1. Introduction
Global energy concerns have led to the rise of renewable energy technologies, thus far primarily land-based operations. More recent forays look to the ocean as well, with marine energy (ME), including wave energy converters (WECs), of increasing interest. The introduction of these novel structures in the marine environment presents new challenges and concerns related to uncertainties surrounding a variety of environmental stressors [
1,
2]. Several stressors related to ME devices, such as noise, electromagnetic fields, changes in habitat, and collision risks, have been identified and received significant attention [
2]. Light has not yet been extensively studied for its role as a stressor in relation to ME installations. While some lighting will be required for safety, navigation, and maintenance purposes—such as the lit buoy shown in
Figure 1—and careful lighting design choices that are informed by ecological concerns can help prevent or mitigate the impacts on wildlife and the environment. Throughout this work, we review the current lighting requirements relevant to ME devices, discuss what may be done to minimize the ecological impacts of necessary lighting, and recommend next steps to address the challenges and potential problems before ME installations go in the water. In this introduction, a general discussion of ME is followed by an overview of light as a stressor to contextualize the latter sections of this review.
1.1. Marine Energy
The development of ME as a key sector contributing to the nation’s sustainable climate goals hinges on its ability to provide significant renewable power with limited environmental impacts at a reasonable levelized cost of energy. The total technical ME resource within U.S. exclusive economic zone waters can provide up to 57% of the energy that was generated by all 50 states in 2019 [
3]. Still, significant challenges remain surrounding the deployment and cycled testing of these novel structures and devices in marine and coastal habitats. Concerns of regulatory and permitting agencies responsible for managing these ecosystems stem from limited deployments of ME devices and a lack of environmental monitoring data in U.S. waters. In this literature review, we begin to address environmental concerns for anthropogenic light in the marine environment used to aid mariner navigation, safety, and device condition monitoring at ME installations. Important groups of marine fauna sensitive to light and found in existing and proposed ME project environments are identified. We also describe next steps for research toward achieving the proactive goal of establishing best practices for ME lighting and technologies that will help reduce the effects of anthropogenic light as a stressor of regulatory concern for ME installations.
1.2. Stressor: Discrete Light Sources
Human-made or anthropogenic lighting has become more prominent as technological advances have created more lighting options and population growth has increased lighting needs. Anthropogenic lighting has increasingly been viewed as a pollutant and ecological stressor, which should be further investigated and addressed with technological solutions and best practices to reduce its potential widespread impacts on wildlife and adjacent habitats [
4,
5,
6,
7]. Studies of terrestrial light pollution have been conducted for a wide variety of organisms, but marine life has been less well studied [
8,
9,
10,
11]. Reviews of lighting effects on marine life largely focus on the effects on coastal regions where cities and towns can be nearby [
12,
13,
14,
15,
16,
17,
18]. Light as a stressor can be broadly categorized as either discrete sources of light, such as individual streetlamps or buoy lights, and skyglow. Skyglow is the diffuse scattered light that brightens the night sky, particularly the anthropogenic portion of skyglow that comes from the light domes of cities and towns, and discussed at length in several reviews [
19,
20,
21,
22]. While skyglow can be a naturally occurring phenomenon, human-made skyglow can substantially brighten the night sky by orders of magnitude in unnatural ways, thereby affecting ecological and biological functions [
22].
Figure 2 shows skyglow from a city as a diffuse dome of light.
Discrete bright sources in the open ocean, such as ships, fishing vessels, or ME devices, introduce additional light that affects local environments. The increased intensity or changed spectrum/color caused by discrete lighting sources can further alter species behavior, physiology, and ecosystem structure beyond the alterations already caused by skyglow [
7,
9,
22]. Additionally, some discrete lighting choices can contribute further to unwanted skyglow. The installation of ME devices, in the formation of an arrayed system, therefore necessitates in-depth consideration of discrete sources of light in the open ocean. Some studies have addressed this topic by looking at light on oil rigs, commercial fishing and container ships, and controlled experiments involving high light levels [
23,
24,
25,
26,
27]. In this work, we primarily focus on discrete sources of light as the main lighting stressor that ME installations introduce.
1.3. Scope of Review
This review of lighting impacts on marine life focuses on discrete lighting sources like those necessary for ME installations. Most of the considerations herein are broadly applicable to ME devices, which are largely underwater but have lighting above the water’s surface. The structures associated with ME installations are typically smaller in subaerial profile than what would be of concern for aircraft—like offshore wind installations, which can easily be over 200 ft/61 m tall and are expected to increase in height in the future [
28]. For this reason, discussion in this review most accurately addresses the interests of ME devices such as WECs and current energy converters, while offshore wind installations have additional concerns not addressed in this review. We use the PacWave site in Oregon as a case study to provide an example of particular species’ interactions and other details such as weather and climate [
29]. PacWave is a fully permitted and licensed U.S. Department of Energy supported WEC testing facility under construction approximately 7 nautical miles (NM) off the coast of Newport, OR, USA.
Section 2 discusses the legal and technical requirements for ME lighting, focusing on the specific case of WECs in U.S. open water areas. These requirements have been determined based on the understanding of WECs size and the distribution of devices into arrays of structures for installations.
Section 3 focuses on the choices available to lighting designers and how these lighting technologies and methods affect wildlife and the environment. The choices available are largely driven by the perspective of lighting scientists, where specific guidance is given through requirements discussed in
Section 2.
Section 4 addresses lighting factors that primarily affect the marine environment. As there is a significant body of literature surrounding anthropogenic lighting in terrestrial environment, these factors are highlighted to promote marine-specific considerations.
Section 5 considers categories of wildlife and the unique ways anthropogenic lighting influences each. The discussion herein is structured to follow generalized groupings of wildlife, as given by regulatory guidelines or research areas. Species that exist in or around the PacWave site in Oregon are explicitly addressed as a case study. Research referenced in this review is largely limited to studies which target open ocean ecosystems or species and provide sufficient conclusions on lighting as a stressor. The lessons from this review can be applied to other sites and ME types as appropriate, taking into consideration the concerns and unique challenges of other environments and ME installations.
2. Requirements for Marine Energy Lighting
Because ME devices have not yet been deployed on a large scale, lighting standards for them have not been extensively explored. Structures that share similarities to ME devices can provide us with insight into lighting requirements, but arrays of these structures will require unique solutions. Minimum requirements mandated by law, safety, and technical needs are identified here, followed by an overview of the associated ecological impacts. In the following sections, these sets of requirements are considered together to make recommendations about how to install lighting that complies with the determined constraints. This is not intended to supersede or completely describe regulations for any project, but rather are intended to be used in conjunction with federal, state, or local guidance to develop best practices for lighting during ME deployments.
