Differences in the Structure and Diversity of Invertebrate Assemblages Harbored by an Intertidal Ecosystem Engineer between Urban and Non-Urban Shores
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling and Sample Procedure
2.2. Data Analysis
3. Results
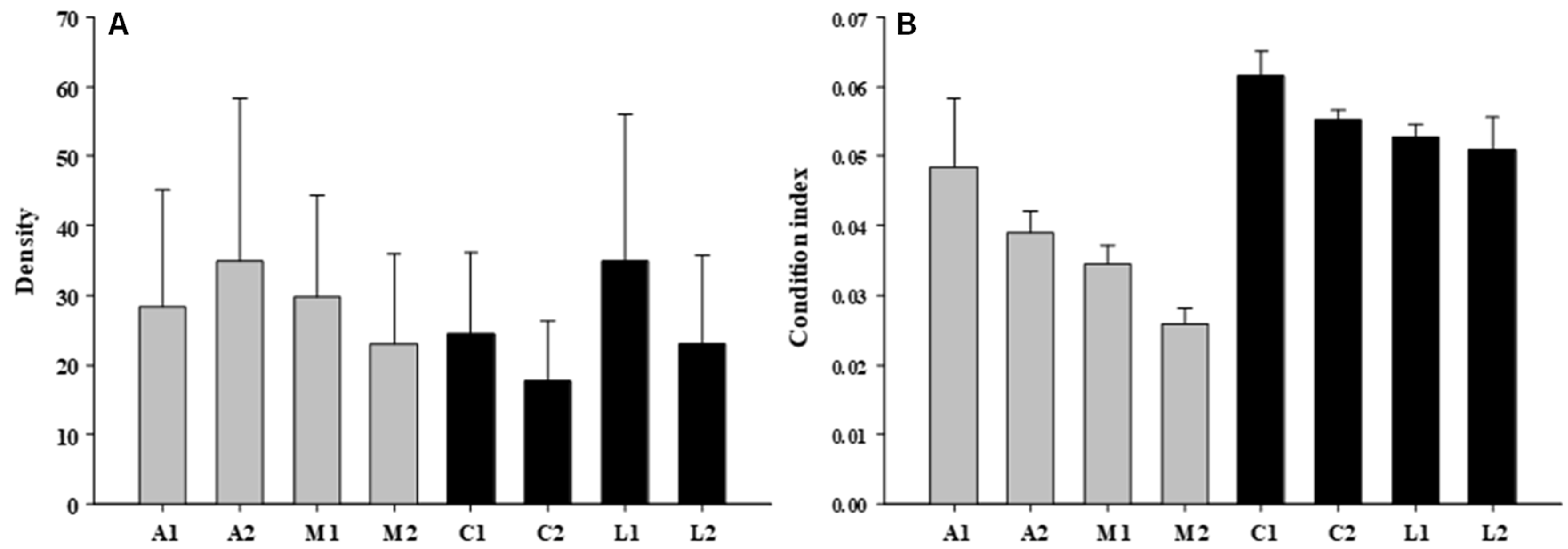
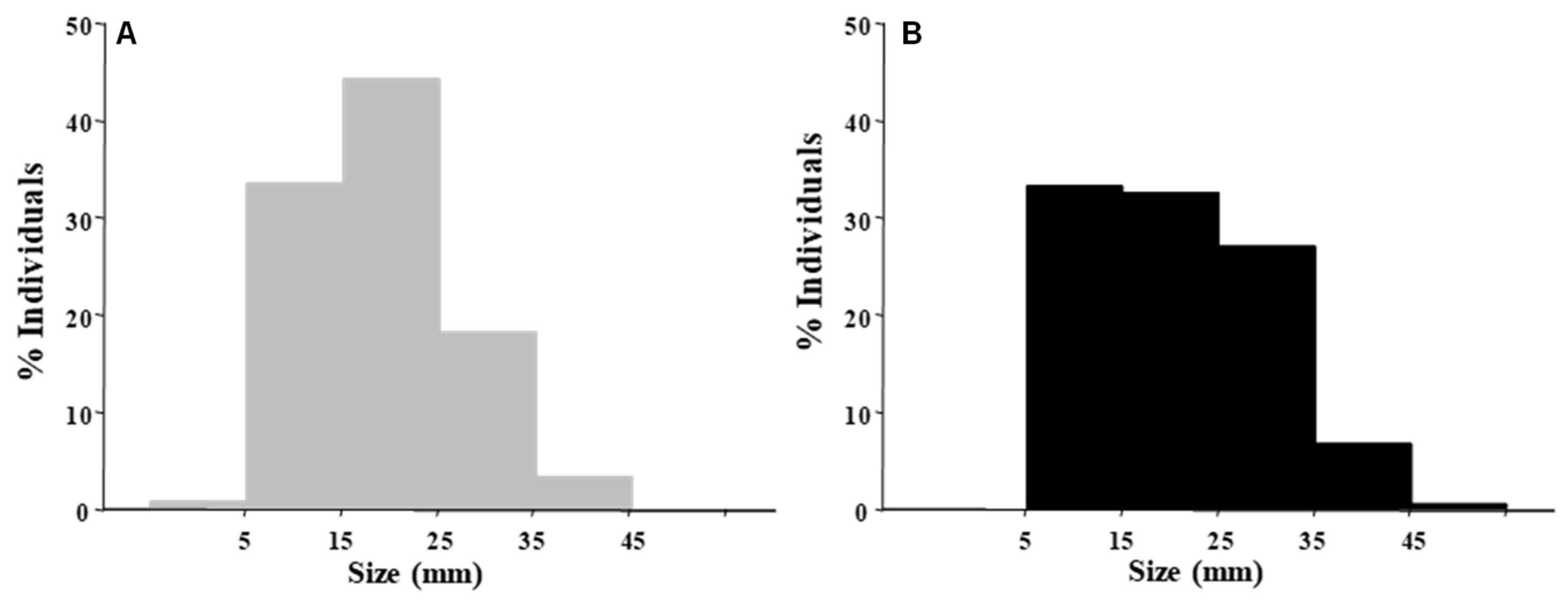
3.1. Mussel’ Attributes
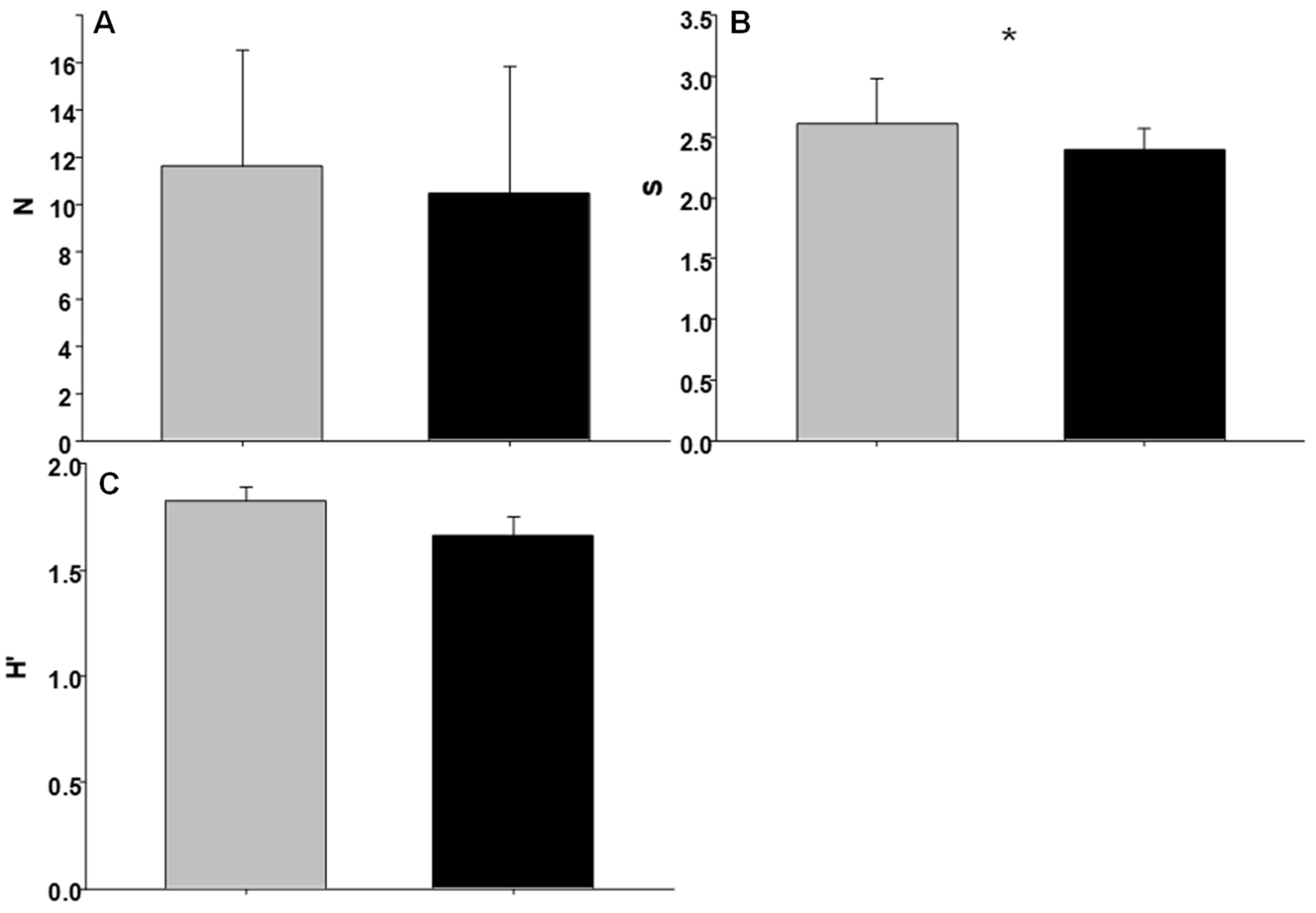
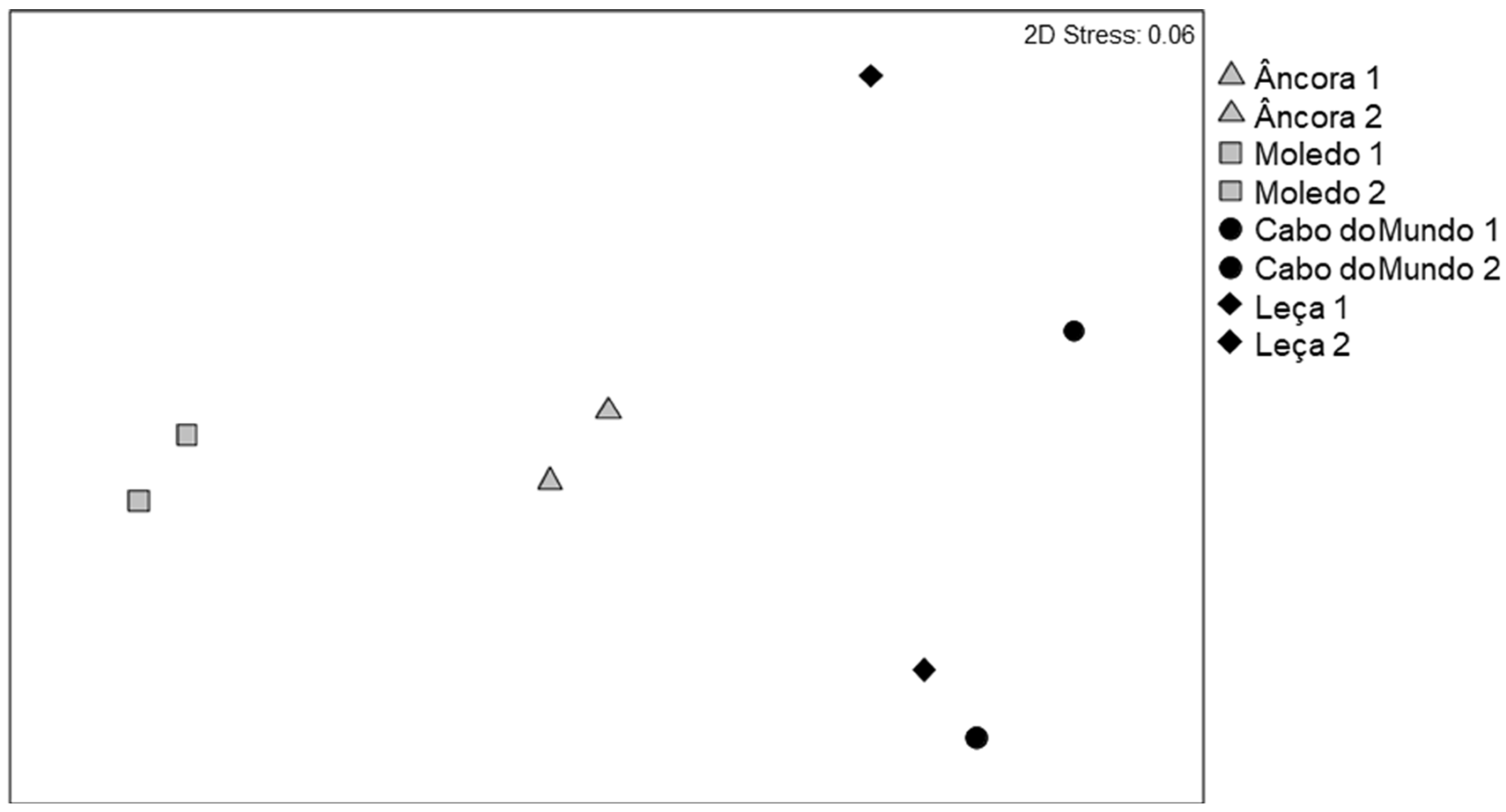
3.2. Invertebrate Assemblages
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Crain, C.M.; Bertness, M.D. Ecosystem engineering across environmental gradients: Implications for conservation and management. Bioscience 2006, 56, 211–218. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Borthagaray, A.I.; Carranza, A. Mussels as ecosystem engineers: Their contribution to species richness in a rocky littoral community. Acta Oecol. 2007, 31, 243–250. [Google Scholar] [CrossRef]
- Arribas, L.P.; Donnarumma, L.; Palomo, M.G.; Scrosati, A. Intertidal mussels as ecosystem engineers: Their associated invertebrate biodiversity under contrasting wave exposures. Mar. Biodivers. 2014, 44, 203–211. [Google Scholar] [CrossRef]
- Norling, P.; Kautsky, N. Structural and functional effects of Mytilus edulis on diversity of associated species and ecosystem functioning. Mar. Ecol. Prog. Ser. 2007, 351, 163–175. [Google Scholar] [CrossRef]
- Singh, G.G.; Markel, R.W.; Martone, R.G.; Salomon, A.K.; Harley, C.D.G.; Chan, K.M.A. Sea otters homogenize mussel beds and reduce habitat provisioning in a rocky intertidal ecosystem. PLoS ONE 2013, 8, e65435. [Google Scholar] [CrossRef] [PubMed]
- Çinar, M.E.; Bakir, K.; Öztürk, B.; Doğan, A.; Açik, S.; Kirkim, F.; Dağli, E.; Kurt, G.; Evcen, A.; Koçak, F.; et al. Spatial distribution pattern of macroinvertebrates associated with the black mussel Mytilus galloprovincialis (Mollusca: Bivalvia) in the Sea of Marmara. J. Mar. Syst. 2020, 211, 103402. [Google Scholar] [CrossRef]
- Hodgson, A.N.; Smith, F.; Smith, P.; Claassens, L. Macrofauna associated with intertidal mussel beds in the Knysna estuarine embayment, South Africa. Afr. Zool. 2021, 56, 44–57. [Google Scholar] [CrossRef]
