Abstract
Among aquaculture activities, shellfish culture is considered more sustainable and beneficial in terms of food security. Currently, only a few bivalve species are reared and there is a need to explore the possibility to introduce new candidates for shellfish farming. Due to the lack of information on bivalve recruitment in the North-Western Adriatic Sea, in this study, the possibility to collect natural spat of commercial species was investigated. Artificial collectors (net bags) were deployed in two sites, Pellestrina and Caleri (North-Western Adriatic Sea), within two commercial mussel parks, during the spring–summer and summer–autumn periods. At both sites, collectors were placed at a distance of 1 m from each other, from 5 to 14 m depth. The influence of season, site and depth on bivalve recruitment was inspected and the presence of invasive species was also evaluated. In all, 28 bivalve taxa were found, and a higher settlement rate was observed in summer–autumn compared to the spring–summer period. Mytilus galloprovincialis, Flexopecten glaber, Mimachlays varia and Aequipecten opercularis were the most abundant species in spring–summer. In the summer–autumn period, in both sites analysed, a very high quantity of Anadara transversa and F. glaber were found. Indeed, these species were dominant at Pellestrina and Caleri, respectively. Another non-indigenous species, Arcuatula senhousia, was also detected. Relevant amounts of Pectinidae spat, F. glaber in particular, were collected and the optimal depth range for the scallop spat collection was found to be between 8 and 14 m. Our results highlight the relevant potential of Pectinidae spat collection along the North-Western Adriatic coasts, even though the presence of invasive species needs to be monitored.
1. Introduction
The natural bivalve populations of the North Adriatic Sea are subject to intense fishing efforts. The main commercial species in the Adriatic Sea (striped venus clam, Chamelea gallina, smooth clam, Callista chione, Mediterranean scallop, Pecten jacobaeus and queen scallop, A. opercularis) are fished by hydraulic dredges and rapido trawls [1,2]. These fishing gears have a heavy impact on the seabed, change its morphology, damage benthic organisms and fauna, tend to over-exploit the target species and significantly increase the mortality of non-target species [3,4]. Bivalves are mainly filter and suspension feeders and exploit phytoplankton and organic particles. Additional foods, as well as pharmaceuticals, are not required to sustain bivalve growth in outdoor rearing plants [5]. Within the aquaculture sector, shellfish culture is more beneficial in terms of food security and environment conservation [6]. As for the latter issue, an increase in cultured shellfish products might contribute in reducing the consumption of fished bivalves, with a consequent reduction in fishing pressure and the negative impacts of fishing gears on the sea bottom [1,2].
Between 1961 and 2016, the global supply of fish for human consumption strongly increased, due to the growth of both population and per capita fish food consumption. Increased global fish production was mainly accountable to the steady increase in aquaculture production since the 1980s, rather than to the increase in capture fishery production, which remained relatively static [7]. Italy is the fifth European state for aquaculture production, preceded by Norway, Spain, the United Kingdom and France [8]. As for Spain and France, Italian mariculture is mainly based on bivalve species. In Italy, shellfish culture represents 63% (in weight) of national aquaculture production and is almost entirely limited to the Mediterranean mussel, M. galloprovincialis (72%), and Manila clams, Ruditapes philippinarum (28%) [9]. Mussels are cultivated mainly in offshore long-line facilities, while clams are grown intensively in the soft-bottoms of confined coastal lagoons, mainly in the Lagoon of Venice [10,11]. The lack of diversification in Italian shellfish culture makes it vulnerable to stochastic events, e.g., new pathology outbreaks, strong sea storms or climatic changes [12]. The availability of seed is one of the basic requirements for shellfish farming [13]. Previous studies showed that wild scallop spat in the Northern Adriatic waters is easy to collect with artificial collectors, where scallop juveniles were the most frequent and abundant among the newly settled bivalves [14,15]. Scallop spat collection is also successful in the Ionian Sea, in particular for F. glaber and M. varia species [12,16,17,18]. The collection of wild spat from local bivalve populations reduces production costs and could allow for the avoidance of risking to introduce new, non-indigenous species and invasion of coastal habitats, by spat translocation from different natural sites [19]. Scallops constitute an interesting prospect for rearing, also, in relation to their considerable market value. For the collection of wild seeds intended for scallop culture, artificial collectors, made by synthetic filaments, contained inside a plastic-mesh bag, are mainly used. Competent larvae (pediveliger or larvae with eye spot) enter the bag, attach themselves to the filaments through the byssus and undergo the metamorphosis. Most scallops, after reaching a size of a few millimetres, lose the byssus and detach themselves from the substrate. Therefore, the openings of the plastic bag should not be greater than 3–5 mm, so as to retain the spat inside the collector after the attached phase, while filament type and collector bag dimensions may vary [20].
Several biotic and abiotic factors (life and reproductive traits of the species, type, orientation and heterogeneity of the substrate, water column turbidity and sedimentation rate) influence the settlement and the formation of fouling communities [21,22,23,24,25]. The settlement of bivalve competent larvae occurs some weeks after the species spawning period. Artificial collectors have to be immersed at an appropriate time, in order to allow the formation of the microfouling layer, which facilitates the invertebrate settlement [26]. Besides spat availability, time of collectors’ deployment and their suitability, the abundance of collected spat is influenced by the post-settlement mortality, due to competition and/or predation [27,28]. Other factors, such as temperature variations and availability of suitable food, could influence post-settlement mortality [29]. Among abiotic factors, hydrodynamic activity is of crucial importance, for both larval settlement and growth, because it influences larval dispersal and spat distribution [30], as well as the plankton community composition [31] that could be fundamental for successful bivalve larvae metamorphosis and post-settlement survival [13].
Marine aquaculture practices are strongly affected by biofouling, which leads to a wide range of significant impacts on production, involving both the cultured species and the related infrastructures [32,33]. Shell mechanical function of cultured bivalve species could be compromised by fouling colonization, with consequent decreased feeding ability or increased susceptibility to predation. Biofouling could affect shellfish growth and condition, leading to biological competition for food and space and/or reducing the water flow and, consequently, the oxygen level and food availability [32,33]. Furthermore, marine fouling communities of artificial structures are known to represent invasion hotspots for non-indigenous species. The introduction of non-indigenous species could threaten the native communities’ stability, through competition for resources, predation, release of toxins, disease transmission and ecosystem engineering [34,35,36]. The monitoring of the biofouling communities on artificial substrates allows us to detect their dynamics, in relation to time and depth, and represents a valuable method to avoid the peak settlement of non-target species in mariculture infrastructures. Furthermore, early detection of non-indigenous species allows for the implementation of management strategies aimed at reducing non-indigenous species’ establishment and spreading [37]. In the Adriatic Sea, recent studies of biofouling communities were focused on natural [38] and artificial substrates [39,40,41,42], also in relation to aquaculture infrastructures [43,44,45]. Along Italian coasts, between 1945 and 2009, the highest number of non-indigenous species were registered in the North Adriatic, with Mollusca as the taxon, having a major number of species [46]. However, the knowledge on bivalve recruitment in the North-Western Adriatic is very limited, even though the bivalve stocks have been heavily exploited for a long time [47].
As previously mentioned, the successful collection of natural seeds depends on many factors. The use of suitable collectors, favourable sites and depths for their immersion and the right recovery time are among the fundamental features to be defined in non-hatchery dependent shellfish culture. In addition, the use of bivalve collectors allows us to fill the gap in data on the presence and abundance of invasive species, and to monitor the presence of invasive species that could alter the structure of local biofouling communities and compromise shellfish farming, by competing for space and resources with reared species. In this study, we analysed the composition and spatial and temporal variation of the bivalve community, present on artificial collectors, located within commercial mussel parks on the north-western coast of the Adriatic Sea. The general hypothesis of this study is that in the North-Western Adriatic Sea, it is possible to collect enough natural spat of commercially relevant bivalve species that could be used to enhance and diversify shellfish rearing. The main purposes of this study were as follows: (a) define the bivalve spat distribution in relation to depth, site and season; (b) inspect the possibility of introducing new local bivalves to be cultured, and (c) highlight the presence of invasive species.
