation and paleo-tectonic setting. J. Southeast. Asian Earth Sci. 2019, 173, 314–335. [Google Scholar] [CrossRef]
- Ding, L.; Qasim, M.; Jadoon, I.A.; Khan, M.A.; Xu, Q.; Cai, F.; Wang, H.; Baral, U.; Yue, Y. The India–Asia collision in north Pakistan: Insight from the U–Pb detrital zircon provenance of Cenozoic foreland basin. Earth Planet. Sci. Lett. 2016, 455, 49–61. [Google Scholar] [CrossRef]
- Jan, I.U.; Iqbal, S.; Davies, S.J.; Zalasiewicz, J.A.; Stephenson, M.H.; Wagreich, M.; Haneef, M.; Hanif, M.; Ahmad, S. A Periglacial Palaeoenvionment in the Upper Carboniferous-Lower Permian Tobra Formation of the Salt Range, Pakistan. Acta Geol. Sin. Engl. Ed. 2017, 91, 1063–1078. [Google Scholar] [CrossRef]
- Khan, U.; Janjuhah, H.T.; Kontakiotis, G.; Rehman, A.; Zarkogiannis, S.D. Natural Processes and Anthropogenic Activity in the Indus River Sedimentary Environment in Pakistan: A Critical Review. J. Mar. Sci. Eng. 2021, 9, 1109. [Google Scholar] [CrossRef]
- Ishfaque, M.; Dai, Q.; Haq, N.U.; Jadoon, K.; Shahzad, S.M.; Janjuhah, H.T. Use of Recurrent Neural Network with Long Short-Term Memory for Seepage Prediction at Tarbela Dam, KP, Pakistan. Energies 2022, 15, 3123. [Google Scholar] [CrossRef]
- Malkani, M.S. Stratigraphy, mineral potential, geological history and paleobiogeography of Balochistan Province, Pakistan. Sindh Univ. Res. J. -SURJ (Sci. Ser.) 2015, 43. Available online: https://sujo-old.usindh.edu.pk/index.php/SURJ/article/view/1383 (accessed on 26 April 2022).
- Garzanti, E.; Liang, W.; Andò, S.; Clift, P.D.; Resentini, A.; Vermeesch, P.; Vezzoli, G. Provenance of Thal Desert sand: Focused erosion in the western Himalayan syntaxis and foreland-basin deposition driven by latest Quaternary climate change. EarthSci. Rev. 2020, 207, 103220. [Google Scholar] [CrossRef]
- Malkani, M.S.; Mahmood, Z. Revised stratigraphy of Pakistan. Geol. Surv. Pak. Rec. 2016, 127, 1–87. [Google Scholar]
- Malkani, M.S.; Mahmood, Z. Stratigraphy of Pakistan. Geological Survey of Pakistan. Memoir 2017, 24, 1–134. [Google Scholar]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Cox, R.; Lowe, D.R.; Cullers, R. The influence of sediment recycling and basement composition on evolution of mudrock chemistry in the southwestern United States. Geochim. Cosmochim. Acta 1995, 59, 2919–2940. [Google Scholar] [CrossRef]