- Boaventura, D.; Ré, P.; Fonseca, L.C.; Hawkins, S.J. Intertidal rocky shore communities of the continental Portuguese coast: Analyses of distribution patterns. Mar. Ecol. 2002, 23, 69–90. [Google Scholar] [CrossRef]
- Ramos-Oliveira, C.; Sampaio, L.; Rubal, M.; Veiga, P. Spatial-temporal variability of Mytilus galloprovincialis Lamarck 1819 populations and their accumulated sediment in northern Portugal. PeerJ 2021, 9, e11499. [Google Scholar] [CrossRef]
- Vinagre, C.; Mendoça, V.; Narciso, L.; Madeira, C. Food web of the intertidal rocky shore of the west Portuguese coast—Determined by stable isotope analysis. Mar. Environ. Res. 2015, 110, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.; Bryan, T.; Chen, W.; Moy, F.E.; Sandman, A.N.; Sundblad, G.; Schneider, S.; Andersen, J.H.; Langaas, S.; Walday, M.G. Ecosystem Services in the Coastal Zone of the Nordic Countries; Nordisk Ministerråd: Copenhagen, Denmark, 2017. [Google Scholar] [CrossRef]
- Romero-Freire, A.; Lassoue, J.; Silva, E.; Calvo, S.; Pérez, F.F.; Bejaou, N.; Babarro, J.M.F.; Cobelo-García, A. Trace metal accumulation in the commercial mussel M. galloprovincialis under future climate change scenarios. Mar. Chem. 2020, 224, 103840. [Google Scholar] [CrossRef]
- Veiga, P.; Moreira, J.; Ramos-Oliveira, C.; Sampaio, L.; Rubal, M. Public perception of ecosystem services provided by the Mediterranean mussel Mytilus galloprovincialis related to anthropogenic activities. PeerJ. 2021, 9, e11975. [Google Scholar] [CrossRef]
- Rius, M.; Cabral, H.N. Human harvesting of Mytilus galloprovincialis Lamarck, 1819, on the central coast of Portugal. Sci. Mar. 2004, 68, 545–551. [Google Scholar] [CrossRef]
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernández Polanco, J.M.; et al. The decline of mussel aquaculture in the European Union: Causes, economic impacts and opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Thompson, R.C.; Crowe, T.P.; Hawkins, S.J. Rocky intertidal communities: Past environmental changes, present status and predictions for the next 25 years. Environ. Conserv. 2002, 29, 168–191. [Google Scholar] [CrossRef]
- Carranza, A.; Defeo, O.; Beck, M.; Castilla, J.C. Linking fisheries management and conservation in bioengineering species: The case of South American mussels (Mytilidae). Rev. Fish Biol. Fish. 2009, 19, 349–366. [Google Scholar] [CrossRef]
