Intestine Explants in Organ Culture: A Tool to Broaden the Regenerative Studies in Echinoderms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Collection and Evisceration
2.2. Explants
2.3. Characterization of Cell Populations in Cultured Explants by Immunocytochemistry
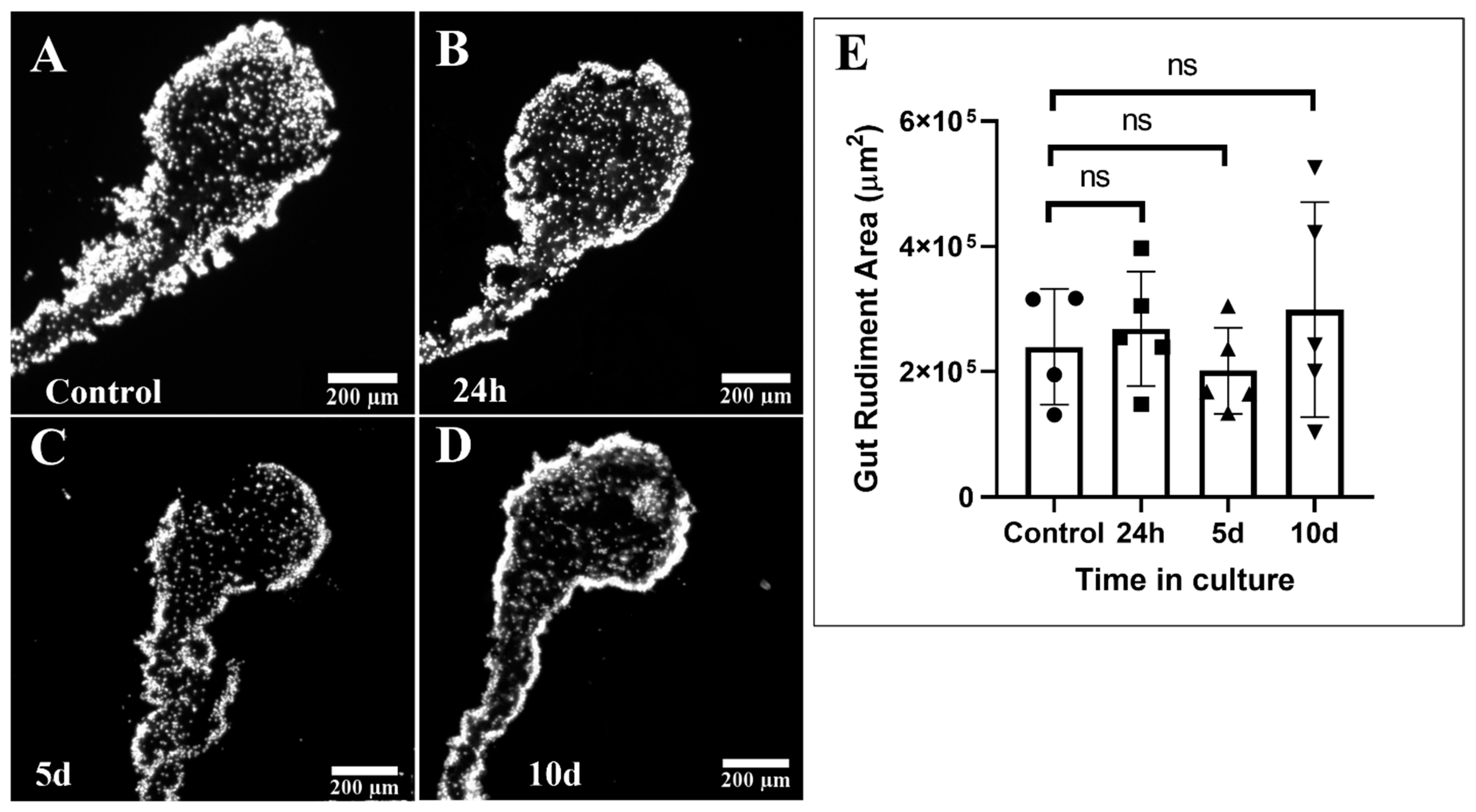
2.4. Gut Rudiment Area
2.5. Muscle and Spindle-like Structures (SLSs)/DAPI Ratio in Cultured Explants
2.6. Cell Proliferation Assays
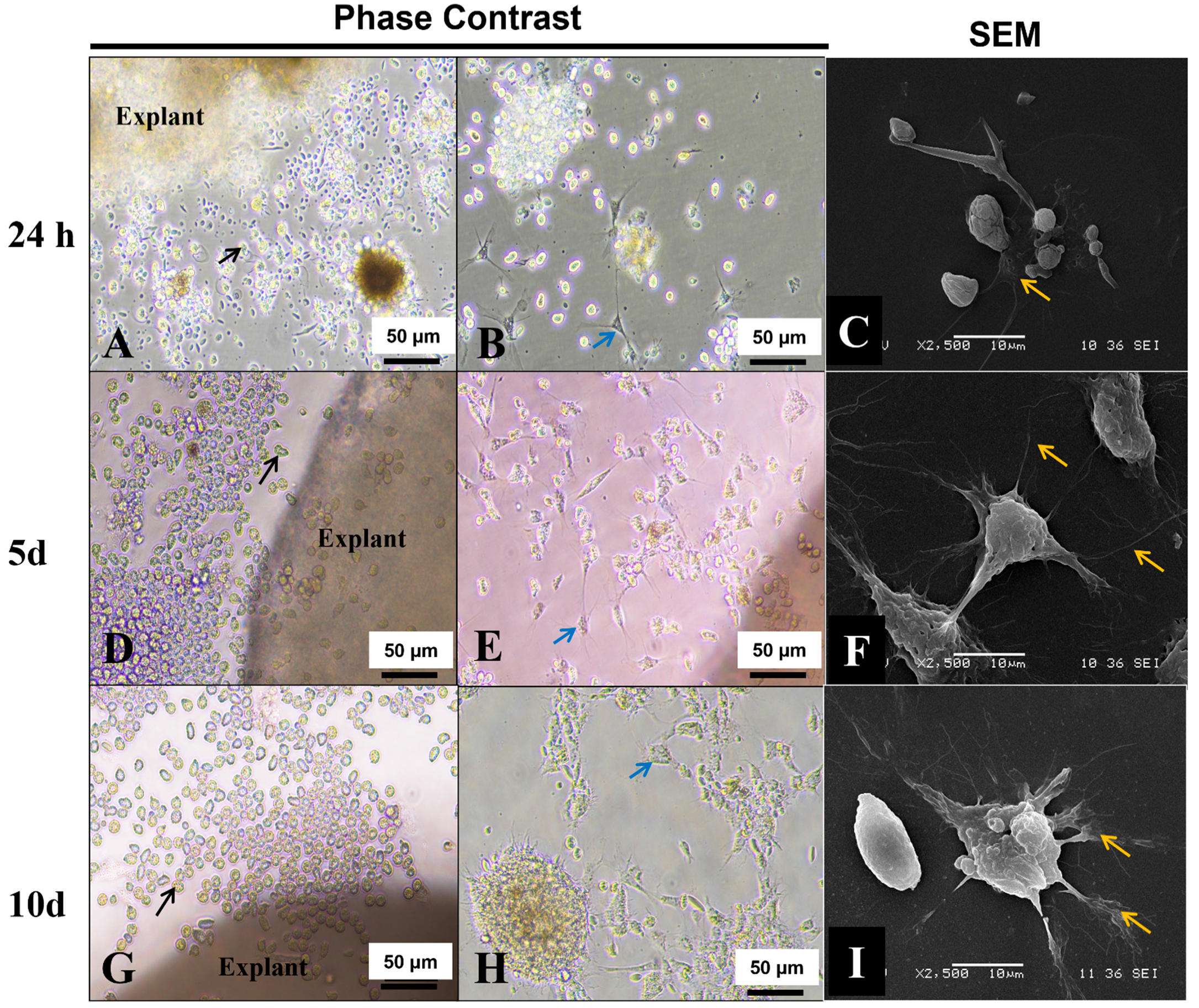
2.7. Morphological Characterization of Explant’s Outgrowths by SEM
2.8. Statistical Analysis
3. Results
3.1. Gut Rudiment Area
3.2. Muscle Dedifferentiation
3.3. Cell Proliferation
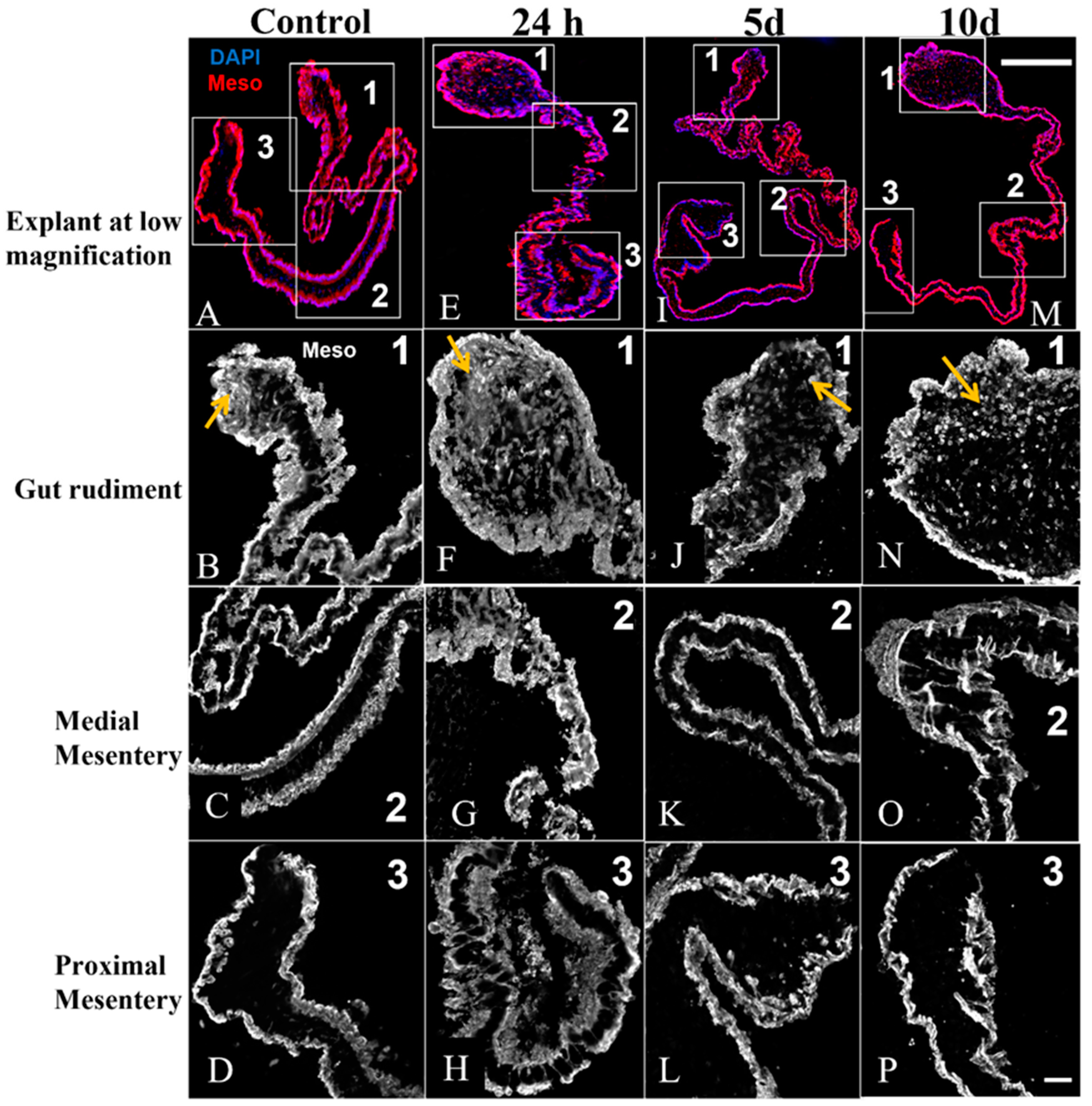
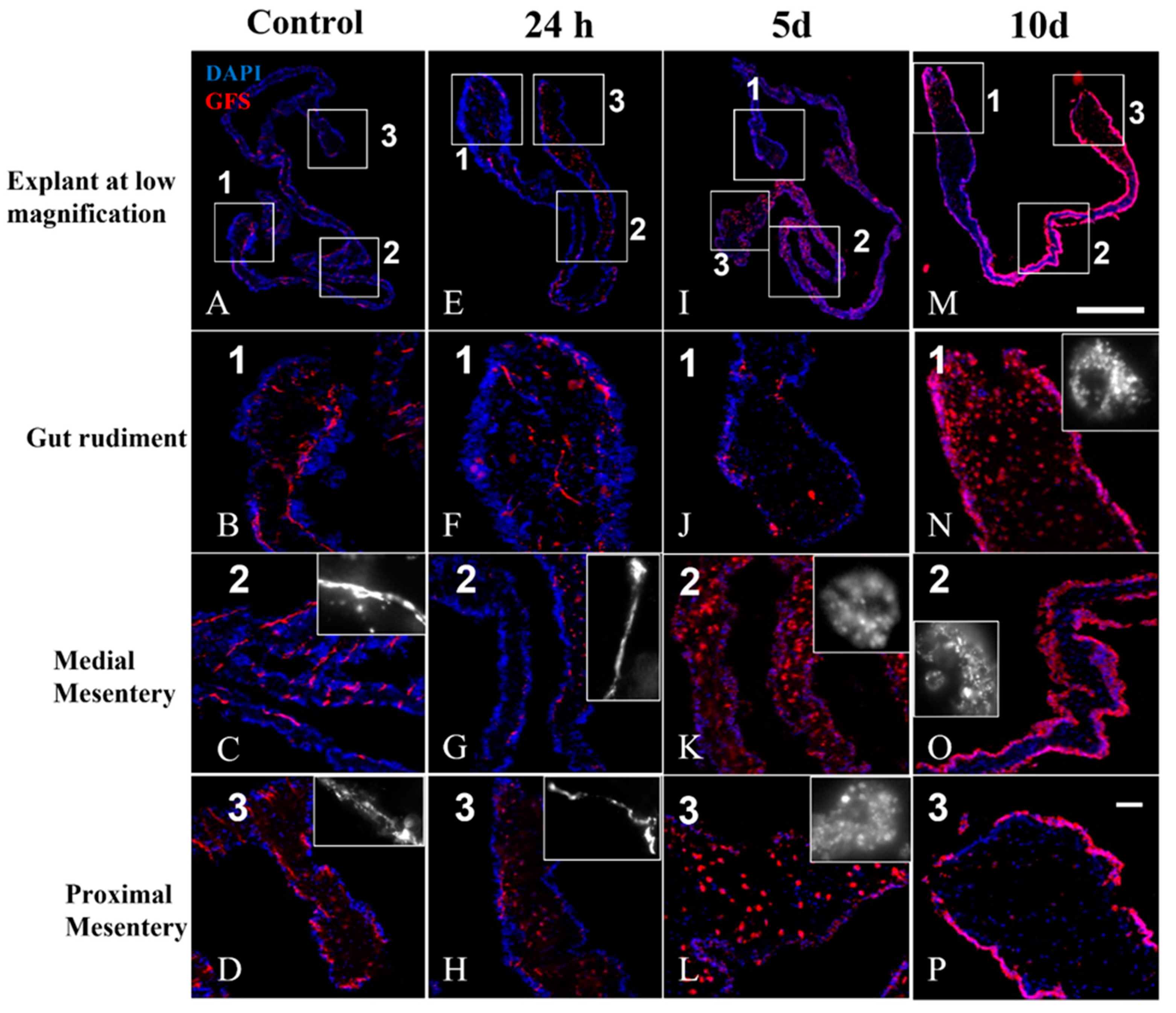
3.4. Mesothelium Labeling in Cultured Explants
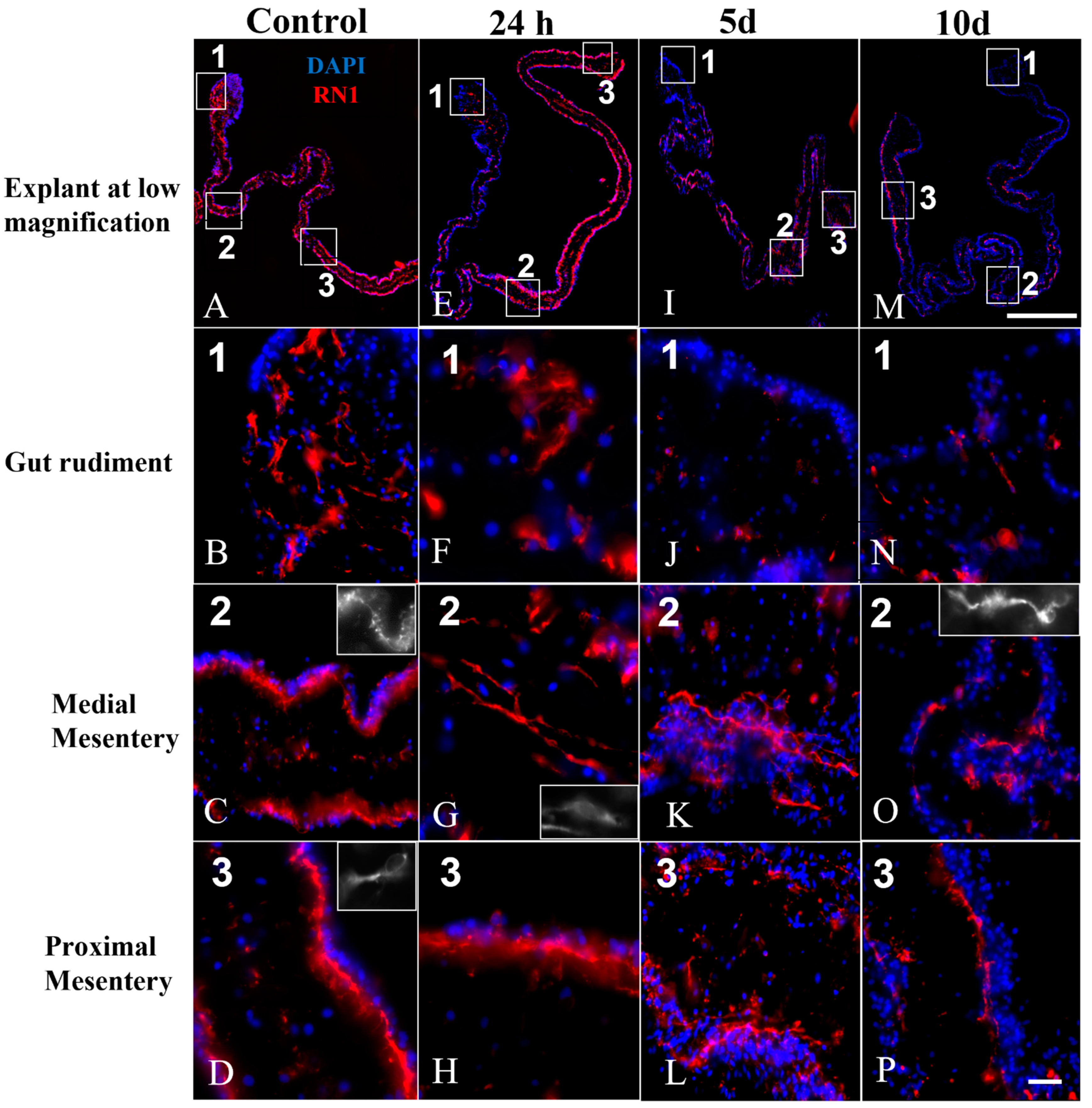
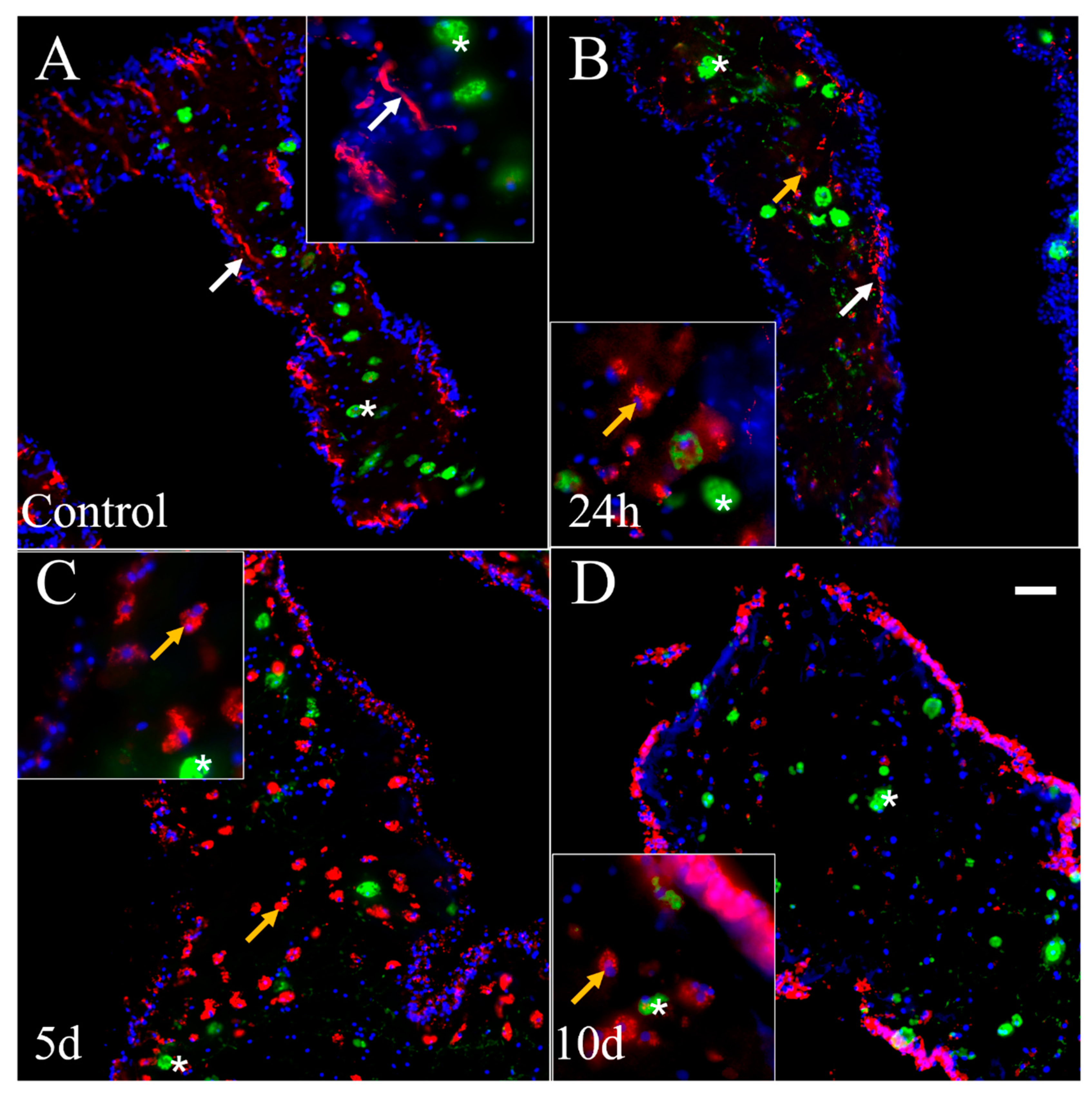
3.5. Nerve Fibers and Neuron-like Cells Labeled by Neuronal Markers in Cultured Explants
3.6. Components of the Connective Tissue in Cultured Explants
3.7. Double Labeling (GFS and Sph3) in Cultured Explants
3.8. Explant Outgrowths
4. Discussion
4.1. RN1 Labeling Suggests That a Subpopulation of Nerve Fibers Is Reduced in Cultured Explants as the Time in Culture Increases
4.2. Anti-GFS Labels a Population of Cells That Resemble Spherulocytes in the Connective Tissue and Mesothelial Cells of Cultured Gut Explants
4.3. The Polarity in the Localization of BrdU-Positive Cell Nuclei Is Lost and the Proliferation Rate Decreases over Time in Cultured Explants