2. Materials and Methods
2.1. Study Area
The artificial collectors used for settlement analyses were deployed in two sites, Pellestrina (P) and Caleri (C), within commercial mussel parks, both located approximately 2 NM off the western coast of the North Adriatic (Figure 1). The geographical coordinates of the mussel farms were 45°15′39″ N, 12°20′13″ E for Pellestrina and 45°05′42″ N, 12°24′12″ E for Caleri. The Northern Adriatic Sea is characterized by shallow waters with maximum depths of 100 m and a mean depth of about 35 m [48]. Its seabed is homogeneous, mostly made of mobile, silty-sandy sediments [49]. The only hard substrates are represented by scattered biogenic outcrops distributed at depths between about 9 and 40 m, at 3 to 13 nautical miles from the North-Western Adriatic coast [50]. The major freshwater input derives from Po river [51] which influences both circulation regime and trophic status of the North-Western Adriatic Sea [52,53]. Pellestrina site is located in front of the Lagoon of Venice and is influenced by outflowing lagoon waters, characterised by higher surface salinity and lower nutrient concentrations than in Caleri site. Indeed, Caleri, located further south, is influenced by large rivers’ discharge (Brenta, Adige and Po) and presents generally lower salinity and high concentration of nutrients and chlorophyll [54,55].
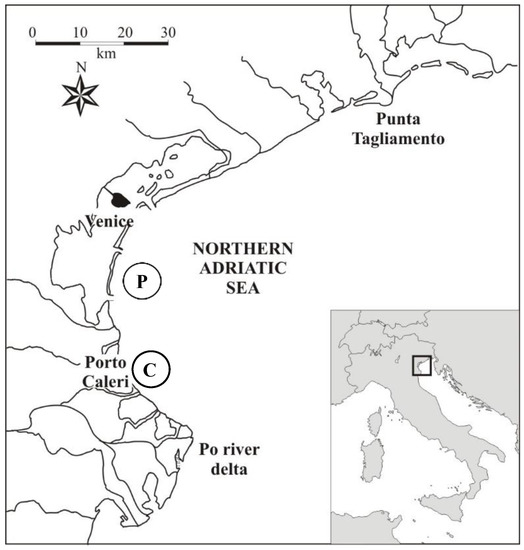
Figure 1.
Sites of net bag collectors deployment in North-Western Adriatic Sea: Pellestrina (P) and Caleri (C).
2.2. Spat Collection
The artificial collectors consisted of 28 cm × 53 cm net bags, with a 4 mm mesh opening (Figure 2a). Net bags were filled internally with 5 m long tubular net having 5 cm mesh opening, which is commonly used for mussel rests. This net, called “filler”, is folded and rolled up on itself, in order to give volume to the collector. Each set of collectors consisted of a rope on which 10 net bags were placed at a distance of 1 m from each other. The upper end of each rope was tied to a longline located at 4 m depth so that the collectors remained submerged from 5 to 14 m depth. A weight of about 12 kg was hung at the lower end of the rope to keep it stretched towards the seabed. Each set of collectors were 1 m distance from each other. At Pellestrina five sets of collectors were submerged in spring–summer and in summer–autumn. At Caleri the collectors’ deployment was granted only in summer–autumn period; due to the accidental loss of a rope, four sets of collectors were recovered. Details are provided in Table 1. During the deployment the collectors were not managed and fouling was not removed until recovery (Figure 2b,c).
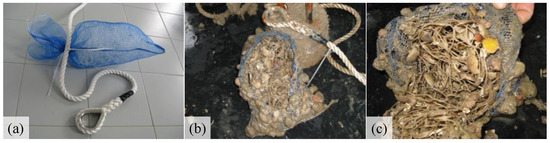
Figure 2.
Net bag collectors before (a) and after (b,c) deployment in Pellestrina (PII) commercial mussel park.

Table 1.
Sampling design: duration of collectors’ immersion periods, and number of collectors used at each site.
2.3. Spat Identification
After recovery, collectors were closed in plastic bags, transported to the laboratory and frozen. Before analysis, each collector was thawed and washed with running water, in order to collect all the material contained in the net bag and on the internal filler. The collected material was sieved with a 2 mm mesh net and stored in 70% alcohol, in polyethylene containers. In the case of particularly abundant samples, half of the total material was analysed. Collected samples were examined under the stereomicroscope (Leica S8 AP0) at a maximum magnification of 80×. Several texts were used for the taxonomic identification [56,57,58,59,60,61,62,63]. The filled and closed bags had a total area useful for settlement of approximately 0.092 m2 (28 cm × 33 cm). The spat abundance was expressed as settlement rate, i.e., number of individuals m−2 day−1.
2.4. Statistical Analyses
To properly compare the samples regardless of the duration of collectors’ immersion, statistical analyses were performed on the number of individuals per square metre per day. NMDS and PERMANOVA multivariate tests were performed using the software package PRIMER 6 PERMANOVA Plus (PRIMER-e Ltd., Plymouth, UK). Differences in species composition among depths, based on square-root transformed abundance data, were assessed for each sampling survey (PI, PII, C) using multivariate analyses based on a Bray–Curtis similarity, combined with visualization by NMDS plots. To compare the two sites in the same seasonal period (summer–autumn), a PERMANOVA (Permutational Multivariate Analysis of Variance) [64] was applied. A three-factor experimental design was used with ‘site’ and ‘depth’ as fixed factors, ‘rope’ as a random factor.
3. Results
In this study 28 bivalve taxa were identified on the 140 collectors analysed and the total number of taxa was similar between sites (21 in Pellestrina I and II, 22 taxa in Caleri) (Table 2). On average, lower settlement was observed in the spring–summer period (Pellestrina I, 58.2 ind m−2 day−1) compared to the summer–autumn period (Pellestrina II and Caleri, 76.8 and 65.1 ind m−2 day−1, respectively). Taxa that settled exclusively in the spring–summer period were P. jacobeus, Pectinidae n.i. and Tellinidae n.i., while Modiolus adriaticus, Mytilaster n.i., Barbatia barbata, Arca noe, Limidae n.i., Pinna nobilis and Varicorbula gibba, were found only in the summer–autumn samples. Tellinidae n.i. were found only in the Pellestrina site in spring–summer samples (Table 2). In all samples, the abundance increased with depth, in particular at PI (Figure 3A–C). Conversely, richness, that is the number of taxa, tended to decrease with increasing depth (Figure 4). In each sample, seven more abundant taxa contributed to about 95% of the total bivalve community abundance (Figure 5). F. glaber and Musculus subpictus have always been among the most abundant species (Table 2, Figure 5 and Figure 6).

Table 2.
List of total bivalve recruits and their relative abundance (m−2 day−1), collected by net bags deployed in spring–summer in Pellestrina (PI) and in summer–autumn in Pellestrina (PII) and Caleri (C). n.i. not identified species. For each taxon the most abundant recruitment is in bold.
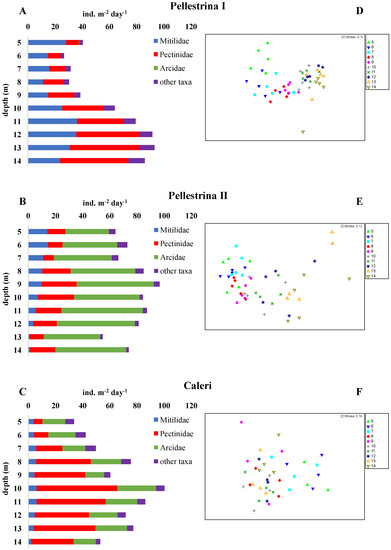
Figure 3.
Settlement rate of the main bivalve families at different depths (A–C) and 2D-MDS plots of bivalve species composition similarities among depths (D–F) over the three sampling surveys.
