New Record of Hydrothermal Vent Squat Lobster (Munidopsis lauensis) Provides Evidence of a Dispersal Corridor between the Pacific and Indian Oceans
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Mapping
2.2. DNA Barcoding
2.3. Estimation of Divergence Time
2.4. Heme-Binding Site of COX1 Sequences
3. Results and Discussion
3.1. Biogeographic Distribution of M. lauensis
3.2. Binding Sites of COX in M. lauensis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, C.T.S.; Vrijenhoek, R.C. Are hydrothermal vent animals living fossils? Trends Ecol. Evol. 2003, 18, 582–588. [Google Scholar] [CrossRef]
- Mullineaux, L.S.; Metaxas, A.; Beaulieu, S.E.; Bright, M.; Gollner, S.; Grupe, B.M.; Herrera, S.; Kellner, J.B.; Levin, L.A.; Mitarai, S.; et al. Exploring the ecology of deep-sea hydrothermal vents in a metacommunity framework. Front. Mar. Sci. 2018, 5, 49. [Google Scholar] [CrossRef]
- McArthur, A.G.; Tunnicliffe, V. Relics and Antiquity Revisited in The Modern Vent Fauna. In Modern Ocean Floor Processes and the Geological Record; Mills, R., Harrison, K., Eds.; Special Publication of the Geological Society of London: Burlington, UK, 1998; pp. 271–291. [Google Scholar]
- Van Dover, C.L. The Ecology of Deep-Sea Hydrothermal Vents; Princeton University Press: Oxford, UK, 2000. [Google Scholar]
- Van Dover, C.L. Ecology of Mid Atlantic Ridge Hydrothermal Vents. In Hydrothermal Vents and Process; Parson, C.M., Dixon, D.R., Eds.; Geological Society of London, Special Publication 887: London, UK, 1995; pp. 257–294. [Google Scholar]
- Gebruk, A.V.; Galkin, S.V.; Vereshchaka, A.L.; Moskalev, L.I.; Southward, A.J. Ecology and biogeography of the hydrothermal vent fauna of the Mid-Atlantic Ridge. Adv. Mar. Biol. 1997, 32, 93–144. [Google Scholar]
- Tunnicliffe, V. The biology of hydrothermal vents: Ecology and evolution. Oceanogr. Mar. Biol. Annu. Rev. 1991, 29, 319–407. [Google Scholar]
- Hashimoto, J.; Ohta, S.; Fujikura, K.; Miura, T. Microdistribution pattern and biogeography of the hydrothermal vent communities of the Minami-Ensie Knoll in the mid-Okinawa trough, Western Pacific. Deep Sea Res. I 1995, 42, 577–598. [Google Scholar] [CrossRef]
- Hashimoto, J.; Ohta, S.; Gamo, T.; Chiba, H.; Yamaguchi, T.; Tsuchida, S.; Okudaira, T.; Watabe, H.; Yamanaka, T.; Kitazawa, M. First hydrothermal vent communities from the Indian Ocean discovered. Zool. Sci. 2001, 18, 717–721. [Google Scholar] [CrossRef]
- Van Dover, C.L.; Humphris, S.E.; Fornari, D.; Cavanaugh, C.M.; Collier, R.; Goffredi, S.K.; Hashimoto, J.; Lilley, M.D.; Reysenbach, A.L.; Shank, T.M.; et al. Biogeography and ecological setting of Indian Ocean hydrothermal vents. Science 2001, 294, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Haney, T.A. Decapod crustaceans from hydrothermal vents and cold seeps: A review through 2005. Zool. J. Linn. Soc. 2005, 145, 445–522. [Google Scholar] [CrossRef]
- Yang, C.H.; Tsuchida, S.; Fujikura, K.; Fujiwara, Y.; Kawato, M.; Chan, T.Y. Connectivity of the squat lobsters Shinkaia crosnieri (Crustacea: Decapoda: Galatheidae) between cold seep and hydrothermal vent habitats. Bull. Mar. Sci. 2016, 92, 17–31. [Google Scholar] [CrossRef]
- Hui, M.; Song, C.; Liu, Y.; Li, C.; Cui, Z. Exploring the molecular basis of adaptive evolution in hydrothermal vent crab Austinograea alayseae by transcriptome analysis. PLoS ONE 2017, 12, e0178417. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.E.; Sha, Z.; Wang, Y. Divergence history and hydrothermal vent adaptation of decapod crustaceans: A mitogenomic perspective. PLoS ONE 2019, 14, e0224373. [Google Scholar]