4.4. The Cellular Processes Underlying Intestine Regeneration Are Halted in Cultured Gut Explants Compared to the In Vivo Counterparts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Freshney, R.I. (Ed.) Primary Culture. In Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 163–186. ISBN 978-0-470-52812-9. [Google Scholar]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell 2017, 168, 1135–1148.e12. [Google Scholar] [CrossRef] [Green Version]
- Carnevali, C.; Bonasoro, F.; Patruno, M.; Thorndyke, M.C. Cellular and molecular mechanisms of arm regeneration in crinoid echinoderms: The potential of arm explants. Dev. Genes Evol. 1998, 208, 421–430. [Google Scholar] [CrossRef]
- Dupont, S.; Thorndyke, M.C. Growth or differentiation? Adaptive regeneration in the brittlestar Amphiura filiformis. J. Exp. Biol. 2006, 209, 3873–3881. [Google Scholar] [CrossRef] [Green Version]
- Burns, G.; Ortega-Martinez, O.; Dupont, S.; Thorndyke, M.C.; Peck, L.S.; Clark, M.S. Intrinsic gene expression during regeneration in arm explants of Amphiura filiformis. J. Exp. Mar. Bio. Ecol. 2012, 413, 106–112. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Parma, L.; Barbaglio, A.; Sugni, M.; Bonasoro, F.; Candia Carnevali, M.D. Echinoderm regeneration: An in vitro approach using the crinoid Antedon mediterranea. Cell Tissue Res. 2014, 358, 189–201. [Google Scholar] [CrossRef]
- Moss, C.; Beesley, P.W.; Thorndyke, M.C.; Bollner, T. Preliminary observations on ascidian and echinoderm neurons and neural explants in vitro. Tissue Cell 1998, 30, 517–524. [Google Scholar] [CrossRef]
- Bello, S.A.; Torres-Gutiérrez, V.; Rodríguez-Flores, E.J.; Toledo-Román, E.J.; Rodríguez, N.; Díaz-Díaz, L.M.; Vázquez-Figueroa, L.D.; Cuesta, J.M.; Grillo-Alvarado, V.; Amador, A.; et al. Insights into intestinal regeneration signaling mechanisms. Dev. Biol. 2020, 458, 12–31. [Google Scholar] [CrossRef]
