Mechanisms for Overpressure Development in Marine Sediments
Abstract
:1. Background
2. Classification of the Mechanisms for Overpressure Development in Marine Sediments
3. Overpressure Development Associated with Physical Processes
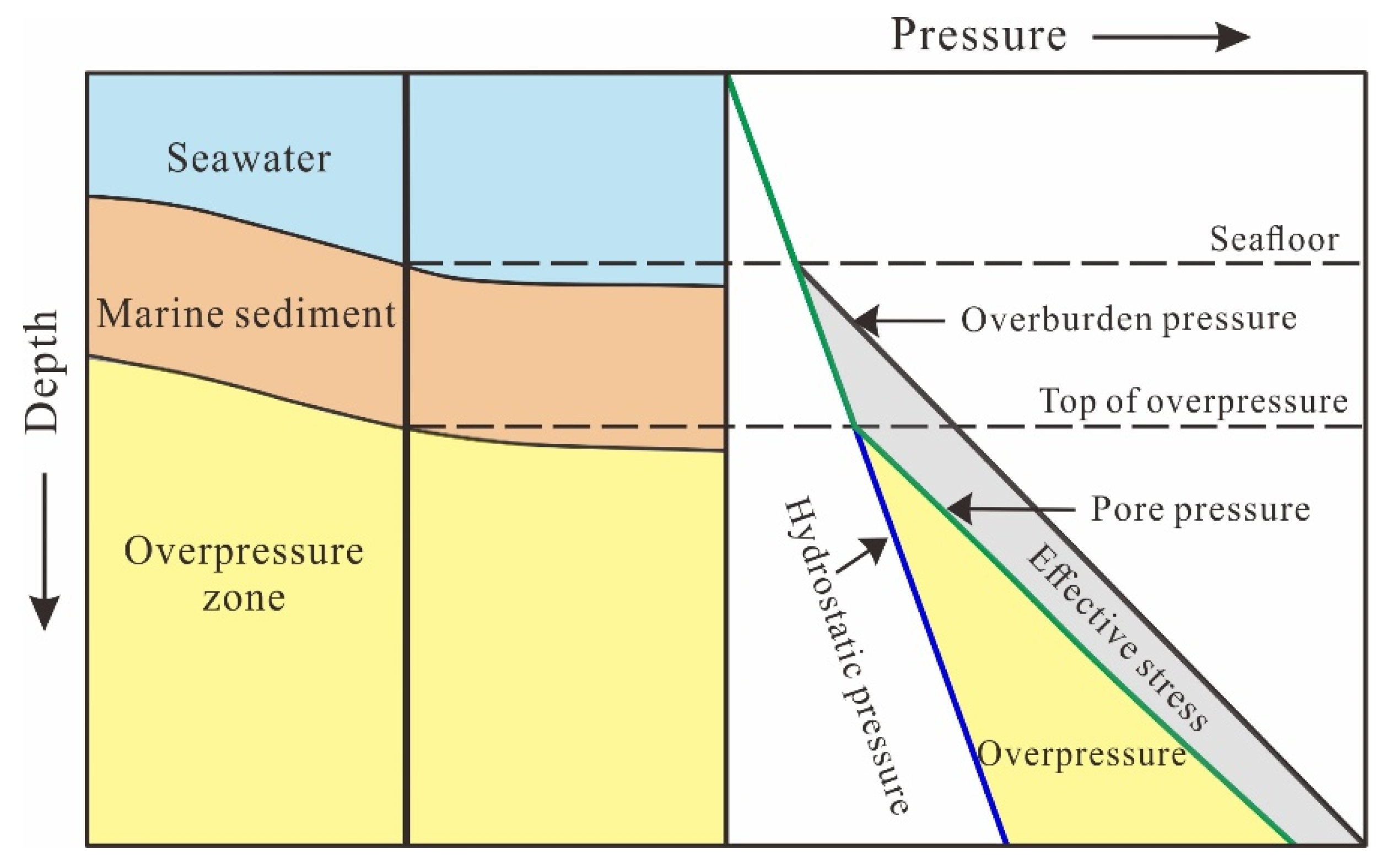
3.1. Disequilibrium Compaction
3.2. Hydrate Formation Sealing
3.3. Degasification
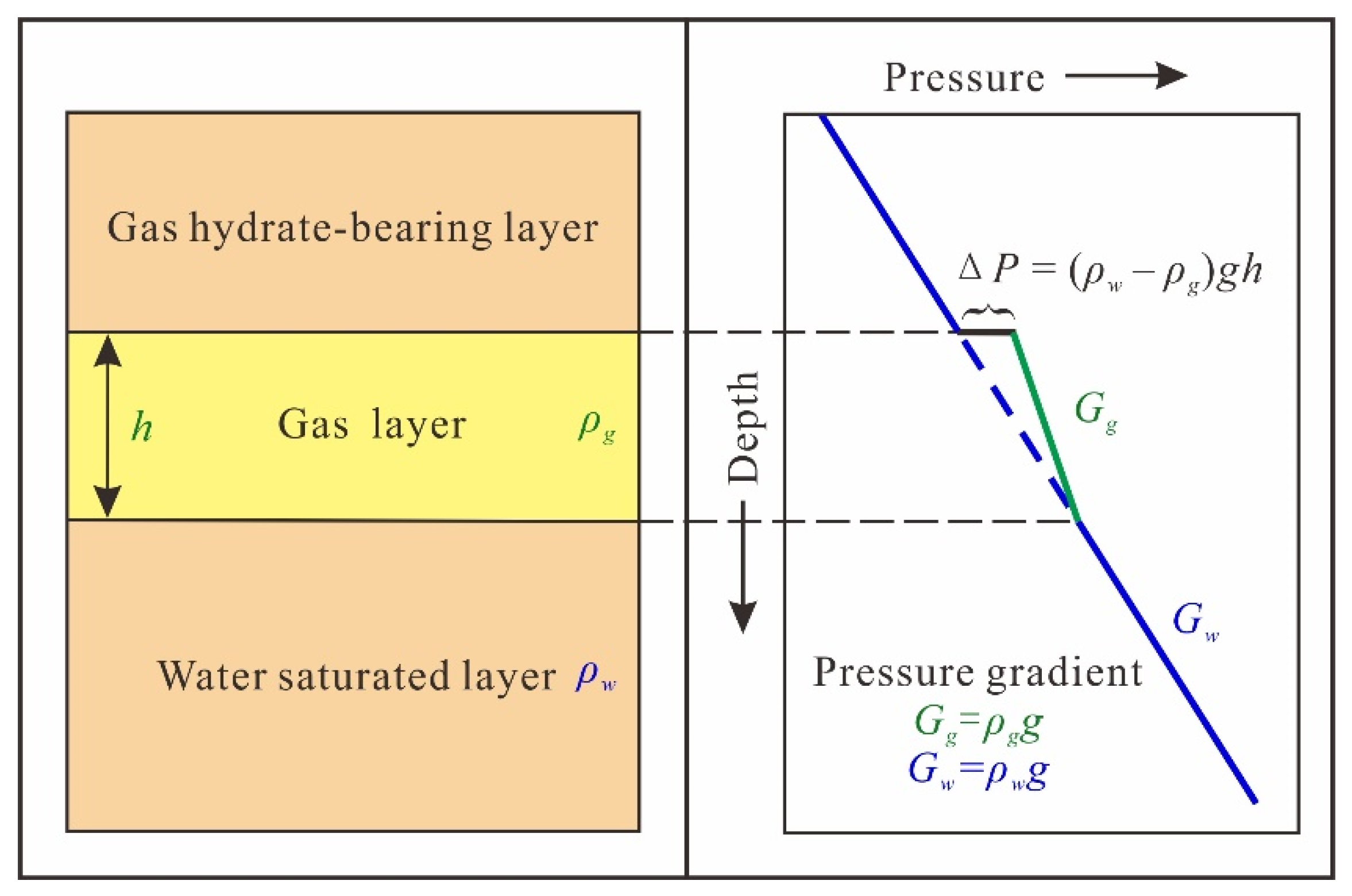
3.4. Buoyancy
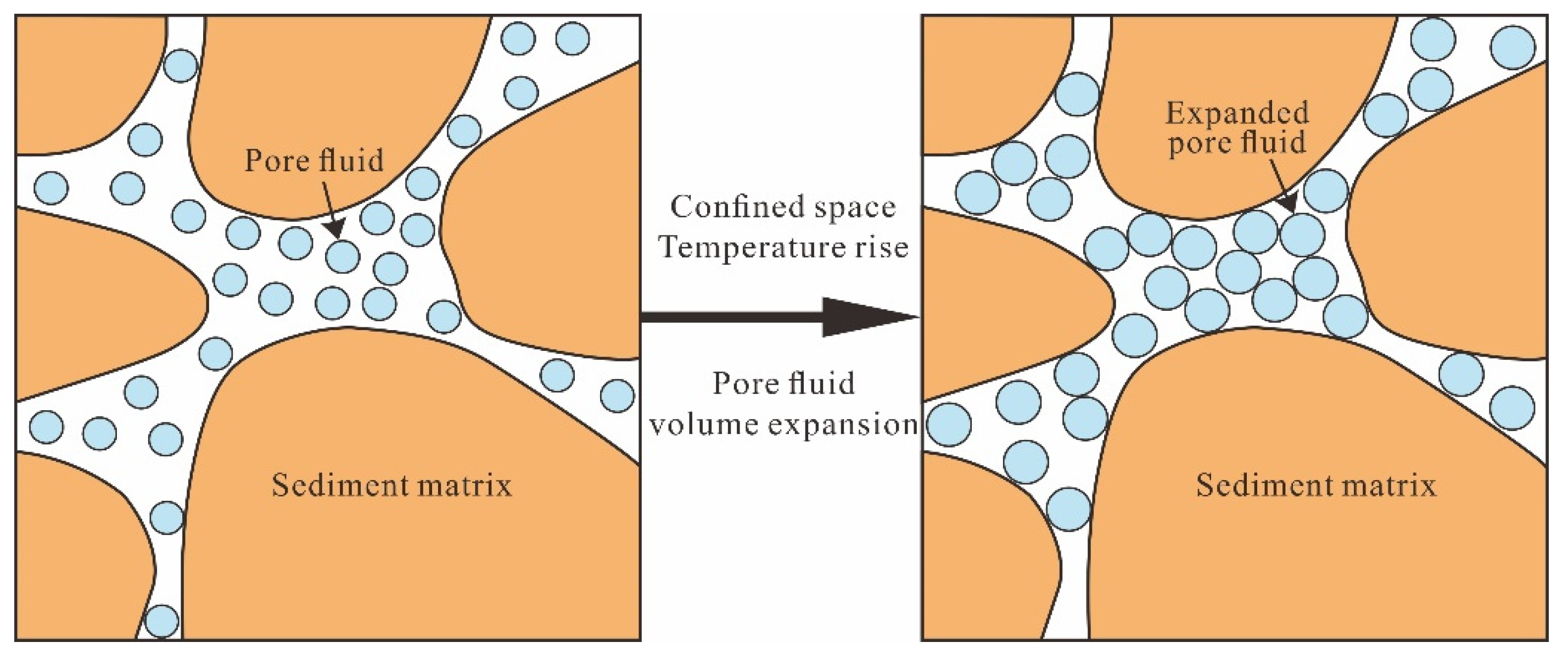
3.5. Hydrothermal Pressuring
3.6. Tectonic Movement
3.7. Overpressure Transfer
4. Overpressure Development Associated with Chemical Process
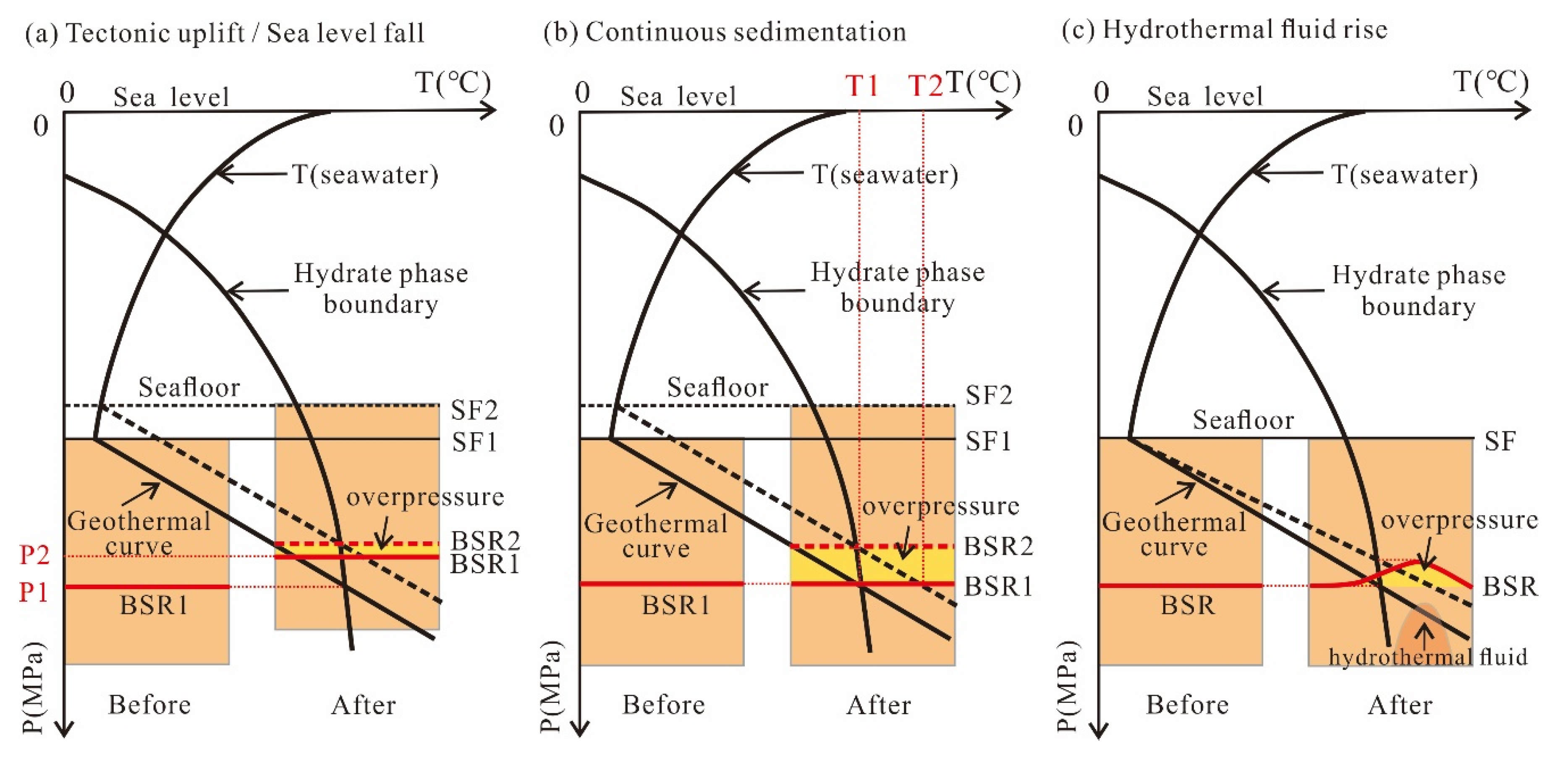
4.1. Hydrate Decomposition
4.2. Diagenesis
4.3. Hydrocarbon Generation
5. Overpressure Development Associated with Biological Activity
5.1. Microbial Gas Production
5.2. Microbial Plugging
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasubramanian, A. Marine Sediments; University of Mysore: Mysore, India, 2017. [Google Scholar]
- Chester, R. Marine Sediments; Springer: Dordrecht, The Netherlands, 1990; pp. 441–467. [Google Scholar]
- Encyclopaedia Britannica. Diagenesis. The Editors of Encyclopaedia Britannica; Encyclopedia Britannica: Scotland, UK, 2017. [Google Scholar]
- Singer, A.; Müller, G. Chapter 3 Diagenesis in Agrillaceous Sediments. Dev. Sedimentol. 1979, 25, 115–212. [Google Scholar]
- Wang, L.; Gu, L.; Lu, H. Sediment permeability change on natural gas hydrate dissociation induced by depressurization—ScienceDirect. China Geol. 2020, 3, 221–229. [Google Scholar]
- Terzaghi, K. Theoretical Soil Mechanics; John Wiley and Sons: Hoboken, NJ, USA, 1943. [Google Scholar]
- Biot, M.A. General Theory of Three-Dimensional Consolidation. J. Appl. Phys. 1941, 12, 155–164. [Google Scholar] [CrossRef]
- Dutta, N.C. Geopressure prediction using seismic data; current status and the road ahead. Geophysics 2002, 67, 2012–2041. [Google Scholar] [CrossRef]
- Tingay, M.R.P.; Hillis, R.R.; Swarbrick, R.E.; Morley, C.K.; Damit, A.R. Origin of overpressure and pore-pressure prediction in the Baram province, Brunei. AAPG Bull. 2009, 93, 51–74. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Luo, X.; Lei, Y.; Yu, L.; Cheng, M.; Wang, Y.; Wang, Z. Overpressure generation by disequilibrium compaction or hydrocarbon generation in the Paleocene Shahejie Formation in the Chezhen Depression: Insights from logging responses and basin modeling. Mar. Pet. Geol. 2021, 133, 105258. [Google Scholar] [CrossRef]
- Duan, M.D.; Ye, J.R.; Wu, J.F.; Tian, Y.H.; Cui, Y. Prediction of pressure distribution and Formation Mechanism in low exploration area: A case study of the Xihu depression in East China Sea Shelf Basin. Bull. Geol. Sci. Technol. 2020, 39, 129–139. [Google Scholar]
- Bowers, G.L. Detecting high overpressure. Lead. Edge 2002, 21, 174–177. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Li, J.; Xu, Z.Y. Advances in the origin of overpressures in sedimentary basins. Acta Pet. Sin. 2017, 38, 973–998. [Google Scholar] [CrossRef]
- Osborne, M.J.; Swarbrick, R.E. Mechanisms for generating overpressure in sedimentary basins: A Reevaluation. AAPG Bull. 1997, 81, 1023–1041. [Google Scholar]
- Luo, X.R.; Yang, J.H.; Wang, Z.F. The Overpressure Mechanisim in Aquifers and Pressure Prediction in Basins. Geol. Rev. 2000, 46, 22–31. [Google Scholar]