- Baba, K. Deep-sea chirostylid and galatheid Crustaceans (Decapoda: Anomura) from the Indo-Pacific, with a list of species. Galathea Rep. 2005, 20, 317. [Google Scholar]
- Macpherson, E. Species of the genus Munidopsis Whiteaves, 1784 from the Indian and Pacific oceans and reestablishment of the genus Galacantha A. Milne-Edwards, 1880 (Crustacea, Decapoda, Galatheidae). Zootaxa 2007, 1417, 1–135. [Google Scholar] [CrossRef]
- Williams, A.B. New Marine decapod crustaceans from waters influenced by hydrothermal discharge, brine and hydrocarbon seepage. Fish. Bull. 1988, 86, 213–287. [Google Scholar]
- Williams, A.B.; Baba, K. New squat lobsters (Galatheidae) from the Pacific Ocean: Mariana Back Arc, East Pacific Rise and Cascadian Basin. Fish. Bull. 1989, 87, 899–910. [Google Scholar]
- Baba, K. A new squat lobster (Decapoda: Anomura: Galatheidae) from an active thermal vent area in the North Fiji Basin, SW Pacific. Crust. Res. 1995, 24, 188–193. [Google Scholar] [CrossRef][Green Version]
- Macpherson, E.; Segonzac, M. Species of genus Munidopsis (Decapoda, Anomura, Galatheidae) from the deep Atlantic Ocean, including cold seeps and hydrothermal vent area. Zootaxa 2005, 1095, 1–60. [Google Scholar] [CrossRef]
- Cubelio, S.S.; Tsuchida, S.; Hendrickx, M.E.; Kado, R.; Watanabe, S. A new species of vent associated Munidopsis (Crustacea: Decapoda: Anomura: Galatheidae) from the Western Pacific, with notes on its genetic identification. Zootaxa 2007, 1435, 25–36. [Google Scholar] [CrossRef]
- Cubelio, S.S.; Tsuchida, S.; Watanabe, S. New Species of Munidopsis (Decapoda: Anomura: Galatheidae from hydrothermal vent areas of Indian and Pacific Oceans. J. Mar. Biol. Assoc. UK 2008, 88, 111–117. [Google Scholar] [CrossRef]
- Cubelio, S.S.; Tsuchida, S.; Watanabe, S. Vent Associated Munidopsis (Decapoda: Anomura: Galatheidae) from Brothers Seamount, Kermadec Arc, Southwest Pacific, with Description of One New Species. J. Crustac. Biol. 2007, 3, 513–519. [Google Scholar] [CrossRef][Green Version]
- Thaler, A.D.; Plouviez, S.; Saleu, W.; Alei, F.; Jacobson, A.; Boyle, E.A.; Van Dover, C.L. Comparative population structure of two deep-sea hydrothermal-vent-associated decapods (Chorocaris sp. 2 and Munidopsis lauensis) from southwestern Pacific back-arc basins. PLoS ONE 2014, 97, e101345. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Macpherson, E.; Poore, G.C.; Ahyong, S.T.; Bermudez, A.; Cabezas, P.; Schnabel, K.E. Catalogue of squat lobsters of the world (Crustacea: Decapoda: Anomura families Chirostylidae, Galatheidae and Kiwaidae). Zootaxa 2008, 1905, 1–220. [Google Scholar] [CrossRef]
- Lin, C.W.; Tsuchida, S.; Lin, S.; Berndt, C.; Chan, T.Y. Munidopsis lauensis Baba & de Saint Laurent, 1992 (Decapoda, Anomura, Munidopsidae), a newly recorded squat lobster from a cold seep in Taiwan. Zootaxa 2013, 92–96. [Google Scholar] [CrossRef]
- Goffredi, S.K.; Warén, A.; Orphan, V.J.; Van Dover, C.L.; Vrijenhoek, R.C. Novel forms of structural integration between microbes and a hydrothermal vent gastropod from the Indian Ocean. Appl. Environ. Microbiol. 2004, 70, 3082–3090. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Shi, P.; Sun, Y.B.; Zhang, Y.P. Relaxation of selective constraint on avian mitochondrial DNA following the degeneration of flight ability. Genome Res. 2009, 19, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Liang, L.; Zhu, Z.H.; Zhou, W.P.; Irwin, D.M.; Zhang, Y.P. Adaptive evolution of energy metabolism genes and the origin of flight in bats. Proc. Natl. Acad. Sci. USA 2010, 107, 8666–8671. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gao, W.; Gao, Y.; Tang, S.; Huang, Q.; Tan, X.; Chen, J.; Huang, T. Mitochondrial genome analysis of Ochotona curzoniae and implication of cytochrome c oxidase in hypoxic adaptation. Mitochondrion 2008, 8, 352–357. [Google Scholar] [CrossRef]