- García-Arrarás, J.E.; Lázaro-Peña, M.I.; Díaz-Balzac, C.A. Holothurians as a Model System to Study Regeneration. In Results and Problems in Cell Differentiation; Springer: Cham, Switzerland, 2018; Volume 65, pp. 255–283. [Google Scholar]
- García-Arrarás, J.E.; Bello, S.A.; Malavez, S. The mesentery as the epicenter for intestinal regeneration. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Alicea-Delgado, M.; García-Arrarás, J.E. Wnt/β-catenin signaling pathway regulates cell proliferation but not muscle dedifferentiation nor apoptosis during sea cucumber intestinal regeneration. Dev. Biol. 2021, 480, 105–113. [Google Scholar] [CrossRef]
- Quesada-Díaz, E.; Figueroa-Delgado, P.; García-Rosario, R.; Sirfa, A.; García-Arrarás, J.E. Dedifferentiation of radial glia-like cells is observed in in vitro explants of holothurian radial nerve cord. J. Neurosci. Methods 2021, 364, 109358. [Google Scholar] [CrossRef]
- Alicea-Delgado, M.; Bello-Melo, S.A.; García-Arrarás, J.E. RNA Interference on Regenerating Holothurian Gut Tissues. In Methods in Molecular Biology; Humana: New York, NY, USA, 2021; pp. 241–252. ISBN 978-1-0716-0973-6. [Google Scholar]
- Bello, S.A.; Abreu-Irizarry, R.J.; García-Arrarás, J.E. Primary cell cultures of regenerating holothurian tissues. Methods Mol. Biol. 2015, 1189, 283–297. [Google Scholar]
- Schacher, S.; Proshansky, E. Neurite regeneration by Aplysia neurons in dissociated cell culture: Modulation by Aplysia hemolymph and the presence of the initial axonal segment. J. Neurosci. 1983, 3, 2403–2413. [Google Scholar] [CrossRef]