- Barragan, J.M.; de Andrés, M. Analysis and trends of the world’s coastal cities and agglomerations. Ocean Coast. Manag. 2015, 114, 11–20. [Google Scholar] [CrossRef]
- Momota, K.; Hosokawa, S. Potential impacts of marine urbanization on benthic macrofaunal diversity. Sci. Rep. 2021, 11, 4028. [Google Scholar] [CrossRef]
- Todd, P.A.; Heery, E.C.; Loke, L.H.L.; Thurstan, R.H.; Kotze, D.J.; Swan, C. Towards an urban marine ecology: Characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 2019, 128, 1215–1242. [Google Scholar] [CrossRef]
- Airoldi, L.; Beck, M.W. Loss, status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol. 2007, 45, 345–405. [Google Scholar]
- Malerba, M.E.; White, C.R.; Dustin, J.; Marshall, D.J. The outsized trophic footprint of marine urbanization. Front. Ecol. Environ. 2019, 17, 400–406. [Google Scholar] [CrossRef]
- Brown, P.J.; Taylor, R.B. Effects of trampling by humans on animals inhabiting coralline algal turf in the rocky intertidal. J. Exp. Mar. Biol. Ecol. 1999, 235, 45–53. [Google Scholar] [CrossRef]
- Chapman, M.G. Paucity of mobile species on constructed seawalls: Effects of urbanisation on biodiversity. Mar. Ecol. Prog. Ser. 2003, 264, 21–29. [Google Scholar] [CrossRef]
- Airoldi, L.; Bulleri, F. Anthropogenic disturbance can determine the magnitude of opportunistic species responses on marine urban infrastructures. PLoS ONE 2011, 6, e22985. [Google Scholar] [CrossRef]
- Brosnan, D.M.; Crumrine, L.L. Effects of human trampling on marine rocky shore communities. J. Exp. Mar. Biol. Ecol. 1994, 177, 79–97. [Google Scholar] [CrossRef]
- Robinson, T.B.; Branch, G.M.; Griffiths, C.L.; Govender, A. Effects of experimental harvesting on recruitment of an alien mussel Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 2007, 345, 1–11. [Google Scholar] [CrossRef]
- Smith, J.R.; Murray, S.N. The effects of experimental bait collection and trampling on a Mytilus californianus mussel bed in southern California. Mar. Biol. 2005, 147, 699–706. [Google Scholar] [CrossRef]
- Veiga, P.; Ramos-Oliveira, C.; Sampaio, L.; Rubal, M. The role of urbanisation in affecting Mytilus galloprovincialis. PLoS ONE 2020, 15, e0232797. [Google Scholar] [CrossRef] [PubMed]
