The SHAPE Project: An Innovative Approach to Understanding Seasonal and Diel Dissolved Oxygen Dynamics in the Marano and Grado Lagoon (Adriatic Sea) under the WFD/2000/60/CE
Abstract
1. Introduction
2. Materials and Methods
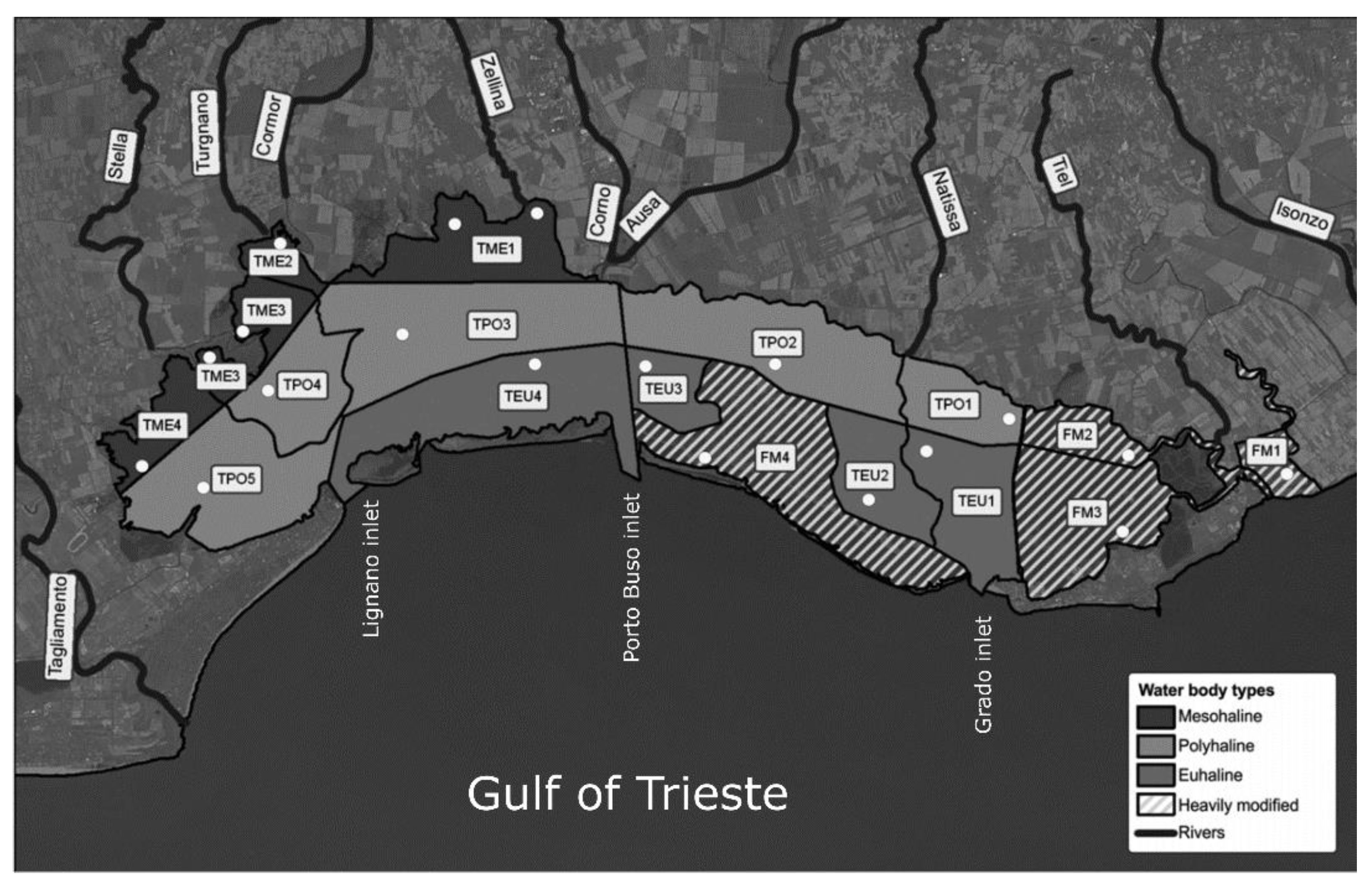
2.1. Environmental Settings
2.2. Multiparameter Probe Characteristics
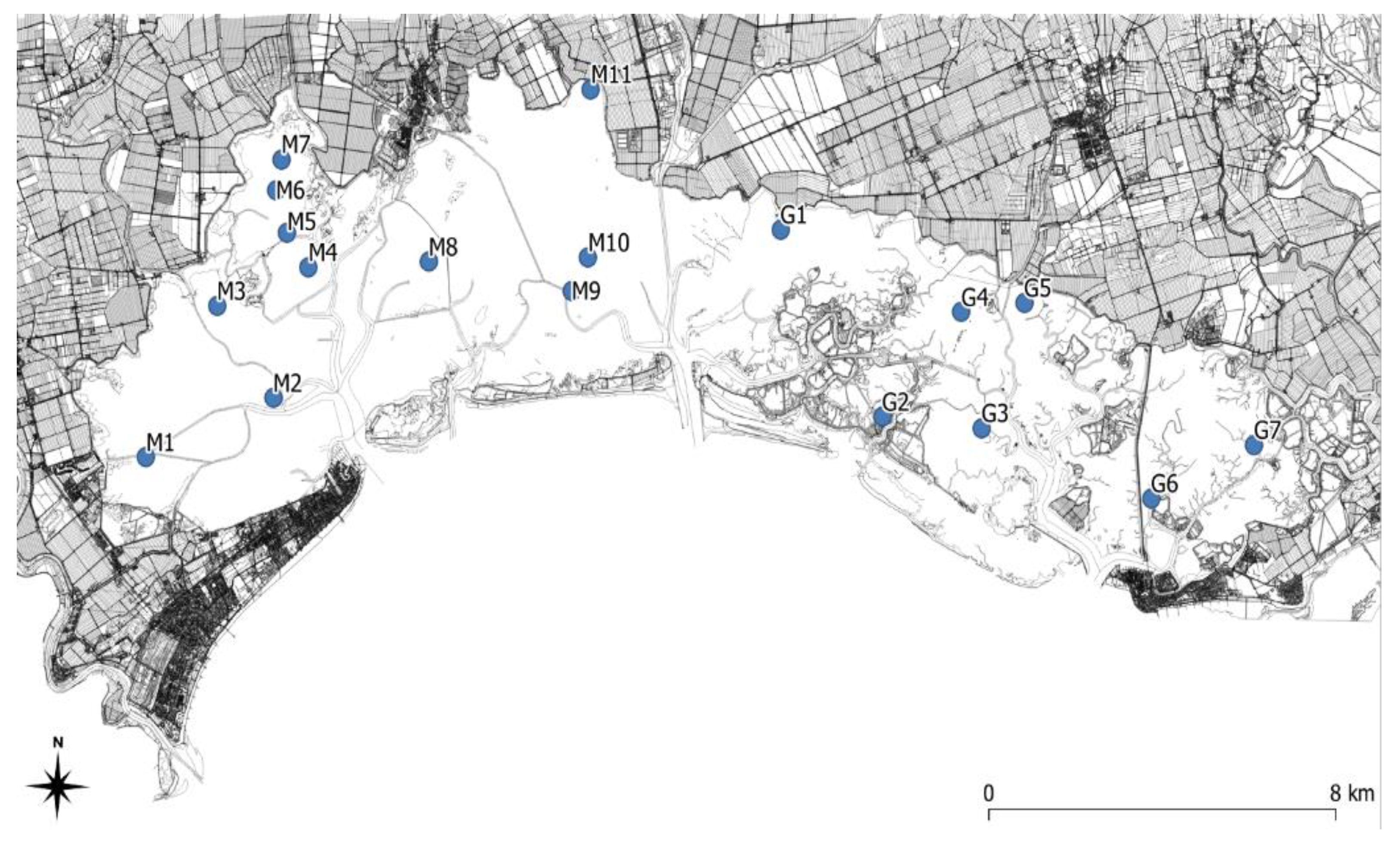
2.3. Sampling Design
2.4. Statistical Assessment and Mapping
3. Results and Discussion
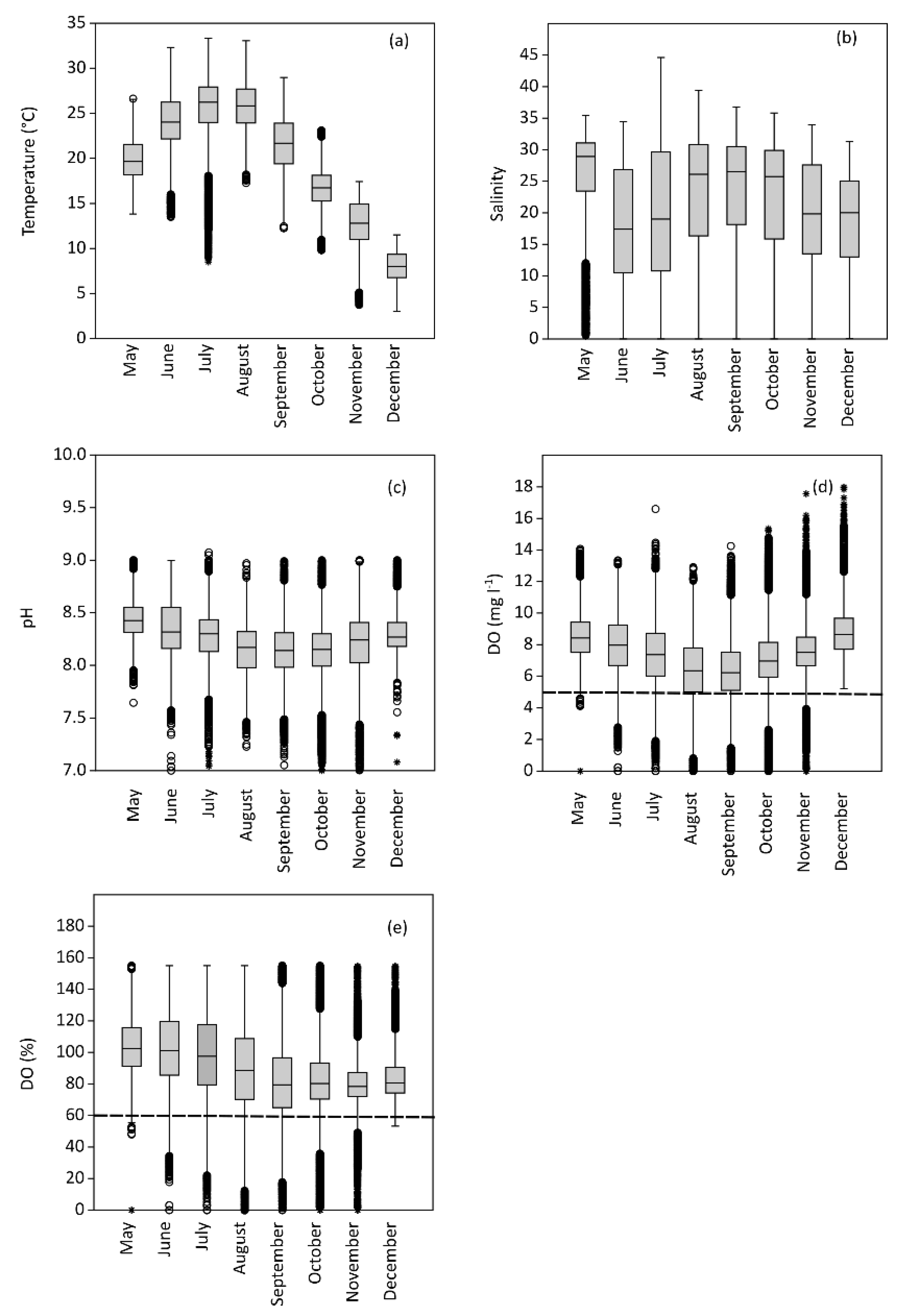
3.1. Complementary Parameters
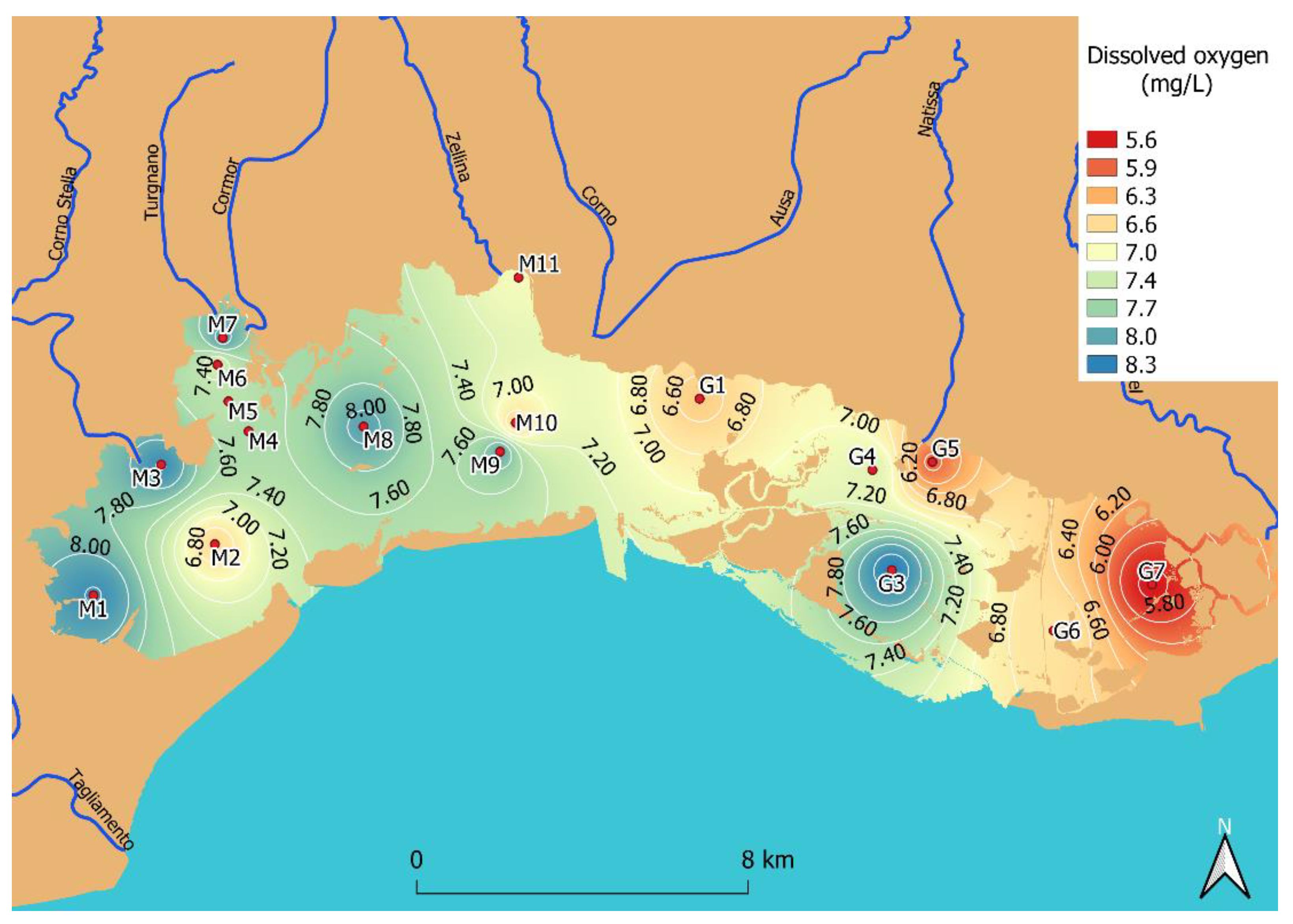
3.2. Dissolved Oxygen
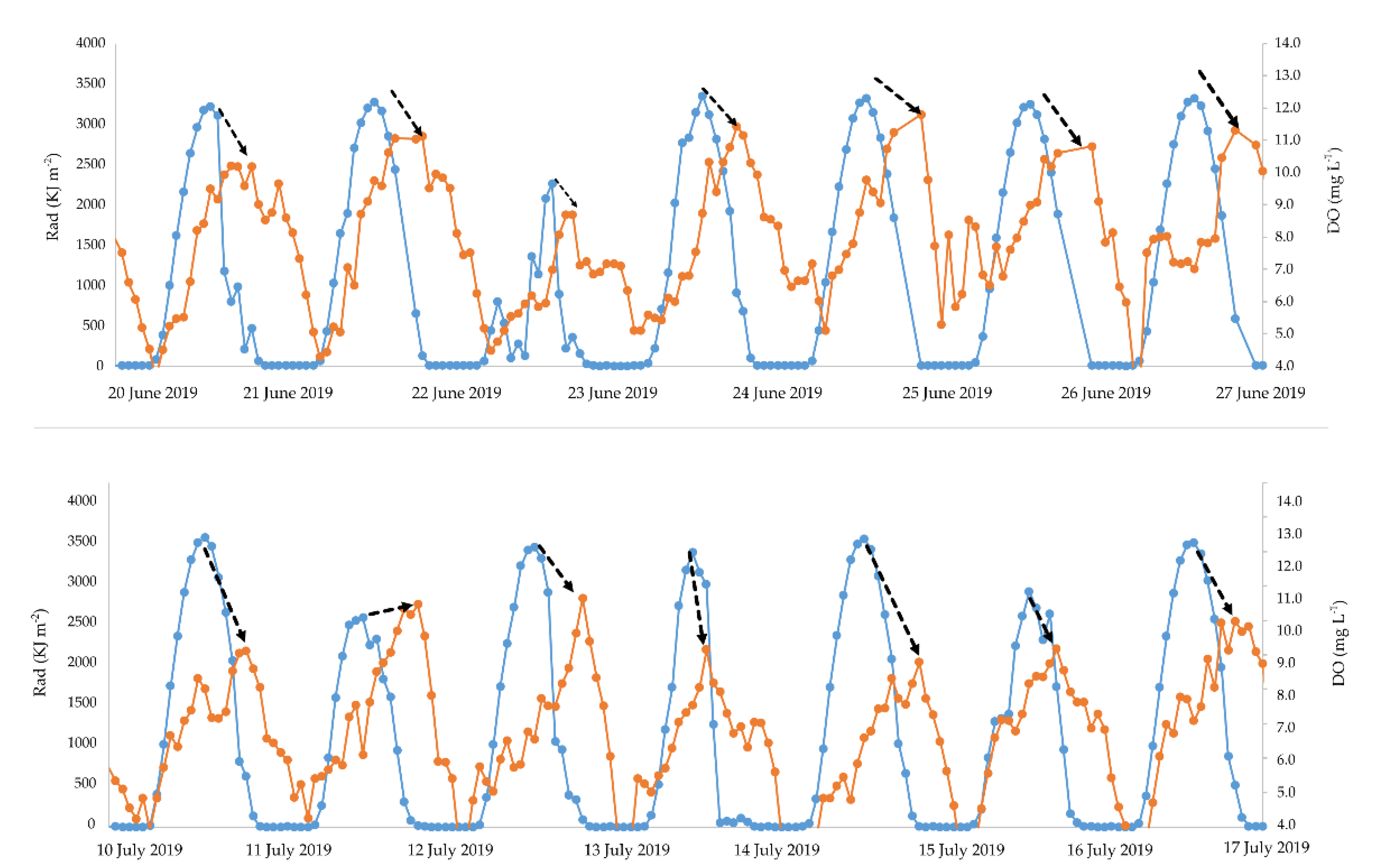
3.3. Influence of Solar Radiation and Tides on DO Content
3.4. Dissolved Oxygen Content and Management in the Context of WFD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razinkovas, A.; Gasiūnaitė, Z.; Viaroli, P.; Zaldívar, J.M. European lagoons—Need for further comparison across spatial and temporal scales. Hydrobiologia 2008, 611, 1–4. [Google Scholar] [CrossRef]
- Gaertner-Mazuoni, N.; De Wit, R. Exploring new issues for coastal lagoons monitoring and management. Estuar. Coast. Shelf Sci. 2012, 114, 1–6. [Google Scholar] [CrossRef]
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Béjaoui, B.; et al. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- WFD. European Commission Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Comm. 2000, L 327, 1–72. [Google Scholar]
- Wu, R.S.S. Hypoxia: From molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002, 45, 35–45. [Google Scholar] [CrossRef]
- Yoann, T.; Flye-Sainte-Marie, J.; Chabot, D.; Aguirre-Velarde, A.; Marques Gonçalo, M.; Pecquerie, L. Effects of hypoxia on metabolic functions in marine organisms: Observed patterns and modelling assumptions within the context of Dynamic Energy Budget (DEB) theory. J. Coast Res. 2019, 143, 231–242. [Google Scholar]
- Riedel, B.; Diaz, R.; Rosenberg, R.; Stachowitsch, M. The ecological consequences of marine hypoxia: From behavioural to ecosystem responses. In Stressors in the Marine Environment: Physiological and Ecological Responses; Societal Implications; Solan, M., Whiteley, N., Eds.; Oxford University Press: Oxford, UK, 2016; Published to Oxford Scholarship Online: May 2016. [Google Scholar] [CrossRef]
- Fortes-Lopes, J.; Silva, C. Temporal and spatial distribution of dissolved oxygen in the Ria de Aveiro lagoon. Ecol. Model 2006, 197, 67–88. [Google Scholar] [CrossRef]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N.; Gilbert, D. Distribution and consequences of hypoxia. In Watersheds, Bays and Bounded Seas; Urban, E., Sundby, B., Malanotte-Rizzoli, P., Melillo, J.M., Eds.; Island Press: Washington, DC, USA, 2009; pp. 209–226. [Google Scholar]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. 1995, 33, 245–303. [Google Scholar]
- EPA. Ambient Aquatic Life Water Quality Criteria for Dissolved Oxygen (Saltwater): Cape Cod to Cape Hatteras; EPA: Washington, DC, USA, 2000; 140p. [Google Scholar]
- Newton, A.; Oliveira, P.S.; Icely, J.D.; Foster, P.A. Monitoring of oxygen condition in the Ria Formosa coastal lagoon, Portugal. J. Environ. Monit. 2010, 12, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Alfonso, A.M.; Carstensen, J.; Conley, D.J. Biogeochemical and environmental drivers of coastal hypoxia. J. Mar. Sys. 2015, 141, 190–199. [Google Scholar] [CrossRef]