2.1. Safety
Several governing bodies provide recommendations and requirements pertaining to lighting for open ocean structures on the Outer Continental Shelf (OCS). These recommendations and requirements are typically categorized based on the structures’ locations and size, in particular their height. The United States Department of the Interior Bureau of Ocean Energy Management Office of Renewable Energy Programs (BOEM), the United States Coast Guard (USCG), and the Federal Aviation Authority (FAA) have published guidance for offshore wind facility lighting [
30,
31,
32,
33]. Lighting standards for other types of renewable ME installations have been less widely issued to date, typically falling under broader categorizations for open ocean structures. By applying the guidelines for offshore wind and other open ocean structures to ME devices, we can better understand the navigation and safety needs of ME installations.
As part of the BOEM application for ME devices, the lighting of the structures should be described [
30]. BOEM regulations require lighting in all facilities to be safe and to not unreasonably interfere with other uses of the OCS. In addition, the best available and safest technology should be used as well as best management practices [
30]. Further practical guidance is needed to understand what this means in the context of a given installation. FAA regulations apply to structures over 200 ft/61 m in height, with the need for aviation-focused lighting on many offshore wind structures. For WECs and other devices expected to be significantly under 200 ft/61 m in height, BOEM and FAA recommend, but do not require, aviation red obstruction lights that are steady burning, flashing, and/or flashing omnidirectional [
30,
31]. The USCG requires structures to have installed private aids to navigation, which typically consist of flashing yellow lights during low-light or restricted visibility conditions [
30,
32,
33]. In the case of ME installations, the specific lighting needs will be dictated in part by the number of devices and their layout in relation to each other. For such an array of structures, the lights on the inner structures can be dimmer and have lower flash rates than the outer structure lighting. These regulations are summarized in
Table 1, which also provides color guidelines as given by relevant authorities [
30,
31,
32,
34].
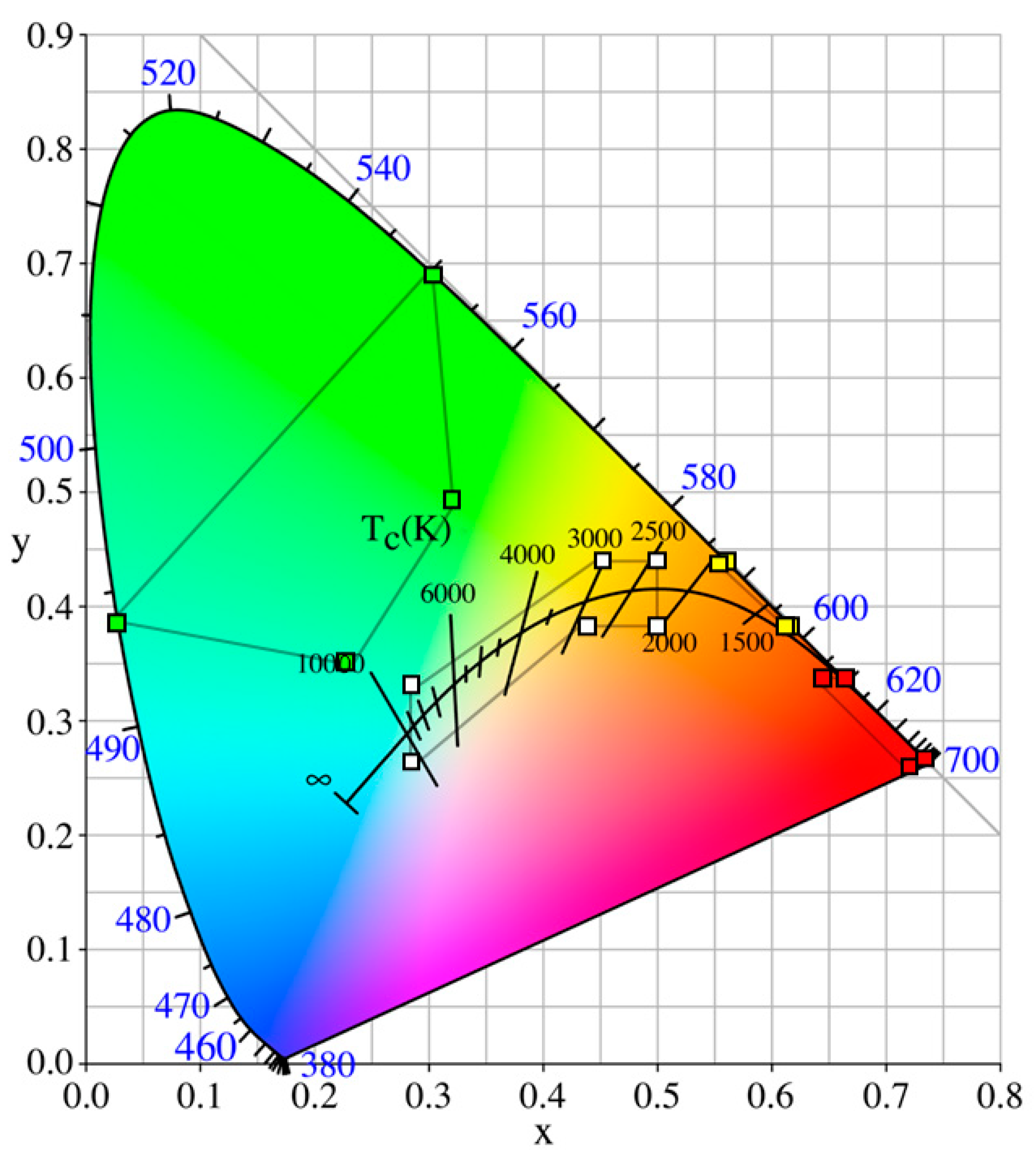
Figure 3 depicts a chromaticity diagram indicating these colors in a graphical format. The International Commission on Illumination (CIE) chromaticity diagram is based on human color vision and therefore does not provide direct spectral information. Similarly, the use of intensity metrics such as candela focuses on a human-centric scale, which has a number of limitations. Updating the guidelines to ensure full spectral information is given would enhance understanding regarding animal-centric, ecological impacts. This is discussed further in
Section 3.
2.2. Maintenance
For the purposes of this review, we have classified lighting that is not used for navigation or external visibility for human safety around ME devices as maintenance lighting. This includes lighting for the purpose of device monitoring as well as operational task lighting. Some smaller WECs may have limited or no maintenance lighting, whereas larger WECs with significant area above the water’s surface may have more. In general, maintenance lighting should have a defined purpose, such as assisting onboard personnel with required tasks. If the lights can be switched off during periods when they are unnecessary, appropriate controls such as switches or timers should be provided to do so. Large area floodlights should be avoided and lighting should not be directed upward or outside the boundaries of the structure.