- Gestoso, I.; Arenas, F.; Rubal, M.; Veiga, P.; Peña, M.; Olabarria, C. Shifts from native to non-indigenous mussels: Enhanced habitat complexity and its effects on faunal assemblages. Mar. Environ. Res. 2013, 90, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Walls, K.; Jeffs, A. Biotic consequences of a shift in invertebrate ecosystem engineers: Invasion of New Zealand rocky shores by a zone-forming ascidian. Mar. Ecol. 2018, 39, e12502. [Google Scholar] [CrossRef]
- Chintiroglou, C.C.; Damianidis, P.; Antoniadou, C.; Lantzouni, M.; Vafidis, D. Macrofauna biodiversity of mussel bed assemblages in Thermaikos Gulf (northern Aegean Sea). Helgol. Mar. Res. 2004, 58, 62–70. [Google Scholar] [CrossRef]
- Çinar, M.E.; Katağan, T.; Koçak, F.; Öztürk, B.; Ergen, Z.; Kocatas, A.; Önen, M.; Kirkim, F.; Bakir, K.; Kurt, G.; et al. Faunal assemblages of the mussel Mytilus galloprovincialis in and around Alsancak Harbour (Izmir Bay, eastern Mediterranean) with special emphasis on alien species. J. Mar. Syst. 2008, 71, 1–17. [Google Scholar] [CrossRef]
- Crain, C.M.; Kroeker, K.; Halpern, B.S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008, 11, 1304–1315. [Google Scholar] [CrossRef]
- Van der Zee, E.M.; Tielens, E.; Holthuijsen, S.; Donadi, S.; Eriksson, B.K.; van der Veer, H.W.; Piersma, T.; Olff, H.; van der Heide, T. Habitat modification drives benthic trophic diversity in an intertidal soft-bottom ecosystem. J. Exp. Mar. Biol. Ecol. 2015, 465, 41–48. [Google Scholar] [CrossRef]
- Creel, L. Ripple Effects: Population and Coastal Regions in Measure Communication; Population Reference Bureau: Washington, DC, USA, 2003. [Google Scholar]
- Rius, M.; Kaehler, S.; McQuaid, C.D. The relationship between human exploitation pressure and condition of mussel populations along the south coast of South Africa. S. Afr. J. Sci. 2006, 102, 130–136. [Google Scholar]
- McPhee, D.P. Urban Recreational Fisheries in the Australian Coastal Zone: The Sustainability Challenge. Sustainability 2017, 9, 422. [Google Scholar] [CrossRef]
- Rubal, M.; Veiga, P.; Reis, P.A.; Bertocci, I.; Sousa-Pinto, I. Effects of subtle pollution at different levels of biological organisation on species-rich assemblages. Environ. Pollut. 2014, 191, 101–110. [Google Scholar] [CrossRef]
