Present and Future of Seaweed Cultivation and Its Applications in Colombia
Abstract
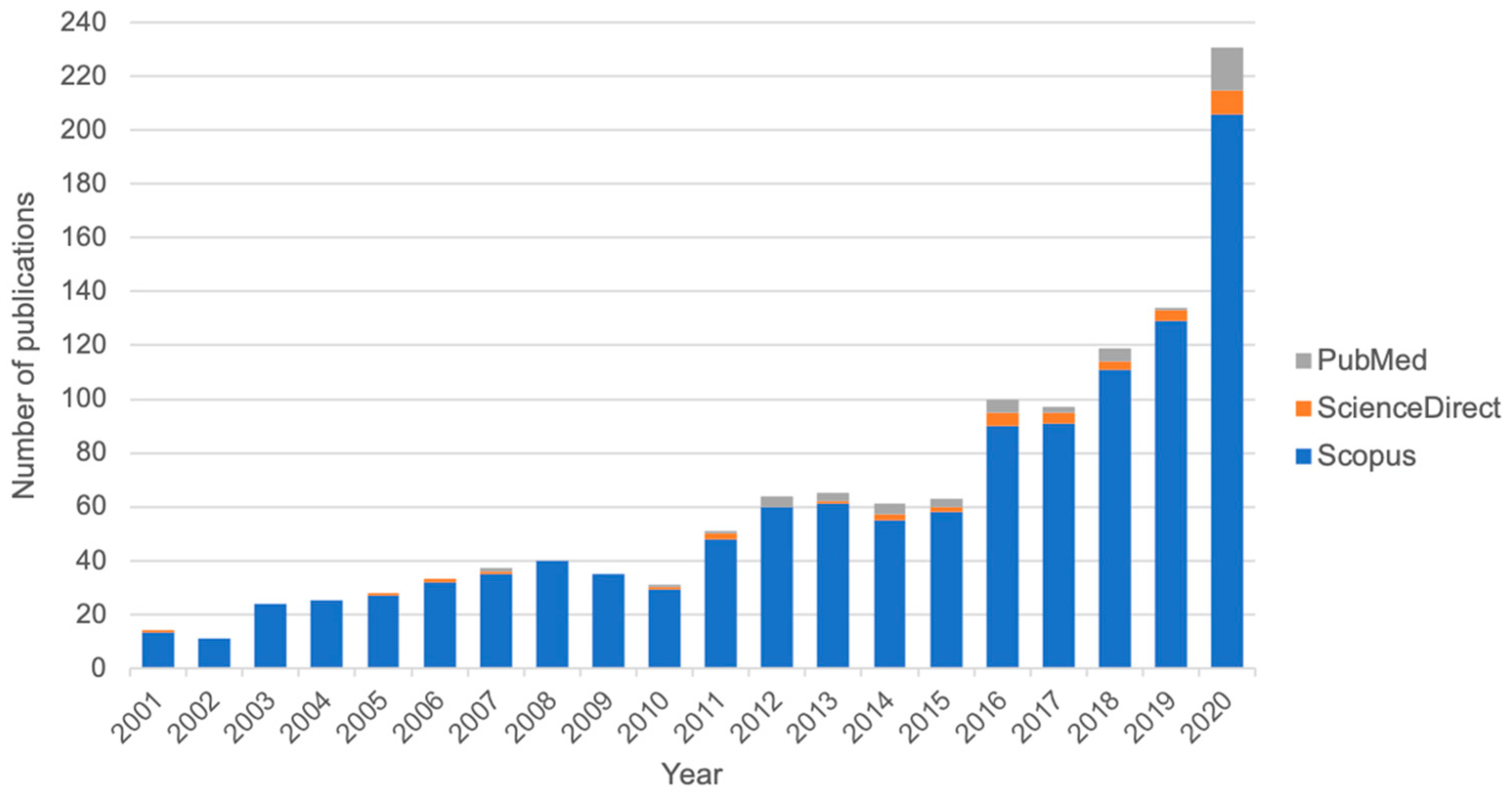
1. Introduction
2. A Global View of Seaweed Use
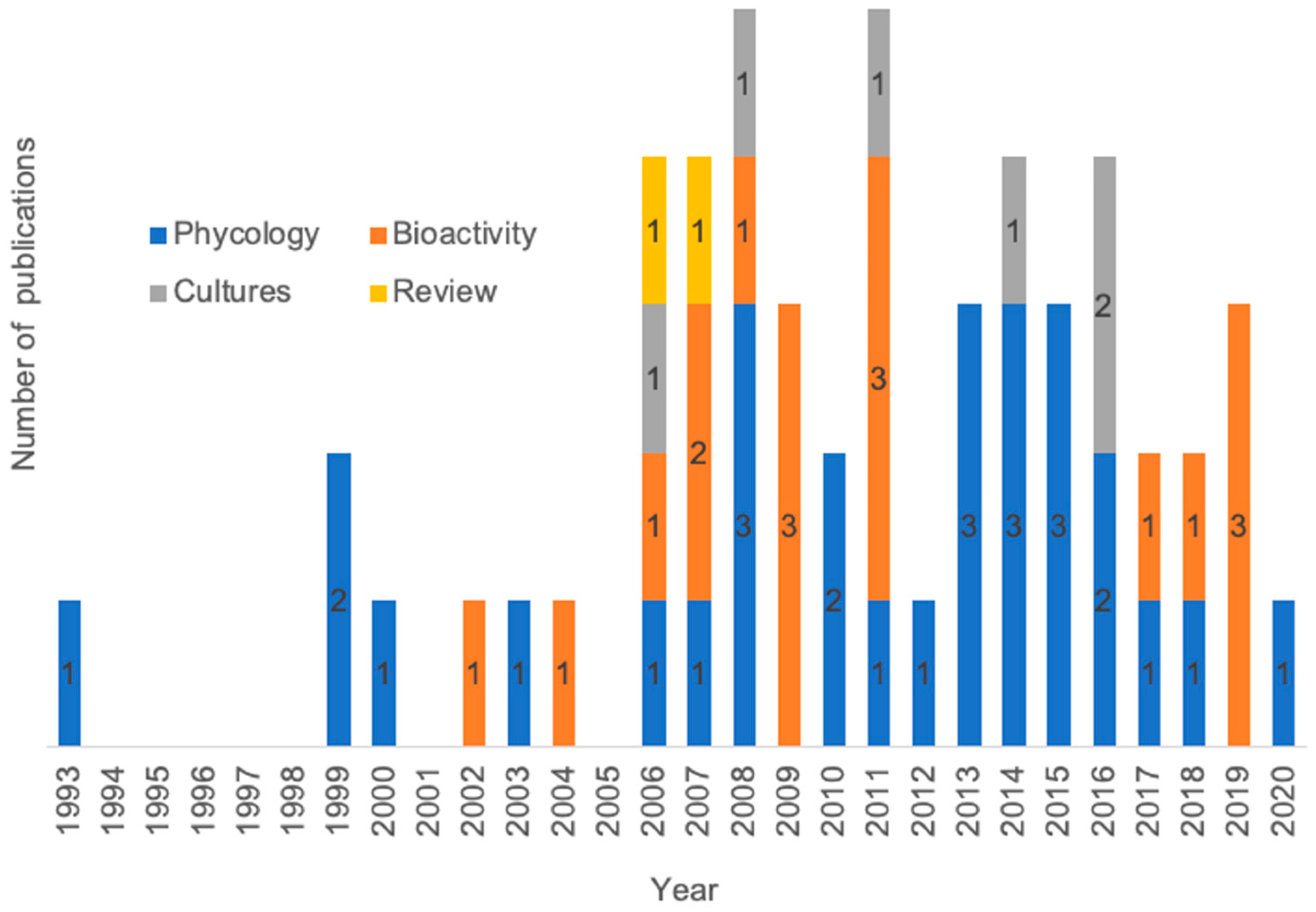
3. Biodiversity of Marine Algae in Colombia
4. Types of Seaweed Cultivation
4.1. Open-Sea Cultivation
4.2. Land-Based Cultivation
4.3. Integrated Multitrophic Aquaculture Systems (IMTA)
5. Seaweed Cultivation Experiences in Colombia
6. Applications of Seaweeds in Colombia
6.1. Fertilizers
6.2. Biological Activity
6.3. Polysaccharides and Pigments
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grand View Research. Global Commercial Seaweeds Market Size Report, 2020–2027. Report Overview. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/commercial-seaweed-market (accessed on 20 November 2020).
- Cioš, A.M.; Jerković, I.; Molnar, M.; Šubarić, D.; Jokić, S. New trends for macroalgal natural products applications. Nat. Prod. Res. 2019, 35, 1180–1191. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Algaebase: Listing the World’s Algae. Available online: http://www.algaebase.org/ (accessed on 12 November 2021).
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 17. [Google Scholar] [CrossRef]
- Duarte, C.M.; Marbá, N.; Holmer, M. Rapid domestication of marine species. Science 2007, 316, 382–383. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Historical use of seaweed as an agricultural fertilizer in the european atlantic area. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; Pereira, L., Bahcevandziev, K., Joshi, N., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–23. [Google Scholar]
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses, 3rd ed.; Springer: Dordrecht, The Netherlands, 1980. [Google Scholar]