- García-Arrarás, J.E.; Valentín-Tirado, G.; Flores, J.E.; Rosa, R.J.; Rivera-Cruz, A.; San Miguel-Ruiz, J.E.; Tossas, K. Cell dedifferentiation and epithelial to mesenchymal transitions during intestinal regeneration in H. glaberrima. BMC Dev. Biol. 2011, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Tossas, K.; Qi-Huang, S.; Cuyar, E.; García-Arrarás, J.E. Temporal and spatial analysis of enteric nervous system regeneration in the sea cucumber Holothuria glaberrima. Regeneration 2014, 1, 10–26. [Google Scholar] [CrossRef]
- Díaz-Balzac, C.A.; Santacana-Laffitte, G.; San Miguel-Ruíz, J.E.; Tossas, K.; Valentín-Tirado, G.; Rives-Sánchez, M.; Mesleh, A.; Torres, I.I.; García-Arrarás, J.E. Identification of nerve plexi in connective tissues of the sea cucumber Holothuria glaberrima by using a novel nerve-specific antibody. Biol. Bull. 2007, 213, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Rosado-Olivieri, E.A.; Ramos-Ortiz, G.A.; Hernández-Pasos, J.; Díaz-Balzac, C.A.; Vázquez-Rosa, E.; Valentín-Tirado, G.; Vega, I.E.; García-Arrarás, J.E. A START-domain-containing protein is a novel marker of nervous system components of the sea cucumber Holothuria glaberrima. Comp. Biochem. Physiol. Part-B Biochem. Mol. Biol. 2017, 214, 57–65. [Google Scholar] [CrossRef]
- Díaz-Miranda, L.; Blanco, R.E.; García-Arrarás, J.E. Localization of the heptapeptide GFSKLYFamide in the sea cucumber holothuria glaberrima (echinodermata): A light and electron microscopic study. J. Comp. Neurol. 1995, 352, 626–640. [Google Scholar] [CrossRef]
