3.1. Superhydrophilic Meshes for Oil-Water Separation
Over the past few years, the development of superhydrophilic meshes has attracted increasing attention, owing to their appealing application in separating oil from water. Superhydrophilic meshes show water permeation and oil repelling behavior as schematically illustrated in
Figure 7. Water can quickly pass through a superhydrophilic mesh, but oil is repelled and prohibited from passing through it. In this way, oil and water can be quickly separated without causing significant blockage of the mesh pores. Functional materials especially metal oxides such as silicon dioxide, titanium dioxide, and zinc oxide nanoparticles, can induce the formation of micro-hierarchical structures and a large number of pores on the mesh surface, making it exhibit superhydrophilicity. Superhydrophilic surfaces have broad applications, including antifogging screens, windows, lenses, antifouling coatings, and foils for food packaging.
In 2012, Yang [
41] developed a superhydrophilic and superoleophobic nanocomposite mesh. Modified with silicon dioxide (SiO
) nanoparticles, the mesh surface attained increased roughness. Another polymer-based coating material, PDDA-PFO (polydially diammonium perfluoroctonate), was also used to attach hydrophilic and oleophobic groups to the mesh surface. In this way, the surface achieved a water contact angle (WCA) of 0
and oil contact angle (OCA) of 155
for different types of oils. This study demonstrated that SiO
has a great potential in the treatment of various types of oils, and the efficiency for removal of oil could be enhanced by varying the concentration of SiO
. The modified meshes became highly efficient in oil removal when the concentration of silica is above 50 wt%. Zhang et al. [
42] in 2013 developed a self-cleaning superhydrophilic and underwater superoleophobic mesh. The mesh was prepared by the layer-by-layer assembly of sodium silicate, and titanium dioxide (TiO
) nanoparticles on a PDDA modified stainless steel mesh. UV-light illumination of the TiO
component can be used as a way of self-cleaning, through which the contaminants accumulated on the mesh are photocatalytically decomposed. The particles of sodium silicate and titanium dioxide on the surface of the mesh can be preserved to give excellent wetting properties. The results of the study showed that the prepared mesh could effectively separate water from oil–water mixtures, while good re-usability was attained through UV-illumination for self-cleaning.
Li et al. [
12] in 2015 developed a zinc oxide (ZnO)-coated mesh with superhydrophilic and underwater superoleophobic performance. They modified a stainless steel mesh by spraying a mixture of ZnO nanoparticles and waterborne polyurethane (PU) on the mesh surface. This spray coating method is now commercialized and used in large-scale application. The high water affinity and strong underwater oil repellence exhibited by this type of mesh make it particularly effective in gravity-driven oil–water separation. Experimental results showed a water/oil separation efficiency as high as 99%. More significantly, the coated mesh maintains a high separation efficiency and recyclability. After 50 cycles of separation, the separation efficiency for the kerosene-water mixture is still above 97.3%.
A self-cleaning nanoscale carbon hybrid system was developed by Shervani et al. in 2019 [
43]. In this work, a steel mesh was coated by layers of polymer-based porous materials and graphene oxide (GO). With this nano-coated mesh, a two-state process was developed to selectively remove hydrocarbons from emulsified water. Reduction in TPHs (total petroleum hydrocarbons) from 290 ppm to less than 1 ppm and more than 99.5% separation efficiency (oil removal from oil–water mixture) have been achieved. It is also worth remarking that no PAHs (polycyclic aromatic hydrocarbons) were detected in the treated water, and the mesh was robust and did not suffer from any fouling problems.
Liu et al. [
44] in 2016 developed an effective and simple method for cleaning up oily wastewater. In this research, a superhydrophilic and underwater superoleophobic mesh was developed by spraying chitosan silica nanoparticles–glutaraldehyde composite (CS-SiO
-GA) on a stainless steel mesh for separation of oil–water mixtures. The developed mesh showed useful properties for oil–water separation, such as biocompatibility, high mechanical, chemical, and thermal strength, good film-forming ability, and hydrophilicity.