2.3. Ecological
While some lighting regulations mention the desire to minimize ecological impacts, this subject necessitates more specific guidance. Overall, the lighting of the water should be limited to minimize impacts on marine life, and bird-friendly lighting should be implemented with no upward emission, which will also limit contributions to skyglow. The environmental recommendations offered by BOEM to minimize the impacts of offshore wind facility lighting on birds, bats, marine mammals, turtles, and fish are also applicable to other ME lighting. Generally, they recommend minimizing the number of lights, their intensity, and lighting duration whenever and wherever possible, provided the lighting remains adequate for safety [
30]. This means using flashing lights with low flash rates instead of steady burning lights, minimizing the lighting of the water surface, directing light only to where it is needed, and using automatic controls to shut off lighting not related to FAA or USCG regulations [
30].
These general guidelines are broadly applicable, allowing specific environmental concerns to be addressed by the lighting designer. Subsequent sections of this review discuss how to manage the effectiveness of lighting with best practices for reducing ecological impacts, focusing on methods for understanding specific installation needs. While some generalizations can be made, attention should be paid to the unique environment and setup, including weather, species demographics, adjacency to land, and the type of ME devices. Additionally, the spectrum of the lighting can be a factor for consideration. Although there are guidelines for lighting color, as given in
Figure 3, this measure of color is based on typical human vision and does not represent what is seen by animals. The spectrum of light between two light sources that achieve the same color point can be different, and therefore may affect wildlife differently. This is discussed further in
Section 3.3.
3. Considerations for Lighting Installations
Given the regulations and requirements for ME device lighting presented in
Section 2, the best-case scenario would meet the minimum safety and legal needs while causing no ecological impact. Although it may be difficult to ensure no environmental or wildlife impacts, lighting designers have a variety of options at their disposal to work toward minimizing impacts. Choices include where the lights are placed and directed, what intensity and color the light sources provide, and how the lights change intensity and direction. Each of these considerations is discussed in turn, focusing on how each choice might be made to achieve the optimal effect. Compared to previous lighting technologies, current and upcoming light-emitting diode (LED) lighting technologies provide opportunities for enhanced control over all these choices. The general guidance for light at night proposed here for ME is in alignment with best practices agreed upon by the Illuminating Engineering Society [
35].
3.1. Location and Direction
One of the most meaningful choices for lighting installations is to incorporate lighting only where necessary. This can be done through proper placement of light sources in addition to directing the light beams effectively. When deciding these factors, the purpose of the light should be clearly understood, while also knowing where unwanted light might cause harmful effects. The amount of lighting should be targeted to achieve minimum required or necessary light levels, by reducing the number of lights or by moving from general area lighting to specifically focused task-based lighting. Light should not be allowed to spill out into the water or up into the sky unless for a particular purpose. For instance, studies of oil rigs using floodlighting indicated the lighted area extended more than 328 ft/100 m past the rig itself; light spill such as this serves no purpose and may increase ecological impacts [
24]. In the case of private aid to navigation lights, vertical beam spread is not specified and intensities are specified by the minimum requirements [
32,
33]. It is therefore prudent to minimize vertical beam spread and intensity while still maintaining what is necessary for safety and navigation.
Figure 4A shows a WEC lit by too many lights, which are undirected and therefore light up the surrounding ocean. In
Figure 4B, the reduction in both the number of lights and the spread of the light beams limits the ocean illumination.
Some lighting on ME devices is necessary for guidance or hazard avoidance of ships or airplanes. In both cases, any light that is not seen by someone on the other craft or detected by navigation systems serves no purpose. To be seen by seacraft, the lights should be largely in the horizontal direction with limited vertical spread either up into the sky or down into the water. For aircraft guidance, horizontal and vertical spread is needed but light directed downward is of limited use in most situations. To maintain high directionality, the lighting fixture chosen should contain proper optics to achieve the spread required with minimal light loss [
36]. Shielding the light to avoid directions beyond the desired angles of interest may also be useful where more precise control is desired. Careful installation to focus the lights in the proper direction is also necessary where directional lights are used instead of wide-angle lights. This is a typical design consideration for general illumination lighting, where the optical distribution and placement of lighting products is considered for achieving targeted light levels [
36].
3.2. Intensity
In addition to controlling the direction of light, another way to limit the amount of light is by reducing its overall intensity. Different light intensities are mandated by various regulations and are often given in units of luminous intensity such as candela, which is a measure of human-perceived light within a solid angle (lumens per steradian) [
30,
31]. Luminous intensity is related to human vision by weighting the power of light at each wavelength by how strongly humans see that wavelength of light. By using this metric, the wavelength of the light cannot be decoupled from the optical power. Human-centric measurements can be useful in understanding how two sources with different light output powers and colors can be compared when seen by humans, but this metric does not translate well to other animals. To retain the most information about a given light source, spectral power distribution should be measured as part of the requirements, as discussed in
Section 3.3. These measurements can then be translated to human visual metrics (lumen, candela) or similar visual metrics for other animals of interest.
Proper choice in lighting technology can be significant in achieving high efficiency for a given intensity need. Intensity can also be a function of lighting directionality. For example, as luminous intensity depends on the solid angle of emission, a high-powered source light spread over a larger area has an effectively lower luminous intensity than the same source light directed in a smaller angle. Therefore, proper control of directionality should be considered first before the power of the light source is determined. In general, lighting should not be of higher intensity than the minimum requirements for a given purpose. In most cases, the effectiveness of lighting is not improved by making lighting brighter, and unintended side-effects are increased. Applying the minimum requirements for a given purpose may involve the use of controls to turn the light off when not needed or to have the option to dim the light. Ambient light sensors and/or timers can control both maintenance and safety lights, which need to switch on during low-light conditions and at nighttime.
3.3. Spectrum
Color choices in lighting are made for a variety of reasons and often are reported in terms of how humans see the light, for example by using the CIE color diagram [
37]. The color visible to the human eye is weighted by how our eyes sense the incoming light and is not necessarily representative of the wavelength. For example, what we view as white light can be a combination of yellow and blue wavelengths or a combination of red and cyan wavelengths. When considering effects for animal responses, transmission of light through air and water, and color contrast/rendering, the discussion of spectrum or spectral power distribution is more helpful. Spectral information should be reported in radiometric units to retain as much information as possible. Depending on the choice of light bulb technology, the spectrum of the light can vary significantly. For instance, high pressure sodium (HPS) lights, typically used in the commercial fishing industry, primarily emit in the yellow-orange portion of the spectrum and cannot be tailored significantly. LEDs are an efficient lighting technology and the spectrum can be decided upon to fit the application needs. While white LEDs have been popularized for general indoor and outdoor lighting, LEDs of nearly any color can be produced—blue or red LEDs are some of the most efficient, while yellow and green LEDs are typically less efficient but still relatively efficient compared to previous technologies [
38]. Infrared (IR) LEDs may also be used in wavelength ranges of 800–900 nm and beyond, which can be detected by onboard sensors of passing vessels but are not visible to the human eye.