- Hart, B.S.; Flemings, P.B.; Deshpande, A. Porosity and pressure: Role of compaction disequilibrium in the development of geopressures in a Gulf Coast Pleistocene basin. Geology 1995, 23, 45–48. [Google Scholar] [CrossRef]
- Wan, Z.F.; Xia, B.; Lin, G.; Li, J.T.; Liu, B.M. Hydrocarbon Accumulation Model for Overpressure Basin: An Example from the Yinggehai Basin. MARINE Geol. Quat. Geol. 2010, 30, 91–97. [Google Scholar]
- Liu, T.; Lu, Y.; Zhou, L.; Yang, X.; Guo, L. Experiment and Analysis of Submarine Landslide Model Caused by Elevated Pore Pressure. J. Mar. Sci. Eng. 2019, 7, 146. [Google Scholar] [CrossRef] [Green Version]
- Flemings, P.B.; Stump, B.B.; Finkbeiner, T.; Zoback, M. Flow focusing in overpressured sandstones: Theory, observations, and applications. Am. J. Sci. 2002, 302, 827. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Zhang, P.; Li, G.; Tian, S.; Lu, J.; Zhang, B. A Solution to Sand Production from Natural Gas Hydrate Deposits with Radial Wells: Combined Gravel Packing and Sand Screen. J. Mar. Sci. Eng. 2022, 10, 71. [Google Scholar] [CrossRef]
- Alberty, M. Cost analysis of SWF preventative, remedial measures in deepwater drilling. Offshore 2000, 60, 58–64. [Google Scholar]
- Hardage, B.A.; Remington, R. Gas hydrate-a source of shallow water flow? Lead. Edge 2006, 25, 634–635. [Google Scholar] [CrossRef]
- Ostermeier, R.M.; Pelletier, J.H.; Winker, C.D.; Nicholson, J.W.; Rambow, F.H.; Cowan, K.M. Dealing with shallow-water flow in the deepwater Gulf of Mexico. In Proceedings of the Offshore Technology Conference One Petro, Houston, TX, USA, 1 May 2000. [Google Scholar]
- Kvenvolden, K.A. Gas hydrates—Geological perspective and global change. Rev. Geophys. 1993, 31, 173–187. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—A potential energy source for the 21st Century. J. Pet. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Delli, M.L.; Grozic, J.L.H. Experimental determination of permeability of porous media in the presence of gas hydrates. J. Pet. Sci. Eng. 2014, 120, 1–9. [Google Scholar] [CrossRef]
- Xu, W. Excess pore pressure resulting from methane hydrate dissociation in marine sediments: A theoretical approach. J. Geophys. Res. Solid Earth 2006, 111, B01104. [Google Scholar] [CrossRef]
- Foucher, J.-P.; Nouzé, H.; Henry, P. Observation and tentative interpretation of a double BSR on the Nankai slope. Mar. Geol. 2002, 187, 161–175. [Google Scholar] [CrossRef]
- Dasgupta, T.; Mukherjee, S. Pore Pressure Determination Methods. Adv. Oil Gas Explor. Prod. 2020, 19–30. [Google Scholar]
- Xie, Y.; Wu, T.; Sun, J.; Zhang, H.; Wang, J.; Gao, J.; Chen, C. Sediment compaction and pore pressure prediction in deepwater basin of the South China Sea: Estimation from ODP and IODP drilling well data. J. Ocean. Univ. China 2018, 17, 25–34. [Google Scholar] [CrossRef]
- Sen, S.; Kundan, A.; Kumar, M. Modeling Pore Pressure, Fracture Pressure and Collapse Pressure Gradients in Offshore Panna, Western India: Implications for Drilling and Wellbore Stability. Nat. Resour. Res. 2020, 29, 2717–2734. [Google Scholar] [CrossRef]
- Singha, D.K.; Chatterjee, R.; Sen, M.K.; Sain, K. Pore pressure prediction in gas-hydrate bearing sediments of Krishna–Godavari basin, India. Mar. Geol. 2014, 357, 1–11. [Google Scholar] [CrossRef]