- García-Arrarás, J.E.; Schenk, C.; Rodrígues-Ramírez, R.; Torres, I.I.; Valentín, G.; Candelaria, A.G. Spherulocytes in the echinoderm Holothuria glaberrima and their involvement in intestinal regeneration. Dev. Dyn. 2006, 235, 3259–3267. [Google Scholar] [CrossRef]
- Quiñones, J.L.; Rosa, R.; Ruiz, D.L.; García-Arrarás, J.E. Extracellular Matrix Remodeling and Metalloproteinase Involvement during Intestine Regeneration in the Sea Cucumber Holothuria glaberrima. Dev. Biol. 2002, 250, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Bello, S.A.; De Jesús-Maldonado, I.; Rosim-Fachini, E.; Sundaram, P.A.; Diffoot-Carlo, N. In vitro evaluation of human osteoblast adhesion to a thermally oxidized γ-TiAl intermetallic alloy of composition Ti-48Al-2Cr-2Nb (at.%). J. Mater. Sci. Mater. Med. 2010, 21, 1739–1750. [Google Scholar]
- Candelaria, A.G.; Murray, G.; File, S.K.; García-Arrarás, J.E. Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima. Cell Tissue Res. 2006, 325, 55–65. [Google Scholar] [CrossRef]
- Dolmatov, I.Y.; Ginanova, T.T. Muscle Regeneration in Holothurians. Microsc. Res. Tech. 2001, 55, 452–463. [Google Scholar] [CrossRef]
- Diaz-Miranda, L.; Price, D.A.; Greenberg, M.J.; Lee, T.D.; Doble, K.E.; Garcia-Arraras, J.E. Characterization of Two Novel Neuropeptides from the Sea Cucumber Holothuria glaberrima. Biol. Bull. 1992, 182, 241–247. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Bradley, J.R.; Pober, J.S. Human organ culture: Updating the approach to bridge the gap from in vitro to in vivo in inflammation, cancer, and stem cell biology. Front. Med. 2017, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Hassan, B.A. The Making and Un-Making of Neuronal Circuits in Drosophila. Out with the Brain: Drosophila Whole-Brain Explant Culture. Neuromethods 2012, 69, 261–268. [Google Scholar]