- Zhang, J.; Gilbert, D.; Gooday, A.J.; Levin, L.A.; Naqvi, W.A.; Middelburg, J.J.; Scranton, M.; Ekau, W.; Peña, A.; Dewitte, B.; et al. Natural and human-induced hypoxia and consequences for coastal areas: Synthesis and future development. Biogeoscience 2010, 7, 1443–1467. [Google Scholar] [CrossRef]
- Chapelle, A.; Ménesguen, A.; Deslous-Paoli, J.M.; Souchu, P.; Mazouni, N.; Vaquer, A.; Millet, B. Modelling nitrogen, primary production and oxygen in a Mediterranean lagoon. Impact of oysters farming and inputs from the watershed. Ecol. Model. 2000, 127, 161–181. [Google Scholar] [CrossRef]
- Como, S.; Magni, P. Temporal changes of a macrobenthic assemblage in harsh lagoon sediments. Estuar. Coast Shelf. Sci. 2009, 83, 638–646. [Google Scholar] [CrossRef]
- Viaroli, P.; Azzoni, R.; Bartoli, M.; Giordani, G.; Naldi, M.; Nizzoli, D. Primary productivity, biogeochemical buffers and factors controlling trophic status and ecosystem processes in Mediterranean coastal lagoons: A synthesis. Adv. Oceanogr. Limnol. 2010, 1, 271–293. [Google Scholar] [CrossRef]
- Avramidis, P.; Bekiari, V.; Christodoulou, D.; Papatheodorou, G. Sedimentology and water column stratification in a permanent anoxic Mediterranean lagoon environment, Aetoliko Lagoon, western Greece. Environ. Earth Sci. 2015, 73, 5687–5701. [Google Scholar] [CrossRef]
- Zilius, M.; Giordani, G.; Petkuviene, J.; Lubiene, I.; Ruginis, T.; Bartoli, M. Phosphorus mobility under short-term anoxic conditions in two shallow eutrophic coastal systems (Curonian and Sacca di Goro lagoons). Estuar. Coast Shelf Sci. 2015, 164, 134–146. [Google Scholar] [CrossRef]
- Brigolin, D.; Rabouille, C.; Demasy, C.; Bombled, B.; Monvoisin, G.; Pastres, R. Early diagenesis in sediments of the Venice Lagoon (Italy) and its relationship to hypoxia. Front. Mar. Sci. 2021, 7, 575547. [Google Scholar] [CrossRef]
- OSPAR. Common Procedure for the Identification of the Eutrophication Status of the OSPAR Maritime Area; Agreement 2005-3; OSPAR Commission: London, UK, 2008. [Google Scholar]
- Covelli, S.; Faganeli, J.; De Vittor, C.; Predonzani, S.; Acquavita, A.; Horvat, M. Benthic fluxes of mercury species in a lagoon environment (Grado lagoon, Northern Adriatic Sea, Italy). Appl. Geochem. 2008, 23, 529–546. [Google Scholar] [CrossRef]
- Covelli, S.; Emili, A.; Acquavita, A.; Koron, N.; Faganeli, J. Benthic biogeochemical cycling of mercury in two contaminated northern Adriatic coastal lagoons. Cont. Shelf Res. 2011, 31, 1777–1789. [Google Scholar] [CrossRef]
- Acquavita, A.; Emili, A.; Covelli, S.; Faganeli, J.; Predonzani, S.; Koron, N.; Carrasco, L. The effects of resuspension on the fate of Hg in contaminated sediments (Marano and Grado Lagoon, Italy): Short-term simulation experiments. Estuar. Coast Shelf Sci. 2012, 13, 32–40. [Google Scholar] [CrossRef]
- De Vittor, C.; Faganeli, J.; Emili, A.; Covelli, S.; Predonzani, S.; Acquavita, A. Benthic fluxes of oxygen, carbon and nutrients in the Marano and Grado Lagoon (northern Adriatic Sea, Italy). Estuar. Coast Shelf Sci. 2012, 113, 57–70. [Google Scholar] [CrossRef]