- Besly, B.; Burley, S.D.; Turner, P. The late Carboniferous ‘Barren Red Bed’ play of the Silver Pit area, Southern North Sea. In Geological Society, London, Petroleum Geology Conference Series; Geological Society of London: London, UK, 1993; Volume 4, pp. 727–740. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.-W.; Tsang, D.C.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.; Ok, Y.S.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Hossain, I.; Roy, K.K.; Biswas, P.K.; Alam, M.; Moniruzzaman, M.; Deeba, F. Geochemical characteristics of Holocene sediments from Chuadanga district, Bangladesh: Implications for weathering, climate, redox conditions, provenance and tectonic setting. Chin. J. Geochem. 2014, 33, 336–350. [Google Scholar] [CrossRef]
- Cox, P.; Wood, R.; Dickson, J.; Al Rougha, H.; Shebl, H.; Corbett, P. Dynamics of cementation in response to oil charge: Evidence from a Cretaceous carbonate field, U.A.E. Sediment. Geol. 2010, 228, 246–254. [Google Scholar] [CrossRef]
- Deru, X.; Xuexiang, G.; Pengchun, L.; Guanghao, C.; Bin, X.; Bachlinski, R.; Zhuanli, H.; Gonggu, F. Mesoproterozoic–Neoproterozoic transition: Geochemistry, provenance and tectonic setting of clastic sedimentary rocks on the SE margin of the Yangtze Block, South China. J. Southeast. Asian Earth Sci. 2007, 29, 637–650. [Google Scholar] [CrossRef]
- Paikaray, S.; Banerjee, S.; Mukherji, S. Geochemistry of shales from the Paleoproterozoic to Neoproterozoic Vindhyan Supergroup: Implications on provenance, tectonics and paleoweathering. J. Southeast. Asian Earth Sci. 2008, 32, 34–48. [Google Scholar] [CrossRef]
- Howarth, R.J. Sources for a history of the ternary diagram. Br. J. Hist. Sci. 1996, 3, 337–356. [Google Scholar] [CrossRef]
- Nagarajan, R.; Madhavaraju, J.; Nagendra, R.; Armstrong-Altrin, J.S.; Moutte, J. Geochemistry of Neoproterozoic shales of the Rabanpalli Formation, Bhima Basin, Northern Karnataka, southern India: Implications for provenance and paleoredox conditions. Rev. Mex. De Cienc. Geológicas 2007, 24, 150–160. [Google Scholar]
- Cullers, R.L.; Barrett, T.; Carlson, R.; Robinson, B. Rare-earth element and mineralogic changes in Holocene soil and stream sediment: A case study in the Wet Mountains, Colorado, U.S.A. Chem. Geol. 1987, 63, 275–297. [Google Scholar] [CrossRef]
- Adriano, D. Trace Elements in Terrestrial Ecosystems; Springer: New York, NY, USA, 1986. [Google Scholar]
- Cullers, R.L. The controls on the major and trace element variation of shales, siltstones, and sandstones of Pennsylvanian-Permian age from uplifted continental blocks in Colorado to platform sediment in Kansas, USA. Geochim. Cosmochim. Acta 1994, 58, 4955–4972. [Google Scholar] [CrossRef]