Wen et al. [
45] in 2013 fabricated a zeolite-coated mesh film (ZCMF) for efficient oil–water separation, which showed excellent superhydrophilic and underwater superoleophobic properties. Due to a gravity-driven mechanism, water could easily pass through the film, whereas oil was retained above the film. Meanwhile, the underwater superoleophobic surface showed a low affinity for oil droplets, thus protecting the film from fouling. Moreover, the films are highly stable under harsh conditions. The results of their study showed that underwater oil contact angle is above 150
, which is an indicative of remarkable underwater superoleophobicity. Because of this, a water cushion can be formed between the oil droplets and the solid surface. The strong repulsive interactions between the water cushion and oil droplets protect the ZCMF surface from fouling by oil during the separation process. Further, the separation efficiency of ZCMF was tested by calculating water flux and intrusion pressure. It was shown that the water flux increases with the increase of the pore size of ZCMF, but the intrusion pressure of oil decreases with increasing pore size of ZCMF.
Agano et al. [
46] in 2021 fabricated a superhydrophilic and underwater superoleophobic CuO/TiO
mesh for oil–water separation. They used the method of electrochemical anodization for coating CuO nanostructures on the copper mesh. After anodization, the mesh was rinsed with distilled water and air-dried. They deposited TiO
on the mesh with a high-rate vacuum coating technique. After deposition, the TiO
coated mesh was annealed in an air atmosphere in a tube furnace with a slow temperature ramp-up to 500
C and maintained at this temperature for 2 h. The annealing step is expected to improve the crystallinity of the TiO
film. Needle-like structures were developed on the anodized mesh, which entrapped water between the needles. The rough surface present on the mesh further improved the affinity for water. The hydrophilic nature of TiO
allowed water to pass through the mesh. A thin layer of water trapped by the TiO
needles further enhanced the surface oleophobicity, thus preventing the oil from passing through the mesh.
Zhang et al. [
47] in 2013 developed nanowire-haired inorganic membranes with superhydrophilicity and underwater superoleophobicity. The nanowire-haired membrane was prepared by surface oxidation of a copper mesh in an alkaline aqueous solution with (NH
)
S
O
. The existence of Cu(OH)
nanowires on the copper mesh greatly enhanced its hydrophilic property. A thin layer of water trapped by the Cu(OH)
nanowires on the copper mesh would further enhance its surface oleophobicity, thus preventing oil from passing through the mesh. The mesh surface showed a WCA of 0
and an OCA of 155
. These nanowire-haired inorganic membranes show great application potential in separating oil from water.
Xue et al. [
48] fabricated a novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil–water separation. The preparation of polyacrylamide (PAM) hydrogel-coated mesh was done by using a photo-initiated polymerization process. After immersing the stainless steel mesh into a monomer solution, UV light was used to start the polymerization process. After polymerization, the mesh was washed to remove excess monomers. Through hydrogel-coating on stainless steel mesh, nanostructured papillae were developed. A water layer was formed at the oil/water/solid interface, which decreased the contact area between oil droplets and the solid surface. This made the oil droplets to easily roll off from the surface. This hydrogel-coated mesh is particularly useful for separating oil from emulsified oil–water mixtures.
Shi et al. [
49] in 2016 prepared a three-dimensional (3D) graphene foam for oil–water separation. In this work, a sea shell-based chemical vapor deposition template function technique was used for fabricating the 3D graphene foam, which showed high purity, high porosity, ultralow density, and superbendability. They used a highly abundant shell of oceanic mollusk called scallop, which comprises 95% calcium carbonate (CaCO
) and 5% organic biopolymer. Through a simple calcination process, the biological CaCO
was converted into calcium oxide (CaO) without destroying its structural framework. After heating the CaO framework, a graphene layer was deposited on the CaO surface, forming a CaO@graphene structure. The oxygen atoms on the CaO template delivered a synergistic effect by absorbing and decomposing hydrocarbons at high temperature and promoting carbon–carbon coupling on the substratum. After chemical etching with dilute HCl and followed by freeze-drying treatment, the 3D graphene foam was generated. The results of the studies showed fast separation efficiency, absorption capacity up to a 250-fold weight gain, and excellent absorption performance towards various oils and organic solvents. This functional material can also be used in other industrial applications such as energy storage and water remediation.
Yu et al. [
50] in 2019 prepared a superhydrophilic and underwater superoleophobic stainless steel mesh treated with Co(NO
)
and H
NCONH
solutions through a simple hydrothermal process and subsequent calcination. By increasing the reaction time, needle-like Co
O
was densely coated on the mesh surface. The as-prepared Co
O
-coated mesh separated various oil/water mixtures with high efficiency (>99.4%) and high flux (>75,000 L·m
h
) even in harsh conditions such as highly acidic, alkaline, and salty solutions. Moreover, the Co
O
-coated mesh showed an outstanding antiabrasion performance; after 40 abrasion cycles with sandpaper, it still retained a very good separation efficiency. The excellent anticorrosion and antiabrasion behavior of this Co
O
-coated mesh makes it resistant to mechanical damages during oil/water separation. The pristine mesh showed hydrophobicity in air (WCA = 126.86 ± 1.56
) and oleophobicity under water (OCA = 138.45 ± 1.45
), but after coating with Co
O
it showed superhydrophilicity in air (WCA ≈ 0
) with an increase in underwater oleophobicity (OCA = 151.19 ± 0.19
).