- Acquavita, A.; Aleffi, I.F.; Benci, C.; Bettoso, N.; Crevatin, E.; Milani, L.; Tamberlich, F.; Toniatti, L.; Barbieri, P.; Licen, S.; et al. Annual characterization of nutrients pattern and trophic state in a Mediterranean coastal lagoon: The Marano and Grado Lagoon, northern Adriatic Sea. Reg. Stud. Mar. Sci. 2015, 2, 132–144. [Google Scholar] [CrossRef]
- Bricker, S.B.; Ferreira, J.G.; Simas, T. An integrated methodology for assessment of estuarine trophic status. Ecol. Model. 2003, 169, 39–60. [Google Scholar] [CrossRef]
- Winkler, L.W. Die Bestimmung des im Wasser gelösten Sauerstoffen. Ber. Dtsche. Chem. Ges. 1888, 21, 2842–2855. [Google Scholar] [CrossRef]
- Garel, E.; Ferreira, O. Monitoring estuaries using non-permanent stations: Practical aspects and data examples. Ocean Dyn. 2011, 61, 891–902. [Google Scholar] [CrossRef]
- D’Autilia, R.; Falcucci, M.; Hull, V.; Parrella, L. Short time dissolved oxygen dynamics in shallow water ecosystems. Ecol. Model. 2004, 179, 297–306. [Google Scholar] [CrossRef]
- Nezlin, N.P.; Kamer, K.; Hyde, J.; Stein, E.D. Dissolved oxygen dynamics in a eutrophic estuary, Upper Newport Bay, California. Estuar. Coast Shelf Sci. 2009, 82, 139–151. [Google Scholar] [CrossRef]
- Friedrich, J.; Janssen, F.; Aleynik, D.; Bange, H.W.; Boltacheva, N.; Çagatay, M.N.; Dale, A.W.; Etiope, G.; Erdem, Z.; Geraga, M.; et al. Investigating hypoxia in aquatic environments: Diverse approaches to addressing a complex phenomenon. Biogeosciences 2014, 11, 1215–1259. [Google Scholar] [CrossRef]
- Cravo, A.; Rosa, A.; Jacob, J.; Correia, C. Dissolved oxygen dynamics in Ria Formosa Lagoon (South Portugal)—A real time monitoring observatory. Mar. Chem. 2020, 223, 10386. [Google Scholar] [CrossRef]
- Brambati, A. Metalli Pesanti Nelle Lagune di Marano e Grado: Piano di Studi Finalizzato All’accertamento della Presenza di Eventuali Sostanze Tossiche Persistenti nel Bacino Lagunare di Marano e Grado ed al Suo Risanamento; Regione Autonoma Friuli-Venezia Giulia, Servizio Idraulica: Trieste, Italy, 1996; 174p. [Google Scholar]
- Ferrarin, C.; Umgiesser, G.; Bajo, M.; Bellafiore, D.; De Pascalis, F.; Ghezzo, M.; Mattassi, G.; Scroccaro, I. Hydraulic zonation of the lagoons of Marano and Grado, Italy. A modeling approach. Estuar. Coast Shelf Sci. 2010, 87, 561–572. [Google Scholar] [CrossRef]
- Triches, A.; Pillon, S.; Bezzi, A.; Lipizer, M.; Gordini, E. Carta Batimetrica della Laguna di Marano e Grado; Regione Autonoma Friuli Venezia Giulia—A Cura di: Autorità di Bacino Regionale del Friuli Venezia Giulia, Commissario Delegato per l’emergenza socio-economico ambientale determinatasi nella Laguna di Marano Lagunare e Grado, Dipartimento di Geoscienze dell’Università di Trieste: Trieste, Italy; OGS—Istituto Nazionale di Oceanografia e di Geofisica Sperimentale: Sgonico, Italy; Arti Grafiche Friulane, Imoco spa (Ud): Fagagna, Italy, 2011. [Google Scholar]
- Ret, M. Bilancio Idrologico e Circolazione Idrica della Laguna di Marano e Grado. Master’s Thesis, Faculty of Engineering, University of Udine, Udine, Italy, 2006; p. 442. (In Italian). [Google Scholar]
- Mancero-Mosquera, I. Water flow in the inlets of the Marano-Grado lagoon system (NE Italy). Il Nuovo Cim. 2013, 36, 132–144. [Google Scholar]
- Dorigo, L. La Laguna di Grado e le Sue Foci. Ricerche e Rilievi Idrografici; Magistrato Alle Acque-Ufficio Idrografico: Venezia, Italy, 1965; 231p. (In Italian) [Google Scholar]