- Singha, D.K.; Shukla, P.K.; Chatterjee, R.; Sain, K. Multi-channel 2D seismic constraints on pore pressure- and vertical stress-related gas hydrate in the deep offshore of the Mahanadi Basin, India. J. Asian Earth Sci. 2019, 180, 103882. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Greenberg, J.; Weare, J.H. The prediction of methane solubility in natural waters to high ionic strength from 0 to 250 °C and from 0 to 1600 bar. Geochim. Cosmochim. Acta 1992, 56, 1451–1460. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Tong, C.; Liu, P.; Wu, H.; Huang, Z. High temperature and high pressure gas enrichment condition, distribution law and accumulation model in central diapir zone of Yinggehai basin. China Offshore Oil Gas. 2015, 27, 1–12. [Google Scholar]
- Herkommer, M.A. How gas buoyancy creates shallow-zone geopressures. World Oil. 1993, 214, 63. [Google Scholar]
- Zhang, J. Pore pressure prediction from well logs: Methods, modifications, and new approaches. Earth-Sci. Rev. 2011, 108, 50–63. [Google Scholar] [CrossRef]
- Gao, G.; Huang, Z.L.; Wang, Z.F.; Quan, Y. Study on the mechanisms of the formation of formation abnormal high-pressure. J. Xi′Shiyou Univ. (Nat. Sci. Ed.) 2005, 20, 1–7. [Google Scholar]
- Barker, C. Aquathermal Pressuring; Role of Temperature in Development of Abnormal-Pressure Zones. AAPG Bull. 1972, 56, 2068–2071. [Google Scholar]
- Magara, K. Importance of Aquathermal Pressuring Effect in Gulf Coast: GEOLOGIC NOTE. AAPG Bull. 1975, 59, 2037–2045. [Google Scholar]
- Sharp, J.M., Jr.; Kort, M.H. Numerical Model of Shale Compaction, Aquathermal Pressuring, and Hydraulic Fracturing: ABSTRACT. AAPG Bull. 1982, 67, 547. [Google Scholar]
- Dai, J.X.; Wang, T.B.; Song, Y.; Zhang, H.N.; Xu, Y.C.; Zhang, Q.M. Formation Conditions and Distribution Law of Large and Medium-Sized Natural Gas Fields in China; Geology Press: Beijing, China, 1997. [Google Scholar]
- Luo, X.R. Quantitative Analysis on Overpressuring Mechanism Resulted from Tectonic Stress. Chin. J. Geophys. 2004, 47, 1086–1093. [Google Scholar] [CrossRef]
- Moore, J.C.; Shipley, T.H.; Goldberg, D.; Ogawa, Y.; Filice, F.; Fisher, A.; Jurado, M.J.; Moore, G.F.; Rabaute, A.; Yin, H. Abnormal fluid pressures and fault-zone dilation in the Barbados accretionary prism: Evidence from logging while drilling. Geology 1995, 23, 605. [Google Scholar] [CrossRef]
- Dugan, B.; Flemings, P.B. Overpressure and Fluid Flow in the New Jersey Continental Slope: Implications for Slope Failure and Cold Seeps. Science 2000, 289, 288–291. [Google Scholar] [CrossRef]
- Yin, X.L.; Li, S.T.; Yang, J.H.; Zhang, Q.M. Correlations between Overpressure Fluid Activity and Fault System in Yinggehai Basin. Acta Geosci. Sin. 2002, 23, 141–146. [Google Scholar]
- Han, S.; Bangs, N.L.; Hornbach, M.J.; Pecher, I.A.; Tobin, H.J.; Silver, E.A. The many double BSRs across the northern Hikurangi margin and their implications for subduction processes. Earth Planet. Sci. Lett. 2021, 558, 116743. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, S.; Wan, Z. The Thermodynamics of Mud Diapir/Volcano Fluid and Its Influence on Gas Hydrate Occurrence. Mar. Geol. Front. 2018, 34, 15–23. [Google Scholar]
- Wang, L.; Sha, Z.; Liang, J.; Lu, J. Analysis of Gas Hydrate Absence Induced by the Late-stage Diapir Domination in the Borehole SH5 of Shenhu Area. Geoscience 2010, 24, 450–456. [Google Scholar]
- Jowett, E.C.; Cathles, L.M.; Davis, B.W. Predicting depths of gypsum dehydration in evaporitic sedimentary basins. AAPG Bull. 1993, 77, 402–413. [Google Scholar]