Sun et al. [
51] in 2020 reported a green and facile method for modification of stainless steel mesh through immersion into an oxidation solution (ammonium persulfate and sodium hydroxide aqueous solution) and a conversion solution (tannic acid with pH of 2.4 adjusted by phosphate acid aqueous solution). In the first step, FeOOH clusters were formed and then converted to a complex with tannic and phosphoric acids to make a film on the surface of mesh. The grafting of tannic and phosphoric acid with plenty of peony-like microspheres in the intervals between the wires of the stainless steel mesh improved the surface energy, superhydrophilicity, and superoleophobicity, which are beneficial for oil–water separation and antifouling performance. Long-term durability and resistance were observed even under harsh corrosion and abrasion conditions with oil rejection above 99%.
Mahmodi and co-workers [
52] in 2020 synthesized a NaA-type zeolite-coated mesh by a secondary growth method. The aluminum/silicon ratio (ASR) was controlled by varying the amounts of sodium hydroxide, sodium aluminate, and sodium metasilicate monohydrate in aqueous solutions. ASR of 1.21 led to a mesh showing the highest oil–water separation efficiency with superhydrophilicity (WCA = 0
) and underwater superoleophobicity (OCA = 163.7
). Changing the ASR from 0 to 1.21 caused a decrease in the pore size of the mesh from 150
m to about 10
m, as a result of the aggregation of NaA zeolite. In the meantime, the roughness of the surface and the oil rejection rate were increased with increasing ASR, while the water flux rate was reduced. Re-usability of the zeolite mesh was demonstrated by implementing a re-calcination process at 550
C for 6 h. The ASR decreased slightly in each cycle of re-calcination, which is due to dealumination of the zeolite along with the burning of organic components. The average oil rejection rate remained more than 99.4% after three cycles of re-calcination. The results indicated that the zeolite-coated mesh is applicable for separating oil/water mixtures.
Zhang et al. [
53] in 2021 developed a novel superhydrophilic/underwater superoleophobic mesh that gave excellent oil/water separation performance. With the use of an inorganic nanomaterial (MnCo
O
), microscale cube-like structures were developed on the surface of a stainless steel mesh through a facile hydrothermal-annealing process. The resulting MnCo
O
-coated stainless steel mesh exhibited good antifouling property, high oil/water separation efficiency, and high flux rate (>63 L·m
s
). The nanostructures on the mesh surface gave rise to special wettability, allowing a water layer to form between the mesh surface and oil. This layer acted as a cushion to prevent the oil from directly contacting the mesh surface, resulting in excellent underwater superoleophobic performance. This modified mesh also showed excellent corrosion resistance, recyclability, and long-term durability under harsh environmental conditions, which are advantageous for application in oily wastewater treatment.
Table 10 summarizes the recent investigations on functional material-based superhydrophilic meshes and their applications in oil–water separation.