- Fontolan, G.; Pillon, S.; Bezzi, A.; Villalta, R.; Lipizer, M.; Triches, A.; D’Aietti, A. Human impact and the historical transformation of saltmarshes in the Marano and Grado Lagoon, northern Adriatic Sea. Estuar. Coast Shelf Sci. 2012, 113, 41–56. [Google Scholar] [CrossRef]
- Acquavita, A.; Covelli, S.; Emili, A.; Berto, D.; Faganeli, J.; Giani, M.; Horvat, M.; Koron, N.; Rampazzo, F. Mercury in the sediments of the Marano and Grado Lagoon (Northern Adriatic Sea): Sources, distribution and speciation. Estuar. Coast Shelf Sci. 2012, 13, 20–31. [Google Scholar] [CrossRef]
- Sladonja, B.; Bettoso, N.; Zentilin, A.; Tamberlich, F.; Acquavita, A. Manila clam (Tapes philippinarum Adams & Reeve, 1852) in the Lagoon of Marano and Grado (Northern Adriatic Sea, Italy): Socio-economic and environmental pathway of a shell farm. In Aquaculture and the Environment—A Shared Destiny; Sladonja, B., Ed.; Tech Open Publishers: Rijeka, Croatia, 2011; pp. 51–78. [Google Scholar]
- Ramieri, E.; Barbanti, A.; Picone, M.; Menchini, G.; Bressan, E.; Dal Forno, E. Integrated plan for the sustainable management of the Lagoon of Marano and Grado. In Proceedings of the Littoral 2010—Adapting to Global Change at the Coast: Leadership, Innovation, and Investment, London, UK, 21–23 September 2010; Available online: http://coastnet-littoral2010.edpsciences.org/articles/litt/pdf/2011/01/litt-05008.pdf (accessed on 10 October 2021).
- Acquavita, A.; Falomo, J.; Predonzani, S.; Bettoso, N.; Mattassi, G. The PAH level, distribution and composition in surface sediments from a Mediterranean Lagoon: The Marano and Grado Lagoon (Northern Adriatic Sea, Italy). Mar. Pollut. Bull. 2014, 81, 234–241. [Google Scholar] [CrossRef]
- Covelli, S.; Langone, L.; Acquavita, A.; Piani, R.; Emili, A. Historical flux of mercury associated with mining and industrial sources in the Marano and Grado Lagoon (northern Adriatic Sea). Estuar. Coast Shelf Sci. 2012, 13, 7–19. [Google Scholar] [CrossRef]
- Saccon, P.; Leis, A.; Marca, A.; Kaiser, J.; Campisi, L.; Böttcher, M.E.; Savarino, J.; Escher, P.; Eisenhauer, A.; Erbland, J. Multi-isotope approach for the identification and characterisation of nitrate pollution sorces in the Marano lagoon (Italy) and parts of its catchment area. App. Geochem. 2013, 34, 75–89. [Google Scholar] [CrossRef]
- Bettoso, N.; Aleffi, I.F.; Faresi, L.; D’Aietti, A.; Acquavita, A. Macrozoobenthos monitoring in relation to dredged sediment disposal: The case of the Marano and Grado Lagoon (northern Adriatic Sea, Italy). Reg. Stud. Mar. Sci. 2020, 33, 100916. [Google Scholar] [CrossRef]
- Torresan, S.; Critto, A.; Rizzi, J.; Marcomini, A. Assessment of coastal vulnerability to climate change hazards at the regional scale: The case study of the North Adriatic Sea. Nat. Hazards Earth Syst. Sci. 2012, 12, 2347–2368. [Google Scholar] [CrossRef]
- Ferrarin, C.; Bajo, M.; Bellafiore, D.; Cucco, A.; De Pascalis, F.; Ghezzo, M.; Umgiesser, G. Toward homogenization of Mediterranean lagoons and their loss of hydrodiversity. Geophys. Res. Lett. 2014, 41, 5935–5941. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Graham, P.; Falconer, L.; Telfer, T.; Mossone, P.; Viale, I. A modelling approach to classify the suitability of shallow Mediterranean lagoons for pacific oyster, Crassostrea gigas (Thunberg, 1793) farming. Ocean Coast Manag. 2020, 192, 105234. [Google Scholar] [CrossRef]