- Newton, L. Animal and human nutrition from seaweed resources in Europe: Uses and potential. In Seaweed Utilization; Sampson Low: London, UK, 1951; pp. 31–57. [Google Scholar]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry, 1st ed.; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Dillehay, T.M.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte verde: Seaweed, food, medicine, and the peopling of South America. Science 2008, 9, 784–786. [Google Scholar] [CrossRef]
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Kelly, M.S.; Dworjanyn, S. The Potential of Marine Biomass for Anaerobic Biogas Production. The Crown Estate: London, UK, 2008. Available online: https://arpa-e.energy.gov/sites/default/files/The%20Potential%20of%20Marine%20Biomass%20for%20Anaerobic%20Biogas%20Production%202008.pdf (accessed on 20 August 2020).
- Qin, Y. Production of seaweed-derived food hydrocolloids. In Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets; Qin, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–69. [Google Scholar]
- Coas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Seaweed application in cosmetics. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–441. [Google Scholar]
- Mrais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy-cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Pangestuti, R.; Kim, S.-K. Seaweeds: Valuable ingredients for the pharmaceutical industries. In Grand Challenges in Marine Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 49–95. [Google Scholar]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Informe del Estado de los Ambientes Marinos y Costeros en Colombia. 2017. Available online: http://www.invemar.org.co/documents/10182/14479/IER_2017_baja_Final.pdf/76690566-f6e1-4610-906f-1c49c610b2c8 (accessed on 20 October 2020).
- Comisión Colombiana del Océano. Reserva de Biósfera Seaflower. Available online: http://www.cco.gov.co/la-reserva.html (accessed on 3 October 2020).
- Seaflower Biosphere Reserve. Available online: https://en.unesco.org/biosphere/lac/seaflower (accessed on 20 March 2020).