Engineering the spectrum of the light to use colors other than white light has been of significant interest to those studying the ecological effects of light. Different wavelengths of light can cause physiological effects and visual responses that vary across species. Humans have their highest eye response in the green region of the spectrum, but the sensitivity is different for other animal species [
39,
40,
41]. In this way, light with certain wavelengths may seem relatively less or more bright to different species. The known reactions of species to light has been employed in various industries, such as the commercial squid fishing industry’s use of green light to attract squid to boats. In a similar fashion, wavelengths of light to which species are less sensitive may cause fewer ecological impacts. Determining species-specific light responses and adjusting the light spectrum could be an effective mitigation for target key species in each environment. One specific case for which this system has been employed is in the use of colored light for illumination of towers to reduce bird strikes [
26,
42,
43].
3.4. Movement and Flashing
A final factor that lighting designers may use is a flashing sequence for lighting. Most aviation obstruction and navigation lights have specified flash rates [
31,
32,
33]. Flashing lights have been shown to reduce bird fatalities and are often recommended as a bird-friendly lighting option [
42]. However, little research has been conducted about how flashing lights affect other marine life, so this area should be further studied. LED lighting technology is inherently dimmable and can be turned on and off instantaneously. A near-infinite range of flashing frequencies, duty cycles, and on/off ramp times can be considered with modern LED lighting technology, and different cycles can be considered based on personnel needs, weather, and time of year (migratory) considerations.
4. Marine-Focused Lighting
While the previous section expanded upon choices available for lighting designers, those factors are general to all lighting installations and do not consider challenges specific to the marine environment. This section serves as a summary of special issues, including weather, surface reflections, and water depth, which are unique to open ocean environments. Suggestions about future work in line with these specific effects are made in this section.
4.1. Reflection, Transmission, and Scattering
When a lighting installation for a ME device is eventually decided upon, the lighting designer will have in mind light location, intensity, and spectrum. However, the reality will often look different from the ideal situation due to weather conditions such as fog and beam spread leading to excess light in unwanted areas. Compounding factors such as reflections off the water and potential movement of the light due to waves could additionally disrupt the intended light design. Any light that is directed downward has the potential to transmit into the water and upward light can contribute to skyglow. In terrestrial applications, light fixtures with blocking components to stop light from going above the horizontal are considered fully shielded and thus do not contribute to skyglow. In maritime applications, even with full upward shielding the reflections off the water may cause additional light to be directed upward while the downward light transmitting into the ocean can affect wildlife.
While fog and other scattering sources are challenging to predict, modeling that accounts for these conditions provides insight into minimizing light that scatters down into the water or up toward the sky. Reducing changes in light direction due to wave motion might be aided by implementation of a gimbal or similar device to hold the light upright, decoupling it from structure motion. Testing temporary lighting in a variety of weather conditions, by measuring the resultant lightscape prior to permanent deployment, allows for design modifications and potential weather-based sensing and response systems to be put in place. Pre-testing should be considered when the environment, ME device, or array design is significantly different from previous installations.
4.2. Depth of Light Penetration
In marine lighting applications, light directed downward reflects but also has the likelihood to transmit into the water where it can affect aquatic life. One study concluded that ship lighting can affect wildlife at a depth of 656 ft/200 m or more [
27]. The water attenuates the light over depth and distance, with the highest intensity occurring at the surface near the light source. Increased particles in the water cause more light to be absorbed and scattered such that the light does not reach as far into the ocean. Additionally, the entrance angle of the light into the water may affect how deep the light travels. As the light passes through the water, some wavelengths penetrate farther than others; short-wavelength visible light transmits the farthest in most conditions [
21,
22]. Choosing light sources that have relatively low intensities of highly transmissive wavelengths can further minimize how much light reaches the underwater environments. Although some studies have extrapolated surface levels of light to find how much light reaches the seafloor, making direct measurements of underwater light levels can be challenging [
22]. Spectrally resolved tools are typically not sensitive enough to detect down to low light levels, particularly in the red area of the spectrum; therefore, more specialized equipment would be necessary [
21]. Measurement of underwater light levels using a spectrometer that can measure absolute spectral radiance over a large angle would be beneficial in obtaining an understanding of how deep in the ocean lights are penetrating. This would enable the measurement of light at levels relatable to the optical sensitivity of animal species near the light installation. Not only are new light source technologies available to mitigate lighting impacts, but new light measurement tools with improved sensitivity on the order of optically sensitive animals can be deployed to better understand potential impacts on such species.
5. Primary Wildlife
Wildlife can have different responses to light, which can also vary depending on the type of light. Where some species may be photopositive, or attracted to light, others can be photonegative, or repelled by light. Other responses to light include disorientation, changes in fitness level, or alterations in predation [
18,
23,
43,
44,
45,
46,
47,
48]. These responses can vary for organisms that seem apparently similar, such as different species of crustacean zooplankton [
49], commercial fish [
50], bats [
51], or coral [
46]. Different life stages of the same species can also show varied responses to light [
52]. These effects can lead to demographic changes within an ecosystem that is affected by unnatural light [
9,
44,
53,
54]. This section categorizes wildlife into several important groups, discussing how each may be affected by lighting changes and using the PacWave site as a case study where appropriate [
29]. Responses to light are not necessarily the same for similar species and this section provides general considerations with evaluation of potential ecosystem-level effects.