- Capaccioni, F.; Contò, M.; Failla, S.; Cataudella, S.; Ciccotti, E. Fatty acid profiles of migrating female silver eel from Mediterranean coastal lagoons as integrative descriptors of spawners biological quality. Estuar. Coast Shelf Sci. 2018, 210, 87–97. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Rieradevall, M.; Farrés-Correl, R.; Newton, A. Annual characterisation of four Mediterranean coastal lagoons subjectedto intense human activity. Estuar. Coast Shelf Sci. 2012, 114, 59–69. [Google Scholar] [CrossRef]
- Zucchetta, M.; Franco, A.; Torricelli, P.; Franzoi, P. Habitat distribution model for European flounder juveniles in the Venice lagoon. J. Sea Res. 2010, 64, 133–144. [Google Scholar] [CrossRef]
- Best, M.A.; Wither, A.W.; Coates, S. Dissolved oxygen as a physico-chemical supporting element in the Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 53–64. [Google Scholar] [CrossRef]
- Gilabert, J. Seasonal plankton dynamics in a Mediterranean hypersaline coastal lagoon: The Mar Menor. J. Plankton Res. 2001, 23, 207–218. [Google Scholar] [CrossRef]
- Diamantopoulou, E.; Dassenakis, M.; Kastritis, A.; Tomara, V.; Paraskevopoulou, V.; Poulos, S. Seasonal fluctuations of nutrients in a hypersaline Mediterranean lagoon. Desalination 2008, 224, 271–279. [Google Scholar] [CrossRef]
- Petranich, E.; Covelli, S.; Acquavita, A.; De Vittor, C.; Faganeli, J.; Contin, M. Benthic nutrient cycling at the sediment-water interface in a lagoon farming system (northern Adriatic Sea, Italy). Sci. Total Environ. 2018, 644, 137–149. [Google Scholar] [CrossRef]
- D’Aietti, A.; Altobelli, A.; Cabrini, M. La Vallicoltura nella laguna di Grado: Impatti e problematiche delle valli da pesca. In Linee Guida e Casi di Studio per la Gestione dei siti della rete Natura 2000 in Ambiente di Transizione; EUT Edizioni Università di Trieste: Trieste, Italy, 2007; pp. 224–242. [Google Scholar]
- Vincenzi, S.; Caramori, G.; Rossi, R.; De Leo, G.A. A GIS-based habitat suitability model for commercial yield estimation of Tapes philippinarum in a Mediterranean coastal lagoon (Sacca di Goro, Italy). Ecol. Model. 2006, 193, 90–104. [Google Scholar] [CrossRef]
- Facchini, A.; Mocenni, C.; Marwan, N.; Vicino, A.; Tiezzi, E. Nonlinear time series analysis of dissolved oxygen in the Orbetello Lagoon (Italy). Ecol. Model. 2007, 203, 339–348. [Google Scholar] [CrossRef]
- Coelho, S.; Gamito, S.; Pérez-Ruzafa, A. Trophic state of Foz de Almargem coastal lagoon (Algarve, South Portugal) based on the water quality and the phytoplankton community. Estuar. Coast Shelf Sci. 2007, 71, 218–231. [Google Scholar] [CrossRef]
- Specchiulli, A.; Focardi, S.; Renzi, M.; Scirocco, T.; Cilenti, L.; Breber, P.; Bastianoni, S. Environmental heterogeneity patterns and assessment of trophic levels in two Mediterranean lagoons: Orbetello and Varano, Italy. Sci. Total Environ. 2008, 402, 285–298. [Google Scholar] [CrossRef]
- Facca, C.; Pellegrino, N.; Ceoldo, S.; Tibaldo, M.; Sfriso, A. Trophic conditions in the Venice Lagoon (Northern Adriatic Sea, Italy). Open Ocean J. 2011, 5, 1–13. [Google Scholar] [CrossRef][Green Version]
- Alp, M.T.; Fakioğlu, Y.E.; Özbay, Ö.; Turan Koçer, M.A. A study on water quality and trophic state of Akgöl Lagoon (Mersin, Turkey). Aquat. Ecosyst. Health Manag. 2016, 19, 58–63. [Google Scholar] [CrossRef]