- Eqani, S.A.M.A.S.; Kanwal, A.; Bhowmik, A.; Sohail, M.; Ullah, R.; Ali, S.M.; Alamdar, A.; Ali, N.; Fasola, M.; Shen, H. Spatial distribution of dust–bound trace elements in Pakistan and their implications for human exposure. Environ. Pollut. 2016, 213, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Ding, L.; Khan, M.A.; Jadoon, I.A.K.; Haneef, M.; Baral, U.; Cai, F.; Wang, H.; Yue, Y. Tectonic Implications of Detrital Zircon Ages From Lesser Himalayan Mesozoic-Cenozoic Strata, Pakistan. Geochem. Geophys. Geosystems 2018, 19, 1636–1659. [Google Scholar] [CrossRef]
- Roser, B.; Korsch, R. Provenance signatures of sandstone-mudstone suites determined using discriminant function analysis of major-element data. Chem. Geol. 1988, 67, 119–139. [Google Scholar] [CrossRef]
- Keskin, Ş. Geochemistry of Çamardı Formation sediments, central Anatolia (Turkey): Implication of source area weathering, provenance, and tectonic setting. Geosci. J. 2011, 15, 185–195. [Google Scholar] [CrossRef]
- Agrawal, S.; Guevara, M.; Verma, S.P. Tectonic Discrimination of Basic and Ultrabasic Volcanic Rocks through Log-Transformed Ratios of Immobile Trace Elements. Int. Geol. Rev. 2008, 50, 1057–1079. [Google Scholar] [CrossRef]
- Verma, S.P.; Pandarinath, K.; Verma, S.K.; Agrawal, S. Fifteen new discriminant-function-based multi-dimensional robust diagrams for acid rocks and their application to Precambrian rocks. Lithos 2013, 168, 113–123. [Google Scholar] [CrossRef]
- Bhatia, M.R. Plate Tectonics and Geochemical Composition of Sandstones. J. Geol. 1983, 91, 611–627. [Google Scholar] [CrossRef]
- Yan, D.; Chen, D.; Wang, Q.; Wang, J. Large-scale climatic fluctuations in the latest Ordovician on the Yangtze block, South China. Geology 2010, 38, 599–602. [Google Scholar] [CrossRef]
- Visser, J.N.; Young, G.M. Major element geochemistry and paleoclimatology of the Permo-Carboniferous glacigene Dwyka Formation and postglacial mudrocks in southern Africa. Palaeogeogr. Palaeoclim. Palaeoecol. 1990, 81, 49–57. [Google Scholar] [CrossRef]
- Singh, P.K.; Khan, M.S. Geochemistry of Palaeoproterozoic Rocks of Aravalli Supergroup: Implications for Weathering History and Depositional Sequence. Int. J. Geosci. 2017, 8, 1278–1299. [Google Scholar] [CrossRef][Green Version]
- Herron, M.M. Geochemical Classification of Terrigenous Sands and Shales from Core or Log Data. J. Sediment. Res. 1988, 58, 820–829. [Google Scholar] [CrossRef]