- Díaz, J.M.; Acero, A. Marine biodiversity in Colombia: Achievements, status of knowledge, and challenges. Gayana 2003, 67, 261–274. [Google Scholar] [CrossRef]
- Márquez, G. Biodiversidad marina: Aproximación con referencia al Caribe. In Ecosistemas Estratégicos y Otros Estudios de Ecología Ambiental; Fondo FEN: Bogotá, Colombia, 1996; pp. 67–102. [Google Scholar]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and microalgae: An overview for unlocking their potential in global aquaculture development. In FAO Fisheries and Aquaculture Circular No. 1229; FAO: Rome, Italy, 2021. [Google Scholar]
- Gacía, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The evolution road of seaweed aquaculture: Cultivation technologies and the industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Olsen, Y.S.; Mayol, E.; Marbà, N.; Duarte, C.M. Global unbalance in seaweed production, research effort and biotechnology markets. Biotechnol. Adv. 2014, 32, 1028–1036. [Google Scholar] [CrossRef]
- Dos Santos Fernandes De Araujo, R. Brief on algae biomass production. In JRC Publications Repository JRC118214; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Buchmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, A.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef]
- Alemañ, A.E.; Robledo, D.; Hayashi, L. Development of seaweed cultivation in Latin America: Current trends and future prospects. Phycologia 2019, 58, 462–471. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Padilla-Vallejos, J. The seaweed resources of Peru. Bot. Mar. 2020, 63, 381–394. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Pliego-Cortés, H.; Robledo, D. Seaweed resources of Mexico: Current knowledge and future perspectives. Bot. Mar. 2019, 62, 275–289. [Google Scholar] [CrossRef]
- Ferdouse, F.; Holdt, L.S.; Smith, R.; Murúa, P.; Yang, Z. The Global Status of Seaweed Production, Trade and Utilization; FAO Globefish Research Programme: Rome, Italy, 2018; Volume 124, p. 120. [Google Scholar]
- Shobika, U.; Chrispin, L.C.; Santhiya, A.V. Global status of mariculture. Biotica Res. Today 2020, 2, 618–621. [Google Scholar]
- Hayashi, L.; Bulboa, C.; Kradolfer, P.; Soriano, G.; Robledo, D. Cultivation of red seaweeds: A latin american perspective. J. Appl. Phycol. 2013, 26, 719–727. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; Jorge Da Silva, G.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Lopez-Santamaria, A.; Miranda, J.M.; Mondragon, A.D.C.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential use of marine seaweeds as prebiotics: A review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-derived ingredients for cosmetic industry—An update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.G.C.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vivo studies and clinical trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, G. Las algas marinas colombianas y su distribucion geografica. Rev. Acad. Colomb. Cienc. Exactas Fís. Nat. 1968, 13, 237–257. [Google Scholar]
- Schnetter, R. Beitrag zur kenntnis der algenflora an der Kolumbianischen küste der karibischen see. Bol. Investig. Mar. Costeras 1969, 30, 49–57. [Google Scholar] [CrossRef]
- Panizzo, L. Evaluación del contenido de nitrógeno y de aminoácidos totales en algunas especies de algas marinas pertenecientes a la región del Magdalena, Colombia. Rev. Acad. Colomb. Cienc. 1976, 14, 31–36. [Google Scholar]
- Comisión Colombiana del Océano. Plan de Expediciones Científicas. Available online: http://www.cco.gov.co/plan-de-expediciones-cientificas.html (accessed on 3 October 2020).
- Albis, M.R.; Gavio, B. Notes on marine algae in the international biosphere reserve seaflower, caribbean colombian I: New records of macroalgal epiphytes on the seagrass Thalassia testudinum. Bot. Mar. 2011, 54, 537–543. [Google Scholar] [CrossRef]
- Albis, M.R.; Gavio, B. Notes on the marine algae of the international biosphere reserve seaflower, caribbean colombia IV: New records of macroalgal epiphytes on the seagrass Thalassia testudinum. Bol. Investig. Mar. Cost 2015, 44, 55–69. [Google Scholar] [CrossRef]
- Gavio, B.; Cifuentes-Ossa, M.A.; Wynne, M.J. Notes on the marine algae of the international biosphere reserve seaflower, caribbean Colombia V: First study of the algal flora of quitasueño bank. Bol. Investig. Mar. Cost 2015, 44, 117–126. [Google Scholar] [CrossRef]
- Reyes-Gómez, V.P.; Gavio, B. Notes on the marine algae of the international biosphere reserve seaflower, Caribbean Colombia VI: New records of Phaeophyceae from old providence and Santa Catalina. Acta Biol. Colomb. 2017, 22, 238–241. [Google Scholar] [CrossRef]
- Reyes-Gómez, V.P.; Gavio, B.; Velasquez, H. Notes on the marine algae of the international biosphere reserve seaflower, caribbean Colombia III. New records of Cyanophyta. Nova Hedwig. 2013, 97, 349–360. [Google Scholar] [CrossRef]
- Rincón-Díaz, M.N.; Gavio, B.; Wynne, M.J.; Santos-Martínez, A. First record of the red alga Griffithsia capitata (Ceramiales, Rhodophyta) in the southwestern Caribbean Sea, Western Atlantic. Mar. Biodivers. Rec. 2016, 9, 537. [Google Scholar] [CrossRef]
- Rincón-Díaz, M.N.; Gavio, B.; Wynne, M.J.; Santos-Martínez, A. Notes on marine algae in the international biosphere reserve seaflower, caribbean Colombia, VII: Additions to the benthic flora of San Andrés Island. Caldasia 2018, 40, 97–111. [Google Scholar] [CrossRef]
- Rincón-Díaz, M.N.; Gavio, B. Diversidad de Macroalgas Marinas del Caribe Colombiano, v2.8; Instituto de Investigaciones Marinas y Costeras—Invemar: Santa Marta, Colombia, 2020. [Google Scholar]
- Diaz, G.; Díaz, M. Diversity of benthic marine algae of the Colombian atlantic. Biota Colomb. 2003, 4, 203–246. [Google Scholar] [CrossRef]
- Reyes-Gómez, V.P.; Velasquez, H.; Gavio, B. Notes on the marine algae of the international biosphere reserve seaflower, Caribbean Colombia VIII: New records of red algae (Rhodophyta) from San Andres, Old Providence, and Saint Cataline, Colombia. Acta Bot. Mex. 2021, 128, e1848. [Google Scholar] [CrossRef]
- Salazar-Forero, C.E.; Gavio, B.; Wynne, M.J. Macroalgae associated with aerial roots of Rhizophora mangle in islas del rosario, Colombia, southwestern Caribbean. Caldasia 2021, 43, 94–104. [Google Scholar] [CrossRef]
- Baos, R.A.; Morales, S. Algae asociated to a mangrove in the Colombian Pacific. Municipio buenaventura—Valle del cauca. Fac. Cienc. Agropecu. 2007, 5, 84–89. [Google Scholar]
- Peña, E.J. Dinámica espacial y temporal de la biomasa algal asociada a las raíces de mangle en la bahía de Buenaventura, costa pacífica de Colombia. Bol. Investig. Mar. Cost 2008, 37, 55–70. [Google Scholar]
- Schnetter, R.; Bula-Meyer, G. Algas Marinas del Litoral Pacífico de Colombia. Chlorophyceae, Phaeophyceae, Rhodophyceae; J. Cramer: Lehre, Germany, 1982. [Google Scholar]
- Marín, H.; Peña, E.J. Seaweed checklist of Tumaco’s bay, Colombian pacific. Hidrobiológica 2016, 26, 299–309. [Google Scholar] [CrossRef]
- Murillo, M.; Peña, E.J. Algas marinas bentónicas de la isla gorgona, costa pacífica colombiana. Rev. Biol. Trop. 2014, 62 (Suppl. 1), 27–41. [Google Scholar] [CrossRef]
- Rincón-Díaz, N.; Gavio, B.; Sánchez, J.V.; Chasqui, L. Crouania mageshimensis Itono, 1977 (Ceramiales, Rhodophyta) and three other species new to the eastern tropical Pacific. Check List 2020, 16, 1171–1180. [Google Scholar] [CrossRef]
- Rincón-Díaz, N.; Sánchez, J.V.; Gavio, B.; Chasqui, L. Diversity of benthic macroalgae in the Colombian pacific: A study of a rocky reef flora. Nova Hedwig. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Fernández, C.; Riosmena, R.; Wysor, B.; Tejada, O.L.; Cortés, J. Checklist of the Pacific marine macroalgae of central America. Bot. Mar. 2011, 54, 53–73. [Google Scholar] [CrossRef]
- Amosu, A.O.; Robertson-Andersson, D.V.; Maneveldt, G.W.; Anderson, R.J.; Bolton, J.J. South african seaweed aquaculture: A sustainable development example for other African coastal countries. Afr. J. Agric. Res. 2013, 8, 5260–5271. [Google Scholar] [CrossRef]
- Seaweed Aquaculture. Available online: https://www.fisheries.noaa.gov/national/aquaculture/seaweed-aquaculture (accessed on 5 May 2021).