- Rigaud, S.; Deflandre, B.; Grenz, C.; Cesbron, F.; Pozzato, L.; Voltz, B.; Grémare, A.; Romero-Ramirez, A.; Mirleau, P.; Meulé, S.; et al. Benthic oxygen dynamics and implication for the maintenance of chronic hypoxia and ecosystem degradation in the Berre lagoon (France). Estuar. Coast Shelf Sci. 2021, 258, 107437. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Exposure to nitrate increases susceptibility to hypoxia in fish. Physiol. Biochem. Zool. 2021, 94, 124–142. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Levin, L.A. Coastal hypoxia and sediment biogeochemistry. Biogeosciences 2009, 6, 1273–1293. [Google Scholar] [CrossRef]
- Emili, A.; Koron, N.; Covelli, S.; Faganeli, J.; Acquavita, A.; Predonzani, S.; De Vittor, C. Does anoxia affect mercury cycling at the sediment-water interface in the Gulf of Trieste (northern Adriatic Sea)? Incubation experiments using benthic flux chambers. App. Geochem. 2011, 26, 194–204. [Google Scholar] [CrossRef]
- Ogrinc, N.; Faganeli, J. Phosphorus regeneration and burial in near-shore marine sediments (the Gulf of Trieste, northern Adriatic Sea). Estuar. Coast Shelf Sci. 2006, 67, 579–588. [Google Scholar] [CrossRef]
- Warnken, K.W.; Gill, G.A.; Griffin, L.L.; Santschi, P.H. Sediment-water exchange of Mn, Fe, Ni and Zn in Galveston Bay, Texas. Mar. Chem. 2001, 73, 215–231. [Google Scholar] [CrossRef]
- Emili, A.; Acquavita, A.; Covelli, S.; Spada, L.; Di Leo, A.; Giandomenico, S.; Cardellicchio, N. Mobility of heavy metals from polluted sediments of a semi-enclosed basin: In situ benthic chamber experiments in Taranto’s Mar Piccolo (Ionian Sea, Southern Italy). Environ. Sci. Pollut. Res. 2016, 23, 12582–12595. [Google Scholar] [CrossRef]
- Rigaud, S.; Radakovitch, O.; Couture, R.M.; Deflandre, B.; Cossa, D.; Garnier, C.; Garnier, J.-M. Mobility and fluxes of trace elements and nutrients at the sediment-water interface of a lagoon under contrasting water column oxygenation conditions. Appl. Geochem. 2013, 31, 35–51. [Google Scholar] [CrossRef]
- Formalewicz, M.M.; Rampazzo, F.; Noventa, S.; Gion, C.; Petranich, E.; Crosera, M.; Covelli, S.; Faganeli, J.; Berto, D. Organotin compounds in touristic marinas of the northern Adriatic Sea: Occurrence, speciation and potential recycling at the sediment-water interface. Environ. Sci. Pollut. Res. 2019, 26, 31142–31157. [Google Scholar] [CrossRef]
- Petranich, E.; Croce, S.; Crosera, M.; Pavoni, E.; Faganeli, J.; Adami, G.; Covelli, S. Mobility of metal(loid)s at the sediment-water interface in two tourist port areas of the Gulf of Trieste (northern Adriatic Sea). Environ. Sci. Pollut. Res. 2018, 25, 26887–26902. [Google Scholar] [CrossRef]
- Emili, A.; Acquavita, A.; Koron, N.; Covelli, S.; Faganeli, J.; Horvat, M.; Žižek, S.; Fajon, V. Benthic flux measurements of Hg species in a northern Adriatic lagoon environment (Marano & Grado Lagoon). Estuar. Coast Shelf Sci. 2012, 13, 71–84. [Google Scholar]
- UNESCO. Oxygen Solubility in Sea Water; UNESCO International Oceanographic Tables; UNESCO: Paris, France, 1973; Volume 2. [Google Scholar]
- UNESCO. Tenth Report of the Joint Panel on Oceanographic Tables and Standards. UNESCO Tech. Pap. Mar. Sci. 1981, 36, 22. [Google Scholar]