- McLennan, S.; Taylor, S.; Eriksson, K. Geochemistry of Archean shales from the Pilbara Supergroup, Western Australia. Geochim. Cosmochim. Acta 1983, 47, 1211–1222. [Google Scholar] [CrossRef]
- Das, B.K.; Haake, B.-G. Geochemistry of Rewalsar Lake sediment, Lesser Himalaya, India: Implications for source-area weathering, provenance and tectonic setting. Geosci. J. 2003, 7, 299–312. [Google Scholar] [CrossRef]
- Tulyaganov, D.; Agathopoulos, S.; Kansal, I.; Valério, P.; Ribeiro, M.; Ferreira, J. Synthesis and properties of lithium disilicate glass-ceramics in the system SiO2–Al2O3–K2O–Li2O. Ceram. Int. 2009, 35, 3013–3019. [Google Scholar] [CrossRef]
- Guagliardi, I.; Cicchella, D.; De Rosa, R. A Geostatistical Approach to Assess Concentration and Spatial Distribution of Heavy Metals in Urban Soils. Water Air Soil Pollut. 2012, 223, 5983–5998. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Pak, E. National Standards for Drinking Water Quality; Pakistan Environmental Protection Agency, Ministry of Environment, Government of Pakistan: Isiamabad, Pakistan, 2008. [Google Scholar]
- Podkovyrov, V.N.; Grazhdankin, D.V.; Maslov, A.V. Lithogeochemistry of the Vendian fine-grained clastic rocks in the southern Vychegda trough. Lithol. Miner. Resour. 2011, 46, 427–446. [Google Scholar] [CrossRef]
- Banerjee, S.; Bansal, U.; Thorat, A.V. A review on palaeogeographic implications and temporal variation in glaucony composition. J. Palaeogeogr. 2016, 5, 43–71. [Google Scholar] [CrossRef]
- Jonell, T.N.; Owen, L.A.; Carter, A.; Schwenniger, J.-L.; Clift, P.D. Quantifying episodic erosion and transient storage on the western margin of the Tibetan Plateau, upper Indus River. Quat. Res. 2017, 89, 281–306. [Google Scholar] [CrossRef]
- Krishna, J. Applicability of the Sequence Framework in IEAP and GTM, with Brief Comments on the Hydrocarbon Prospects in the Indian Basins. In The Indian Mesozoic Chronicle; Springer: Singapore, 2017; pp. 367–459. [Google Scholar] [CrossRef]
- Gakkhar, R.A.; Bechte, A.; Gratzer, R. Source-rock potential and origin of hydrocarbons in the Cretaceous and Jurassic sediments of the Punjab Platform (Indus Basin, Pakistan). Pak. J. Hydrocarb. Res. 2011, 21, 1–17. [Google Scholar]
- Ghose, N.C.; Chatterjee, N.; Windley, B.F. Subaqueous early eruptive phase of the late Aptian Rajmahal volcanism, India: Evidence from volcaniclastic rocks, bentonite, black shales, and oolite. Geosci. Front. 2017, 8, 809–822. [Google Scholar] [CrossRef]