- Dias, J.M.A.; Gonzalez, R.; Garcia, C.; Diaz-del Rio, V. Sediment distribution patterns on the Galicia-Minho continental shelf. Prog. Oceanogr. 2002, 52, 215–231. [Google Scholar] [CrossRef]
- Lemos, R.T.; Pires, H.O. The upwelling regime off the west Portuguese coast, 1941–2000. Int. J. Climatol. 2004, 24, 511–515. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variances, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods, 1st ed.; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Mayer-Pinto, M.; Cole, V.J.; Johnston, E.L.; Bugnot, A.; Hurst, H.; Airoldi, L.; Glasby, T.M.; Dafforn, K.A. Functional and structural responses to marine urbanisation. Environ. Res. Lett. 2018, 13, 014009. [Google Scholar] [CrossRef]
- Rubal, M.; Torres, A.C.; Veiga, P. Low diversity of intertidal canopy-forming macroalgae at urbanized areas along the North Portuguese coast. Diversity 2020, 12, 211. [Google Scholar] [CrossRef]
- Puccinelli, E.; Noyon, M.; McQuaid, C.D. Does proximity to urban centres affect the dietary regime of marine benthic filter feeders? Estuar. Coast. Shelf Sci. 2016, 169, 147–157. [Google Scholar] [CrossRef]
- Conde, A.; Pacheco, J.; Marques, S.; Afonso, A.S.; Leandro, S.; Maranhão, P. Ecological features of a rocky intertidal community exposed to sewage effluent. Mar. Pollut. Bull. 2020, 158, 111391. [Google Scholar] [CrossRef]
- Sarà, G.; Zenone, A.; Tomasello, A. Growth of Mytilus galloprovincialis (Mollusca, Bivalvia) close to fish farms: A case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 2009, 636, 129–136. [Google Scholar] [CrossRef]
- Lander, T.R.; Robinson, S.M.C.; MacDonald, B.A.; Martin, J.D. Enhanced growth rates and condition index of blue mussels (Mytilus edulis) Held at integrated multitrophic aquaculture sites in the Bay of Fundy. J. Shellfish Res. 2012, 31, 997–1007. [Google Scholar] [CrossRef]
- Krishnakumar, P.K.; Casillas, E.; Varanasi, U. Effects of environmental contaminants on the health of Mytilus edulis from Puget Sound, Washington, USA. I. Cytochemical measures of lysosomal responses in the digestive cells using automatic image analysis. Mar. Ecol. Prog. Ser. 1994, 106, 249–261. [Google Scholar] [CrossRef]
- Beiras, R.; Durán, I.; Parra, S.; Urrutia, M.B.; Besada, V.; Bellas, J.; Viñas, L.; Sánchez-Marín, P.; González-Quijano, A.; Franco, M.A.; et al. Linking chemical contamination to biological effects in coastal pollution monitoring. Ecotoxicology 2012, 21, 9–17. [Google Scholar] [CrossRef]