5.1. Primary Producers
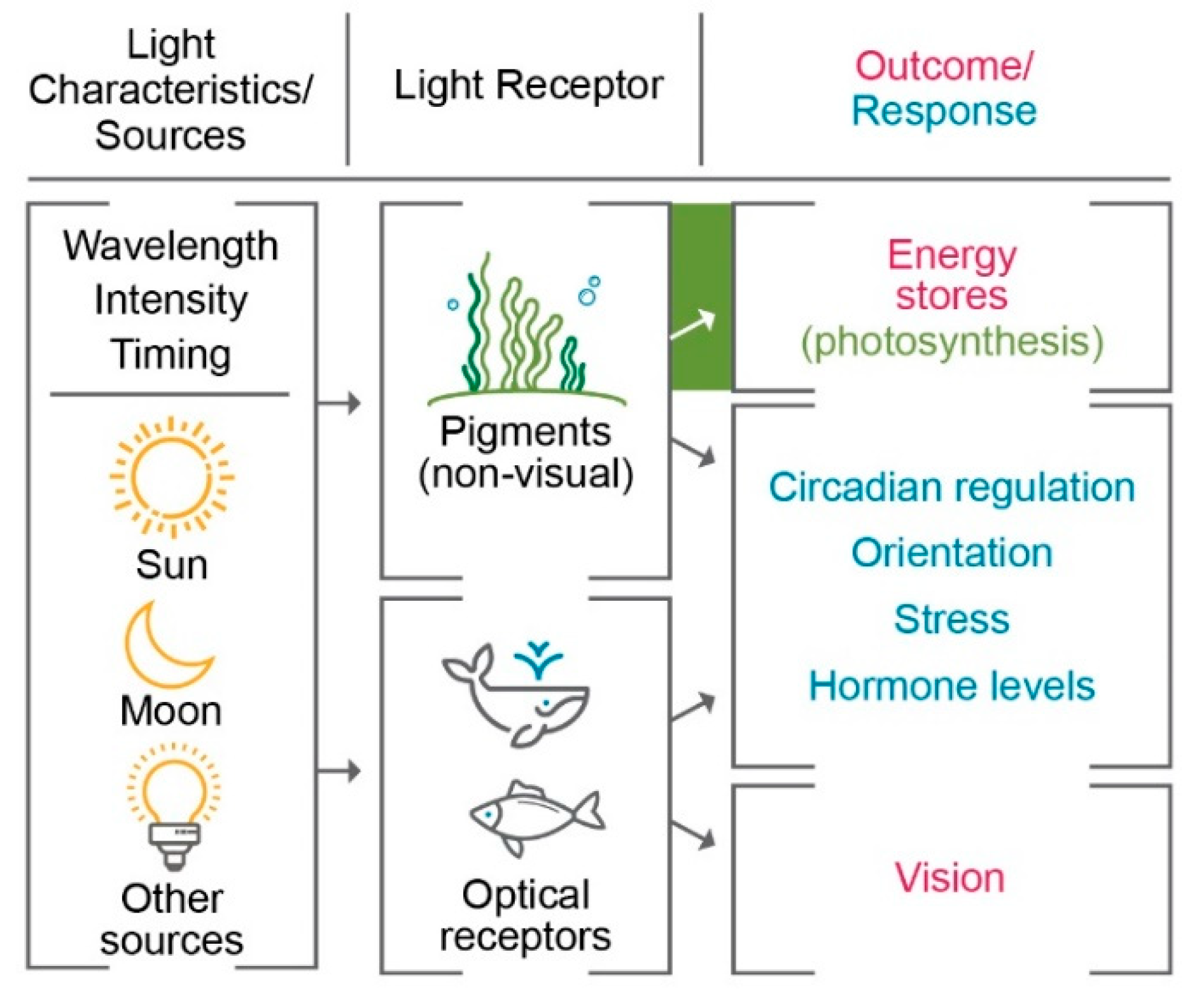
The effects of light on the amount and variety of organisms at the lower levels of the food chain can create bottom-up ecosystem changes. Typically, the way organisms use lighting can be broken down into two main categories: light as energy input and light for information [
7].
Figure 5 indicates some ways that light can be used by an organism, starting with either detection by optical receptors or by other, non-visual pigments. In the case of photosynthetic marine organisms, sufficient amounts of anthropogenic light can induce photosynthesis during naturally dark times of day, thereby introducing additional energy into the ecosystem, which can lead to additional, human-induced growth. However, other effects of constant light on species fitness have the potential to outweigh additional energy input [
55], as some studies have shown decrease in primary producers with the addition of light at night [
47]. Over longer periods of time, changes in the makeup of community structure due to light at night have been seen to lead to an increase in ecosystem production [
56]. Accordingly, increases or decreases in biomass and species of primary producers can cause changes in the rest of the ecosystem.
5.2. Larvae and Plankton
Near the bottom of the food chain, changes in larvae and plankton can affect the adult stages of marine life as well as predators who feed on these creatures. Significant research efforts have been directed toward determining the light sensitivity of zooplankton, primarily due to these species often undergoing diel vertical migration (DVM)—the daily movement of zooplankton from daytime lower water depths to nighttime near-surface depths and vice versa [
19]. This phenomenon is thought to allow zooplankton to feed in lower predation-level conditions when light levels are low and is primarily triggered by changes in light levels [
57]. The response of zooplankton to light has been of particular interest in polar climates where long winter nights mean minimal changes in light level [
19,
25,
27,
58]. Additional light at night can alter the DVM and cause the zooplankton to migrate less, with fewer individuals migrating to, on average, deeper depths during nighttime [
25,
49]. Because zooplankton make up the primary prey of many other species, changes in their daily movements may accordingly cause predators to modify behavior and/or feeding levels [
57,
59].
Further considering the PacWave site, we can find some examples of how larvae and zooplankton can affect other marine life, giving insight into which organisms should potentially be monitored. Leatherback sea turtles (
Dermochelys coriacea), a federally endangered species, have listed critical habitat within the PacWave South area [
29]. Aside from direct effects of lighting on this species, their primary prey is jellyfish, which can themselves be affected by introduction of light into the area. Changes in jellyfish abundance or depth may affect the feeding habits of leatherback sea turtles in the area. As an example with more commercial impact, Dungeness crab (
Metacarcinus magister) larvae could be within the PacWave project area, where light impacts on their development may not be well understood.
5.3. Fish
With broad categorizations of wildlife, it can be beneficial to find common trends rather than focusing on each individual or species in the group. In the case of species that have been designated as being particularly important, for instance fish that are threatened/endangered or make up the primary prey of other threatened/endangered species, species-specific response studies may be warranted. However, as various fish species will see or use light differently, it would not be practical to analyze each species in turn to understand ecosystem-level responses to light. With this limitation in mind, lighting tailored to reduce impacts on the most critical species may be the best approach. This species-specific method has been under consideration by the fish industry, where high-intensity lighting is used to intentionally alter fish behavior, fitness, or productivity in fish farms [
48,
60]. For fishing boats using high-intensity lights to catch fish, understanding photopositive or photonegative responses to different colors of light, which are unique for different fish species, can enable more selective fishing [
50].
As lighting is actively used by the fishing industry to elicit responses in fish, it is important to avoid similar effects in the natural environment. However, even lights at lower intensities that are not intended to affect fish can cause ecosystem changes. One study found that additional light at night led to a lower presence of predatory fish but an overall increase in predatory activity [
44]. Another study found an increase in large predatory fish as well as small shoaling fish in water illuminated by floodlighting from a nearby structure, indicating the dangers of changing wildlife behavior when marine lighting goes unchecked [
23]. Overall, additional illumination of the water under typically dark times can benefit visual predators and skew the ecosystem balance toward these species. However, lab results have shown that, while fish lit at night can grow bigger and heavier, they also die earlier and can experience more predation [
45].