- Shen, T.; Wang, G.; Leloup, P.H.; van der Beek, P.; Bernet, M.; Cao, K.; Wang, A.; Liu, C.; Zhang, K. Controls on Cenozoic exhumation of the Tethyan Himalaya from fission-track thermochronology and detrital zircon U-Pb geochronology in the Gyirong basin area, southern Tibet. Tectonics 2016, 35, 1713–1734. [Google Scholar] [CrossRef]
- Naeem, M.; Burg, J.-P.; Ahmad, N.; Chaudhry, M.N.; Khalid, P. U-Pb zircon systematics of the Mansehra Granitic Complex: Implications on the early Paleozoic orogenesis in NW Himalaya of Pakistan. Geosci. J. 2016, 20, 427–447. [Google Scholar] [CrossRef]
- Fulekar, M.H.; Jadia, C.D. Phytoremediation: The application of vermicompost to remove zinc, cadmium, copper, nickel and lead by sunflower plant. Environ. Eng. Manag. J. 2008, 7, 547–558. [Google Scholar] [CrossRef]
- Dadar, M.; Adel, M.; Saravi, H.N.; Fakhri, Y. Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev. 2017, 36, 222–227. [Google Scholar] [CrossRef]
- Sarapää, O.; Lauri, L.S.; Ahtola, T.; Al-Ani, T.; Grönholm, S.; Kärkkäinen, N.; Lintinen, P.; Torppa, A.; Turunen, P. Discovery Potential of Hi-Tech Metals and Critical Minerals in Finland; Geological Survey of Finland: Espoo, Finland, 2015. [Google Scholar]
- Karasakal, A. Determination of Trace and Major Elements in Vegan Milk and Oils by ICP-OES after Microwave Digestion. Biol. Trace Element Res. 2020, 197, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: Cambridge, MA, USA, 1979. [Google Scholar]
- de Caritat, P.; Reimann, C.; Team, N.P.; Team, G.P. Comparing results from two continental geochemical surveys to world soil composition and deriving Predicted Empirical Global Soil (PEGS2) reference values. Earth Planet. Sci. Lett. 2012, 319, 269–276. [Google Scholar] [CrossRef]
- Cassanego, M.B.B.; Goldoni, A.; Heldt, F.H.; Osório, D.M.; Windisch, P.G.; Droste, A. Germination and sporophytic development of Regnellidium diphyllum Lindm. (Marsileaceae) in the presence of copper. Acta Bot. Bras. 2013, 27, 26–30. [Google Scholar] [CrossRef][Green Version]
- Pandey, G.; Madhuri, S. Heavy metals causing toxicity in animals and fishes. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Sathawara, N.G.; Parikh, D.J.; Agarwal, Y.K. Essential Heavy Metals in Environmental Samples from Western India. Bull. Environ. Contam. Toxicol. 2004, 73, 756–761. [Google Scholar] [CrossRef]
- Massa, F.; Monory, K. Endocannabinoids and the gastrointestinal tract. J. Endocrinol. Investig. 2006, 29, 47–57. [Google Scholar]
