Photoaging Characteristics of Disposable Masks under UV Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. UV Photoaging Procedure
2.3. Mechanical Strength Test
2.4. Morphological Analysis
2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
3. Results and Discussion
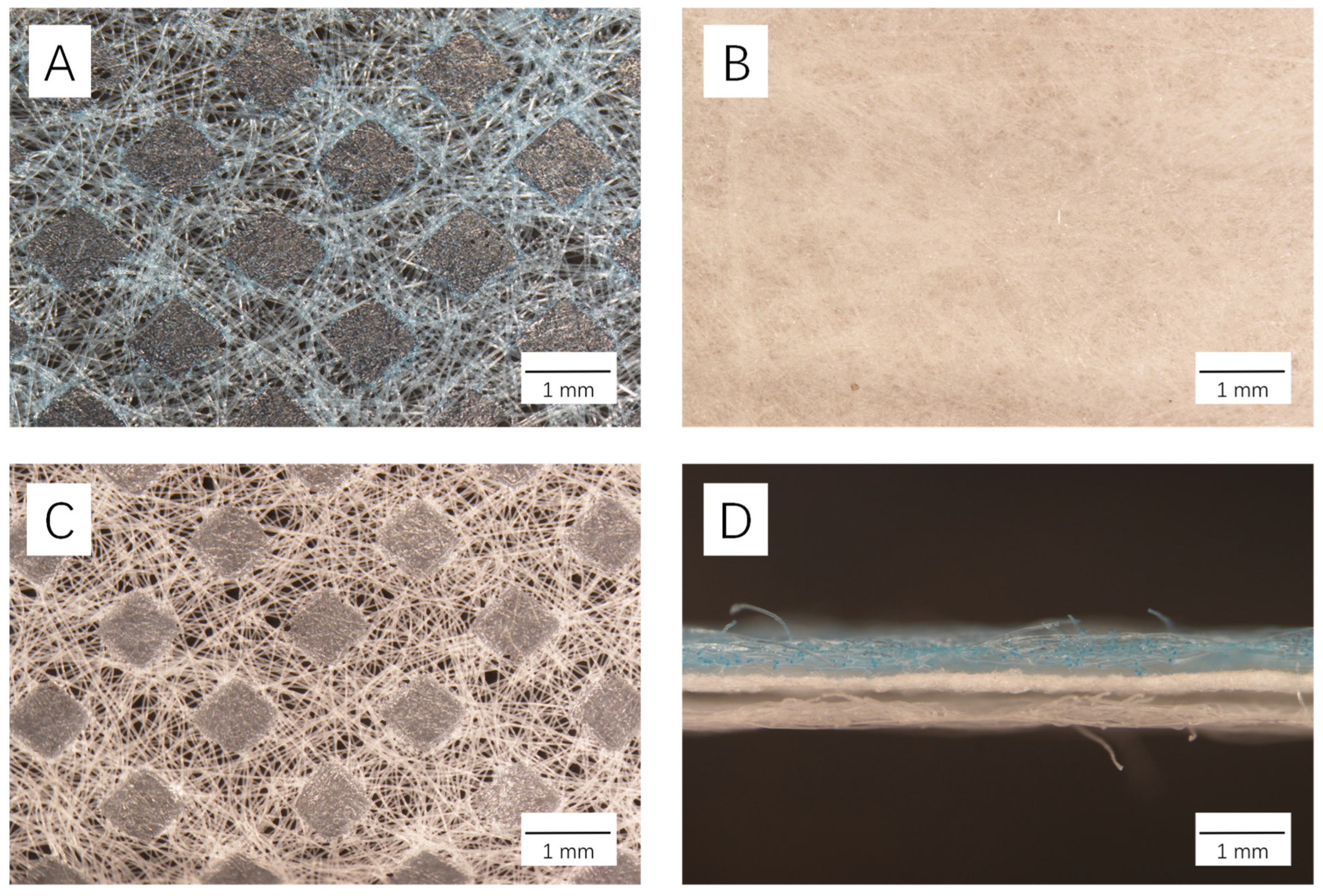
3.1. Morphological Features of Disposable Masks under Light Microscope
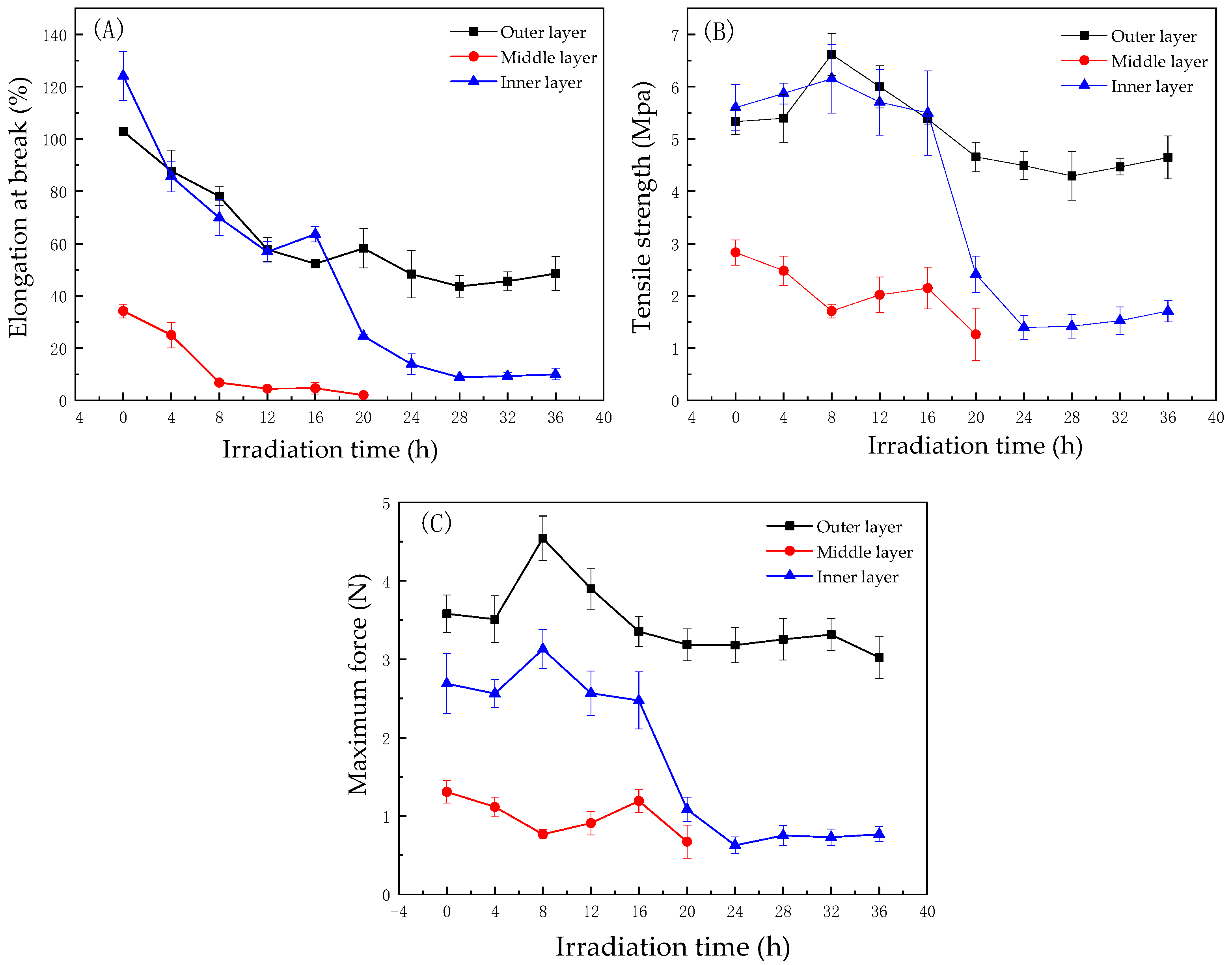
3.2. Changes in the Mechanical Strength of the Masks during UV Degradation
3.3. Surface Morphology of Disposable Masks during UV Irradiation
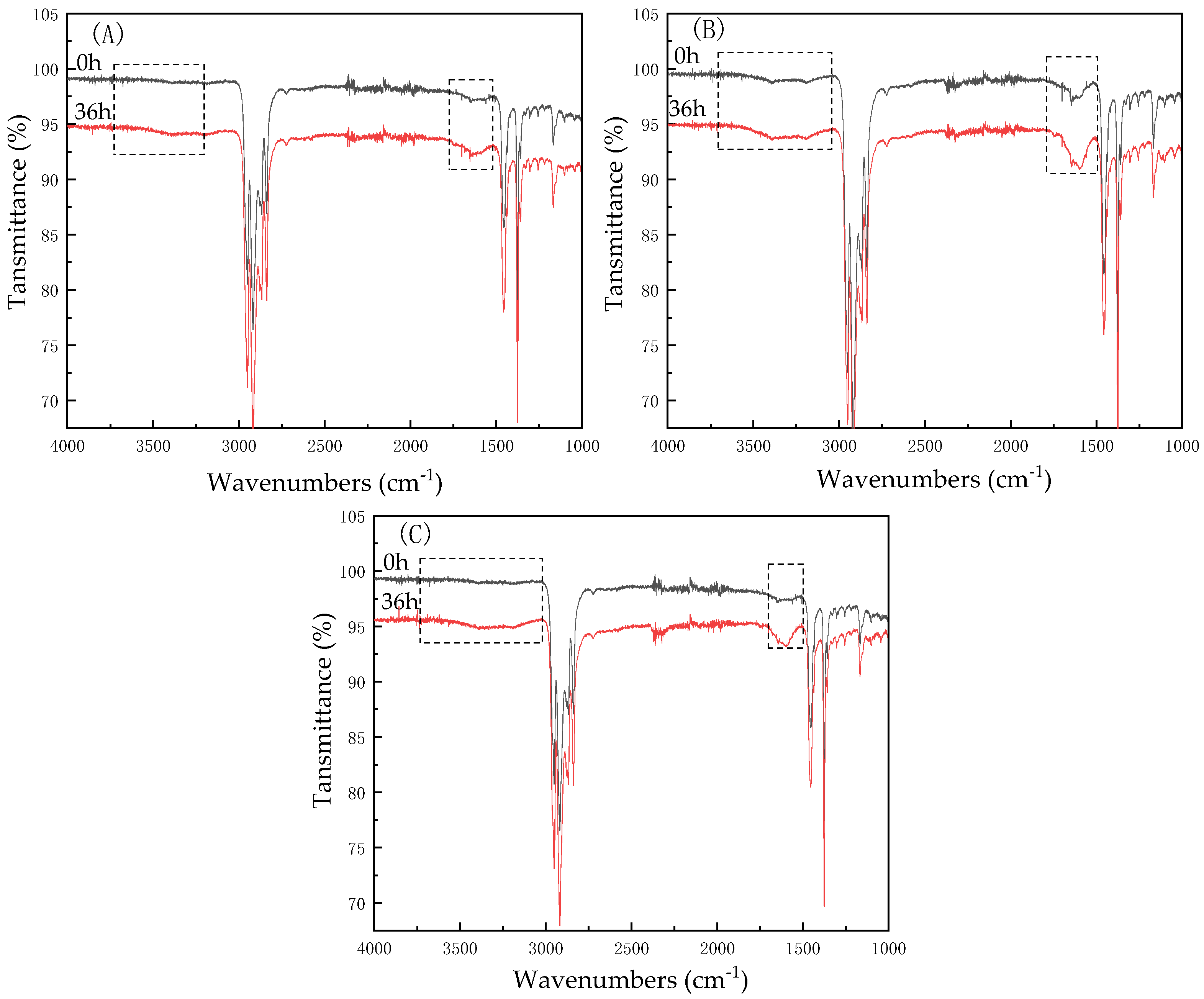
3.4. FT-IR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, M.; Cheng, W.; Goh, S.; Kong, J.; Li, B.; Lim, J.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face masks in the new COVID-19 normal: Materials, testing, and perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Wexler, A.; Cappa, C.; Barreda, S.; Bouvier, N.; Ristenpart, W. Aerosol emission and super emission during human speech increase with voice loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, B.; Wang, Y.; Ye, X.; Zhang, F.; Peng, Y. Impact of structural features on dynamic breathing resistance of healthcare face mask. Sci. Total Environ. 2019, 689, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ko, N.; Chang, Y.; Wu, C.; Lu, W.; Yen, C. Subjective deterioration of physical and psychological health during the COVID-19 pandemic in Taiwan: Their association with the adoption of protective behaviors and mental health problems. Int. J. Environ. Res. Public Health 2020, 17, 6827. [Google Scholar] [CrossRef]
- Vander Sande, M.; Teunis, P.; Sabel, R. Professional and home-made face masks reduce exposure to respiratory infections among the general population. PLoS ONE 2008, 3, e2618. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Burbine, S.; Kodihalli Shivaprakash, N.; Mead, J. 3D-printable PP/SEBS thermoplastic elastomeric blends: Preparation and properties. Polymers 2019, 11, 347. [Google Scholar] [CrossRef] [Green Version]