- Shaw, H.F.; Primmer, T.J. Diagenesis in shales from a partly overpressured sequence in the Gulf Coast, Texas, USA. Mar. Pet. Geol. 1989, 6, 121–128. [Google Scholar] [CrossRef]
- Helset, H.M.; Lander, R.H.; Matthews, J.C.; Reemst, P.; Bonnell, L.M.; Frette, I. The role of diagenesis in the formation of fluid overpressures in clastic rocks. Nor. Pet. Soc. Spec. Publ. 2002, 11, 37–50. [Google Scholar]
- Meissner, F.F. Petroleum Geology of the Bakken Formation Williston Basin, North Dakota and Montana. In Proceedings of the Montana Geological Society: Twenty-Fourth Annual Conference: 1978 Williston Basin Symposium: The Economic Geology of Williston Basin, Denver, CO, USA, 24–27 September 1978. [Google Scholar]
- Hunt, J.M.; Whelan, J.K.; Eglinton, L.B.; Cathles, L.M.I. Gas generation—A major cause of deep Gulf Coast overpressures. Oil Gas. J. 1994, 92, 59–63. [Google Scholar]
- Dasgupta, S.; Chatterjee, R.; Mohanty, S.P. Magnitude, mechanisms, and prediction of abnormal pore pressure using well data in the Krishna-Godavari Basin, east coast of India. AAPG Bull. 2016, 100, 1833–1855. [Google Scholar] [CrossRef]
- Cobbold, P.R.; Mourgues, R.; Boyd, K. Mechanism of thin-skinned detachment in the Amazon Fan: Assessing the importance of fluid overpressure and hydrocarbon generation. Mar. Pet. Geol. 2004, 21, 1013–1025. [Google Scholar] [CrossRef]
- Rice, D.D.; Claypool, G.E. Generation, Accumulation, and Resource Potential of Biogenic Gas. AAPG Bull. 1981, 65, 5–25. [Google Scholar]
- Li, X.; Zhang, S.; Zhu, G.; Liang, Y. Types and Research Direction of Biogenic Gas in China. Nat. Gas Geosci. 2005, 16, 477–484. [Google Scholar]
- Helal, E.N.; Lala, A.; Ahmed, S.; Amr, T. The impact of gas chimneys on the reservoir characteristics, offshore Nile Delta, Egypt. Arab J. Geosci 2015, 8, 7929–7939. [Google Scholar] [CrossRef]
- Su, M.; Yang, R.; Wu, N.; Wang, H.; Liang, J.; Sha, Z.; Cong, X.; Qiao, S. Structural Characteristics in the Shenhu Area, Northern Continental Slope of South China Sea, and Their Influences on Gas Hydrate. Acta Geol Sin. Engl. 2014, 88, 318–326. [Google Scholar]
- Dou, Q.L.; Chen, J.F.; Wang, J.; Zhang, D.W. Advances in researches and outlook for microbial enhanced oil recovery. Nat. Gas. Geosci. 2004, 15, 559–563. [Google Scholar]
- Hata, T.; Saracho, A.C.; Haigh, S.K.; Yoneda, J.; Yamamoto, K. Microbial-induced carbonate precipitation applicability with the Methane Hydrate-bearing layer microbe. J. Nat. Gas. Sci. Eng. 2020, 81, 103490. [Google Scholar] [CrossRef]
- Li, C.; Luo, X.; Zhang, L.; Wang, B.; Lei, Y. Overpressure Generation Mechanisms and Its Distribution in the Paleocene Shahejie Formation in the Linnan Sag, Huimin Depression, Eastern China. Energies 2019, 12, 3183. [Google Scholar] [CrossRef] [Green Version]
- Mann, D.M.; Mackenzie, A.S. Prediction of pore fluid pressures in sedimentary basins. Mar. Pet. Geol. 1990, 7, 55–65. [Google Scholar] [CrossRef]
- Kida, M.; Khlystov, O.; Zemskaya, T.; Takahashi, N.; Minami, H.; Sakagami, H.; Krylov, A.; Hachikubo, A.; Yamashita, S.; Shoji, H. Coexistence of structure I and II gas hydrates in Lake Baikal suggesting gas sources from microbial and thermogenic origin. Geophys. Res. Lett. 2006, 33, L24603. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Canuel, E.A.; Chapman, N.R.; Spence, G.D.; Coffin, R.B. The origin of thermogenic gas hydrates on the northern Cascadia Margin as inferred from isotopic (13C/12C and D/H) and molecular composition of hydrate and vent gas. Org. Geochem. 2005, 36, 703–716. [Google Scholar] [CrossRef]