- Fernand, F.; Israel, A.; Skjermo, J.; Wichard, T.; Timmermans, K.R.; Golberg, A. Offshore macroalgae biomass for bioenergy production: Environmental aspects, technological achievements and challenges. Renew. Sustain. Energy Rev. 2017, 75, 35–45. [Google Scholar] [CrossRef]
- Hafting, J.T.; Critchley, A.T.; Cornish, M.L.; Hubley, S.A.; Archibald, A.F. On-land cultivation of functional seaweed products for human usage. J. Appl. Phycol. 2012, 24, 385–392. [Google Scholar] [CrossRef]
- Mendoza, W.; Mendola, D.; Kim, J.; Yarish, C.; Velloze, A.; Mitchell, B.G. Land-based drip-irrigated culture of Ulva compressa: The effect of culture platform design and nutrient concentration on biomass production and protein content. PLoS ONE 2018, 13, e0201675. [Google Scholar] [CrossRef]
- Levy, I. Technology for Cultivation of Porphyra and Other Seaweeds in Land-Based Ponds; World Intellectual Property Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Algaplus. Available online: https://www.algaplus.pt/ (accessed on 20 October 2020).
- Seakura Sea of Life. Available online: https://www.seakura.co.il/en/ (accessed on 20 October 2020).
- AgriFutures Australia. Australian Seaweed Industry Blueprint. Available online: https://www.agrifutures.com.au/wp-content/uploads/2020/09/20-072.pdf (accessed on 20 August 2020).
- Algas Chile. Available online: https://www.algaschile.com (accessed on 20 October 2020).
- Craigie, J.S.; Cornish, M.L.; Deveau, L.E. Commercialization of Irish moss aquaculture: The Canadian experience. Bot. Mar. 2019, 62, 411–432. [Google Scholar] [CrossRef]
- Lawton, R.J.; Sutherland, J.E.; Glasson, C.R.K.; Magnusson, M.E. Selection of temperate Ulva species and cultivars for land-based cultivation and biomass applications. Algal Res. 2021, 56, 102320. [Google Scholar] [CrossRef]
- Praeger, C.; Vucko, M.J.; de Nys, R.; Cole, A. Maximising the productivity of the attached cultivation of Ulva tepida in land-based systems. Algal Res. 2019, 40, 101507. [Google Scholar] [CrossRef]
- Shpigel, M.; Shauli, L.; Odintsov, V.; Ashkenazi, N.; Ben-Ezra, D. Ulva lactuca biofilter from a land-based integrated multi trophic aquaculture (IMTA) system as a sole food source for the tropical sea urchin Tripneustes gratilla elatensis. Aquaculture 2018, 496, 221–231. [Google Scholar] [CrossRef]
- Demetropoulos, C.L.; Langdon, C.J. Enhanced production of Pacific dulse (Palmaria mollis) for co-culture with abalone in a land-based system: Nitrogen, phosphorus, and trace metal nutrition. Aquaculture 2004, 235, 433–455. [Google Scholar] [CrossRef]
- Grote, B. Recent developments in aquaculture of Palmaria palmata (Linnaeus) (Weber & Mohr 1805): Cultivation and uses. Rev. Aquac. 2017, 11, 25–41. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Turan, G.; Neori, A. Intensive seaweed aquaculture: A potent solution against global warming. In Seaweeds and Their Role in Globally Changing Environments; Israel, A., Einav, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 357–372. [Google Scholar]
- Álvarez, R.; Pardo, C.M.; Trespalacios, A.A. Evaluación y utilización potencial de las macroalgas marinas del caribe y el pacífico de Colombia: Estado actual de su conocimiento. Biosalud 2007, 6, 113–129. [Google Scholar]
- Molina, J.N.; Álvarez, R. Resultados preliminares del cultivo experimental de Gracilaria Verrucosa (Hudson) Papenfuss (=G. caudata J. Agardh) (Rhodophyta: Gracilariaceae) en la costa caribe de Colombia. Rev. Acad. Colomb. Cienc. 2014, 38, 79–87. [Google Scholar]
- Peña, E.J.; Álvarez, R. Experiencias en el cultivo experimental de algas rojas en el caribe y pacífico de Colombia. Rev. Luna Azul 2006, 23, 16–20. [Google Scholar]
- Gallo, H.M.; Rincones, R.E. Factibilidad del Cultivo de Algas Marinas. Proyecto Corpoguajira/IIRBAvH/FAO. Fortalecimiento Para el Desarrollo de Empresas Rurales a Partir de Productos de la Biodiversidad en el Cabo de la Vela, Departamento de La Guajira; Consultoría Fase II: Bogotá, Colombia, 2003. [Google Scholar]
- Montaña, J.C. Ensayos de Cultivo en Medio Natural de la Macroalga Hypnea Musciformis (Wulfen) Lamoroux en las Areas de Taganga y Puerto Luz (Santa Marta). Bachelor’s Thesis, Universidad Jorge Tadeo Lozano, Cundinamarca, Colombia, 2006. [Google Scholar]