3.2. Superhydrophobic Meshes for Oil-Water Separation
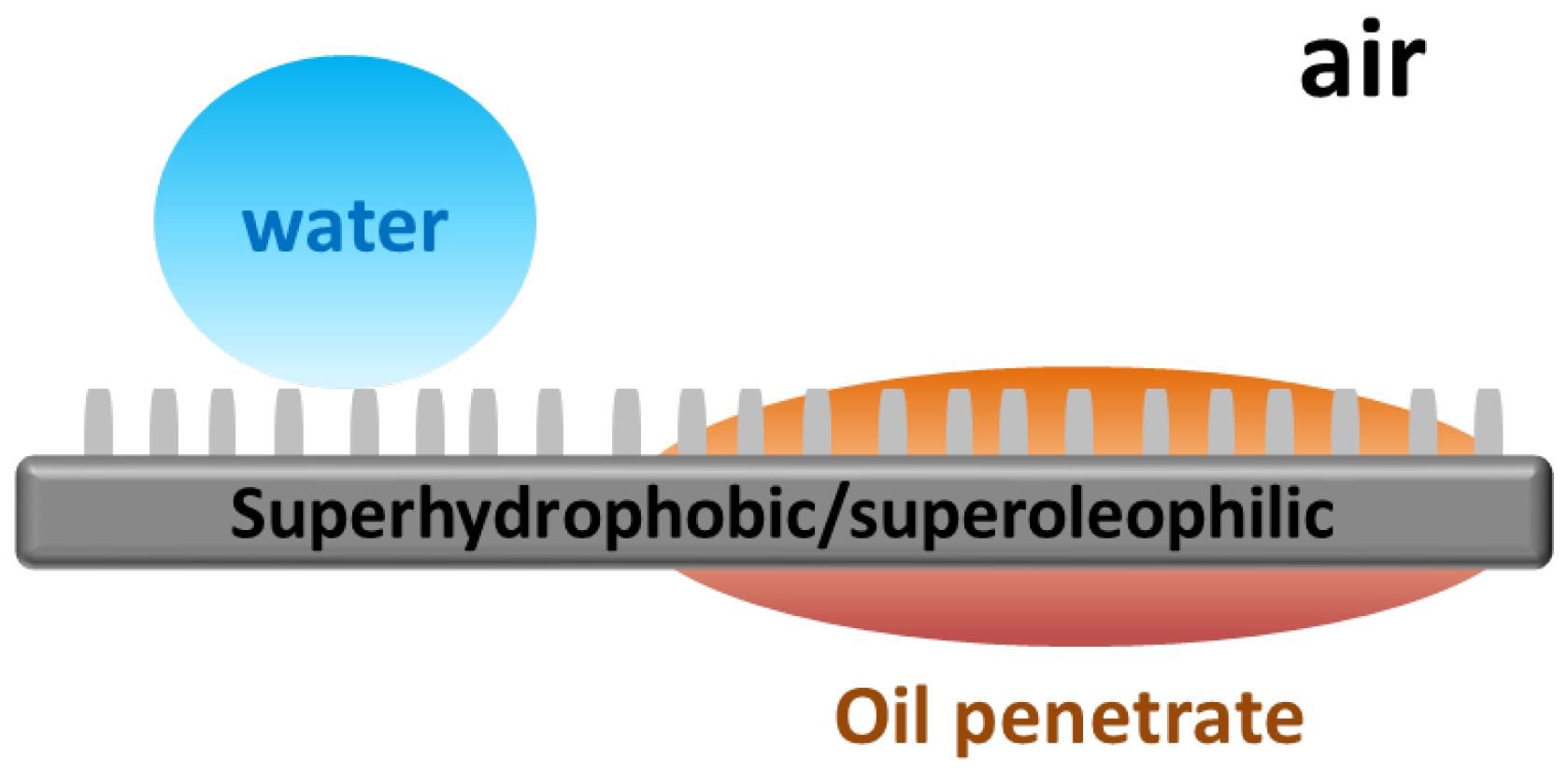
A superhydrophobic surface shows repellance to water but affinity for hydrophobic oil. When a mesh is coated with a superhydrophobic material, it can give oil–water separation performance as illustrated in
Figure 8. Unlike the superhydrophilic meshes discussed above, a superhydrophobic mesh allows oil to penetrate but rejects the passage of water.
Wang et al. [
54] in 2015 prepared superhydrophobic and superoleophilic fabrics for separation of oil–water mixtures using polydopamine as an adhesive agent. In this research work, nanoparticles such as silica were immobilized onto the surface of cotton fabrics through mussel adhesive proteins such as polydopamine. Dopamine, through self-polymerization, formed strong covalent and non-covalent interfacial interactions with all types of substances. The strong bioadhesion of PDMS/silica (SiO
) composite allowed superhydrophobic and superoleophilic coatings to be strongly bound to the surface of the fabrics. The modified fabrics showed superhydrophobicity, low surface energy, and micro/nano-hierarchical structures on their surfaces. A mesh made of these fabrics gave a water contact angle of more than 153
C. Water droplets could be completely bounced off from the surface of the fabrics without leaving any residual water on it. However, when an oil such as toluene or linseed was applied on the prepared fabrics, the oil would quickly wet the surface by forming a large spreading radius as a result of oleophilicity. The mesh led to oil–water separation efficiency as high as 99% up to 90 cycles of ultrasonic treatment. This bioadhesion method can also be used to prepare superhydrophobic oil containment boom, candle soot-coated nickel foam, SiO
/polystyrene-coated filter paper.