- Christison, T.T.; Rohrer, J.S. Direct determination of free cyanide in drinking water by ion chromatography with pulsed amperometric detection. J. Chromatogr. A 2007, 1155, 31–39. [Google Scholar] [CrossRef] [PubMed]




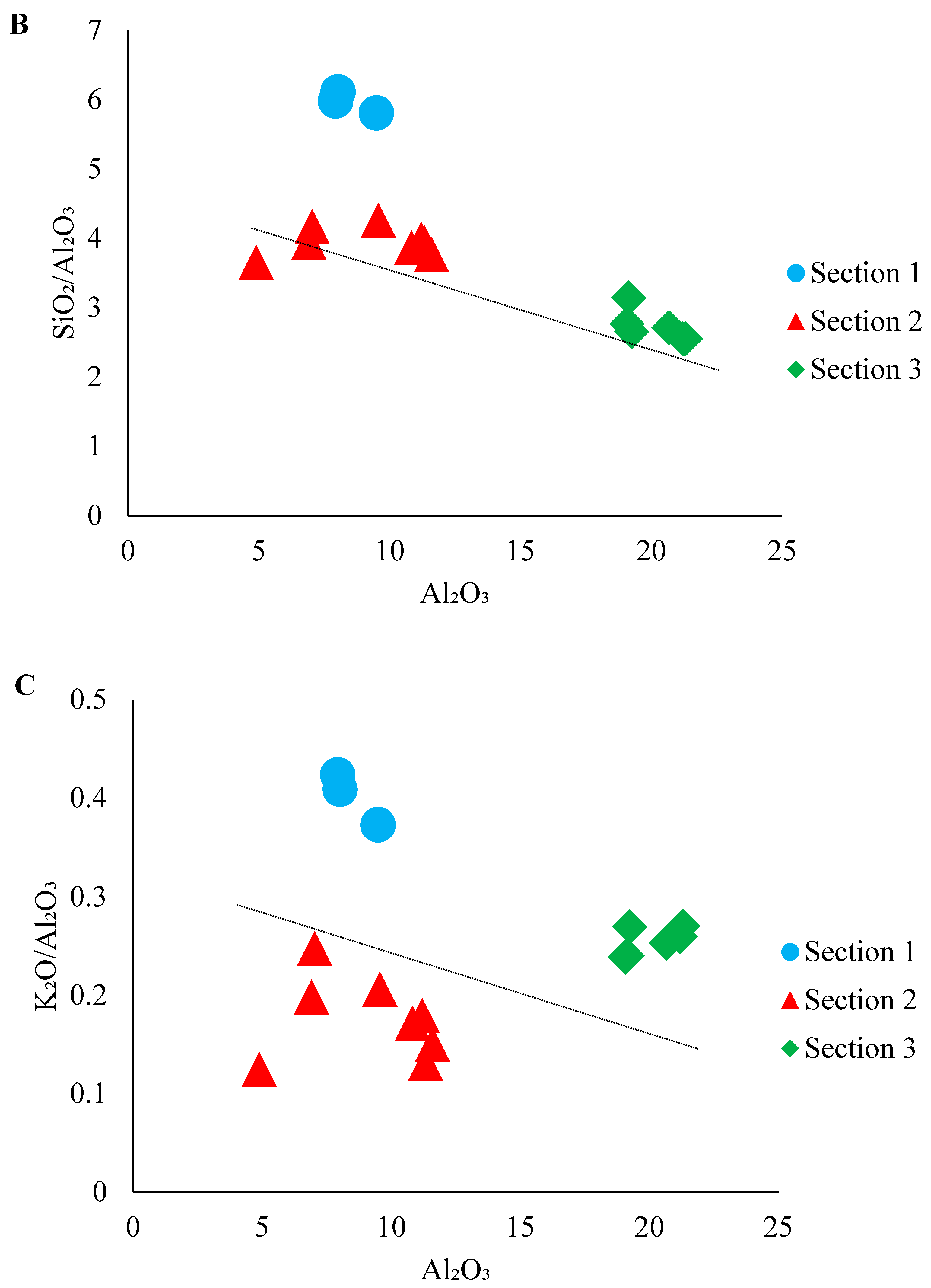
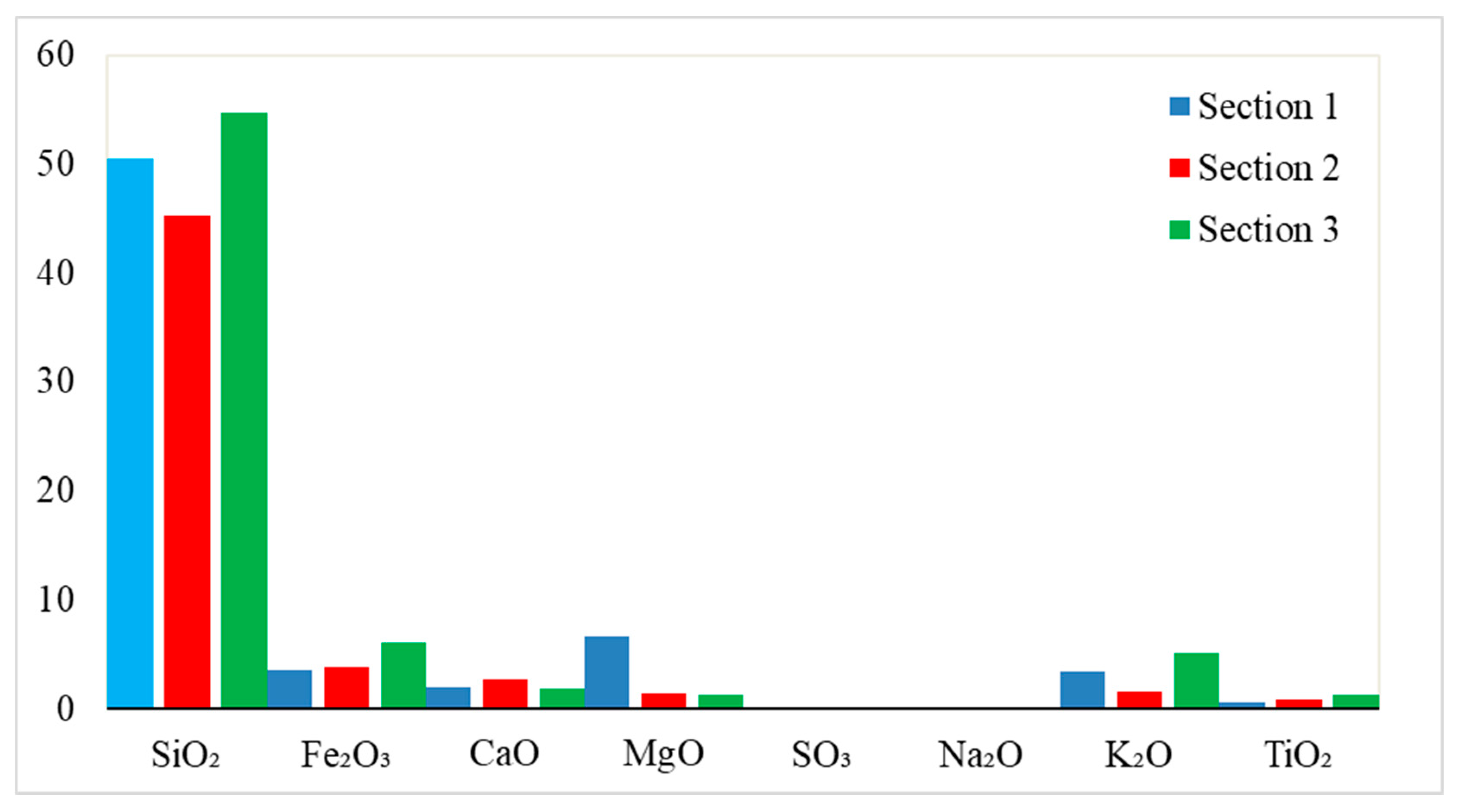
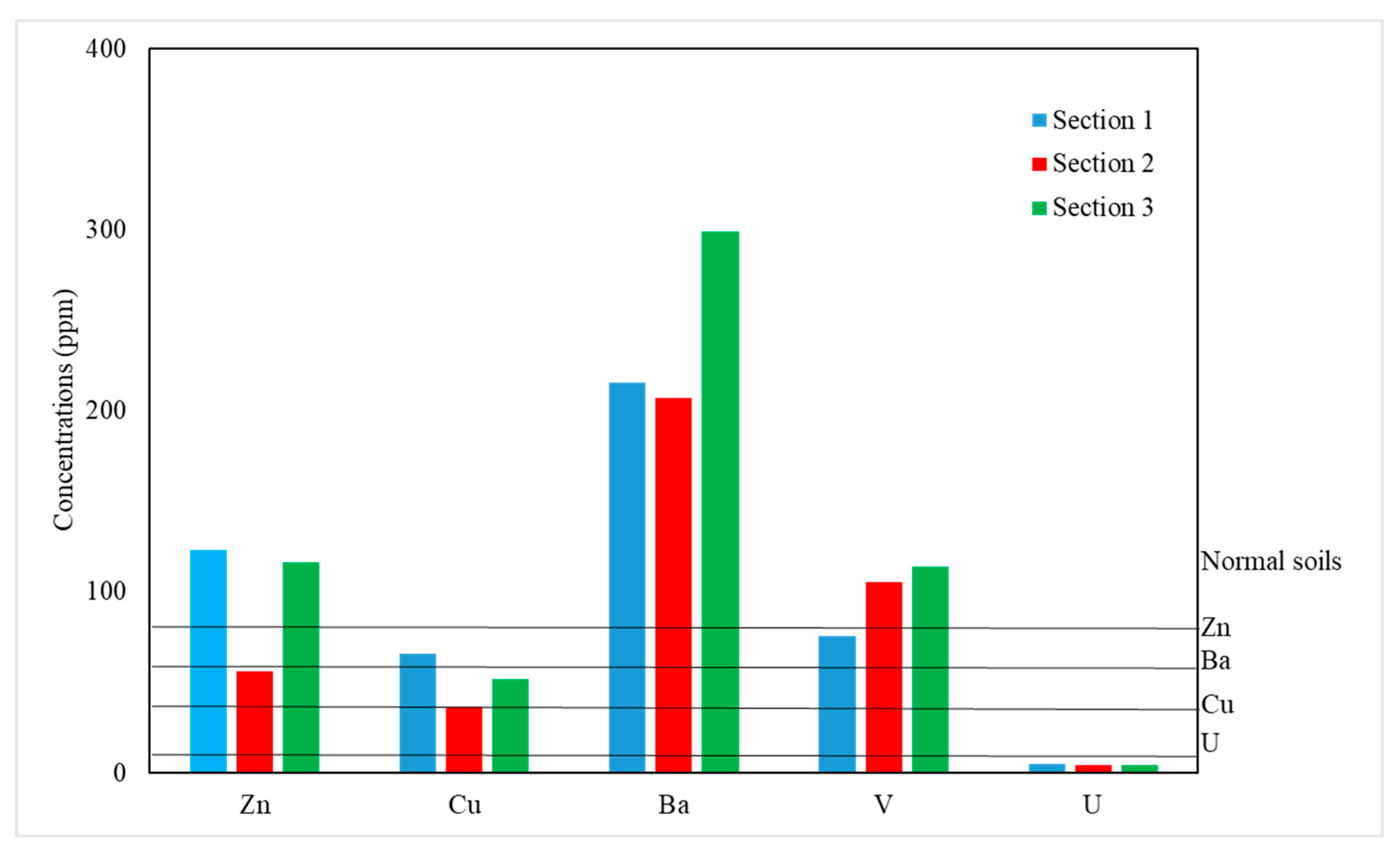
| Section 1 | Section 2 | Section 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major Oxides/Trace Elements | RC-1 | RC-2 | RC-3 | TC-1 | TC-2 | TC-3 | TC-4 | TC-5 | TC-6 | TC-7 | TC-8 | HC-1 | HC-2 | HC-3 | HC-4 | HC-5 | HC-6 |
| SiO2 | 55.1 | 47.4 | 49.0 | 29.3 | 27.2 | 44.6 | 43.7 | 44.6 | 41.8 | 40.7 | 17.8 | 54.1 | 56.0 | 54.3 | 60.1 | 52.8 | 51.0 |
| Al2O3 | 9.49 | 7.93 | 8.02 | 7.03 | 6.91 | 11.2 | 11.6 | 11.3 | 10.8 | 9.56 | 4.89 | 21.1 | 20.6 | 21.2 | 19.1 | 19.0 | 19.2 |
| Fe2O3 | 3.51 | 3.74 | 3.61 | 3.74 | 3.17 | 4.75 | 3.24 | 4.77 | 4.82 | 4.58 | 1.36 | 5.9 | 6 | 5.7 | 5.34 | 4.57 | 9.27 |
| CaO | 2.04 | 0.99 | 1.77 | 1.24 | 2.06 | 1 | 1.83 | 1.9 | 1.66 | 1.52 | 2.88 | 1.02 | 1.77 | 1.47 | 1.73 | 1.48 | 1.79 |
| MgO | 5.85 | 7.27 | 7.1 | 1.4 | 1.26 | 1.52 | 1.82 | 1 | 1.46 | 1.54 | 1.34 | 1.4 | 1.39 | 1.42 | 1.09 | 1.14 | 1.22 |
| SO3 | 0.03 | 0.05 | 0.04 | 0.01 | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0 | 0.05 | 0.03 | 0.07 | 0.01 | 0.1 | 0.06 |
| Na2O | 0.04 | 0.05 | 0.05 | 0.01 | 0.01 | 0.03 | 0.02 | .012 | 0.12 | 0.01 | 0.01 | 0.13 | 0.13 | 0.1 | 0.17 | 0.13 | 0.11 |
| K2O | 3.54 | 3.36 | 3.28 | 1.74 | 1.37 | 2.01 | 1.74 | 1.48 | 1.86 | 1.97 | 0.61 | 5.5 | 5.23 | 5.75 | 4.6 | 4.55 | 5.19 |
| TiO2 | 0.67 | 0.53 | 0.5 | 0.57 | 0.5 | 1 | 0.97 | 1.05 | 1.07 | 1.05 | 0.53 | 1.27 | 1.17 | 1.38 | 1.48 | 1.42 | 1.4 |
| CIA | 62.8 | 64.3 | 61.1 | 70.1 | 66.7 | 78.6 | 76.3 | 76.9 | 74.8 | 73.2 | 58.2 | 76.1 | 74.3 | 74.4 | 74.6 | 75.5 | 73.0 |
| ICV | 2.03 | 2.01 | 2.03 | 1.24 | 1.21 | 0.92 | 0.83 | 0.9 | 1.01 | 1.12 | 1.38 | 0.72 | 0.76 | 0.74 | 0.75 | 0.7 | 0.99 |
| Sc | 5.36 | 7 | 9.64 | 11.8 | 16 | 14.9 | 11 | 15.3 | 11.7 | 12.2 | 13.4 | 14.1 | 7.75 | 6.37 | 8.73 | 11.9 | 4.1 |
| Cr | 149 | 120 | 125 | 95.7 | 127 | 122 | 125 | 135 | 129 | 94 | 94 | 122 | 132 | 142 | 91 | 85 | 112 |
| V | 64 | 65 | 97 | 138 | 28 | 150 | 130 | 70 | 112 | 119 | 97 | 110 | 82 | 67 | 83 | 270 | 70 |
| Co | 6.41 | 4.21 | 3.71 | 5.99 | 5.03 | 6.05 | 4.46 | 5.54 | 3.9 | 4.17 | 5.01 | 11.4 | 7.96 | 6.16 | 9.01 | 5.29 | 8.39 |
| Ni | 9 | 9 | 10 | 15 | 19 | 11 | 18 | 22 | 20 | 19 | 21 | 28 | 14 | 13 | 17 | 12 | 21 |