- Elías, R.; Rivero, M.S.; Vallarino, E.A. Sewage impact on the composition and distribution of Polychaeta associated to intertidal mussel beds of the Mar del Plata rocky shore, Argentina. Iheringia Sér. Zool. 2003, 93, 309–318. [Google Scholar] [CrossRef]
- O’Connor, N.E.; Crowe, T.P. Biodiversity among mussels: Separating the influence of sizes of mussels from the ages of patches. J. Mar. Biol. Assoc. 2007, 87, 551–557. [Google Scholar] [CrossRef]
- Johnston, E.L.; Roberts, D.A. Contaminants reduce the richness and evenness of marine communities: A review and meta-analysis. Environ. Pollut. 2009, 157, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Parapar, J.; Martínez-Ansemil, E.; Caramelo, C.; Collado, R.; Schmelz, R. Polychaetes and oligochaetes associated with intertidal rocky shores in a semi-enclosed industrial and urban embayment, with the description of two new species. Helgol. Mar. Res. 2009, 63, 293–308. [Google Scholar] [CrossRef][Green Version]
- Crowe, T.P.; Smith, E.; Donkin, P.; Barnaby, D.; Rowland, S.J. Measurements of sublethal effects on individual organisms indicate community-level impacts of pollution. J. Appl. Ecol. 2004, 41, 114–123. [Google Scholar] [CrossRef]
- Ridall, A.; Ingels, J. Suitability of free-living marine nematodes as bioindicators: Status and future considerations. Front. Mar. Sci. 2021, 8, 685327. [Google Scholar] [CrossRef]
- Naylor, E. The diet and feeding mechanism of Idotea. J. Mar. Biol. Assoc. UK 1955, 34, 347–355. [Google Scholar] [CrossRef]
- Suchanek, T.H. Oil impacts on marine invertebrate populations and communities. Am. Zool. 1993, 33, 510–523. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Byran, G.W.; Pascoe, P.L.; Burt, G.R. The use of the dogwhelk Nucella lapillus, as an indicator of tributyltin (TBT) contamination. J. Mar. Biol. Assoc. UK 1987, 67, 507–523. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Gibbs, P.E.; Pope, N.D.; Burt, G.R.; Chesman, B.S.; Bray, S.; Proud, S.V.; Spence, S.K.; Southward, A.J.; Langston, W.J. Recovery of polluted ecosystems: The case for long-term studies. Mar. Environ. Res. 2002, 54, 215–222. [Google Scholar] [CrossRef]