- Lorenzen, U.S.; Hansen, G.H.; Danielsen, E.M. Organ culture as a model system for studies on Enterotoxin interactions with the intestinal epithelium. Methods Mol. Biol. 2016, 1396, 159–166. [Google Scholar]
- Taylor, V.; Hicks, J.; Ferguson, C.; Willey, J.; Danelson, K. Effects of tissue culture on the biomechanical properties of porcine meniscus explants. Clin. Biomech. 2019, 69, 120–126. [Google Scholar] [CrossRef]
- Sun, X.; Fu, X.; Du, M.; Zhu, M.J. Ex vivo gut culture for studying differentiation and migration of small intestinal epithelial cells. Open Biol. 2018, 4, 170256. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.V.; Martin, K.R. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3503–3512. [Google Scholar] [CrossRef]
- Müller, B.; Wagner, F.; Lorenz, B.; Stieger, K. Organotypic cultures of adult mouse retina: Morphologic changes and gene expression. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1930–1940. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M. The ultrastructure of the morula cells of Eupentacta quinquesemita (Echinodermata: Holothuroidea) and their role in the maintenance of the extracellular matrix. J. Morphol. 1986, 188, 179–189. [Google Scholar] [CrossRef]
- Zallen, J.A. Planar Polarity and Tissue Morphogenesis. Cell 2007, 129, 1051–1063. [Google Scholar] [CrossRef] [Green Version]
- Robertson, H.; Wheeler, J.; Morley, A.R. Culture and in vitro bromodeoxyuridine-labelling of mouse kidney explants. J. Tissue Cult. Methods 1994, 16, 49–55. [Google Scholar] [CrossRef]