A number of federally threatened or endangered fish species may exist within the PacWave site, including eulachon (
Thaleichthys pacificus), steelhead (
Oncorhynchus mykiss), Chinook salmon (
O. tshawytscha), green sturgeon (
Acipenser medirostris), and coho salmon (
O. kisutch) [
29]. Of these, coho salmon and green sturgeon have critical habitat within the PacWave site, and green sturgeon is a migratory species. Chinook salmon, a federally endangered species, are a primary prey for orcas (
Orcinus orca), another federally endangered species likely to migrate through the project area. Specific studies of the effects of light on these fish species would help enhance understanding of their photopositive or photonegative behaviors, in addition to any potential impacts on their physiology or fitness. More broadly, studies of lighting effects on a larger set of fish species might assist in understanding trends in what causes behaviors to differ for different species, thereby allowing prediction of light responses.
5.4. Migratory Birds and Bats
With increasing construction of tall structures such as buildings and windmills, human-made obstacles have been affecting birds to a larger degree. Bird mortality from striking structures has been a particular area of concern, alongside the potential for birds to become disorientated and/or waste energy, primarily due to the lighting of these structures [
26,
61]. Even in the case of shorter structures, excessive lighting can still contribute to disorientation of migratory species. Anthropogenic lighting can inhibit birds’ use of visual cues as well as magnetic ones when they are migrating. Tailoring the lighting spectrum or using flashing lights have been promoted as means of minimizing lighting impacts on birds [
42,
43]. While ME devices may cover small areas, their impact on migratory wildlife passing through the area can effectively increase the ecological effects. At the PacWave site, in particular, the federally threatened marbled murrelet (
Brachyramphus marmoratus) along with some species of bats are expected to migrate through the area. Flashing lights, specifically on taller structures, would be recommended to avoid bird strikes. Special consideration should be given to turning off maintenance lighting during migratory times of year. In regard to the migratory bats, terrestrial studies of bats have indicated species-dependent light responses, including some bats particularly avoiding lighting [
51]. This may be of benefit to prevent bats from roosting on the site, although the effects should be understood for different species, similar to the case for fish species.
5.5. Migratory Mammals and Sea Turtles
A number of marine mammals and reptiles can be affected either directly or indirectly by offshore lighting. These consist of both migratory and nonmigratory species of whales, dolphins, sea lions, otters, seals, and sea turtles. A significant concern is changes in feeding habits and energy acquisition, either through indirect influences such as differences in prey location and abundance or direct influences such as using lighting to assist in prey capture. Seals have been observed to use anthropogenic lighting to assist in visually hunting fish at nighttime [
62]. Minimizing light levels in the water should decrease this behavior and further studies informing spectral effects on predation behavior in marine mammals could further alleviate the problem. At the PacWave site, three federally endangered species are expected to have critical habitat or feeding areas in or near the site (humpback whales (
Megaptera novaeangliae), orcas, and leatherback sea turtles) [
29]. Harbor (
Phocoena phocoena) and Dall’s (
Phocoenoides dalli) porpoises live in the area and orcas, gray (
Eschrichtius robustus), minke (
Balaenoptera acutorostrata), fin (
B. physalus), blue (
B. musculus), and humpback whales are likely to migrate and forage through the project area at various times in the year. Studies of these migratory species have not thoroughly characterized anthropogenic light responses; beyond the impacts on prey abundance it is unclear whether lighting will disorient, attract, or repulse the marine mammals.
6. Conclusions
ME installations or individual WECs will be small in comparison to the size of the oceans in which they exist, but arrays of structures and an increase in the number of installations could cause further impacts if not studied properly. A variety of stressors have been characterized for these ME devices, but lighting has yet to be fully considered [
2]. While the undesirable effects of coastal anthropogenic lighting cover a much larger geographic scope and should be mitigated, the introduction of light in the open ocean, where little direct human activity typically reaches, could have significant impacts on the local subaerial and underwater ecosystems. By using good lighting practices such as eliminating unnecessary lights, using timers or dimmers, lowering lighting intensities to minimum requirements, reducing uplight, avoiding water illumination, and employing flashing or spectrum-tailored wildlife-friendly lighting, anthropogenic lighting effects on the ecosystem can be minimized. When used appropriately, LED lighting provides an environmentally friendly option in its relatively high efficiency, long lamp lifetime, tailorable spectrum, and ability to be dimmed. These recommended practices along with suggestions about future work are summarized in
Table 2.
To implement these best practices, clear practical guidance and regulatory guidelines should be updated, considering the capabilities of the most relevant technology, and the reporting of full optical and spectral information should be part of the design process for all ME installations. Current guidelines rely on human-centric color and brightness measurements, which cannot be translated to ecosystem impacts without full spectral information. The further study of light in the environment should be similarly supported by spectrometers with high sensitivities that can operate underwater with efficient spectral measurement of biologically important wavelengths of light. Researchers conducting animal response studies should partner with tool designers to direct capabilities of tools such that sensitivities can reach the minimum lighting levels that affect organisms. The further study of animal responses to light across the spectrum can enable more spectrum-tailored lighting to be implemented going forward. Increased understanding of differences in species’ responses to light could also help predict the effects of employing lights prior to implementation of ME installations. Pairing pre-planned lighting choices with a testing phase to characterize how the actual lightscape differs from the intended one can minimize the ecological impacts of lighting in naturally undisturbed environments. This can be done without degrading the maritime safety and navigational function of the lighting.
Although some lighting on ME installations will be necessary for safety and navigation purposes, careful thought about where lighting can be modified may decrease ecological impacts. Marine wildlife have varying responses to light, such that difficult-to-predict, ecosystem-level changes can occur with the improper implementation of lighting. Our overall assessment is that with careful lighting design, lighting on ME devices, such as WECs and project infrastructure, can be employed in ways that do not significantly disrupt the environment. These findings can be translated to many types of ME devices, where various concerns may be more or less important depending on the size of the device and need for maintenance on the structures. As more research is conducted on the light responses of marine animals, more specific guidance may be determined for any given environment, thereby further reducing impacts of light on ocean ecosystems.
Author Contributions
Conceptualization, A.M.A., J.H.H. and P.M.P.; methodology, C.E.R.; validation, C.E.R.; formal analysis, C.E.R. and J.L.; investigation, C.E.R. and J.L.; writing—original draft preparation, C.E.R.; writing—review and editing, C.E.R., J.L., A.M.A., G.J.S., J.H.H. and P.M.P.; visualization, C.E.R. and A.M.A.; photography, A.M.A.; supervision, A.M.A., J.H.H. and P.M.P.; project administration, C.E.R. and A.M.A.; funding acquisition, A.M.A., J.H.H. and P.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States Department of Energy Water Power Technologies Office, contract number DE-AC05-76RL01830.