- Leung, K.M.Y.; Morgan, I.J.; Wu, R.S.S.; Lau, T.C.; Svavarsson, J.; Furness, R.W. Growth rate as a factor confounding the use of the dogwhelk Nucella lapillus as biomonitor of heavy metal contamination. Mar. Ecol. Prog. Ser. 2001, 221, 145–159. [Google Scholar] [CrossRef]
- Murray, S.N.; Denis, T.G.; Kido, J.S.; Smith, J.R. Human visitation and the frequency and potential effects of collecting on rocky intertidal populations in southern California marine reserves. CalCOFI Rep. 1999, 40, 100–106. [Google Scholar]
- Wigham, G.D.; Graham, A. Marine Gastropods 3: Neogastropoda; Synopses of the British Fauna 62; The Linnean Society of London: London, UK, 2018. [Google Scholar]
- Díaz, S.; Fargione, J.; Stuart Chapin, F., III; Tilman, D. Biodiversity loss threatens human well-being. PLoS Biol. 2006, 4, e277. [Google Scholar] [CrossRef] [PubMed]
- Roe, D. Biodiversity loss—More than an environmental emergency. Lancet Planet. Health 2019, 3, e287–e289. [Google Scholar] [CrossRef]
| Source of Variation | Df | Density | |
|---|---|---|---|
| MS | F | ||
| Condition | 1 | 110.24 | 0.76 |
| Shore | 2 | 145.66 | 1.01 |
| Site | 4 | 144.04 | 1.29 |
| Residual | 32 | 111.59 | |
| Total | 39 | ||
| Cochran’s test | 0.31 (ns) | ||
| Transformation | Sqrt (X + 1) | ||
| Source of Variation | Df | Condition Index | |
|---|---|---|---|
| MS | F | ||
| Condition | 1 | 0.0277 | 70.97 |
| Shore | 1 | 0.0073 | 8.54 |
| Site | 4 | 0.0009 | 0.88 |
| Quadrat | 32 | 0.0010 | 1.64 |
| Co x Sh | 1 | 0.0004 | 0.46 |
| Residual | 360 | 0.0006 | |
| Total | 399 | ||
| Cochran’s test | 0.8620 (s) | ||
| Transformation | None | ||
| Source of Variation | Df | S | N | H’ | |||
|---|---|---|---|---|---|---|---|
| MS | F | MS | F | MS | F | ||
| Condition | 1 | 0.3231 | 36.83 * | 12.4339 | 10.39 | 0.2738 | 1.39 |
| Shore | 2 | 0.0088 | 0.21 | 1.1972 | 0.05 | 0.1966 | 0.55 |
| Site | 4 | 0.2343 | 1.27 | 23.0901 | 1.15 | 0.3550 | 4.17 ** |
| Residual | 32 | 0.1845 | 20.1177 | 0.0852 | |||
| Total | 39 | ||||||
| Cochran’s test | 0.3708 (ns) | 0.3130 (ns) | 0.2376 (ns) | ||||
| Transformation | Ln (X) | Sqrt (X + 1) | None | ||||
| Source of Variation | Df | Total Assemblage | ||
|---|---|---|---|---|
| MS | Pseudo-F | Unique Perms | ||
| Condition | 1 | 13928 | 3.9564 * | 3 |
| Shore | 2 | 3520.4 | 1.0776 | 296 |