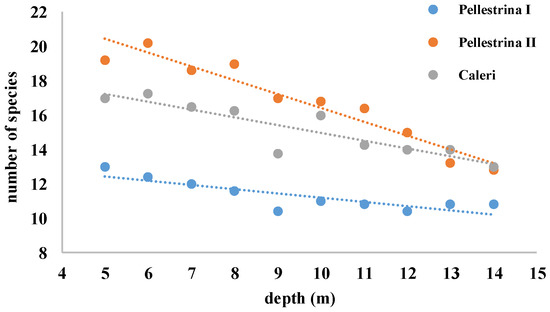
Figure 4.
Richness found in Pellestrina I, Pellestrina II and Caleri samples at different depths.
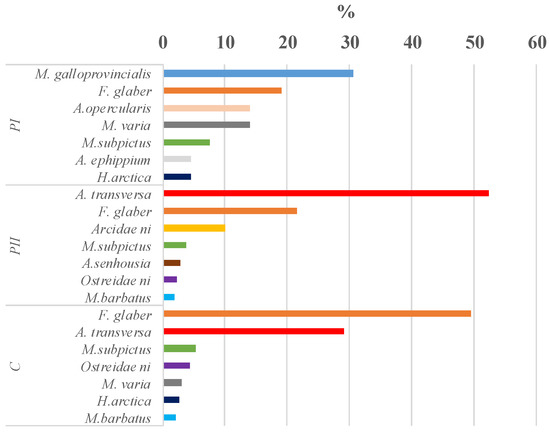
Figure 5.
Percentage contribution to total abundance of the first seven more abundant species in each sample: Pellestrina I (PI), Pellestrina II (PII) and Caleri (C).
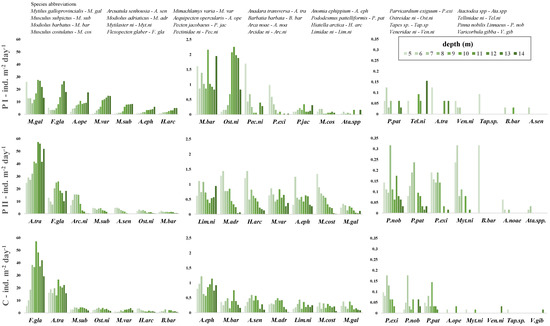
Figure 6.
Settlement rates of each taxon at the various depths over the three sampling surveys: Pellestrina I (PI), Pellestrina II (PII) and Caleri (C). From left to right, taxa with decreasing total abundance are shown. Note the different scales on the y-axes.
In Pellestrina I, a clear increase in settlement with depth was observed, in particular, starting from 10 m downwards (Figure 3A). Depth influenced the settlement in Caleri also, where a higher abundance of bivalve spat was found between 8 and 13 m depths (Figure 3C). Conversely, in Pellestrina II, the settlement rate was high and quite constant between depths (Figure 3B).
In Pellestrina I, Pectinidae (47.9%) and Mytilidae (40.6%) were the dominant families, with a slightly greater presence of Pectinidae (Table 2, Figure 3A). Instead, in Pellestrina II and in Caleri, one family was notably dominant, Arcidae (52.4%) and Pectinidae (52.4%), respectively (Table 2, Figure 3B,C). In terms of abundance, the main settlement rate of Mytilidae was found in Pellestrina I (23.6 ind. m−2 day−1), while for Pectinidae and Arcidae, the settlement was more abundant in Caleri (34.1 ind. m−2 day−1) and Pellestrina II (34.1 ind. m−2 day−1).
For each sampling survey, the separation between superficial and deeper samples is evident in MDS plots, due to both lower total settlement in surface layers and different variations in single taxa abundances with depth (Figure 3D–F). The PERMAVOVA, performed on summer–autumn settlement data, from Pellestrina and Caleri, showed significant effects for both ‘site’ and ‘depth’ factors (Table 3). The same p-value (0.001) was found for these fixed factors, but the pseudo-F value was much higher for ‘site’ (37.18) than for ‘depth’ (4.51), meaning that recruitment differed more between sites than among depths. The interaction between ‘site’ and ‘depth’ was also significant, underlining that at the same depths, different taxa with different abundances were present in the two sites. For example, at 9 m depth at PII, the abundances of F. glaber and A. transversa were 25 and 42 ind. m−2 day−1, respectively, while at the same depth at the C site, the abundances of the same species were 36 and 14 ind. m−2 day−1. Another species, B. barbata, was found only at 5 m, with very small abundance (0.32 ind. m−2 day−1) at PII, while it was the seventh most abundant species (up to 2.79 ind. m−2 day−1), distributing at all depths at the C site.

Table 3.
PERMANOVA test on Bray–Curtis similarities of square root-transformed data according to site (2 levels, fixed: Pellestrina and Caleri), depth (10 levels from 5 to 14 m, fixed) and rope (random).
3.1. Mytilidae
In Pellestrina I, the Mytilidae family was very abundant, clearly more abundant than in the Pellestrina II and Caleri samples. In spring–summer samples (PI), M. galloprovincialis (17.9 ind. m−2 day−1) and M. subpictus (4.4 ind. m−2 day−1) were the most abundant species, even though in exiguous quantity, other species found in PI were Modiolus barbatus, Musculus costulatus and A. senhousia (Table 2). A higher abundance of M. galloprovincialis was observed at 5 and between 11 and 13 m depths, with a mean abundance of 25.69 ± 2.52 ind. m−2 day−1, while M. subpictus settled mainly on deeper collectors, starting from 10 m up, to a maximum abundance of 8.35 ind. m−2 day−1 at 14 m (Figure 6).
In the summer–autumn samples (PII and C), two other taxa were identified, i.e., M. adriaticus and Mytilaster ni (Table 2). The settlement rates of the single taxa were consistent with those observed in PI, with the exception of M. galloprovincialis, which showed very low settlement in summer–autumn (Table 2). In Pellestrina II, the most abundant species were M. subpictus (2.88 ind. m−2 day−1) and A. senhousia (2.20 ind. m−2 day−1), while in Caleri, the highest settlement rate was observed for M. subpictus (3.39 ind. m−2 day−1), followed by M. barbatus (0.35 ind. m−2 day−1) and A. senhousia (0.35 ind. m−2 day−1), with a quite significantly lower settlement rate (Table 2, Figure 6). Interestingly, the settlement observed in the two sites appeared more influenced by the depth; in PII, the settlement of the most abundant species was shown to decrease with increasing depth, while in Caleri, it appeared not to be affected by depth (Figure 6).
3.2. Pectinidae
In spring–summer (Pellestrina I), F. glaber was the most abundant species (11.1 ind. m−2 day−1), with a slightly higher settlement rate compared to M. varia and A. opercularis (8.1 ind. m−2 day−1 for both species). Although very low in quantity, P. jacobeus spat was found only in Pellestrina spring–summer samples (Table 2). Pectinidae species, mainly F. glaber and M. varia, showed an increased settlement rate with depth, starting from 10 m, specifically (Figure 6).
In summer–autumn samples, F. glaber was a dominant and very abundant species, with a higher settlement rate observed at Caleri (32.2 ind. m−2 day−1), compared to both Pellestrina II (16.5 ind. m−2 day−1) and Pellestrina I (11.1 ind. m−2 day−1) (Table 2). The settlement rate appeared to increase with depth, starting from 8 m at both sites. In particular, higher settlement was observed at 9–10 m depth at Pellestrina II and at 10–11 m depth at Caleri (Figure 6).
3.3. Arcidae
This family was almost totally represented by the invasive species A. transversa, which was found in all samples analysed. However, the settlement rate observed in spring–summer (0.02 ind. m−2 day−1) was negligible but very high in summer–autumn. A. transversa was the dominant species in Pellestrina II (40.2 ind. m−2 day−1) and showed the maximum settlement rate observed during this study for a single species (Table 2). In Pellestrina II, a slight increase in settlement with depth was observed, starting from 9 m, while in Caleri the settlement showed similar values among depths, with a peak at 10 m (Figure 6). In addition to A. transversa, a second non-indigenous species detected in this study was A. senhousia. This species was present in all samples, with higher abundance in summer–autumn and maximum settlement in Pellestrina II (Table 2). Indeed, in Pellestrina II, A. senhousia was the fifth most abundant species (Figure 5).