- Gering, E.J.; Opazo, J.C.; Storz, J.F. Molecular evolution of cytochrome b in high- and low-altitude deer mice (genus Peromyscus). Heredity 2009, 102, 226–235. [Google Scholar] [CrossRef][Green Version]
- Scott, G.R.; Schulte, P.M.; Egginton, S.; Scott, A.L.; Richards, J.G.; Milsom, W.K. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 2011, 28, 351–363. [Google Scholar] [CrossRef]
- Wang, Z.; Yonezawa, T.; Liu, B.; Ma, T.; Shen, X.; Su, J.; Guo, S.; Hasegawa, M.; Liu, J. Domestication relaxed selective constraints on the yak mitochondrial genome. Mol. Biol. Evol. 2011, 28, 1553–1556. [Google Scholar] [CrossRef]
- Gu, M.; Dong, X.; Shi, L.; Shi, L.; Lin, K.; Huang, X.; Chu, J. Differences in mtDNA whole sequence between Tibetan and Han populations suggesting adaptive selection to high altitude. Gene 2012, 496, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Shen, X.; Irwin, D.M.; Shen, Y.; Zhang, Y. Mitogenomic analyses propose positive selection in mitochondrial genes for high-altitude adaptation in galliform birds. Mitochondrion 2014, 18, 70–75. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, R.R.; Johnson, W.E.; O’Brien, S.J.; Ramos, M.J.; Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genomics 2008, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Siebenaller, J.F.; Garrett, D.J. The effects of the deep-sea environment on transmembrane signaling. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 131, 675–694. [Google Scholar] [CrossRef]
- Hebert, P.D.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Santana, M.; Teixeira, M. A novel scenario for the evolution of heme–copper oxygen reductases. Biochim. Biophys. Acta Bioenerg. 2001, 1505, 185–208. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Andreakis, N.; Taylor, J. Mitochondrial phylogeny of the deep-sea squat lobsters, Munidopsidae (Galatheoidea). Zool. Anz. 2011, 250, 367–377. [Google Scholar] [CrossRef][Green Version]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acid. Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A Simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Ho, S.Y.W.; Phillips, M.J.; Rambaut, A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006, 4, e88. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, P.; Rieb, E.; Abrami, G.; Lücking, A.; Mehler, A. TreeAnnotator: Versatile Visual Annotation of Hierarchical Text Relations. In Proceedings of the Eleventh International Conference on Language Resources and Evaluation (LREC 2018, European Language Resources Association (ELRA)), Paris, France, 7–12 May 2018. [Google Scholar]
- Sievers, F.; Higgins, D.G. Clustal omega. Curr. Protoc. Bioinform. 2014, 48, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gouet, P.; Robert, X.; Courcelle, E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 2003, 31, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Server, S.M. SWISS-MODEL SERVER (https://swissmodel. expasy. org). Int. J. Mol. Sci. 2014, 15, S10. [Google Scholar]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 3 January 2022).