- Madsen, P.; Madsen, R. A study of disposable surgical masks. Am. J. Surg. 1967, 114, 431–435. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Li, M.; Qian, X.; Dai, S. Mask or no mask for COVID-19: A public health and market study. PLoS ONE 2020, 15, e0237691. [Google Scholar] [CrossRef]
- Makison Booth, C.; Clayton, M.; Crook, B.; Gawn, J. Effectiveness of surgical masks against influenza bioaerosols. J. Hosp. Infect. 2013, 84, 22–26. [Google Scholar] [CrossRef]
- Prata, J.; Silva, A.; Walker, T.; Duarte, A.; Rocha-Santos, T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Fadare, O.; Okoffo, E. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Xu, G.; Jason Ren, Z. Proventing masks from being the next plastic problem. Eviron. Sci. Eng. 2021, 15, 125. [Google Scholar]
- Lusher, A.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivar do Sul, J.; Costa, M.; Barletta, M.; Cysneiros, F. Pelagic microplastics around an archipelago of the Equatorial Atlantic. Mar. Pollut. Bull. 2013, 75, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A.; et al. International pellet watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef]
- Holmes, L.; Turner, A.; Thompson, R. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Rockman, C.; Hentschel, B.; Teh, S. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE 2014, 9, e85433. [Google Scholar] [CrossRef] [Green Version]
- Avio, C.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Jasmine, A.; Piriya, S.; Wenzel, M.N.; Gavin, H.; Christopher, W. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. Polymers 2020, 20, 369–381. [Google Scholar]