4. Discussion
In the present study, from May to December, 28 bivalve taxa were found on net bag collectors deployed along the north-western coasts of the Adriatic Sea. Most of the species are reported in checklists of sessile bivalves of the North Adriatic [65] and are components of benthic biocenoses, on rocky substrates of biogenic concretions (tegnùe), which are not far from the study area [50,66,67,68]. A low number of individuals belonging to mobile substrate taxa, such as Cardiidae, Veneridae, Mesodesmatidae and Tellinidae, were found. Indeed, the bivalve community of the North Adriatic Sea, described following a hydraulic dredge survey, showed a higher diversity (54 taxa) [69]. This confirms that net bags are more suitable for sessile bivalve recruitment. Compared to other artificial collectors, such as net panels, “Chinese caps” or tiles, the diversity of the bivalves settled on net bags was higher [14,15,38,39,43,45,70]. The three sampling surveys produced very similar results in total taxa number but differed in total abundance and taxa type, dominance and distribution along depth. Despite the bivalve taxa diversity, in all samples, seven taxa constituted about 95% of total settled individuals.
4.1. Seasonal Settlement Patterns
In spring–summer, the bivalve community was dominated by Mytilidae and Pectinidae, while in summer–autumn, by Arcidae and Pectinidae.
M. galloprovincialis was the most abundant species in spring–summer and its settlement was negligible in summer–autumn. For this species, very limited spawning events are possible, even in July–August; the main spawning occurs in January–February [71,72]. Spring–summer recruitment detected in this study is consistent with the spawning period of M. galloprovincialis and with settlement previously observed in the North Adriatic [43,73]. As for the Mytilidae family, M. subpictus was very abundant in both periods, with higher settlement in spring–summer.
The second most abundant species in spring–summer was F. glaber, followed by M. varia and A. opercularis. Of the Pectinidae family, P. jacobeus was found only in this period, but was among the less abundant species. The recruitment of F. glaber was even higher in summer–autumn; indeed, this species was dominant together with A. transversa. Our settlement data support the evidence that, for F. flaber, there are two spawning events in the Adriatic Sea. A minor one occurs in April and May and the main one between July and September [74]. For this species, the same increased recruitment in summer–autumn, compared with the winter–spring period, was also observed in the Ionian Sea [16]. M. varia and A. opercularis were recruited in both periods considered, but mainly in spring–summer. For the Mediterranean Sea, the recruitment of both species was observed almost all year round, with a peak in spring–summer. A. opercularis recruitment was recorded, also in autumn [16,75] For both species, spawning activity is likely to occur throughout the year, except in winter for M. varia [76,77,78,79], and our settlement data suggested main spawning events in spring and early summer. In the Adriatic Sea, P. jacobeus was observed to have more restricted spawning periods, which occur in May, August and December, with summer as the main spawning season [76]. However, it is to be noted that in the Mediterranean Sea, the settlement was observed in January–March and April–July [16,75]. In our samples, P. jacobeus recruitment was very low in spring–summer and was even absent in autumn, while the winter period was not investigated.
The settlement of the arcid clam, A. transversa, was very high in summer–autumn, whereas the low recruitment observed in spring–summer suggested the presence of scarce spawning events in spring. These findings are in agreement with previous observations by other authors and support the hypothesis that the main spawning occurs in late summer [43,80,81]. Higher spat abundance of Ostreidae was found in summer–autumn, which is consistent with the spawning period of Crassostrea gigas and settlement of Ostrea edulis in the Adriatic Sea [43,82]. Based on our observations on low abundant taxa, species detected in all samples, but prevailed mainly in the spring–summer samples, were Anomia ephippium, Hiatella arctica and Parvicardium exiguum, while M. costulatus and A. senhousia were mainly summer–autumn settlers. Taxa found exclusively in the summer–autumn period were M. adriaticus, Mytilaster n.i., B. barbata, A. noae, Limidae n.i., Pinna nobilis and V. gibba, while Tellinidae settled exclusively in spring–summer. Data in the literature on M. barbatus, P. nobilis and H. arctica reproduction and settlement are consistent with our findings [83,84,85,86].
It is to note that the patterns of seasonal variations in the main hydrological parameters, shown in Figure 7, are consistent with those reported in the literature for the North-Western Adriatic Sea [87,88], and no atypical trend was observed during the study period (data recorded at the CNR Oceanographic Platform “Acqua Alta”).
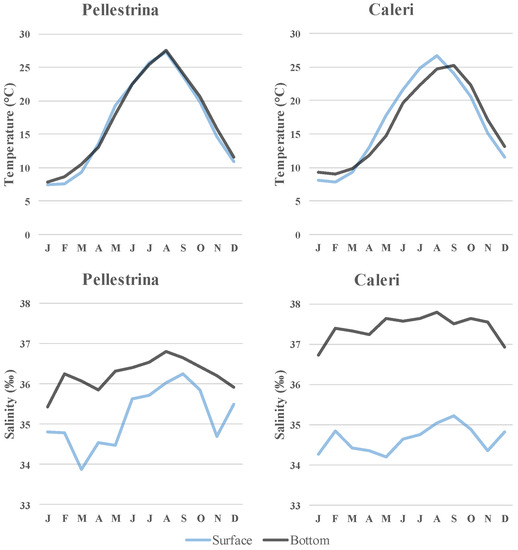
Figure 7.
Mean monthly values of temperature and salinity at Pellestrina and Caleri. Data from E.U. Copernicus Marine Service Information (CMEMS) https://marine.copernicus.eu/ accessed on 2 December 2021.
4.2. Influence of Depth and Site on Settlement Patterns
In all samples, the total settlement rate increased with depth. Our study was focused on the bivalve community; therefore, other taxa, which usually account for most of the biomass in the Adriatic Sea biofouling communities, such as algae, ascidians and crustaceans [44,45], were not examined. The reduced abundance of bivalve recruits at shallower depths may be due to the lower presence of competent larvae in this part of the water column, but also to the higher competition with other fouling species, including phototrophs, which are more abundant at lower depths [89].
In the spring–summer period, this settlement pattern was evident for dominant taxa, such as M. galloprovincialis and Pectinidae (F. glaber, A. opercularis, M. varia), and for most of the less abundant species (M. subpictus, A. ephippium, H. arctica, Ostreidae n.i., P. exiguum, P. jacobeus). M. galloprovincialis settlement was observed to occur mainly at shallower depths [90,91,92]. Our collectors were placed starting from 5 m depth, where high settlement was observed, but we registered an increase in M. galloprovincialis spat also at 10–13 m depth. The increased settlement of Pectinidae at higher depths was expected, since high recruitment of scallops is usually ensured by deploying the collectors near the bottom, at a depth ranging from approximately 10 to 30 m [12,16,78,93,94].
Contrarily to what was observed in spring–summer, in summer–autumn samples, the increase in settlement with depth was detected for two dominant species, A. transversa and F. glaber, while the other less abundant species mainly exhibited the opposite trend. Increased settlement at low depth was particularly noticeable at Caleri, compared to Pellestrina. In this regard, it cannot be excluded that different environmental conditions, mainly concerning stratification of the water column and salinity vertical profile, can influence the settlement at the studied sites (Figure 7). Of the two sites, Caleri was found to be more suitable for scallop spat collection. Indeed, despite the high presence of invasive A. transversa at both sites, in the summer–autumn period, F. glaber was the most abundant species at Caleri and M. varia recruitment was higher than at Pellestrina. In addition, when comparing the settlement rates of the species found in the two sets of summer–autumn samples, H. arctica and B. barbata were more abundant at Caleri, A. senhousia and M. barbatus at Pellestrina. Information on the status of natural bivalve stocks in the North-Western Adriatic Sea is scarce and outdated. For this reason, no specific relationship between settlement data in the study areas and broodstock abundance in the proximity can be inferred. In a previous study, performed in 1995, to assess Pectinidae abundance in an area of about 10 km2, located 40 km south-west of Venice (45°13.5′ N 12°47.1′ E), A. opercularis was found to be particularly abundant, with an estimated population density of 2.8 individuals m−2, while P. jacobeus was less abundant (0.05–0.08 individuals m−2), and very low amounts of M. varia and F. glaber were found [95]. Conversely, a dominance (96.52%) of F. glaber was observed in the adult pectinid stocks at the Gulf of Manfredonia (South-Western Adriatic Sea) [96]. The relevant abundance of F. glaber spat detected in the present study suggests the need for future research addressed to the evaluation of adult Pectinidae abundance in the study areas.