- German, C.R.; Baker, E.T.; Mevel, C.; Tamaki, K. Hydrothermal activity along the southwest Indian ridge. Nature 1998, 395, 490–493. [Google Scholar] [CrossRef]
- Kim, M.; Kang, J.-H.; Kim, D. Holoplanktonic and meroplanktonic larvae in the surface waters of the Onnuri Vent Field in the Central Indian Ridge. J. Mar. Sci. Eng. 2022, 10, 158. [Google Scholar] [CrossRef]
- Wikström, M.; Krab, K.; Sharma, V. Oxygen activation and energy conservation by cytochrome c oxidase. Chem. Rev. 2018, 118, 2469–2490. [Google Scholar] [CrossRef] [PubMed]

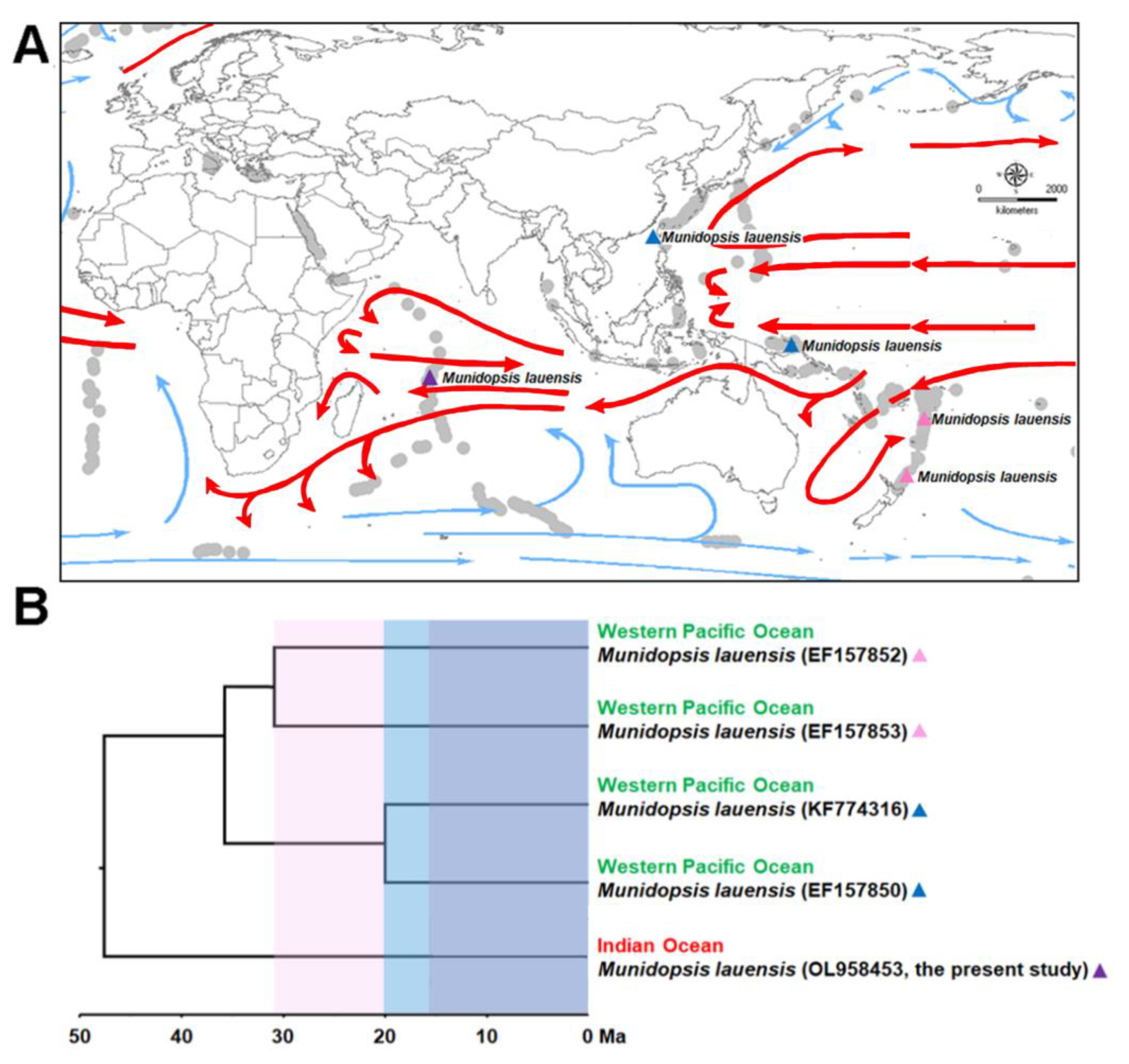
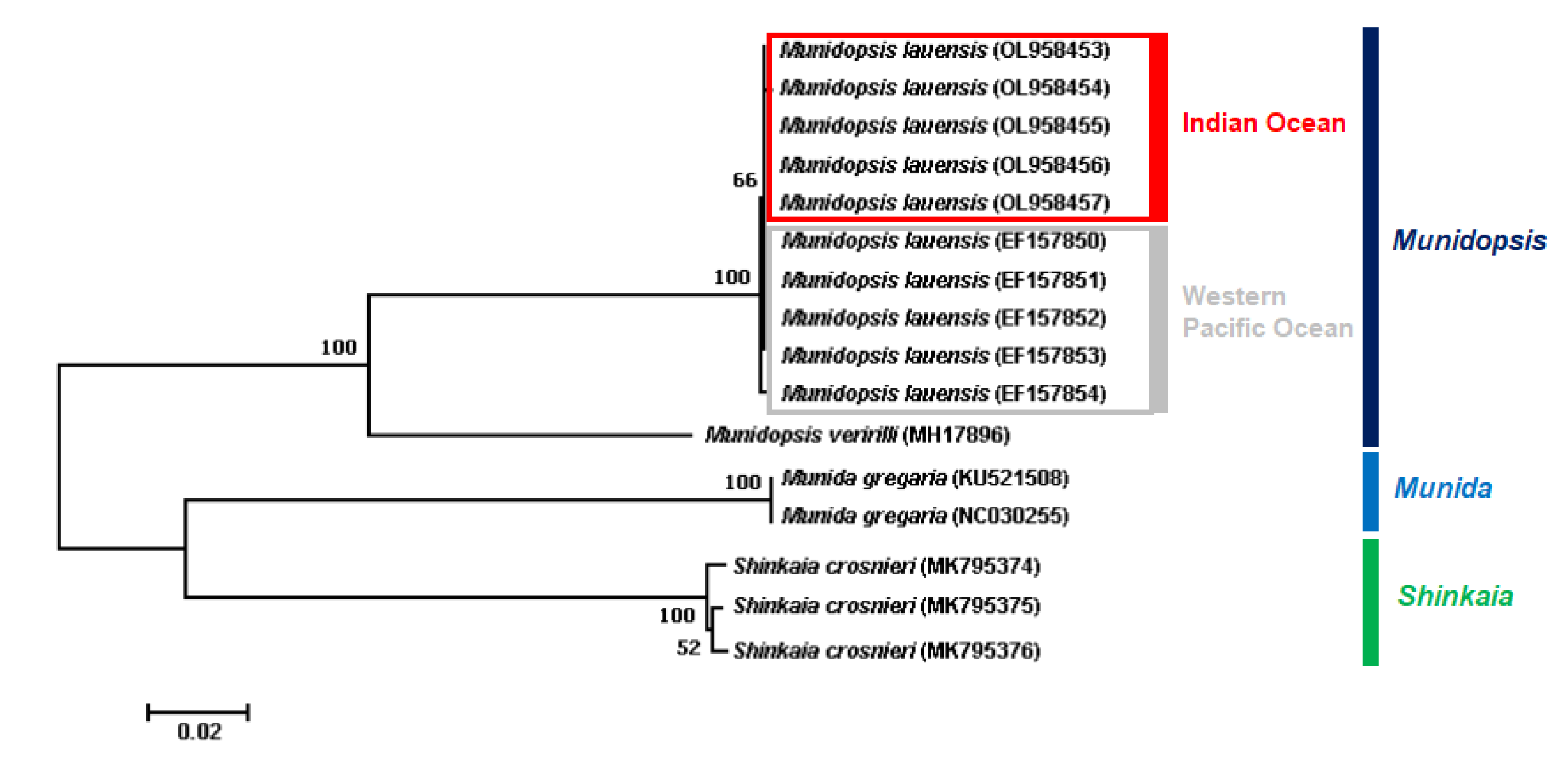
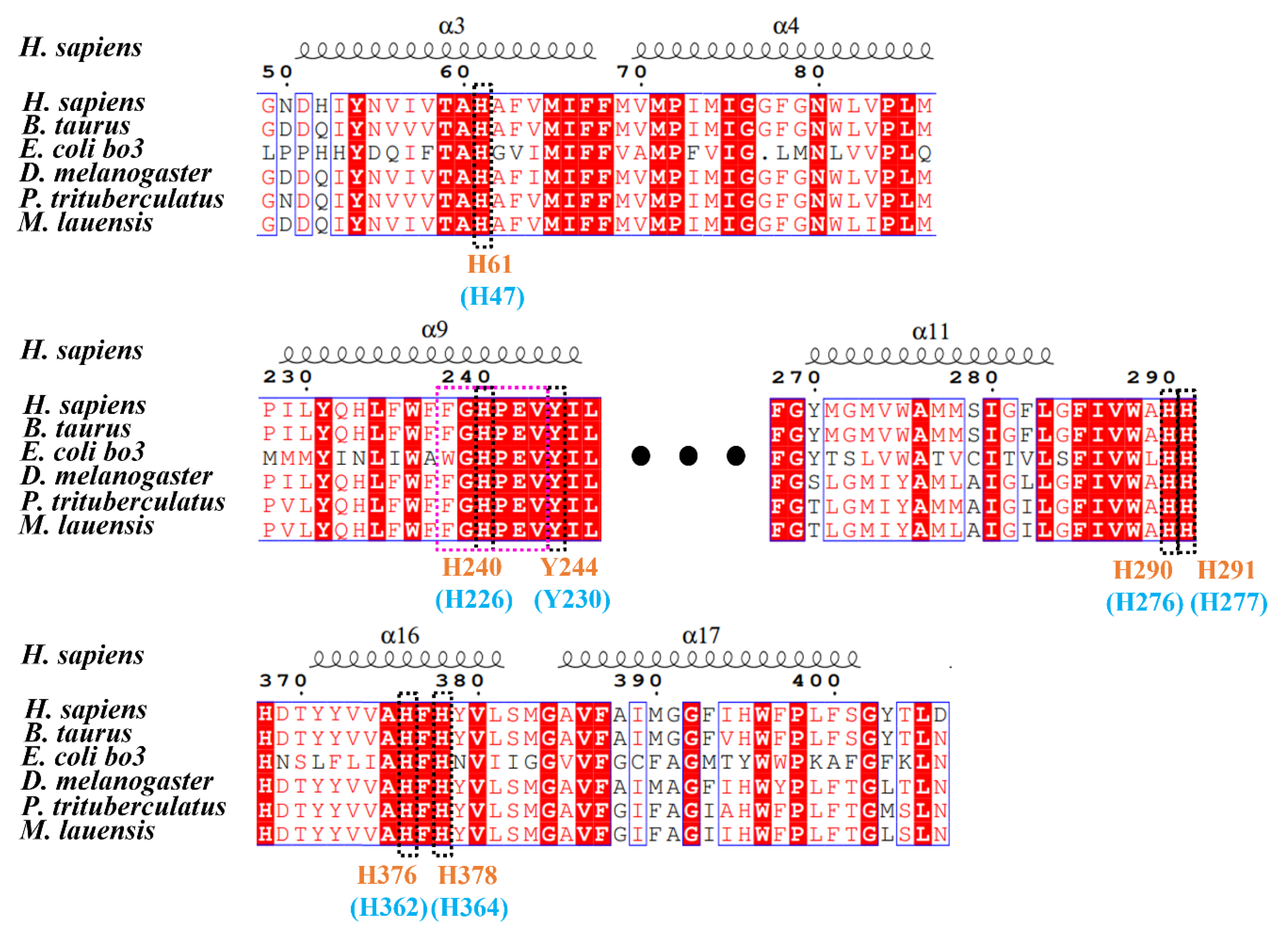

| Ocean | Hydrothermal Vent | Depth | Species | Reference |
|---|---|---|---|---|
| Western Pacific Ocean | Brother Seamount (34°51′45.4″ S, 179°03′28.6″ E) | 1649–1750 m | M. lauensis | [23] |
| 1649–1992 m | M. sonne | |||