- Rincones, R.E.; Gallo, H. Proyecto Jimoula: El Cultivo de Algas Marinas Como Alternativa Sustentable Para las Comunidades Costeras de la Península de la Guajira; BIOTACOL Ltda.: Rioacha, Colombia, 2004. [Google Scholar]
- Delgadillo, O.; Newmark, F. Cultivo piloto de macroalgas rojas (Rhodophyta) en Bahía Portete, La Guajira, Colombia. Bol. Investig. Mar. Cost 2008, 37, 7–26. [Google Scholar] [CrossRef]
- Rincones, R.E.; Moreno, D.A. Aspectos técnicos y económicos para el establecimiento comercial del maricultivo de algas en Colombia: Experiencias en la península de la Guajira. Ambiente Desarro. 2011, 28, 1–52. [Google Scholar]
- Peña, E.J.; Palacios, M.L. Introducción del alga roja Kappaphycus alvarezzi (Doty) en Colombia y experiencias de cultivo en la península de la guajira, caribe Colombiano. In Guiίa de las Especies Introducidas Marinas y Costeras de Colombia; Gracia, A., Medelliίn, J., Gil, D.L., Puentes, V., Eds.; INVEMAR. Ministerio de Ambiente y Desarrollo Sostenible: Santa Marta, Colombia, 2011; pp. 93–103. [Google Scholar]
- Camacho, O.; Montaña, J. Cultivo experimental en el mar del alga roja Hypnea musciformis en el area de Santa Marta, Caribe Colombiano. Bol. Investig. Mar. Cost. 2012, 41, 29–46. [Google Scholar] [CrossRef]
- Mosquera, Z.; Peña, E.J. Effect of salinity on growth of the green alga Caulerpa sertularioides (Bryopsidales, Chlorophyta) under laboratory conditions. Hidrobiológica 2016, 26, 277–282. [Google Scholar] [CrossRef]
- Islam, M.; Khan, S.; Hasan, J.; Mallick, D.; Hoq, E. Seaweed Hypnea sp. culture in Cox’s Bazart coast, Bangladesh. Bangladesh J. Zool. 2017, 45, 37–46. [Google Scholar] [CrossRef]
- Pereira, S.; Kimpara, J.; Valentin, W. A simple substrate to produce the tropical epiphytic algae Hypnea pseudomusciformis. Aquac. Eng. 2020, 89, 102066. [Google Scholar] [CrossRef]
- Ganesan, M.; Thiruppathi, S.; Jha, B. Mariculture of Hypnea musciformis (Wulfen) Lamouroux in South east coast of India. Aquaculture 2006, 256, 201–211. [Google Scholar] [CrossRef]
- Gómez, A.; Millán, J. Cultivo experimental de Gracilaria dentata Agardh y de Gracilariopsis tenuifrons (Bird et Oliveira) (Rhodophyta: Gigartinales) en la isla de Margarita, Venezuela. Rev. Biol. Mar. Oceanogr. 1997, 32, 137–144. [Google Scholar]
- Bermejeo, R.; Cara, C.; Macías, M.; Sánchez-García, J.; Hernández, I. Growth rates of Gracilariopsis longissima, Gracilaria bursa-pastoris and Chondracanthus teedei (Rhodophyta) cultured in ropes: Implication for N biomitigation in Cadiz Bay (Southern Spain). J. Appl. Phycol. 2020, 32, 1879–1891. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L.; Brun, F.G.; Andría, J.; Vergara, J.J. Seasonal and tidal variability of environmental carbon related physico-chemical variables and inorganic C acquisition in Gracilariopsis longissima and Enteromorpha intestinalis from Los Toruños salt marsh (Cádiz Bay, Spain). J. Exp. Mar. Biol. Ecol. 2004, 304, 183–201. [Google Scholar] [CrossRef]
- Jiang, H.; Zou, D.; Lou, W.; Chen, W.; Yang, Y. Growth and photosynthesis by Gracilariopsis lemaneiformis (Gracilariales, Rhodophyta) in response to di!erent stocking densities along Nan’ao Island coastal waters. Aquaculture 2019, 501, 279–284. [Google Scholar] [CrossRef]
- Hayashi, L.; Reis, R.P.; Alves, A.; Castelar, B.; Robledo, D.; de Vega, G.B.; Msuya, F.E.; Eswaran, K.; Yasir, S. The cultivation of Kappaphycus and Eucheuma in tropical and sub-tropical waters. In Tropical Seaweed Farming Trends, Problems and Opportunities; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 55–90. [Google Scholar]
- Hurtado, A.Q.; Reis, R.P.; Loureiro, R.R.; Critchley, A.T. Kappaphycus (Rhodophyta) cultivation: Problems and the impacts of acadian marine plant extract powder. In Marine Algae; Pereira, L., Neto, J.M., Eds.; Taylor & Francis Group LLC: Oxfordshire, UK, 2014; pp. 251–299. [Google Scholar]
- Hayashi, L.; Hurtado, A.Q.; Msuya, F.E.; Bleicher, G.; Critchley, A.T. A review of Kappaphycus farming: Prospects and constraints. In Seaweeds and Their Role in Globally Changing Enviorments; Israel, A., Einav, R., Seckbach, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 251–283. [Google Scholar]
- Chandrasekaran, S.; Nagendran, N.A.; Pandiaraja, D.; Krishnankutty, N.; Kamalakannan, B. Bioinvasion of Kappaphycus alvarezii on corals in the Gulf of Mannar, India. Curr. Sci. 2008, 94, 1167–1172. Available online: https://www.jstor.org/stable/24100697 (accessed on 2 December 2021).