4.3. Occurence of Alien Species
Two alien species, A. transversa and A. senhousia, were found in this study. A. transversa is a Lessepsian Indo-Pacific species, reported for the first time in the Mediterranean, on the Turkish coasts, in the port of Izmir [97], and subsequently found in the North Aegean Sea [98]. In Italy, it was reported for the first time in 2001, at Cesenatico [99], and then in the area of Ancona [100]. The presence of this species in the North Adriatic, and more precisely in the Lagoon of Venice, dates back to 2002 [101], but the significant increase in abundance, confirmed also by our study, indicates the acclimatization of A. transversa and spread in the marine environment [43,102,103]. The abundance of this species on the collectors in summer–autumn may represent a serious problem for a fruitful collection of the Pectinidae spat, F. glaber in particular, in the same period of the year.
A. senhousia is an allochthonous species, of Asian origin, which caused profound changes in the benthic community of Sacca di Goro, a transitional environment in the river Po delta [104]. As in the case of A. transversa, its abundance was greater at Pellestrina than at Caleri. These differences can be related to both variability between sites and competition with other species. Since the abundance of A. senhousia detected offshore in this study was lower than in the Sacca di Goro, a milder impact on the other settled species is hopefully expected at sea collection sites.
4.4. Scallop Spat Abundance
In accordance with our observations, the optimal depth range for the scallop spat collection resulted between 8 and 14 m. Unfortunately, in both seasons evaluated, the presence of competing species emerged. It is likely that M. galloprovincialis, in spring–summer, and A. transversa, in summer–autumn, have reduced the settlement of scallops. Despite the presence of competitors, net bags deployed in May allowed the collection of considerable quantities of scallop spat, belonging to three species (F. glaber: 989 ± 641 sd ind. m−2; M. varia: 766 ± 298 sd ind. m−2 and A. opercularis: 696 ± 246 sd ind. m−2), while collectors deployed in July collected the highest quantity of F. glaber (PII: 2640 ± 733 sd ind. m−2, C: 5639 ± 1245 sd ind. m−2) and minor amounts of M. varia spat (PII: 70 ± 26 sd ind. m−2, C: 325 ± 113 sd ind. m−2).
In the Ionian Sea, spat of F. glaber (average density ranging from 19.3 to 306.1 ind. m−2) were found on net bags immersed for 7 months, near the bottom, together with M. varia (3.3–35.6 ind. m−2) and a negligible amount of A. opercularis spat [12]. Even if the dominance of the species is consistent with our findings, the spat abundance of F. glaber and M. varia was clearly higher in the Adriatic Sea. However, in the Western Mediterranean, A. opercularis and M. varia showed slightly higher recruitment values that were 0.25–99 ind. bag−1 and 0–101 ind. bag−1, respectively, corresponding to about 1–412 ind. m−2 and 0–421 ind. m−2 [75].
5. Conclusions
Our results highlight the relevant potential of Pectinidae spat, F. glaber in particular, collection along the North-Western Adriatic coasts, in the perspective of introducing new cultivations of commercial bivalve species. Optimal seasonal and depth ranges have been defined. In this regard, the present work represents an essential premise to analyse potential effects of global change in future research assessing bivalve recruitment in the study area. As recently highlighted [105], global change is among the main threats to aquaculture. Although the presence of invasive species needs to be monitored, scallop spat abundance in the study area was similar to, or higher than, other coastal areas of the Mediterranean. It is important to consider that our collectors were deployed inside commercial mussel farms; therefore, mussel spat abundance and competition is likely to be reduced if areas specifically devoted to the collection of scallop spat can be set up.
Author Contributions
Conceptualization, T.M., M.G.M. and M.B.; methodology, T.M., M.G.M. and M.B.; formal analysis, T.M., M.G.M. and M.B.; investigation, T.M. and M.B.; resources, M.G.M. and M.B.; data curation, T.M. and M.B.; writing—original draft preparation, T.M. and V.F.C.; writing—review and editing, T.M., M.G.M. and M.B.; funding acquisition, M.G.M. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was part of the project CLODIA, funded by the Veneto Region (Italy) Law 15/2007 (DGR no 4069).
Acknowledgments
The authors wish to acknowledge Francesco M. Falceri for his support in processing data from E.U. Copernicus Marine Service Information.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ardizzone, G.D. An attempt of a global approach for regulating the fishing effort in Italy. Biol. Mar. Mediterr. 1994, 1, 109–113. [Google Scholar]
- Ezgeta-Balić, D.; Peharda, M.; Richardson, C.A.; Kuzmanić, M.; Vrgoč, N.; Isajlović, I. Age, growth, and population structure of the smooth clam Callista chione in the eastern Adriatic Sea. Helgol. Mar. Res. 2011, 65, 457–465. [Google Scholar] [CrossRef]
- Brambati, A.; Fontolan, G. Sediment resuspension induced by clam fishing with hydraulic dredges in the Gulf of Venice (Adriatic Sea). A preliminary experimental approach. Boll. Ocean. Teor. Appl. 1990, 8, 113–121. [Google Scholar]
- Pranovi, F.; Raicevich, S.; Franceschini, G.; Torricelli, P.; Giovanardi, O. Discard analysis and damage to non-target species in the “rapido” trawl fishery. Mar. Biol. 2001, 139, 863–875. [Google Scholar]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global Production of Marine Bivalves. Trends and Challenges. In Goods and Services of Marine Bivalves; Smaal, A., Ferreira, J., Grant, J., Petersen, J., Strand, Ø., Eds.; Springer: Cham, Switzerland, 2019; pp. 7–26. [Google Scholar]
- Suplicy, F.M. A review of the multiple benefits of mussel farming. Rev. Aquacult. 2020, 12, 204–223. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018. In Meeting the Sustainable Development Goals; Food and Agricultural Organization of the United Nations: Rome, Italy, 2018; pp. 2–75. [Google Scholar]
- Hough, C. Regional Review on Status and Trends in Aquaculture Development in Europe—2020; FAO Fisheries and Aquaculture Circular No. 1232/1; FAO: Rome, Italy, 2022; p. 43. [Google Scholar]
- Marino, G.; Crosetti, D.; Petochi, T. Fisheries and Aquaculture Division [Online]; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/fishery/en/countrysector/it/en (accessed on 10 December 2021).