- Xie, H. Photoaging and Degradation of Polypropylene Nonwovens. Master’s Thesis, South China University of Technology, Guangzhou, China, 2012. (In Chinese). [Google Scholar]
- Wang, D.; Qian, X. Crystal transformation and aging resistance properties of polypropylene under UV irradiation. China Plast. 2009, 23, 51–54. (In Chinese) [Google Scholar]
- Arencon, D.; Ignacio Velasco, J. Fracture toughness of polypropylene-based particulate composites. Materials 2009, 2, 2046–2094. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Zhang, J. Study on UV degradation of polypropylene with different structures. Mod. Plast. Prog. Appl. 2014, 26, 8–12. (In Chinese) [Google Scholar]
- Obadal, M.; Cermak, R.; Raab, M.; Verney, V.; Commereuc, S.; Fraisse, F. Structure evolution of α- and β-polypropylenes upon UV irradiation: A multiscale comparison. Polym. Degrad. Stab. 2005, 88, 532–539. [Google Scholar] [CrossRef]
- Physical Sciences Laboratory. Available online: https://psl.noaa.gov/data/gridded/data.ncep.reanalysis.derived.html (accessed on 28 October 2021).
- Jiang, F.; Xie, L.; Sun, C.; Zhang, Y.; Li, J.; Ju, P. The characteristic change of plastic film from common used packing bags under UV photodegradation. Chin. Sci. Bull. 2021, 66, 1571–1579. (In Chinese) [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Q.; Chen, X.; Sun, Y.; She, Z. Studies on aging phenomenon of modified plastic under typical natural environment. Environ. Tech. 2020, 38, 45–51. (In Chinese) [Google Scholar]
- Zhou, C.; Fan, M.; Ding, Y.; Gu, C.; Wang, C. Solar photo-aging of common microplastics. Environ. Chem. 2021, 40, 1741–1748. (In Chinese) [Google Scholar]
- Ma, S.; Li, S.; Guo, X. Studying progress on aging properties, mechanisms and influence on pollutant adsorption of microplastics. China Environ. Sci. 2020, 40, 3992–4003. (In Chinese) [Google Scholar]