- Benson, B.B.; Krause, D., Jr. The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnol. Oceanogr. 1984, 29, 620–632. [Google Scholar] [CrossRef]
- Obernosterer, I.; Catala, P.; Reinthaler, T.; Herndl, G.J.; Lebaron, P. Enhanced heterotrophic activity in the surfacemicrolayer of the Mediterranean Sea. Aquat. Microb. Ecol. 2005, 39, 293–302. [Google Scholar] [CrossRef]
- Morán, X.A.G.; García, F.C.; Røstad, A.; Silva, L.; Al-Otaibi, N.; Irigoien, X.; Calleja, M.L. Diel dynamics of dissolved organic matter and heterotrophic prokaryotes reveal enhanced growth at the ocean’s mesopelagic fish layer during daytime. Sci. Total Environ. 2022, 804, 150098. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J. Photosynthesis and photorespiration in marine phytoplankton. Aquat. Bot. 1989, 34, 105–130. [Google Scholar] [CrossRef]
- Hull, V.; Parrella, L.; Falcucci, M. Modelling dissolved oxygen dynamics in coastal lagoons. Ecol. Model. 2011, 211, 468–480. [Google Scholar] [CrossRef]
- Borja, Á.; Rodríguez, J.G. Problems associated with the ‘one-out, all-out’ principle, when using multiple ecosystem components in assessing the ecological status of marine waters. Mar. Pollut. Bull. 2010, 60, 1143–1146. [Google Scholar] [CrossRef]
- Falace, A.; Curiel, D.; Sfriso, A. Study of the macrophyte assemblages and application of phytobenthic indices to assess the Ecological Status of the Marano-Grado Lagoon (Italy). Mar. Ecol. 2009, 30, 480–494. [Google Scholar] [CrossRef]
- Bettoso, N.; Acquavita, A.; D’Aietti, A.; Mattassi, G. The Marano and Grado Lagoon: A brief synopsis on the aquatic fauna and fisheries resources. Ann. Ser. Hist. Nat. 2013, 23, 135–142. [Google Scholar]
- Bettoso, N.; Aleffi, I.F.; Faresi, L.; Rossin, P.; Mattassi, G. Evaluation on the ecological status of the macrozoobenthic communities in the Marano and Grado Lagoon (northern Adriatic Sea). Ann. Ser. Hist. Nat. 2010, 20, 193–206. [Google Scholar]


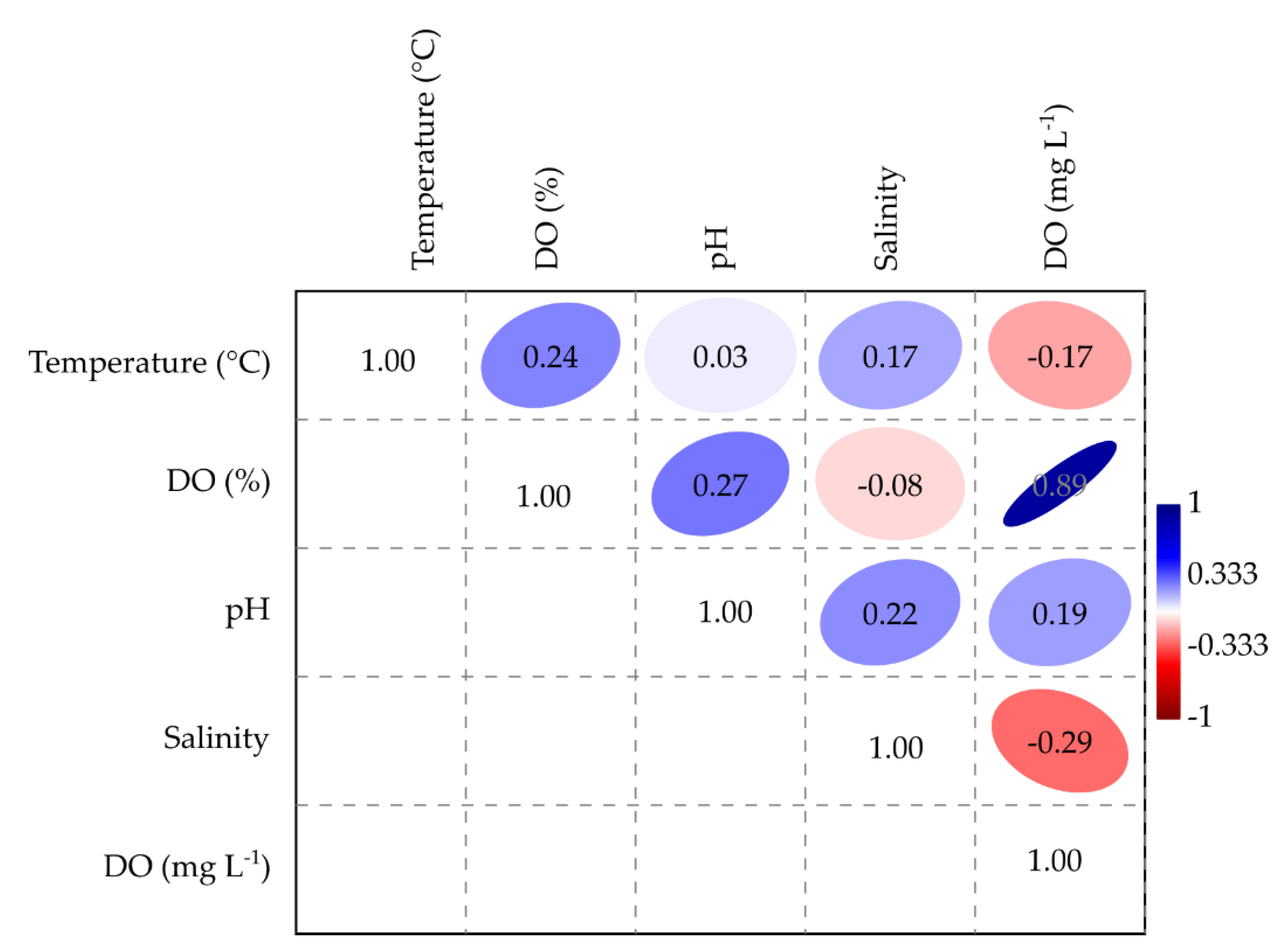
| Site | DO (%) | DO (mg L−1) | References |
|---|---|---|---|
| Ria de Aveiro (Portugal) | n.a. | <1–12 | [8] |
| Fogliano Lagoon (Italy) | n.a. | 2–11.5 | [32] |
| Ria Formosa Lagoon (Portugal) | 30–140 | 1.9–10.9 | [35] |
| Korissia Lagoon (Greece) | n.a. | 3.5–11.2 | [61] |
| Orbetello Lagoon (Italy) | 26.39–144 ± 44.0 | n.a. | [64] |
| Orbetello Lagoon (Italy) | n.a. | 0.2–8.65 | [65] |
| Foz de Almargem Lagoon (Portugal) | 69.88–121.8 | 5.49–12.65 | [66] |
| Orbetello Lagoon (Italy) | 20.10 ± 9.30/89.60 ± 9.00 (mean ± s.d) | n.a. | [67] |
| Varano Lagoon (Italy) | 77.46 ± 4.40/95.48 ± 6.39 (mean ± s.d) | n.a. | [67] |
| Venice Lagoon (Italy) | 112±34.5/133 ± 36.6 (mean ± s.d) | n.a. | [68] |
| Akgöl Lagoon (Turkey) | n.a. | 7.5–8.2 (mean) | [69] |
| Berre Lagoon (France) | 40–100 | n.a. | [70] |
| Marano and Grado Lagoon (Italy) | <5–155 | <0.5–17.99 | This work |
| Site | <2 mg L−1 | <5 mg L−1 | 5%ile | Salinity | Classification |
|---|---|---|---|---|---|
| M1 | 35 | 464 | 5.6 | 12.6 | Good |
| M2 | 3 | 855 | 4.7 | 21.5 | Good |
| M3 | 3 | 148 | 6.1 | 8.9 | Good |
| M4 | 2 | 504 | 4.5 | 17.7 | Moderate |
| M5 | 21 | 763 | 4.1 | 13.6 | Moderate |
| M6 | 16 | 1721 | 4.2 | 17.9 | Moderate |
| M7 | 40 | 847 | 4.6 | 9.4 | Moderate |
| M8 | 1 | 151 | 5.7 | 17.8 | Good |
| M9 | 6 | 457 | 5.2 | 28.5 | Good |
| M10 | 141 | 1295 | 4.1 | 28.0 | Moderate |