- Parisi, G.; Centoducati, G.; Gasco, L.; Gatta, P.P.; Moretti, V.M.; Piccolo, G.; Roncarati, A.; Terova, G.; Pais, A. Molluscs and echinoderms aquaculture: Biological aspects, current status, technical progress and future perspectives for the most promising species in Italy. Ital. J. Anim. Sci. 2012, 11, 397–413. [Google Scholar] [CrossRef]
- Prioli, G. La molluschicoltura in Italia. In Estado Actual del Cultivo y Manejo de Moluscos Bivalvos y su Proyección Futura: Factores que Afectan su Sustentabilidad en América Latina. Taller Técnico Regional de la FAO, 20–24 August 2007, Puerto Montt, Chile; FAO Actas de Pesca y Acuicultura, No. 12; Lovatelli, A., Farías, A., Uriarte, I., Eds.; FAO: Roma, Italy, 2008; pp. 159–176. [Google Scholar]
- Prato, E.; Biandolino, F.; Parlapiano, I.; Gianguzza, P.; Fanelli, G. The recruitment of scallops (and beyond) by two different artificial collectors (Gulf of Taranto, Mediterranean Sea). Aquac. Res. 2015, 47, 3319–3331. [Google Scholar] [CrossRef]
- Lagarde, F.; Roque d’orbcastel, E.; Ubertini, M.; Mortreux, S.; Bernard, I.; Fiandrino, A.; Chiantella, C.; Bec, B.; Roques, C.; Bonnet, D.; et al. Recruitment of the Pacific oyster Crassostrea gigas in a shellfish-exploited Mediterranean lagoon: Discovery, driving factors and a favorable environmental window. Mar. Ecol. Prog. Ser. 2017, 578, 1–17. [Google Scholar] [CrossRef]
- Chinellato, A.; Bressan, M.; Pellizzato, M. Insediamento, reclutamento ed accrescimento di bivalvi eduli su strutture in sospensione nell’area del campo sperimentale. In Campo Sperimentale in Mare: Prime Esperienze nel Veneto Relative Ad Elevazioni dal Fondale con Materiale Inerte; Regione Veneto, ARPAV Osservatorio Alto Adriatico: Venezia, Italy, 2006; pp. 144–164. [Google Scholar]
- Chinellato, A.; Pellizzato, M.; Bressan, M. Insediamento di larve di bivalvi su collettori artificiali in un’area a barriere artificiali nel Nord Adriatico. Biol. Mar. Mediterr. 2006, 13, 1072–1076. [Google Scholar]
- Papa, L.; Prato, E.; Biandolino, F.; Parlapiano, I.; Fanelli, G. Strategies for successful scallops spat collection on artificial collectors in the Taranto Gulf (Mediterranean Sea). Water 2021, 13, 462. [Google Scholar] [CrossRef]
- Prato, E.; Biandolino, F.; Parlapiano, I.; Papa, L.; Denti, G.; Fanelli, G. Estimation of growth parameters of the black scallop Mimachlamys varia in the gulf of taranto (Ionian Sea, Southern Italy). Water 2020, 12, 3342. [Google Scholar] [CrossRef]
- Tsotsios, D.; Tzovenis, I.; Katselis, G.; Geiger, S.P.; Theodorou, J.A. Spat settlement of the smooth scallop Flexopecten glaber (Linnaeus, 1758) and variegated scallop Chlamys varia (Linnaeus, 1758) in Amvrakikos Gulf, Ionian Sea (Northwestern Greece). J. Shellfish Res. 2016, 35, 467–474. [Google Scholar] [CrossRef]
- Mckindsey, C.W.; Landry, T.; O’Beirn, F.X.; Davies, I.M. Bivalve aquaculture and exotic species: A review of ecological considerations and management issues. J. Shellfish Res. 2007, 26, 281–294. [Google Scholar] [CrossRef]
- Lodeiros, C.; Davidson, L.-A.; Dadswell, M.; Rupp, G.S.; Mazón-Suástegui, J.M. Scallop aquaculture. In Molluscan Shellfish Aquaculture: A Practical Guide; Shumway, S., Ed.; 5m Publishing: Great Easton, UK, 2021. [Google Scholar]
- Khalaman, V.V. Life strategies of marine sessile organisms as an approach for exploration of structure and succession of fouling communities. In Biofouling: Types, Impact and Anti-Fouling; Chan, J., Wong, S., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 1–33. [Google Scholar]
- Glasby, T.M.; Connell, S.D. Orientation and position of substrata have large effects on epibiotic assemblages. Mar. Ecol. Prog. Ser. 2001, 214, 127–135. [Google Scholar] [CrossRef]
- Ushiama, S.; Smith, J.A.; Suthers, I.; Lowry, M.; Johnston, E.L. The effects of substratum material and surface orientation on the developing epibenthic community on a designed artificial reef. Biofouling 2016, 32, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, L.; Bourget, E. Influence of substratum heterogeneity scales and complexity on a temperate epibenthic marine community. Mar. Ecol. Prog. Ser. 1999, 189, 159–170. [Google Scholar] [CrossRef]
- Maughan, B.C. The effects of sedimentation and light on recruitment and development of a temperate, subtidal, epifaunal community. J. Exp. Mar. Biol. Ecol. 2001, 256, 59–71. [Google Scholar] [CrossRef]
- Wieczorek, S.K.; Todd, C.D. Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling 1998, 12, 81–118. [Google Scholar] [CrossRef]
- Hunt, H.L.; Scheibling, R.E. Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Mar. Ecol. Prog. Ser. 1997, 155, 269–301. [Google Scholar] [CrossRef]
- Nydam, M.; Stachowicz, J.J. Predator effects on fouling community development. Mar. Ecol. Prog. Ser. 2007, 337, 93–101. [Google Scholar] [CrossRef]
- Avendaño, M.; Cantillánez, M.; Thouzeau, G. Effects of water depth on survival and growth of Argopecten purpuratus (Lamarck, 1819) spat in northern Chile. Aquacult. Int. 2008, 16, 377–391. [Google Scholar] [CrossRef]
- Bandelj, V.; Solidoro, C.; Laurent, C.; Querin, S.; Kaleb, S.; Gianni, F.; Falace, A. Cross-scale connectivity of macrobenthic communities in a patchy network of habitats: The Mesophotic Biogenic Habitats of the Northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2020, 245, 106978. [Google Scholar] [CrossRef]
- Millet, B.; Cecchi, P. Wind-induced hydrodynamic control of the phytoplankton biomass in a lagoon ecosystem. Limnol. Oceanogr. 1992, 37, 140–146. [Google Scholar] [CrossRef]
- Adams, C.M.; Shumway, S.E.; Whitlatch, R.B.; Getchis, T. Biofouling in marine molluscan shellfish aquaculture: A survey assessing the business and economic implications of mitigation. J. World Aquac. Soc. 2011, 42, 242–252. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Witlatch, R.B.; Osman, R.W. Species diversity and invasion resistance in a marine ecosystem. Science 1999, 286, 1577–1579. [Google Scholar] [CrossRef]
- Galil, B.S.; Zenetos, A. A sea change. Exotics in the Eastern Mediterranean. In Invasive Aquatic Species of Europe: Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 325–326. [Google Scholar]
- Zenetos, A.; Koutsoumbas, D.; Vardala-Theodorou, E. Origin and vectors of introduction of exotic molluscs in Greek waters. Belg. J. Zool. 2005, 135, 279–286. [Google Scholar]
- Gartner, H.N.; Clarke Murray, C.; Frey, M.A.; Nelson, J.C.; Larson, K.J.; Ruiz, G.M.; Therriault, T.W. Non-indigenous invertebrate species in the marine fouling communities of British Columbia, Canada. BioInvasions Rec. 2016, 5, 205–212. [Google Scholar] [CrossRef]
- Ponti, M.; Fava, F.; Abbiati, M. Spatial-temporal variability of epibenthic assemblages on subtidal biogenic reefs in the northern Adriatic Sea. Mar. Biol. 2011, 158, 1447–1459. [Google Scholar] [CrossRef]
- Fortič, A.; Mavrič, B.; Pitacco, V.; Lipej, L. Temporal changes of a fouling community: Colonization patterns of the benthic epifauna in the shallow northern Adriatic Sea. Reg. Stud. Mar. Sci. 2021, 45, 101818. [Google Scholar] [CrossRef]