- Davie, M.K.; Zatsepina, O.Y.; Buffett, B. Methane solubility in marine hydrate environments. Mar. Geol. 2004, 203, 177–184. [Google Scholar] [CrossRef]
- Lu, H.; Lorenson, T.D.; Moudrakovski, I.L.; Ripmeester, J.A.; Collett, T.S.; Hunter, R.B.; Ratcliffe, C.I. The characteristics of gas hydrates recovered from the Mount Elbert gas hydrate stratigraphic test well, Alaska North Slope. Mar. Pet. Geol. 2011, 28, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Kida, M.; Suzuki, K.; Kawaraura, T.; Oyama, H.; Nagao, J.; Ebinuma, T.; Narita, H.; Suzuki, H.; Sakagami, H.; Takahashi, N. Characteristics of Natural Gas Hydrates Occurring in Pore-Spaces of Marine Sediments Collected from the Eastern Nankai Trough, off Japan. Energy Fuel 2009, 23, 5580–5586. [Google Scholar] [CrossRef]
- Kumar, A.; Maini, B.; Bishnoi, P.R.; Clarke, M.; Zatsepina, O.; Srinivasan, S. Experimental determination of permeability in the presence of hydrates and its effect on the dissociation characteristics of gas hydrates in porous media. J. Pet. Sci. Eng. 2010, 70, 114–122. [Google Scholar] [CrossRef]
- Song, Y.C.; Huang, X.; Liu, Y.; Yang, M.J. Experimental study of permeability of porous medium containing methane hydrate. J. Therm. Sci. Technol. 2010, 9, 51–57. [Google Scholar]
- Yamawaki, M.; Takeyama, N.; Katsumura, Y. Numerical study on permeability hysteresis during hydrate dissociation in hot water injection. Jpn. J. Appl. Phys. 2008, 47, 1104–1109. [Google Scholar] [CrossRef]
- Li, C.-H.; Zhao, Q.; Xu, H.-J.; Feng, K.; Liu, X.-W. Relation between relative permeability and hydrate saturation in Shenhu area, South China Sea. Appl. Geophys. 2014, 11, 207–214. [Google Scholar] [CrossRef]
- Konno, Y.; Yoneda, J.; Egawa, K.; Ito, T.; Jin, Y.; Kida, M.; Suzuki, K.; Fujii, T.; Nagao, J. Permeability of sediment cores from methane hydrate deposit in the Eastern Nankai Trough. Mar. Pet. Geol. 2015, 487–495. [Google Scholar] [CrossRef]
- Katoh, A.; Nakayama, K.; Baba, K.; Uchida, T. Model simulation for generation and migration of methane hydrate. Energy Explor. Exploit. 2000, 18, 401–421. [Google Scholar] [CrossRef]
- Posewang, J.; Mienert, J. The enigma of double BSRs: Indicators for changes in the hydrate stability field? Geo-Mar. Lett. 1999, 19, 157–163. [Google Scholar] [CrossRef]
- Andreassen, K.; Hart, P.E.; Grantz, A. Seismic studies of a bottom simulating reflection related to gas hydrate beneath the continental margin of the Beaufort Sea. J. Geophys. Res. Solid Earth 1995, 100, 12659–12673. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, J.Q.; Su, P.B.; Wang, L.F.; Lin, L.; Huang, W.A.; Wei, J.G.; Liang, J. Research progress and prospect of relationship between double bottom simulating reflector and the accumulation of gas hydrates. Geol. China 2020, 47, 29–42. [Google Scholar]
- Duan, Z.H.; Wei, Q. Model for the Calculation of the Solubility of CH_4, H_2S and CO_2 in Aqueous Solutions. Acta Geol. Sin. Engl. 2011, 85, 1079–1093. [Google Scholar]
- Wang, M.Z.; Niu, Y.B.; Wang, B.Y. Discussion about Relationship between Water′s Salinity and Methane′s Solubility. Coal Technol. 2016, 35, 178–180. [Google Scholar]