- Mulyani, S.; Tuwo, A.; Syamsuddin, R.; Jompa, J. Effect of seaweed Kappaphycus alvarezii aquaculture on growth and survival of coral Acropora Muricata. AACL Bioflux 2018, 11, 1792–1798. [Google Scholar]
- Rajaram, R.; Rameshumar, S.; Ahmad, B.; Albeshr, M.F. Impacts of cultivation of red algae Kappaphycus alvarezii on planktonic and benthic faunal density in relation to environmental and hydrobiological parameters in tropical coastal ecosystem. Acta Ecol. Sin. 2021, 41, 39–49. [Google Scholar] [CrossRef]
- Reis, R.P.; Castelar, B.; Moura, A.L.; Kirk, R. Invasive potential of Kappaphycus alvarezii off the south coast of Rio de Janeiro state, Brazil: A contribution to environmentally secure cultivation in the tropics. Bot. Mar. 2009, 52, 283–289. [Google Scholar] [CrossRef]
- Chen, S.; Ganapin, D. Polycentric coastal and ocean management in the Caribbean sea large marine ecosystem: Harnessing community-based actions to implement regional frameworks. Environ. Dev. 2016, 17 (Suppl. 1), 264–276. [Google Scholar] [CrossRef]
- Chen, S.; Akhtar, T.; Currea, A.M. Sustainable seaweed production, Belize. In Scaling up Community Actions for International Water Management—Experiences from the GEF Small Grants Programme; The GEF Small Grants Programme: Port Louis, Mauritius, 2016; Available online: https://www.thegef.org/sites/default/files/publications/IW_PublicationCaseStudiesMay-Digital.pdf (accessed on 20 October 2020).
- Gracilarias de Panamá. Available online: https://gracilarias.org (accessed on 10 May 2021).
- Chung, I.K.; Sondak, C.F.A.; Beardall, J. The future of seaweed aquaculture in a rapidly changing world. Eur. J. Phycol. 2017, 52, 495–505. [Google Scholar] [CrossRef]
- INVEMAR. La calidad ambiental marina y costera en Colombia. In Technical Report: Informe de Estado de los Ecosistemas Marinos y Costeros; Invemar: Santa Mata, Colombia, 2004; pp. 39–74. [Google Scholar]
- Rodríguez, L. Programa Nacional de Investigación, Evaluación, Prevención, Reducción y Control de Fuentes Terrestres y Marinas de Contaminación al Mar—Actualización del Plan de Acción 2014–2019; Comisión Colombiana Del Océano (CCO): Bogotá, Colombia, 2016; p. 70.
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Vélez, L.; Álvarez-León, R. Comparación bromatológica de las algas nativas (Gracilariopsis tenuifrons, Sargassum filipendula) y exóticas (Kappaphycus alvarezii) del caribe Colombiano. Bol. Cient. Mus. Hist. Nat. Univ. Caldas 2009, 13, 17–25. [Google Scholar]
- Bula-Meyer, G. Marine macroalgae in the agronomy and potential use of floating Sargassum for manure production in the San Andres and Providencia Archipelago, Colombia. Rev. Intropica 2004, 1, 91–103. [Google Scholar]
- Ospina, S.P.; López, J.B.; Márquez, M.E. Efecto antimitótico in vitro en el extracto metanólico de macroalgas marinas de la costa caribe Colombiana. Vitae 2007, 14, 84–89. [Google Scholar]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Arteaga, M.; De Silvestri, J. Estudio de las sustancias con propiedades antimicrobianas extraordinarias de algas marinas pertenecientes al litoral atlántico colombiano. Rev. Colomb. Cienc. Químico Farm. 1985, 4, 47–52. [Google Scholar]
- Rozo, G.; Rozo, C.; Plazas, E.; Patiño, O. Fraccionamiento bioguiado del extracto etanólico de Hypnea musciformis en búsqueda de compuestos con actividad antioxidante. Vitae 2011, 18 (Suppl. 2), S162–S163. [Google Scholar]
- Echavarría, B.; Franco, A.; Martínez, A. Evaluación de la actividad antioxidante y determinación del contenido de compuestos fenólicos en extractos de macroalgas del caribe Colombiano. Vitae 2009, 16, 126–131. [Google Scholar]
- Díaz, M.C.; Bula-Meyer, G.; Zea, S.; Martínez, A. Ensayos de actividad biológica y ecología química de extractos orgánicos de macroalgas del caribe Colombiano. Bol. Investig. Mar. Cost 2006, 35, 241–247. [Google Scholar]
- Martínez, A.; Arias, L.A.; Rueda, J.L.; Diaz, M.C.; Bula-Meyer, G. Antimicrobial activity study for alcohol extracts of some macroalgae from Colombian Caribbean Sea. Vitae 2002, 9, 49–55. [Google Scholar]
- Nuñez, E.; Arteaga, M.; Castro, M. Investigación de actividad antibacteriana de extractos de algunas algas marinas colombianas. Rev. Colomb. Cienc. Químico Farm. 1972, 2, 123–130. [Google Scholar]