- Jelić-Mrčelić, G.; Slišković, M.; Antolić, B. Biofouling communities on test panels coated with TBT and TBT-free copper based antifouling paints. Biofouling 2006, 22, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jelić-Mrčelić, G.; Slišković, M.; Antolić, B. Macroalgae fouling community as quality element for the evaluation of the ecological status in Vela Luka Bay, Croatia. Acta Soc. Bot. Pol. 2012, 81, 159–165. [Google Scholar] [CrossRef][Green Version]
- Spagnolo, A.; Auriemma, R.; Bacci, T.; Balković, I.; Bertasi, F.; Bolognini, L.; Cabrini, M.; Cilenti, L.; Cuicchi, C.; Cvitković, I.; et al. Non-indigenous macrozoobenthic species on hard substrata of selected harbours in the Adriatic Sea. Mar. Pollut. Bull. 2019, 147, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nerlović, V.; Perić, L.; Slišković, M.; Jelić-Mrčelić, G. The invasive Anadara transversa (Say, 1822) (Mollusca: Bivalvia) in the biofouling community of northern Adriatic mariculture areas. Manag. Biol. Invasions 2018, 9, 239–251. [Google Scholar] [CrossRef]
- Slišković, M.; Jelić-Mrčelić, G.; Antolić, B.; Aničić, I. The fouling of fish farm cage nets as bioindicator of aquaculture pollution in the Adriatic Sea (Croatia). Environ. Monit. Assess. 2011, 173, 519–532. [Google Scholar] [CrossRef]
- Pica, D.; Bloecher, N.; Dell’Anno, A.; Bellucci, A.; Pinto, T.; Pola, L.; Puce, S. Dynamics of a biofouling community in finfish aquaculture: A case study from the South Adriatic Sea. Biofouling 2019, 35, 696–709. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A.; Marchini, A.; Cantone, G.; Castelli, A.; Chimenz, C.; Cormaci, M.; Froglia, C.; Furnari, G.; Gambi, M.C.; Giaccone, G.; et al. Alien species along the Italian coasts: An overview. Biol. Invasions 2011, 13, 215–237. [Google Scholar] [CrossRef]
- Kennedy, V.S.; Bolognini, L.; Dulčić, J.; Woodland, R.J.; Wilberg, M.J.; Harris, L.A. Status of Fish and Shellfish Stocks. In Coastal Ecosystems in Transition; Malone, T.C., Malej, A., Faganeli, J., Eds.; American Geophysical Union: Washington, DC, USA, 2020. [Google Scholar]
- Artegiani, A.; Bregant, D.; Paschini, E.; Pinardi, N.; Raicich, F.; Russo, A. The Adriatic Sea general circulation. Part I: Air-sea interactions and water mass structure. J. Phys. Oceanogr. 1997, 27, 1492–1514. [Google Scholar] [CrossRef]
- Russo, A.; Artegiani, A. Adriatic Sea hydrography. Sci. Mar. 1996, 60, 33–43. [Google Scholar]
- Casellato, S.; Stefanon, A. Coralligenous habitat in the northern Adriatic Sea: An overview. Mar. Ecol. 2008, 29, 321–341. [Google Scholar] [CrossRef]
- Marini, M.; Jones, B.H.; Campanelli, A.; Grilli, F.; Lee, C.M. Seasonal variability and Po River plume influence on biochemical properties along western Adriatic coast. J. Geophys. Res. Oceans 2008, 113, C05S90. [Google Scholar] [CrossRef]
- Artegiani, A.; Bregant, D.; Paschini, E.; Pinardi, N.; Raicich, F.; Russo, A. The Adriatic Sea general circulation. Part II: Baroclinic circulation structure. J. Phys. Oceanogr. 1997, 27, 1515–1532. [Google Scholar] [CrossRef]
- Degobbis, D.; Precali, R.; Ivancic, I.; Smodlaka, N.; Fuks, D.; Kveder, S. Long-term changes in the northern Adriatic ecosystem related to anthropogenic eutrophication. Int. J. Environ. Pollut. 2000, 13, 495–533. [Google Scholar] [CrossRef]
- Bernardi Aubry, F.; Berton, A.; Bastianini, M.; Socal, G.; Acri, F. Phytoplankton succession in a coastal area of the NW Adriatic, over a 10-year sampling period (1990–1999). Cont. Shelf Res. 2004, 24, 97–115. [Google Scholar] [CrossRef]
- Solidoro, C.; Bandelj, V.; Barbieri, P.; Cossarini, G.; Fonda Umani, S. Understanding dynamic of biogeochemical properties in the northern Adriatic Sea by using self-organizing maps and k-means clustering. J. Geophys. Res. 2007, 112, C07S90. [Google Scholar] [CrossRef]
- Cossignani, T.; Di Nisio, A.; Passamonti, M. Atlante Delle Conchiglie del Medio Adriatico; L’Informatore Piceno Editore: Ancona, Italy, 1992; pp. 1–120. [Google Scholar]
- Cesari, P. I Molluschi Della Laguna di Venezia; Arsenale Editrice: Venice, Italy, 1994; pp. 1–189. [Google Scholar]
- Doneddu, M.; Trainito, E. Conchiglie del Mediterraneo; Il Castello Editore: Milano, Italy, 2005; pp. 1–256. [Google Scholar]
- Schiaparelli, S. Checklist della flora e della fauna dei mari italiani (Parte I). Bivalvia. Biol. Mar. Mediterr. 2008, 15, 296–314. [Google Scholar]
- Sullivan, C.M. Bivalve larvae of Malpeque Bay. J. Fish. Res. Board Can. 1948, 77, 1–36. [Google Scholar]
- Loosanoff, V.L.; Davis, H.C.; Chanley, P.E. Dimensions and shapes of larvae of some marine bivalve mollusks. Malacologia 1966, 4, 351–435. [Google Scholar]
- Rose, R.A.; Dix, T.G. Larval and juvenile development of the doughboy scallop Chlamys asperrimus (Lamark) (Mollusca: Pectinidae). Aust. J. Mar. Freshw. Res. 1984, 35, 315–323. [Google Scholar] [CrossRef]
- Hodgson, C.A.; Burke, R.D. Development and larval morphology of the spiny scallop, Chlamys hastate. Biol. Bull. 1988, 174, 303–318. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; pp. 15–85. [Google Scholar]
- Hrs-Brenko, M.; Legac, M. Inter- and intra-species relationships of sessile bivalves on the eastern coast of the Adriatic Sea. Nat. Croat. 2006, 15, 203–230. [Google Scholar]
- Casellato, S.; Sichirollo, E.; Cristofoli, A.; Masiero, L.; Soresi, S. Biodiversità delle ‘‘tegnúe’’ di Chioggia, zona di tutela biologica del Nord Adriatico. Biol. Mar. Mediterr. 2005, 12, 69–77. [Google Scholar]
- Casellato, S.; Masiero, L.; Sichirollo, E.; Soresi, S. Hidden secrets of the Northern Adriatic: ‘‘Tegnúe’’, peculiar reefs. Cent. Eur. J. Biol. 2007, 2, 122–136. [Google Scholar] [CrossRef]
- Gabriele, M.; Bellot, A.; Gallotti, D.; Brunetti, R. Sublittoral hard substrate communities of the northern Adriatic Sea. Cah. Biol. Mar. 1999, 40, 65–76. [Google Scholar]
- Peharda, M.; Ezgeta-Balić, D.; Vrgoč, N.; Isajlović, I.; Bogner, D. Description of bivalve community structure in the Croatian part of the Adriatic Sea—Hydraulic dredge survey. Acta Adriat. 2010, 51, 141–158. [Google Scholar]
- Fava, F.; Ponti, M.; Abbiati, M. Role of recruitment processes in structuring coralligenous benthic assemblages in the northern adriatic continental shelf. PLoS ONE 2016, 11, e0163494. [Google Scholar] [CrossRef]
- Da Ros, L.; Bressan, M.; Marin, M.G. Reproductive cycle of the mussel (Mytilus galloprovincialis Lmk) in Venice Lagoon (North Adriatic). Boll. Zool. 1985, 52, 223–229. [Google Scholar] [CrossRef]
- Orban, E.; Di Lena, G.; Nevigato, T.; Casini, I.; Marzetti, A.; Caproni, R. Seasonal changes in meat content, condition index and chemical composition of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem. 2002, 77, 57–65. [Google Scholar] [CrossRef]
- Ceccherelli, V.U.; Rossi, R. Settlement, growth and production of the Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 1984, 16, 173–184. [Google Scholar] [CrossRef]