- Usugane, M.; Fujita, M.; Lipkin, M.; Palmer, R.; Friedman, E.; Augenlicht, L. Cell proliferation in explant cultures of human colon. Digestion 1982, 24, 225–233. [Google Scholar] [CrossRef]
- Cao, J.; Poss, K.D. Explant culture of adult zebrafish hearts for epicardial regeneration studies. Nat. Protoc. 2016, 11, 872–881. [Google Scholar] [CrossRef]
- Bello-Melo, S.A. Determination of the Expression Levels of axin, β-catenin and myc Genes by Quantitative Real Time PCR in Explants Treated with Modulators of the Wnt Signaling Pathway. In Setting-Up an In Vitro Model to Study the Signaling Mechanisms Associated with Intestine Regeneration in the Sea Cucumber Holothuria glaberrima. Ph.D. Thesis, Rio Piedras Campus, University of Puerto Rico, San Juan, PR, USA, 2019; pp. 216–220. [Google Scholar]
- Díaz-Díaz, L.M.; Rosario-Meléndez, N.; Rodríguez-Villafañe, A.; Figueroa-Vega, Y.Y.; Pérez-Villafañe, O.A.; Colón-Cruz, A.M.; Rodríguez-Sánchez, P.I.; Cuevas-Cruz, J.M.; Malavez-Cajigas, S.J.; Maldonado-Chaar, S.M.; et al. Antibiotics Modulate Intestinal Regeneration. Biology 2021, 10, 236. [Google Scholar] [CrossRef]





| Antigen (Antibody Name) | Raised in | Immunogen | Cells/Structures Labeled | Source | Dilution |
|---|---|---|---|---|---|
| Unknown (Meso-1) | Mouse (monoclonal) | Homogenate of the regenerating mesothelium | Peritoneocytes and myocytes of the mesothelium | Dr. García-Arrarás lab. [16] | 1:50 |
| β-Tubulin (Anti- β-Tubulin clone | Mouse (monoclonal) | Tubulin from S. purpuratus sperm axonemes | Subpopulation of neuron-like cells | Sigma-Aldrich (T-293) [17] | 1:500 |
| HgSTARD10 protein (RN1) | Mouse (monoclonal) | Homogenate of the radial nerve of H. glaberrima | Subpopulation of neuron-like cells | Dr. García-Arrarás lab. [18,19] | 1:50,000 |