- Sreenivasa, P.; Parekh, K. Antibacterial activity of Indian seaweed extracts. Bot. Mar. 1981, 24, 577–582. [Google Scholar] [CrossRef]
- Valle, H.; Ospina, S.; Galeano, E.; Martínez, A.; Márquez, M.E.; López, J.B. Obtención de una fracción antimitótica del extracto etanólico de la acroalga Digenia simplex. Bol. Investig. Mar. Cost 2009, 38, 109–117. [Google Scholar]
- Valle, H.; Ospina, S.; Galeano, E.; Martínez, A.; Marquez, M.E.; López, J.B. Componentes de la fracción antimitótica del extracto etanólico de la macroalga Digenia simplex. Vitae 2008, 15, 141–149. [Google Scholar]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef]
- Molina, J.N.; de la Rosa, C.R.; Olivares, L.A.; Parra, X.J.; Serrano, A. Evaluación de las condiciones de extracción de agar-agar a partir de Gracilaria verrucosa en el balneario de Santa Verónica en el departamento del atlántico. Vitae 2011, 18 (Suppl. 2), S219. [Google Scholar]
- Monsalve-Bustamante, Y.; Rincón-Valencia, S.; Puertas-Mejía, M.; Moreno-Tirado, D.; Mejía-Giraldo, J.; Restrepo-Moná, Y. Potencial material de encapsulación a partir del alga Gracilariopsis tenuifrons del mar caribe Colombiano. Vitae 2018, 25 (Suppl. 1), 153–155. [Google Scholar]
- Camacho, O.; Hernández-Carmona, G. Phenology and alginates of two Sargassum species from the Caribbean coast of Colombia. Cienc. Mar. 2012, 38, 381–393. [Google Scholar] [CrossRef][Green Version]
- Restrepo, N. Extracción, Purificación Y Análisis del Contenido de Fucoxantina en Algas Pardas del Caribe Colombiano. Bachelor’s Thesis, Universidad de Bogotá Jorge Tadeo Lozano, Cundinamarca, Colombia, 2015. [Google Scholar]
- Monsalve-Bustamante, Y.; Rincón-Valencia, S.; Mejía-Giraldo, J.; Moreno-Tirado, D.; Puertas-Mejía, M. Screening of the UV absorption capacity, proximal and chemical characterization of extracts, and polysaccharide fractions of the Gracilariopsis tenuifrons cultivated in Colombia. J. Appl. Pharm. Sci. 2019, 9, 103–109. [Google Scholar] [CrossRef][Green Version]
- Vargas, P.A. Efectos Fotoprotectores y Humectantes de Formulaciones Cosméticas que Contienen Extractos de Hypnea Musciformis Recolectadas en el Caribe Colombiano. Bachelor’s Thesis, Universidad de Bogotá Jorge Tadeo Lozano, Cundinamarca, Colombia, 2020. [Google Scholar]
- Ferrara, L. Seaweeds: A food for our future. J. Food Chem. Nanotechnol. 2020, 6, 56–64. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, J.; Grimm, D. Algae and their potential for a future bioeconomy, landless food production, and the socio-economic impact of an algae industry. Org. Agric. 2021, 11, 261–267. [Google Scholar] [CrossRef]
- Figueroa, V.; Farfán, M.; Aguilera, J.M. Seaweeds as novel foods and source of culinary flavors. Food Rev. Int. 2021, 37, 1–26. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Rhatigan, P.; Pérez, J.L. World cuisine of seaweeds: Science meets gastronomy. Int. J. Gastron. Food Sci. 2018, 14, 55–65. [Google Scholar] [CrossRef]
- Colombia Potencia Bioceánica Sostenible 2030—Documento CONPES 3990. Consejo Nacional de Politica Economica y Social—Republica de Colombia 91. 2020. Available online: https://www.co.undp.org/content/colombia/es/home/sustainable-development-goals.html (accessed on 30 May 2021).
- Objetivos de Desarrollo Sostenible|El PNUD en Colombia. United Nations Development Programme. 2015. Available online: https://www.co.undp.org/content/colombia/es/home/sustainable-development-goals/background/ (accessed on 30 March 2021).
- Autoridad Nacional de Acuicultura y Pesca. Plan Nacional Para el Desarrollo de la Acuicultura Sostenible en Colombia—PlaNDAS. 2014. Available online: https://www.aunap.gov.co/wp-content/uploads/2016/04/Plan-Nacional-para-el-Desarrollo-de-la-Acuicultura-Sostenible-Colombia.pdf. (accessed on 30 May 2021).
- Cikoš, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Acadian Seaplants. A Sustainable Solutions for Healthier Plants, Animals and People. Available online: https://www.acadianseaplants.com/ (accessed on 20 May 2021).
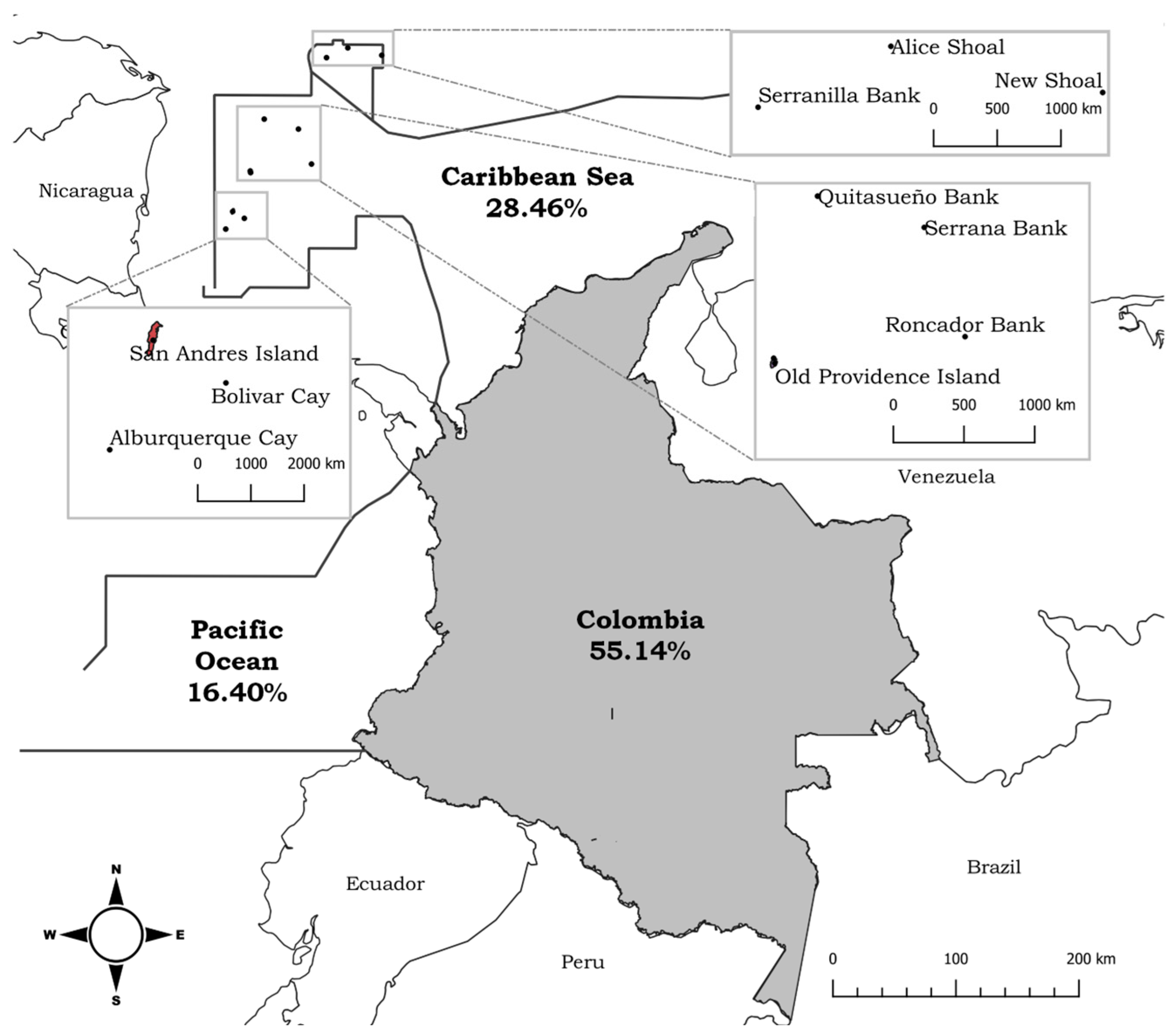
| Caribbean | Pacific | |
|---|---|---|