| 1649 m | M. kermadec | |||
| North Fiji Basin (16°59′00.0″ S, 173°55′00.0″ E) | no record | M. lauensis | [21] | |
| M. sonne | ||||
| M. starmer | ||||
| Lau Basin (20°59′21.0″ S, 176°34′06.0″ W) | 1750 m | M. lauensis | [21,24] | |
| Izu-Bonin arc (32°06.25′ N, 139°52.17′E) | 1288–1625 m | M. myojinensis | [21] | |
| Manus Basin (3°43′49.4″ S, 151°40′27.5″ E) | no record | M. lauensis | [21] | |
| Mariana (18°11′00.0″ N, 144°45′00.0″ E) | no record | M. marianica | [21] | |
| Formosa Ridge (22°06′54.0″ N 119°17′06.0″ E) | 1750–2000 m | M. lauensis | [25] | |
| Indian Ocean | Onnuri Vent Field(11°24′54.9″ S, 66°25′24.0″ E) | 2023 m | M. lauensis | the present study |
| Kairei Field (25°19′14.4″ S, 70°02′24.0″ E) | 2422–2435 m | M. larticorpus | [22] | |
| Forecast Vent Field/Mariana Back Arc Basin (13°23.07′ N 145°55.02′ E) | 1450 m | M. gracilis | [22] | |