| GFSKLYFamide (GFS) | Rabbit (polyclonal) | Synthetic peptide GFSKLYFamide | Subpopulation of neuron-like cells | Dr. García-Arrarás lab. [20] | 1:1000 |
| Unknown (Sph3) | Mouse (monoclonal) | Holothurian homeobox peptide coupled to bovine serum albumin | Spherulocytes | Dr. García-Arrarás lab. [21] | Undiluted |
| Fibrous collagen (Hg-fCOL) | Mouse (monoclonal) | Insoluble intestinal fibrous collagen extract of H. glaberrima | Fibrous collagen | Dr. García-Arrarás lab. [22] | 1:10 |
| Bromodeoxyuridine (Anti-BrdU) | Mouse (monoclonal) | BrdU | Cell nuclei of replicating cells | Millipore-Sigma (GERPN202) | 1:5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, S.A.; García-Arrarás, J.E. Intestine Explants in Organ Culture: A Tool to Broaden the Regenerative Studies in Echinoderms. J. Mar. Sci. Eng. 2022, 10, 244. https://doi.org/10.3390/jmse10020244
Bello SA, García-Arrarás JE. Intestine Explants in Organ Culture: A Tool to Broaden the Regenerative Studies in Echinoderms. Journal of Marine Science and Engineering. 2022; 10(2):244. https://doi.org/10.3390/jmse10020244
Chicago/Turabian StyleBello, Samir A., and José E. García-Arrarás. 2022. "Intestine Explants in Organ Culture: A Tool to Broaden the Regenerative Studies in Echinoderms" Journal of Marine Science and Engineering 10, no. 2: 244. https://doi.org/10.3390/jmse10020244
APA StyleBello, S. A., & García-Arrarás, J. E. (2022). Intestine Explants in Organ Culture: A Tool to Broaden the Regenerative Studies in Echinoderms. Journal of Marine Science and Engineering, 10(2), 244. https://doi.org/10.3390/jmse10020244