| Rhodophyta | 60% | 63% |
| Chlorophyta | 28% | 20% |
| Phaeophyceae | 12% | 17% |
| Specie | Region | Type of Culture 1 | DGR 2 | Reference | |
|---|---|---|---|---|---|
| Rhodophyta | |||||
| Order Gracilariales | |||||
| Grateloupia sp. | Guajira | B, R | - | [96] | |
| Gracilariopsis tenuifrons | Guajira | B, R | 0.59 | [97] | |
| Gracilaria cervicornis | Guajira | B, R | 0.44 | [96] | |
| Gracilaropsis longissima (as Gracilaria verrucosa) | Magdalena | B | - | [91] | |
| Crassiphycus corneus (as Hydropuntia cornea) Kappaphycus alvarezii * | Guajira Guajira | B, R R | 0.97, 0.51 5.1 | [96,97] [98] | |
| Order Gigartinales | |||||
| Hypnea musciformis | Guajira-Santa Marta | B, R | 2.66, - | [96,99] | |
| Eucheumatopsis isiformis (as Eucheuma isiforme) | Guajira | B, R | 1.58 | [97] | |
| Order Halymeniales | |||||
| Grateloupia filicina | Magdalena | - | - | [90] | |
| Chlorophyta | |||||
| Order Bryopsidales | |||||
| Caulerpa sertularioides | Nariño | P | 4.82 | [100] | |
| Species | Biological Activity | Reference | |
|---|---|---|---|
| Rhodophyta | |||
| Order Gelidiales | |||
| Gelidiella acerosa | Cytotoxic | [124] | |
| Order Corallinales | |||
| Amphiroa fragilissima | Cytotoxic | [124] | |
| Order Gracilariales | |||
| Gracilaria mammillaris | Antioxidant | [125] | |
| Order Gigartinales | |||
| Hypnea musciformis | Antibacterial—Phenolic and steroidal compounds | [126,127] | |
| Order Ceramiales | |||
| Digenea simplex | Cytotoxic | [124] | |
| Laurencia sp. | Antioxidant | [128] | |
| Laurencia microcladia | Antibacterial | [129,130] | |
| Chlorophyta | |||
| Order Ulvales | |||
| Ulva sp. (as Enteromorpha sp.) | Antibacterial | [126] | |
| Order Bryopsidales | |||
| Caulerpa mexicana | Antibacterial—Antioxidant | [126,128] | |
| C. sertularioides | Cytotoxic | [124] | |
| Ochrophyta | |||
| Order Dictyotales | |||
| Dictyota bartayresiana | Antibacterial—Feeding inhibitor | [129,130] | |
| Dictyota pulchella | Antibacterial-Cytotoxic—Feeding inhibitor | [129,130] | |
| Dictyota sp. | Antioxidant | [128] | |
| Padina boergesenii | Antibacterial | [129,130] | |
| Order Fucales | |||
| Sargassum cymosum | Antibacterial-Cytotoxic – Feeding inhibitor | [124,128,129] | |
| Sargassum sp. | Antioxidant | [128] | |
| Sargassum schnetteri (as Cladophyllum schnetteri) | Feeding inhibitor | [129] | |
| Species | Extract/Compound | Reference | ||
|---|---|---|---|---|
| Rhodophyta | ||||
| Order Gracilariales | ||||
| Gracilariopsis longissima (as Gracilaria verrucosa) | Agar-agar | [136] | ||
| Gracilariopsis tenuifrons | Agar and carrageenan | [137] | ||
| Ochrophyta | ||||
| Order Fucales | ||||
| Sargassum sp. | Alginate | [138] | ||
| S. cymosum | Alginate | [138] | ||
| S. filipendula | Fucoxanthin | [139] | ||
| Turbinaria spp. | Fucoxanthin | [139] | ||
| S. polyceratium | Fucoxanthin | [139] | ||
| Order Dictyotales | ||||
| Dictyota caribaea | Fucoxanthin | [139] | ||
| D. pinnatifida | Fucoxanthin | [139] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Echeverri, J.P.; Zapata-Ramírez, P.A.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Present and Future of Seaweed Cultivation and Its Applications in Colombia. J. Mar. Sci. Eng. 2022, 10, 243. https://doi.org/10.3390/jmse10020243
Arias-Echeverri JP, Zapata-Ramírez PA, Ramírez-Carmona M, Rendón-Castrillón L, Ocampo-López C. Present and Future of Seaweed Cultivation and Its Applications in Colombia. Journal of Marine Science and Engineering. 2022; 10(2):243. https://doi.org/10.3390/jmse10020243
Chicago/Turabian StyleArias-Echeverri, Juan Pablo, Paula Andrea Zapata-Ramírez, Margarita Ramírez-Carmona, Leidy Rendón-Castrillón, and Carlos Ocampo-López. 2022. "Present and Future of Seaweed Cultivation and Its Applications in Colombia" Journal of Marine Science and Engineering 10, no. 2: 243. https://doi.org/10.3390/jmse10020243
APA StyleArias-Echeverri, J. P., Zapata-Ramírez, P. A., Ramírez-Carmona, M., Rendón-Castrillón, L., & Ocampo-López, C. (2022). Present and Future of Seaweed Cultivation and Its Applications in Colombia. Journal of Marine Science and Engineering, 10(2), 243. https://doi.org/10.3390/jmse10020243