Wang et al. [
55] in 2013 prepared a superhydrophobic copper mesh film, analogous to the performance of butterfly wings, for rapid oil/water separation. In their study, the copper mesh surface was modified by an environmentally-friendly electrochemical deposition method. The electroplating of copper nanoparticles was performed on the as-cleaned copper mesh film and followed by a thiol grafting process to fabricate superhydrophobic surface on the surface of the copper mesh. Such modified surface showed high roughness, low surface energy, and excellent superhydrophobicity.
Salehabadi et al. [
56] in 2017 developed superhydrophobic and superoleophilic polyurethane sponges, the surface of which was decorated with nanosilica particles. The effect of nanosilica concentration on the final wetting behavior of the sponges was investigated. They used a simple dipping process for the treatment of sponges with modified and unmodified nanosilica particle suspension. After this treatment, a uniform layer of silica nanoparticles was formed on the surface of the sponge. For the untreated sponge, the WCA value is 120
. Despite this high value, it cannot efficiently separate oil/water mixtures due to a high level of water drop stickiness (sliding angle = 180
). In order to increase superhydrophobicity, different loads of hydrophobic nanosilica were used. By the addition of nanosilica, two simultaneous effects (reduction of surface energy and enhancement of surface roughness) occur. Both effects help increase the WCA value and reduce the sliding angle value. This work showed that nanosilica particles have a great potential in separating different oils and organic solvents from water, and the coating of nanosilica is a simple, efficient, and inexpensive method.
Rasouli and co-workers [
57] in 2021 fabricated a superhydrophobic and superoleophilic stainless steel mesh-based membrane, using a facile one-stage dip-coating technique, and investigated the effects of silane alkyl chain size, and the ratio of micro-to-nano particles in the coating solid fraction. Silane compounds with short- and long-alkyl chains were used as surface energy modifiers. Increasing the concentration of nanoparticles to 100% led to a decrease in hydrophobicity, and this effect was found to be more pronounced for the short-chain silane modified surface. Flower-like hierarchical microstructures were obtained using the coating solution of silanes with only nanoparticles. The average pore opening for the mesh was decreased from 76 mm in the bare mesh to 48 mm for the coated mesh. Using this type of mesh, a separation efficiency >99% was achieved for kerosene–water mixtures. The influence of solid composition in the coating with hydrophobic nanoparticles (NPs) and microparticles (MPs) on the wetting characteristics of the mesh was also investigated. Modifications of the surface with long silane chains led to greater hydrophobicity than with short-chain silane.
Yue and co-workers [
58] in 2020 fabricated a silicone-modified stainless steel mesh via coating the mesh surface with silicone upon transient heating. The electrothermal effect was employed for heating the water-in-crude oil emulsion, reducing the viscosity of the water-in-crude oil emulsion and thus leading to efficient viscous emulsion separation. The as-prepared silicone-modified stainless steel mesh showed combined superhydrophobic/superoleophilic properties and good re-usability.
Du et al. [
59] in 2019 prepared a superhydrophobic stainless steel mesh, the surface of which was coated with hierarchical micro/nanostructures. The coating materials were nontoxic graphite and environmentally friendly titanium dioxide, which were applied on the mesh surface by simple brush-painting method. Polydimethylsiloxane and ethyl cellulose were used as adhesives to have the hydrophobic coating bound to the surface of the mesh. The unique inlay-gated structure on the pores of the mesh resulted in good separation efficiency for various water-in-oil nanoemulsions; an separation efficiency higher than 99.9% was reported with the residual droplets smaller than 8 nm. Furthermore, the wettability of the as-prepared mesh showed good resistance to acids, alkaline, salts, organic solvents, and exposure to UV light. After the mesh was abraded by sandpaper, its separation capability was barely affected, indicating good antiabrasion performance.
Zhang et al. [
60] in 2020 reported a superhydrophobic stainless steel mesh coated with fly-ash. In their method, fly-ash was first modified by stearic acid, and then bonded to the surface of a stainless steel mesh with the aid of an epoxy resin solution. The coated mesh exhibited superhydrophobicity with a WCA of 153
. The superhydrophobicity could be ascribed to the formation of a protective barrier against moisture/water on the surface of the stainless steel mesh. Oil/water mixtures such as
n-hexane/water were successfully separated by this mesh, giving an efficiency of over 97% even after 30 cycles of separation. The simplicity, eco-friendliness, and low cost of this method make it practically useful for preparing large-scale superhydrophobic surfaces.
Table 11 summarizes the recent studies of superhydrophobic material-coated meshes for separation applications.