- Marčeta, T.; Da Ros, L.; Marin, M.G.; Codognotto, V.F.; Bressan, M. Overview of the biology of Flexopecten glaber in the North Western Adriatic Sea (Italy): A good candidate for future shellfish farming aims? Aquaculture 2016, 462, 80–91. [Google Scholar] [CrossRef]
- Peña, J.B.; Canales, J.; Adsuara, J.M.; Sos, M.A. Study of seasonal settlements of five scallop species in the western Mediterranean. Aquac. Int. 1996, 4, 253–261. [Google Scholar] [CrossRef]
- Castagnolo, L. La pesca e la riproduzione di Pecten jacobaeus (L). e di Aequipecten opercularis (L.) nell’alto Adriatico. Boll. Malacol. 1991, 27, 39–48. [Google Scholar]
- Reddiah, K. The sexuality and spawning of Manx pectinids. J. Mar. Biol. Assoc. U. K. 1962, 42, 683–703. [Google Scholar] [CrossRef]
- Román, G.; Campos, M.J.; Acosta, C.P. Relationships among environment, spawning and settlement of queen scallop in the Ria de Arosa (Galicia, NW Spain). Aquacult. Int. 1996, 4, 225–236. [Google Scholar] [CrossRef]
- Shafee, M.S. Seasonal changes in the biochemical composition and calorific content of the black scallop Chlamys varia (L) from Lanveoc, Bay of Brest. Oceanol. Acta 1981, 4, 331–341. [Google Scholar]
- Morello, E.B.; Solustri, C.; Froglia, C. The alien bivalve Anadara demiri (Arcidae): A new invader of the Adriatic Sea, Italy. J. Mar. Biol. Ass. U. K. 2004, 84, 1057–1064. [Google Scholar] [CrossRef]
- Solustri, C.; Morello, E.; Froglia, C. Osservazioni su Anadara demiri (Piani, 1981) (Bivalvia: Arcidae) epibionte di alcune specie di molluschi. Biol. Mar. Mediterr. 2003, 10, 622–625. [Google Scholar]
- Ezgeta Balić, D.; Radonić, I.; Bojanić Varezić, D.; Zorica, B.; Arapov, J.; Stagličić, N.; Jozić, S.; Peharda, M.; Briski, E.; Lin, Y.; et al. Reproductive cycle of a non-native oyster, Crassostrea gigas, in the Adriatic Sea. Mediterr. Mar. Sci. 2020, 21, 146–156. [Google Scholar] [CrossRef]
- Mladineo, I.; Peharda, M.; Orhanović, S.; Bolotin, J.; Pavela-Vrančić, M.; Treursić, B. The reproductive cycle, condition index and biochemical composition of the horse-bearded mussel Modiolus barbatus. Helgol. Mar. Res. 2007, 61, 183. [Google Scholar] [CrossRef]
- Cabanellas-Reboredo, M.; Deudero, S.; Alós, J.; Valencia, J.; March, D.; Hendriks, I.; Álvarez, E. Recruitment of Pinna nobilis (Mollusca: Bivalvia) on artificial structures. Mar. Biodivers. Rec. 2009, 2, E126. [Google Scholar] [CrossRef]
- Richardson, C.A.; Peharda, M.; Kennedy, H.; Kennedy, P.; Onofri, V. Age, growth rate and season of recruitment of Pinna nobilis (L.) in the Croatian Adriatic determined from Mg: Ca and Sr: Ca shell profiles. J. Exp. Mar. Biol. Ecol. 2004, 299, 1–16. [Google Scholar] [CrossRef]
- Guijarro Garcia, E.; Thorarinsdóttir, G.G.; Ragnarsson, S.A. Settlement of bivalve spat on artificial collectors in Eyjafjordur, North Iceland. Hydrobiologia 2003, 503, 131–141. [Google Scholar] [CrossRef]
- Solidoro, C.; Bastianini, M.; Bandelj, V.; Codermatz, R.; Cossarini, G.; Canu, D.M.; Ravagnan, E.; Salon, S.; Trevisani, S. Current state, scales of variability, and trends of biogeochemical properties in the northern Adriatic Sea. J. Geophys. Res. 2009, 114, C07S91. [Google Scholar] [CrossRef]
- Grilli, F.; Accoroni, S.; Acri, F.; Bernardi Aubry, F.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Cronin, E.R.; Cheshire, A.C.; Clarke, S.M.; Melville, A.J. An investigation into the composition, biomass and oxygen budget of the fouling community on a tuna aquaculture farm. Biofouling 1999, 13, 279–299. [Google Scholar] [CrossRef]
- Fuentes, J.; Molares, J. Settlement of the mussel Mytilus galloprovincialis on collectors suspended from rafts in the Ría de Arousa (NW of Spain): Annual pattern and spatial variability. Aquaculture 1994, 122, 55–62. [Google Scholar] [CrossRef]
- Halla, M.I.; Kassila, J.; Chattou, E.M.A.; Ouaggajou, Y.; El Aamri, F.; Benbani, A.; Nhhala, H. Depth and seasonal effects on the settlement density of two mussel species (Perna perna and Mytilus galloprovincialis) in offshore, Agadir (Morocco). Eur. Sci. J. 2018, 14, 229–240. [Google Scholar] [CrossRef]
- Yildiz, H.; Berber, S. Depth and seasonal effects on the settlement density of Mytilus galloprovincialis L. 1819 in the Dardanelles. J. Anim. Vet. Adv. 2010, 9, 756–759. [Google Scholar] [CrossRef]
- Pearce, C.M.; Gallager, S.M.; Manuel, J.L.; Manning, D.A.; O’Dor, R.K.; Bourget, E. Settlement of larvae of the giant scallop, Placopecten magellanicus, in 9-m deep mesocosms as a function of temperature stratification, depth, food, and substratum. Mar. Biol. 1996, 124, 693–706. [Google Scholar] [CrossRef]
- Thouzeau, G. Experimental collection of postlarvae of Pecten maximus (L.) and other benthic macrofaunal species in the Bay of Saint-Brieuc, France. II. Reproduction patterns and postlarval growth of five mollusc species. J. Exp. Mar. Biol. Ecol. 1991, 148, 181–200. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Froglia, C.; Atkinson, R.J.A.; Moore, P.G. The impact of Rapido trawling for scallops, Pecten jacobaeus (L.), on the benthos of the Gulf of Venice. ICES J. Mar. Sci. 1999, 56, 111–124. [Google Scholar] [CrossRef]
- Vaccarella, R.; Paparella, P.; Bello, G.; Marano, G. The smooth scallop, Chlamys glabra, fishery in the Gulf of Manfredonia (south-western Adriatic Sea). Rapp. Comm. Int. Mer. Médit. 1998, 35, 500–501. [Google Scholar]
- Demir, M. On the presence of Arca (Scapharca) amygdalum Philippi, 1847 (Mollusca: Bivalvia) in the harbour of Izmir, Turkey. J. Fac. Sci. Istanb. Univ. 1977, 42, 197–202. [Google Scholar]
- Zenetos, A. Scapharca demiri (Piani, 1981): Primo ritrovamento nel nord Egeo. La Conchiglia 1994, 271, 37–38. [Google Scholar]
- Rinaldi, E. Segnalazione faunistica n. 41. Quad. Studi Nat. Romagna 2001, 14, 127–128. [Google Scholar]
- Morello, E.; Solustri, C. First record of Anadara demiri (Piani 1981) (Bivalvia: Arcidae) in Italian waters. Boll. Malacol. 2001, 37, 231–234. [Google Scholar]
- Mizzan, L. Biodiversita’ della Laguna di Venezia. Segnalazioni (1–143) –18. Anadara demiri. Boll. Mus. Civ. St. Nat. Venezia 2002, 53, 265–266. [Google Scholar]
- Mizzan, L.; Vianello, C. Biodiversità della Laguna di Venezia e della costa nord adriatica veneta. Segnalazioni 189–201. Boll. Mus. Civ. St. Nat. Venezia 2007, 58, 319. [Google Scholar]
- Nerlović, V.; Doğan, A.; Perić, L. First record of Anadara transversa (Mollusca: Bivalvia: Arcidae) in Croatian waters (Adriatic Sea). Acta Adriat. 2012, 53, 139–144. [Google Scholar]
- Mistri, M. The non-indigenous mussel Musculista senhousia in an Adriatic lagoon: Effects on benthic community over a ten year period. J. Mar. Biol. Assoc. U. K. 2003, 83, 1277–1278. [Google Scholar] [CrossRef]
- Halley, E.F.; Koehn, J.Z.; Holsman, K.K.; Halpern, B.S. Emerging trends in science and news of climate change threats to and adaptation of aquaculture. Aquaculture 2022, 549, 737812. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).