| Mid-Atlantic Ridge | Mid-Atlantic Ridge (1°38′50.7″ N 19°42′41.1″ W) | no record | M. acutispina | [22] |
| Mid-Atlantic Ridge (2°18′10.9″ N 25°38′33.9″ W) | no record | M. exuta | [21,26] | |
| East Pacific Rise S of Baja California (20°49′36.0″ N 109°06′00.0″ W) | 3502 m | M. lentigo | ||
| Northern Pacific Ocean | Galapagos Rift (eastern Pacific 13° N and 21° N areas) | no record | M. subsquamosa | [25] |
| Limbo Vent, Juan De Fuca Ridge (46°00′02.0″ N 129°59′59.1″ W) | 1545–2008 m | M. alvisca | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-s.; Cho, B.; Cho, J.; Park, B.; Kim, T. New Record of Hydrothermal Vent Squat Lobster (Munidopsis lauensis) Provides Evidence of a Dispersal Corridor between the Pacific and Indian Oceans. J. Mar. Sci. Eng. 2022, 10, 400. https://doi.org/10.3390/jmse10030400
Hwang H-s, Cho B, Cho J, Park B, Kim T. New Record of Hydrothermal Vent Squat Lobster (Munidopsis lauensis) Provides Evidence of a Dispersal Corridor between the Pacific and Indian Oceans. Journal of Marine Science and Engineering. 2022; 10(3):400. https://doi.org/10.3390/jmse10030400
Chicago/Turabian StyleHwang, Hee-seung, Boongho Cho, Jaemin Cho, Beomseok Park, and Taewon Kim. 2022. "New Record of Hydrothermal Vent Squat Lobster (Munidopsis lauensis) Provides Evidence of a Dispersal Corridor between the Pacific and Indian Oceans" Journal of Marine Science and Engineering 10, no. 3: 400. https://doi.org/10.3390/jmse10030400
APA StyleHwang, H.-s., Cho, B., Cho, J., Park, B., & Kim, T. (2022). New Record of Hydrothermal Vent Squat Lobster (Munidopsis lauensis) Provides Evidence of a Dispersal Corridor between the Pacific and Indian Oceans. Journal of Marine Science and Engineering, 10(3), 400. https://doi.org/10.3390/jmse10030400