| Cu | 65 | 65 | 67 | 57 | 42 | 45 | 24 | 44 | 18 | 36 | 29 | 46 | 33 | 32 | 56 | 79 | 66 |
| Zn | 112 | 169 | 89 | 86 | 41 | 78 | 47 | 42 | 57 | 46 | 51 | 81 | 89 | 121 | 114 | 154 | 139 |
| Rb | 190 | 119 | 193 | 198 | 169 | 248 | 184 | 199 | 131 | 175 | 183 | 151 | 194 | 159 | 213 | 211 | 227 |
| Sr | 130 | 211 | 148 | 232 | 261 | 103 | 200 | 142 | 350 | 212 | 222 | 144 | 234 | 124 | 135 | 136 | 139 |
| Zr | 211 | 347 | 321 | 331 | 259 | 100 | 300 | 200 | 190 | 241 | 210 | 388 | 485 | 476 | 438 | 454 | 327 |
| Nb | 74 | 64 | 54 | 45 | 47 | 85 | 39 | 55 | 70 | 57 | 48 | 68 | 50 | 61 | 73 | 44 | 54 |
| Sb | 0.6 | 0.38 | 0.25 | 0.23 | 0.65 | 0.15 | 0.46 | 0.32 | 0.39 | 0.36 | 0.41 | 0.59 | 0.37 | 0.29 | 0.39 | 0.3 | 0.37 |
| Cs | 2.02 | 2.99 | 2.59 | 3.63 | 2.42 | 2.46 | 2.5 | 2.47 | 2.8 | 2.72 | 2.45 | 3.59 | 2.16 | 2.26 | 2.19 | 3.62 | 2.43 |
| Ba | 299 | 153 | 194 | 184 | 292 | 243 | 208 | 189 | 176 | 165 | 198 | 205 | 187 | 272 | 631 | 210 | 290 |
| La | 21.1 | 29.3 | 19.8 | 17.6 | 23.5 | 18.5 | 15.6 | 33.5 | 27.8 | 31.9 | 34.1 | 17.8 | 26.9 | 17.4 | 21.3 | 39.9 | 30.3 |
| Ce | 306 | 157 | 77.9 | 116 | 120 | 135 | 152 | 139 | 118 | 144 | 137 | 76.6 | 54.9 | 117 | 154 | 63 | 117 |
| Nd | 125 | 51.9 | 37.2 | 56.4 | 52.6 | 63.6 | 73.4 | 59.5 | 55.6 | 61.3 | 51.8 | 30 | 21 | 51 | 78.6 | 30.7 | 42.9 |
| Sm | 19 | 7.98 | 5.06 | 8.56 | 7.51 | 9.38 | 10.5 | 8.09 | 9.7 | 7.98 | 8.32 | 6.23 | 4.3 | 7.97 | 11.2 | 4.3 | 8.45 |
| Eu | 3.17 | 1.38 | 1.41 | 1.58 | 1.34 | 1.61 | 2.03 | 1.46 | 1.7 | 1.33 | 0.98 | 1.2 | 0.9 | 1.46 | 2.2 | 0.86 | 1.61 |
| Gd | 9.7 | 5.13 | 5.31 | 4.17 | 4.29 | 5.75 | 4.49 | 5.44 | 5.24 | 4.9 | 4.2 | 4.49 | 2.73 | 4.63 | 7.97 | 2.67 | 5.04 |
| Tb | 1.65 | 0.92 | 0.96 | 0.81 | 0.79 | 0.99 | 0.73 | 0.89 | 0.94 | 0.85 | 0.81 | 1.04 | 0.62 | 1 | 1.25 | 0.58 | 1 |
| Tm | 0.89 | 0.6 | 0.61 | 0.41 | 0.45 | 0.48 | 0.61 | 0.53 | 0.58 | 0.57 | 0.51 | 0.64 | 0.42 | 0.64 | 0.75 | 0.35 | 0.64 |
| Yb | 6.57 | 4.16 | 3.99 | 3.38 | 3.5 | 4.21 | 3.78 | 3.6 | 3.2 | 4.1 | 3.9 | 4.48 | 2.73 | 4.21 | 5.47 | 2.4 | 4.35 |
| Lu | 1.03 | 0.64 | 0.61 | 0.54 | 0.55 | 0.65 | 0.56 | 0.57 | 0.54 | 0.61 | 0.59 | 0.68 | 0.44 | 0.65 | 0.83 | 0.39 | 0.66 |
| Hf | 3.46 | 5.29 | 3.33 | 5.29 | 3.33 | 3.74 | 2.9 | 4.1 | 2.76 | 3.12 | 3.4 | 3.62 | 4.7 | 2.61 | 3.6 | 7.64 | 5.72 |
| Ta | 5.03 | 4.03 | 3.35 | 3.08 | 3.15 | 5.9 | 2.17 | 3.77 | 4.23 | 2.78 | 3.12 | 4.93 | 3.09 | 4.27 | 4.58 | 2.32 | 3.6 |
| Th | 38.2 | 28.4 | 29 | 23 | 22.4 | 30 | 22.3 | 28.6 | 27.3 | 19.8 | 22.3 | 29.6 | 24.2 | 24.9 | 28.7 | 15.1 | 22.7 |