| Site | 4 | 3266.9 | 1.6877 * | 998 |
| Residual | 32 | 1935.7 | ||
| Total | 39 | |||
| Taxon | Average Abundance | δi | δi% | δi/SD (δi) | |
|---|---|---|---|---|---|
| Non-Urban | Urban | ||||
| Nematoda | 48.00 | 16.15 | 17.29 | 23.80 | 1.34 |
| Nucella lapillus | 8.75 | 36.20 | 13.21 | 18.18 | 1.15 |
| Idotea pelagica | 4.85 | 16.70 | 7.71 | 10.61 | 1.02 |
| Hyale spp. | 11.95 | 3.45 | 4.65 | 6.40 | 1.03 |
| Lasaea rubra | 11.75 | 6.65 | 4.52 | 6.22 | 0.84 |
| Oligochaeta | 1.95 | 12.15 | 4.40 | 6.06 | 0.72 |
| Brachystomia scalaris | 9.30 | 4.05 | 3.62 | 4.98 | 1.02 |
| Jaera praehirsuta | 6.00 | 3.80 | 2.99 | 4.12 | 0.86 |
| Steromphala umbilicalis | 4.40 | 2.40 | 2.31 | 3.18 | 0.91 |
| Sabellaria alveolata | 6.50 | 0.05 | 1.66 | 2.28 | 0.34 |
| Apohyale prevostii | 2.25 | 1.10 | 1.45 | 2.00 | 0.75 |
| Syllis pulvinata | 2.15 | 0.35 | 1.22 | 1.68 | 0.36 |
| Patella depressa | 2.65 | 0.75 | 1.08 | 1.49 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, A.C.; Rubal, M.; Costa-Garcia, R.; Sousa-Pinto, I.; Veiga, P. Differences in the Structure and Diversity of Invertebrate Assemblages Harbored by an Intertidal Ecosystem Engineer between Urban and Non-Urban Shores. J. Mar. Sci. Eng. 2022, 10, 242. https://doi.org/10.3390/jmse10020242
Torres AC, Rubal M, Costa-Garcia R, Sousa-Pinto I, Veiga P. Differences in the Structure and Diversity of Invertebrate Assemblages Harbored by an Intertidal Ecosystem Engineer between Urban and Non-Urban Shores. Journal of Marine Science and Engineering. 2022; 10(2):242. https://doi.org/10.3390/jmse10020242
Chicago/Turabian StyleTorres, Ana Catarina, Marcos Rubal, Ricardo Costa-Garcia, Isabel Sousa-Pinto, and Puri Veiga. 2022. "Differences in the Structure and Diversity of Invertebrate Assemblages Harbored by an Intertidal Ecosystem Engineer between Urban and Non-Urban Shores" Journal of Marine Science and Engineering 10, no. 2: 242. https://doi.org/10.3390/jmse10020242
APA StyleTorres, A. C., Rubal, M., Costa-Garcia, R., Sousa-Pinto, I., & Veiga, P. (2022). Differences in the Structure and Diversity of Invertebrate Assemblages Harbored by an Intertidal Ecosystem Engineer between Urban and Non-Urban Shores. Journal of Marine Science and Engineering, 10(2), 242. https://doi.org/10.3390/jmse10020242