- Lu, W.; Chou, I.M.; Burruss, R.C. Determination of methane concentrations in water in equilibrium with sI methane hydrate in the absence of a vapor phase by in situ Raman spectroscopy. Geochim. Cosmochim. Acta 2008, 72, 412–422. [Google Scholar] [CrossRef]
- Rui, S.; Duan, Z. An accurate model to predict the thermodynamic stability of methane hydrate and methane solubility in marine environments. Chem. Geol. 2007, 244, 248–262. [Google Scholar]
- Tsimpanogiannis, I.N.; Economou, I.G.; Stubos, A.K. Methane solubility in aqueous solutions under two-phase (H–Lw) hydrate equilibrium conditions. Fluid Phase Equilibria 2014, 371, 106–120. [Google Scholar] [CrossRef]
- Xu, W. Modeling dynamic marine gas hydrate systems. Am. Miner. 2004, 89, 1271–1279. [Google Scholar] [CrossRef]
- Sahagian, D.L.; Proussevitch, A.A. Bubbles in volcanic systems. Nature 1992, 359, 485. [Google Scholar] [CrossRef]
- Wan, Z.F.; Xia, B.; He, J.X.; Liu, B.M. Analogy analysis on petroleum geological characteristics of the foreland basins between western China and central Asia. Nat. Gas Geosci. 2007, 18, 219–223. [Google Scholar]
- Guo, Z.F.; Zhen, L.; Peng, L.; Liu, W.C. Experimental analysis of aquathermal pressuring under high temperature conditions and its geological implications. Pet. Geol. Exp. 2016, 38, 836–841. [Google Scholar]
- Daines, S.R. Aquathermal pressuring and geopressure evaluation. AAPG Bull. 1982, 66, 931–939. [Google Scholar]
- Sharp, J.M., Jr. Permeability controls on aquathermal pressuring. AAPG Bull. 1983, 67, 2057–2061. [Google Scholar]
- Luo, X.; Vasseur, G. Contributions of compaction and aquathermal pressuring to geopressure and the influence of environmental conditions. AAPG Bull. 1992, 76, 1550–1559. [Google Scholar]
- Shi, X.; Cheng, Y.F.; Mei, W. Method for Formation Pore Pressure Prediction Based on Logging Data. J. Oil Gas Technol. 2012, 34, 94–98. [Google Scholar]
- Yardley, G.S.; Swarbrick, R.E. Lateral transfer: A source of additional overpressure? Mar. Pet. Geol. 2000, 17, 523–537. [Google Scholar] [CrossRef]
- Tingay, M.; Hillis, R.R.; Swarbrick, R.E.; Morley, C.K.; Damit, A.R. ‘Vertically transferred’ overpressures in Brunei: Evidence for a new mechanism for the formation of high-magnitude overpressure. Geology 2007, 35, 1023–1026. [Google Scholar] [CrossRef]
- Finkbeiner, T.; Zoback, M.; Flemings, P.; Stump, B. Stress, pore pressure, and dynamically constrained hydrocarbon columns in the South Eugene Island 330 Field, northern Gulf of Mexico. AAPG Bull. 2001, 85, 1007–1031. [Google Scholar]
- Ma, G.; Zhan, L.; Lu, H.; Hou, G. Structures in Shallow Marine Sediments Associated with Gas and Fluid Migration. J. Mar. Sci. Eng. 2021, 9, 396. [Google Scholar] [CrossRef]
- Mienert, J.; Posewang, J. Evidence of shallow- and deep-water gas hydrate destabilizations in North Atlantic polar continental margin sediments. Geo-Mar. Lett. 1999, 19, 143–149. [Google Scholar] [CrossRef]
- Xu, W. Phase balance and dynamic equilibrium during formation and dissociation of methane gas hydrate. In Proceedings of the Fourth International Conference on Gas Hydrates, Yokohama, Japan, 19–23 May 2002. [Google Scholar]
- Ashi, J.; Tokuyama, H.; Taira, A. Distribution of methane hydrate BSRs and its implication for the prism growth in the Nankai Trough. Mar. Geol. 2002, 187, 177–191. [Google Scholar] [CrossRef]