| U | 5.3 | 4.76 | 4.53 | 3.41 | 4.18 | 5.61 | 2.95 | 5.19 | 4.2 | 2.4 | 5.2 | 5.1 | 4.5 | 5 | 5.15 | 2.72 | 4.5 |
| Elemental Ratio | Range of Shales from the Chichali Formation | Range of Sediments (Based on Cullers [27]). | |
|---|---|---|---|
| Felsic Rocks | Mafic Rocks | ||
| Th/Sc | 1.26–7.12 | 0.84–20.5 | 0.05–0.22 |
| Th/Co | 2.52–7.81 | 0.67–19.4 | 0.04–1.4 |
| Th/Cr | 0.17–0.31 | 0.13–2.7 | 0.018–0.046 |
| La/Sc | 1.24–7.39 | 2.5–16.3 | 0.43–0.86 |
| Parameter | Mean Sec-1 | Mean Sec-2 | Mean Sec-3 | Min–Max Sec-1 | Min–Max Sec-2 | Min–Max Sec-3 | Standard (Soils)A | (MCL)B | (MCL)C | (MCL)D |
|---|---|---|---|---|---|---|---|---|---|---|
| Copper (Cu) | 65.6 | 36.8 | 52 | 65–67 | 18–57 | 32–66 | 20–40 | 1.3 | 1 | 1 |
| Chromium (Cr) | 131.3 | 115.2 | 114 | 120–149 | 94–135 | 85–142 | 90 | - | 0.05 | 0.05 |
| Nickel (Ni) | 9.33 | 18.1 | 17.5 | 44,814 | 44,887 | 44,923 | 40 | - | 0.02 | <0.02 |
| Zinc (Zn) | 123.3 | 56 | 116.3 | 89–169 | 41–86 | 81–154 | 44,880 | 5 | 5 | 5 |
| Barium (Ba) | 215.3 | 206.8 | 299 | 153–299 | 165–243 | 187–631 | - | - | 0.7 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazal, A.G.; Umar, M.; Shah, F.; Miraj, M.A.F.; Janjuhah, H.T.; Kontakiotis, G.; Jan, A.K. Geochemical Analysis of Cretaceous Shales from the Hazara Basin, Pakistan: Provenance Signatures and Paleo-Weathering Conditions. J. Mar. Sci. Eng. 2022, 10, 800. https://doi.org/10.3390/jmse10060800
Fazal AG, Umar M, Shah F, Miraj MAF, Janjuhah HT, Kontakiotis G, Jan AK. Geochemical Analysis of Cretaceous Shales from the Hazara Basin, Pakistan: Provenance Signatures and Paleo-Weathering Conditions. Journal of Marine Science and Engineering. 2022; 10(6):800. https://doi.org/10.3390/jmse10060800
Chicago/Turabian StyleFazal, Abdul Ghaffar, Muhammad Umar, Faisal Shah, Muhammad Armaghan Faisal Miraj, Hammad Tariq Janjuhah, George Kontakiotis, and Abdul Khaliq Jan. 2022. "Geochemical Analysis of Cretaceous Shales from the Hazara Basin, Pakistan: Provenance Signatures and Paleo-Weathering Conditions" Journal of Marine Science and Engineering 10, no. 6: 800. https://doi.org/10.3390/jmse10060800
APA StyleFazal, A. G., Umar, M., Shah, F., Miraj, M. A. F., Janjuhah, H. T., Kontakiotis, G., & Jan, A. K. (2022). Geochemical Analysis of Cretaceous Shales from the Hazara Basin, Pakistan: Provenance Signatures and Paleo-Weathering Conditions. Journal of Marine Science and Engineering, 10(6), 800. https://doi.org/10.3390/jmse10060800