Acknowledgments
We acknowledge Stephanie King for her graphic design work on
Figure 4 and
Figure 5.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bonar, P.A.J.; Bryden, I.G.; Borthwick, A.G.L. Social and ecological impacts of marine energy development. Renew. Sustain. Energy Rev. 2015, 47, 486–495. [Google Scholar] [CrossRef]
- Copping, A.E.; Hemery, L.G. OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development Around the World; Ocean Energy Systems: Richland, WA, USA, 2020; pp. 1–295. [Google Scholar] [CrossRef]
- Kilcher, L.; Fogarty, M.; Lawson, M. Marine Energy in the United States: An Overview of Opportunities Marine; National Renewable Energy Laboratory: Golden, CA, USA, 2021.
- Davies, T.W.; Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol. 2018, 24, 872–882. [Google Scholar] [CrossRef] [Green Version]
- Cinzano, P.; Falchi, F.; Elvidge, C.D. The first World Atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001, 328, 689–707. [Google Scholar] [CrossRef] [Green Version]
- Longcore, T.; Rich, C. Ecological light pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 2014, 12, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Zapata, M.J.; Sullivan, S.M.P.; Gray, S.M. Artificial Lighting at Night in Estuaries—Implications from Individuals to Ecosystems. Estuaries Coasts 2019, 42, 309–330. [Google Scholar] [CrossRef] [Green Version]
- Kamrowski, R.L.; Limpus, C.; Moloney, J.; Hamann, M. Coastal light pollution and marine turtles: Assessing the magnitude of the problem. Endanger. Species Res. 2012, 19, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Depledge, M.H.; Godard-Codding, C.A.J.; Bowen, R.E. Light pollution in the sea. Mar. Pollut. Bull. 2010, 60, 1383–1385. [Google Scholar] [CrossRef]
- Witherington, B.E.; Bjorndal, K.A. Influences of artificial lighting on the seaward orientation of hatchling loggerhead turtles Caretta caretta. Biol. Conserv. 1991, 55, 139–149. [Google Scholar] [CrossRef]
- Garratt, M.J.; Jenkins, S.R.; Davies, T.W. Mapping the consequences of artificial light at night for intertidal ecosystems. Sci. Total Environ. 2019, 691, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.L.; Branch, L.C.; Miller, D.L. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 2004, 18, 1435–1439. [Google Scholar] [CrossRef]
- Tuxbury, S.M.; Salmon, M. Competitive interactions between artificial lighting and natural cues during seafinding by hatchling marine turtles. Biol. Conserv. 2005, 121, 311–316. [Google Scholar] [CrossRef]
- Rivas, M.L.; Tomillo, P.S.; Uribeondo, J.D.; Marco, A. Leatherback hatchling sea-finding in response to artificial lighting: Interaction between wavelength and moonlight. J. Exp. Mar. Biol. Ecol. 2015, 463, 143–149. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Bearhop, S.; Campbell, H.A.; Bryant, D.M. Shedding light on light: Benefits of anthropogenic illumination to a nocturnally foraging shorebird. J. Anim. Ecol. 2013, 82, 478–485. [Google Scholar] [CrossRef]
- Manríquez, P.H.; Jara, M.E.; Diaz, M.I.; Quijón, P.A.; Widdicombe, S.; Pulgar, J.; Manríquez, K.; Quintanilla-Ahumada, D.; Duarte, C. Artificial light pollution influences behavioral and physiological traits in a keystone predator species, Concholepas concholepas. Sci. Total Environ. 2019, 661, 543–552. [Google Scholar] [CrossRef]
- Båtnes, A.S.; Miljeteig, C.; Berge, J.; Greenacre, M.; Johnsen, G. Quantifying the light sensitivity of Calanus spp. During the polar night: Potential for orchestrated migrations conducted by ambient light from the sun, moon, or aurora borealis? Polar Biol. 2015, 38, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Barranco, C.; Hughes, L.E. Effects of light pollution on the emergent fauna of shallow marine ecosystems: Amphipods as a case study. Mar. Pollut. Bull. 2015, 94, 235–240. [Google Scholar] [CrossRef]
- Tamir, R.; Lerner, A.; Haspel, C.; Dubinsky, Z.; Iluz, D. The spectral and spatial distribution of light pollution in the waters of the northern Gulf of Aqaba (Eilat). Sci. Rep. 2017, 7, 42329. [Google Scholar] [CrossRef] [Green Version]
- Davies, T.W.; McKee, D.; Fishwick, J.; Tidau, S.; Smyth, T. Biologically important artificial light at night on the seafloor. Sci. Rep. 2020, 10, 12545. [Google Scholar] [CrossRef]
- Becker, A.; Whitfield, A.K.; Cowley, P.D.; Järnegren, J.; Næsje, T.F. Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J. Appl. Ecol. 2013, 50, 43–50. [Google Scholar] [CrossRef]
- Keenan, S.F.; Benfield, M.C.; Blackburn, J.K. Importance of the artificial light field around offshore petroleum platforms for the associated fish community. Mar. Ecol. Prog. Ser. 2007, 331, 219–231. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsen, M.; Berge, J.; Geoffroy, M.; Cohen, J.H.; De La Torre, P.R.; Nornes, S.M.; Singh, H.; Sørensen, A.J.; Daase, M.; Johnsen, G. Use of an autonomous surface vehicle reveals small-scale diel vertical migrations of zooplankton and susceptibility to light pollution under low solar irradiance. Sci. Adv. 2018, 4, eaap9887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, F.K.; Montevecchi, W.A.; Davoren, G.K.; Huettmann, F.; Diamond, A.W.; Linke, J. Seabirds at risk around offshore oil platforms in the North-west Atlantic. Mar. Pollut. Bull. 2001, 42, 1285–1290. [Google Scholar] [CrossRef]
- Berge, J.; Geoffroy, M.; Daase, M.; Cottier, F.; Priou, P.; Cohen, J.H.; Johnsen, G.; McKee, D.; Kostakis, I.; Renaud, P.E.; et al. Artificial light during the polar night disrupts Arctic fish and zooplankton behaviour down to 200 m depth. Commun. Biol. 2020, 3, 102. [Google Scholar] [CrossRef] [Green Version]
- Hartman, L. Top 10 Things You Didn’t Know about Offshore Wind Energy. Available online: https://www.energy.gov/eere/wind/articles/top-10-things-you-didn’t-know-about-offshore-wind-energy (accessed on 1 August 2021).
- Federal Energy Regulatory Commission. Environmental Assessment for Hydropower License; PacWave South Project; Federal Energy Regulatory Commission: Washington, DC, USA, 2020.
- Bureau of Ocean Energy Management. Guidelines for Lighting and Marking of Structures Supporting Renewable Energy Development; Bureau of Ocean Energy Management: Washington, DC, USA, 2021.
- Federal Aviation Administration, U.S. Department of Transportation. Advisory Circ–lar—Obstruction Marking and Lighting; Federal Aviation Administration: Washington, DC, USA, 2015.