| M11 | 44 | 1859 | 4.3 | 13.0 | Moderate |
| G1 | 195 | 3057 | 3.2 | 28.4 | Moderate |
| G2 | 1545 | 3197 | 0.1 | 26.5 | Bad |
| G3 | 2 | 652 | 5.0 | 31.3 | Good |
| G4 | 14 | 518 | 3.8 | 29.4 | Moderate |
| G5 | 50 | 4431 | 3.8 | 25.8 | Moderate |
| G6 | 3 | 1386 | 4.5 | 31.5 | Moderate |
| G7 | 1510 | 6919 | 1.1 | 30.4 | Bad |
| Water Body | Phytoplankton | Macrophyte | Benthos | Fish Fauna | Nutrients | Chemical | DO |
|---|---|---|---|---|---|---|---|
| TEU 1 | Good | High | Good | Good | Moderate | Poor | n.c. |
| TEU 2 | High | High | Moderate | High | Moderate | Good | n.c. |
| TEU 3 | High | High | Good | High | Moderate | Poor | n.c. |
| TEU 4 | High | High | Moderate | Good | Moderate | Good | Good |
| TPO 1 | Good | Moderate | Good | Good | Moderate | Good | n.c. |
| TPO 2 | High | Good | Good | Good | Moderate | Good | Good |
| TPO 3 | Good | Moderate | High | Good | Moderate | Good | Moderate |
| TPO 4 | Good | Moderate | Good | High | Moderate | Poor | n.c. |
| TPO 5 | Good | Poor | Good | Good | Moderate | Poor | Good |
| TME 1 | Good | Poor | Good | Good | Good | Poor | n.c. |
| TME 2 | Moderate | Poor | Moderate | Moderate | Moderate | Good | n.c. |
| TME 3 | Good | Poor | Good | Moderate | Moderate | Good | Moderate |
| TME 4 | Good | Poor | Good | Moderate | Moderate | Poor | Good |
| FM 1 | Good | Poor | Moderate | n.c. | Good | Good | n.c. |
| FM 2 | High | High | Moderate | Moderate | Good | Poor | n.c. |
| FM 3 | High | Good | Good | Moderate | Good | Poor | Moderate |
| FM 4 | High | High | Poor | High | Moderate | Poor | n.c. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pittaluga, F.; Aleffi, I.F.; Bettoso, N.; Blasutto, O.; Celio, M.; Codarin, A.; Cumani, F.; Faresi, L.; Guiatti, D.; Orlandi, C.; et al. The SHAPE Project: An Innovative Approach to Understanding Seasonal and Diel Dissolved Oxygen Dynamics in the Marano and Grado Lagoon (Adriatic Sea) under the WFD/2000/60/CE. J. Mar. Sci. Eng. 2022, 10, 208. https://doi.org/10.3390/jmse10020208
Pittaluga F, Aleffi IF, Bettoso N, Blasutto O, Celio M, Codarin A, Cumani F, Faresi L, Guiatti D, Orlandi C, et al. The SHAPE Project: An Innovative Approach to Understanding Seasonal and Diel Dissolved Oxygen Dynamics in the Marano and Grado Lagoon (Adriatic Sea) under the WFD/2000/60/CE. Journal of Marine Science and Engineering. 2022; 10(2):208. https://doi.org/10.3390/jmse10020208
Chicago/Turabian StylePittaluga, Federico, Ida Floriana Aleffi, Nicola Bettoso, Oriana Blasutto, Massimo Celio, Antonio Codarin, Francesco Cumani, Lisa Faresi, Denis Guiatti, Claudia Orlandi, and et al. 2022. "The SHAPE Project: An Innovative Approach to Understanding Seasonal and Diel Dissolved Oxygen Dynamics in the Marano and Grado Lagoon (Adriatic Sea) under the WFD/2000/60/CE" Journal of Marine Science and Engineering 10, no. 2: 208. https://doi.org/10.3390/jmse10020208
APA StylePittaluga, F., Aleffi, I. F., Bettoso, N., Blasutto, O., Celio, M., Codarin, A., Cumani, F., Faresi, L., Guiatti, D., Orlandi, C., Zanello, A., & Acquavita, A. (2022). The SHAPE Project: An Innovative Approach to Understanding Seasonal and Diel Dissolved Oxygen Dynamics in the Marano and Grado Lagoon (Adriatic Sea) under the WFD/2000/60/CE. Journal of Marine Science and Engineering, 10(2), 208. https://doi.org/10.3390/jmse10020208