- Nathan, L.B.B.; Musgrave, R.J.; Tréhu, A.M. Upward shifts in the southern Hydrate Ridge gas hydrate stability zone following postglacial warming, offshore Oregon. J. Geophys. Res. Solid Earth 2005, 110, B03102. [Google Scholar]
- Yang, H.; Yu, S.; Lu, H. Iron-coupled anaerobic oxidation of methane in marine sediments: A review. J. Mar. Sci. Eng. 2021, 9, 875. [Google Scholar] [CrossRef]
- Weaver, C.E. Potassium, illite and the ocean. Geochim. Cosmochim. Acta 1967, 31, 2181–2196. [Google Scholar] [CrossRef]
- Lahann, R.W.; Swarbrick, R.E. Overpressure generation by load transfer following shale framework weakening due to smectite diagenesis. Geofluids 2011, 11, 362–375. [Google Scholar] [CrossRef]
- Bols, H.; Hermanrud, C.; Teige, G. Origin of overpressures in shales: Constraints from basin modeling. AAPG Bull. 2004, 88, 193–211. [Google Scholar]
- Vidar, S.; Ivar, B. Identifying time, temperature, and mineralogical effects on chemical compaction in shales by rock physics relations. Lead. Edge 2008, 27, 750–756. [Google Scholar]
- Chilingar, G.V.; Robertsonjr, J.; Riekeiii, H. Chapter 2 Origin of abnormal formation pressures. Dev. Pet. Sci. 2002, 50, 21–67. [Google Scholar]
- Wangen, M. A quantitative comparison of some mechanisms generating overpressure in sedimentary basins. Tectonophysics 2001, 334, 211–234. [Google Scholar] [CrossRef]
- Osborne, M.J.; Swarbrick, R.E. How Overpressure and Diagenesis Interact in Sedimentary Basins—Consequences for Porosity Preservation in HPHT Reservoir Sandstones. Indones. Pet. Assoc. 1997, 947–954. [Google Scholar]
- Ma, Y.; Huang, Y.; Yao, G.; Tao, C.; Pan, S. Effecting of Overpressure on Diagenesis of Huangliu Formation in DX Area, Yinggehai Basin. Geol. Sci. Technol. Inf. 2015, 34, 7–14. [Google Scholar]
- Tissot, B.P.; Welte, D.H. From Kerogen to Petroleum. in Petroleum Formation and Occurrence; Springer: Berlin/Heidelberg, Germany, 1984; pp. 160–198. [Google Scholar]
- Shurr, G.W.; Ridgley, J.L. Unconventional shallow biogenic gas systems. AAPG Bull. 2002, 86, 1939–1969. [Google Scholar]
- Wu, N.; Zhang, H.; Yang, S.; Zhang, G.; Liang, J.; Lu, J.A.; Su, X.; Schultheiss, P.; Holland, M.; Zhu, Y. Gas Hydrate System of Shenhu Area, Northern South China Sea: Geochemical Results. J. Geol. Res. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.-X.; Zhang, Q.-F.; He, S.-H.; Zhang, A.; Shan, H.-F.; Xia, T.-D. Variation in Micro-Pores during Dynamic Consolidation and Compression of Soft Marine Soil. J. Mar. Sci. Eng. 2021, 9, 750. [Google Scholar] [CrossRef]
- Wang, W. Laboratory research and field trials of microbial oil recovery technique. Oil Drill. Prod. Technol. 2012, 34, 107–113. [Google Scholar]
- Yang, J.; Ye, S.J.; Wu, J.C. Study on the influence of bioclogging on permeability of saturated porous media by experiments and models. Environ. Sci. 2011, 32, 1364–1371. [Google Scholar]
- Cheng, H.Y.; Wang, L.; Zhang, J.; Zhang, R.Y. Impacts of in situ microorganism growth and multiplication on permeability in porous medium. Spec. Oil Gas Reserv. 2010, 17, 98–101. [Google Scholar]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Paassen, L.A.V.; Qabany, A.A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Geotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Xu, T.; Shang, S.; Tian, H.; Cao, Y.; Wang, J.; Shi, Z.; Liu, X. Laboratory Experimental Study on the Formation of Authigenic Carbonates Induced by Microbes in Marine Sediments. J. Mar. Sci. Eng. 2021, 9, 479. [Google Scholar] [CrossRef]