- Bertoldo, M.; Bronco, S.; Cappelli, C.; Gragnoli, T.; Andreotti, L. Combining theory and experiment to study the photooxidation of polyethylene and polypropylene. Phys. Chem. 2003, 107, 10880–10888. [Google Scholar] [CrossRef]
- Rjeb, A.; Letarte, S.; Tajounte, L.; Idrissi, M.; Adnot, A.; Roy, D.; Claire, Y.; Kaloustian, J. Polypropylene natural aging studied by X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2000, 107, 221–230. [Google Scholar] [CrossRef]
- Grossetete, T.; Gonon, L.; Verney, V. Submicrometric characterization of the heterogeneous photooxidation of polypropylene by microthermal analysis. Polym. Degrad. Stab. 2002, 78, 203–210. [Google Scholar] [CrossRef]
- Castejón, M.; Tiemblo, P.; Gómez-Elvira, J. Photo-oxidation of thick isotactic polypropylene films I. Characterization of the heterogeneous degradation kinetics. Polym. Degrad. Stab. 2000, 70, 357–364. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cao, W.; Xie, L.; Sun, C.; Jiang, F. Photoaging Characteristics of Disposable Masks under UV Irradiation. J. Mar. Sci. Eng. 2022, 10, 170. https://doi.org/10.3390/jmse10020170
Liu X, Cao W, Xie L, Sun C, Jiang F. Photoaging Characteristics of Disposable Masks under UV Irradiation. Journal of Marine Science and Engineering. 2022; 10(2):170. https://doi.org/10.3390/jmse10020170
Chicago/Turabian StyleLiu, Xinhao, Wei Cao, Linqing Xie, Chengjun Sun, and Fenghua Jiang. 2022. "Photoaging Characteristics of Disposable Masks under UV Irradiation" Journal of Marine Science and Engineering 10, no. 2: 170. https://doi.org/10.3390/jmse10020170
APA StyleLiu, X., Cao, W., Xie, L., Sun, C., & Jiang, F. (2022). Photoaging Characteristics of Disposable Masks under UV Irradiation. Journal of Marine Science and Engineering, 10(2), 170. https://doi.org/10.3390/jmse10020170