- U.S. Coast Guard. Part 66—Private Aids to Navigation; U.S. Coast Guard: Washington, DC, USA, 2021; pp. 152–160. [Google Scholar]
- U.S. Coast Guard. Part 67—Aids to Navigation on Artificial Islands and Fixed Structures; U.S. Coast Guard: Washington, DC, USA, 2021; pp. 160–174. [Google Scholar]
- Federal Aviation Administration, U.S. Department of Transportation. Advisory Circ–lar—Specification for Obstruction Lighting Equipment; Federal Aviation Administration: Washington, DC, USA, 2019.
- Liebel, B.; Hartley, R. IDA and IES Announce Strategic Collaboration to Advance Quality Lighting to Reduce Light Pollution; Illuminating Engineering Society: New York, NY, USA, 2020. [Google Scholar]
- Pattison, M.; Hansen, M.; Norman, B.; Clay, E.; Kyung, L.; Lee, K.; Pattison, L.; Tsao, J. 2019 Lighting R&D Opportunities; U.S. Department of Energy: Washington, DC, USA, 2020.
- Chromaticity Diagram from Newton to the CIE 1931 Standard System. In Standard Colorimetry; Oleari, C. (Ed.) John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 237–251. [Google Scholar]
- Pust, P.; Schmidt, P.J.; Schnick, W. A revolution in lighting. Nat. Mater. 2015, 14, 454–458. [Google Scholar] [CrossRef]
- Kremers, J.; Baraas, R.C.; Marshall, N.J. Human Color Vision; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Marshall, J.; Carleton, K.L.; Cronin, T. Colour vision in marine organisms. Curr. Opin. Neurobiol. 2015, 34, 86–94. [Google Scholar] [CrossRef]
- Gehring, J.; Kerlinger, P.; Manville, A.M. Communication towers, lights, and birds: Successful methods of reducing the frequency of avian collisions. Ecol. Appl. 2009, 19, 505–514. [Google Scholar] [CrossRef]
- Poot, H.; Ens, B.J.; de Vries, H.; Donners, M.A.H.; Wernand, M.R.; Marquenie, J.M. Green Light for Nocturnally Migrating Birds. Ecol. Soc. 2008, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Bolton, D.; Mayer-Pinto, M.; Clark, G.F.; Dafforn, K.A.; Brassil, W.A.; Becker, A.; Johnston, E.L. Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci. Total Environ. 2017, 576, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.J.; Fobert, E.K.; Besson, M.; Jacob, H.; Lecchini, D. Live fast, die young: Behavioural and physiological impacts of light pollution on a marine fish during larval recruitment. Mar. Pollut. Bull. 2019, 146, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Ayalon, I.; Marangoni, L.F.D.B.; Benichou, J.I.C.; Avisar, D.; Levy, O. Red Sea corals under Artificial Light Pollution at Night (ALAN) undergo oxidative stress and photosynthetic impairment. Glob. Chang. Biol. 2019, 25, 4194–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubisic, M. Waters under Artificial Lights: Does Light Pollution Matter for Aquatic Primary Producers? Limnol. Oceanogr. Bull. 2018, 27, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Oppedal, F.; Dempster, T.; Stien, L.H. Environmental drivers of Atlantic salmon behaviour in sea-cages: A review. Aquaculture 2011, 311, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.V.; Pierce, S.M.; Walsh, H.M.; Kvalvik, S.K.; Lim, J.D. Urban light pollution alters the diel vertical migration of Daphnia. Int. Ver. Theor. Angew. Limnol. Verh. 2000, 27, 779–782. [Google Scholar] [CrossRef]
- Marchesan, M.; Spoto, M.; Verginella, L.; Ferrero, E.A. Behavioural effects of artificial light on fish species of commercial interest. Fish. Res. 2005, 73, 171–185. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Chang. Biol. 2012, 18, 2458–2465. [Google Scholar] [CrossRef]
- Lynn, K.D.; Quintanilla-Ahumada, D.; Anguita, C.; Widdicombe, S.; Pulgar, J.; Manríquez, P.H.; Quijón, P.A.; Duarte, C. Artificial light at night alters the activity and feeding behaviour of sandy beach amphipods and pose a threat to their ecological role in Atlantic Canada. Sci. Total Environ. 2021, 780, 146568. [Google Scholar] [CrossRef]
- Davies, T.W.; Coleman, M.; Griffith, K.M.; Jenkins, S.R. Night-time lighting alters the composition of marine epifaunal communities. Biol. Lett. 2015, 11, 20150080. [Google Scholar] [CrossRef]
- Davies, T.W.; Bennie, J.; Inger, R.; de Ibarra, N.H.; Gaston, K.J. Artificial light pollution: Are shifting spectral signatures changing the balance of species interactions? Glob. Chang. Biol. 2013, 19, 1417–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulin, C.; Bruyant, F.; Laprise, M.H.; Cockshutt, A.M.; Vandenhecke, J.M.-R.; Huot, Y. The impact of light pollution on diel changes in the photophysiology of Microcystis aeruginosa. J. Plankton Res. 2014, 36, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Hölker, F.; Wurzbacher, C.; Weißenborn, C.; Monaghan, M.T.; Holzhauer, S.I.J.; Premke, K. Microbial diversity and community respiration in freshwater sediments influenced by artificial light at night. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, G.C. A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 2003, 503, 163–170. [Google Scholar] [CrossRef]
- Cohen, J.H.; Berge, J.; Moline, M.A.; Sørensen, A.J.; Last, K.; Falk-Petersen, S.; Renaud, P.E.; Leu, E.S.; Grenvald, J.; Cottier, F.; et al. Is ambient light during the high Arctic polar night sufficient to act as a visual cue for zooplankton? PLoS ONE 2015, 10, e0126247. [Google Scholar] [CrossRef]
- Kelly, T.B.; Davison, P.C.; Goericke, R.; Landry, M.R.; Ohman, M.D.; Stukel, M.R. The Importance of Mesozooplankton Diel Vertical Migration for Sustaining a Mesopelagic Food Web. Front. Mar. Sci. 2019, 6, 508. [Google Scholar] [CrossRef] [Green Version]
- Migaud, H.; Cowan, M.; Taylor, J.; Ferguson, H.W. The effect of spectral composition and light intensity on melatonin, stress and retinal damage in post-smolt Atlantic salmon, Salmo salar. Aquaculture 2007, 270, 390–404. [Google Scholar] [CrossRef]
- Merkel, F.R.; Johansen, K.L. Light-induced bird strikes on vessels in Southwest Greenland. Mar. Pollut. Bull. 2011, 62, 2330–2336. [Google Scholar] [CrossRef]
- Yurk, H.; Trites, A.W. Experimental Attempts to Reduce Predation by Harbor Seals on Out-Migrating Juvenile Salmonids. Trans. Am. Fish. Soc. 2000, 129, 1360–1366. [Google Scholar] [CrossRef] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).