- Bao, M.T.; Yuan, S.W.; Li, X.M.; Song, Z.Y.; Guo, L.Y. Effects of permeability of porous medium on the growth and metabolism of microorganism in reservoir. J. Shenzhen Univ. Sci. Eng. 2011, 28, 35–40. [Google Scholar]
- Swarbrick, R.E.; Osborne, M.J.; Yardley, G.S. Comparison of overpressure magnitude resulting from the main generating mechanisms. AAPG Mem. 2002, 76, 1–12. [Google Scholar]




| Classification | Mechanism | Intrinsic Driving Force | Cases Reported | References |
|---|---|---|---|---|
| Overpressure development associated with physical process | Disequilibrium compaction | Deposition rate greater than drainage rate in compaction | Gulf of Mexico; Baram province, Brunei; South China Sea; Nile Delta; Offshore Mumbai, Western India | [8,9,13,16,31,32] |
| Hydrate formation sealing | Permeability reduction | Mahanadi basin, India; Krishna-Godavari basin, India | [5,27,33,34] | |
| Degasification | Gas solubility change | Central diapir zone of the Yinggehai basin | [35,36] | |
| Buoyancy | Density difference between fluids | Dabis oilfield in the Malay basin | [37,38,39] | |
| Hydrothermal pressuring | Difference in thermal expansion coefficients of fluids | Gulf Coast | [40,41,42] | |
| Tectonic movement | Stress related permeability reduction | Barbados accretionary prism | [43,44,45] | |
| Overpressure transfer | Overpressured fluid migration due to permeability difference | New Jersey continental slope; Yinggehai basin; Baram province, Brunei | [9,19,46,47] | |
| Overpressure development associated with chemical process | Hydrate decomposition | Fluid volume expansion by gas released from hydrate dissociation | Nankai Trough; Hikurangi margin; Shenhu in the northern South China Sea | [28,29,48,49,50] |
| Diagenesis | Fluid volume expansion or permeability reduction by authigenic mineral formation | Gulf Coast; Halten Terrace offshore mid-Norway and the Gulf of Mexico | [13,51,52,53] | |
| Hydrocarbon generation | Fluid volume expansion by oil/gas generation | Gulf Coast; Amazon Fan; Krishna-Godavari basin, India | [13,54,55,56,57] | |
| Overpressure development associated with biological activity | Microbial gas production | Generation of biogenic gas | Offshore Nile Delta, Egypt; Shenhu area in the northern South China Sea | [58,59,60,61] |
| Microbial plugging | Permeability reduction | Nankai Trough | [62,63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhan, L.; Lu, H. Mechanisms for Overpressure Development in Marine Sediments. J. Mar. Sci. Eng. 2022, 10, 490. https://doi.org/10.3390/jmse10040490
Li C, Zhan L, Lu H. Mechanisms for Overpressure Development in Marine Sediments. Journal of Marine Science and Engineering. 2022; 10(4):490. https://doi.org/10.3390/jmse10040490
Chicago/Turabian StyleLi, Chong, Linsen Zhan, and Hailong Lu. 2022. "Mechanisms for Overpressure Development in Marine Sediments" Journal of Marine Science and Engineering 10, no. 4: 490. https://doi.org/10.3390/jmse10040490
APA StyleLi, C., Zhan, L., & Lu, H. (2022). Mechanisms for Overpressure Development in Marine Sediments. Journal of Marine Science and Engineering, 10(4), 490. https://doi.org/10.3390/jmse10040490






