Daily Rhythmicity of Hepatic Rhythm, Lipid Metabolism and Immune Gene Expression of Mackerel Tuna (Euthynnus affinis) under Different Weather
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Sample Collection
2.3. Total RNA Extraction and cDNA Synthesis
2.4. Real-Time qPCR Analysis
2.5. Statistical Analysis
3. Results
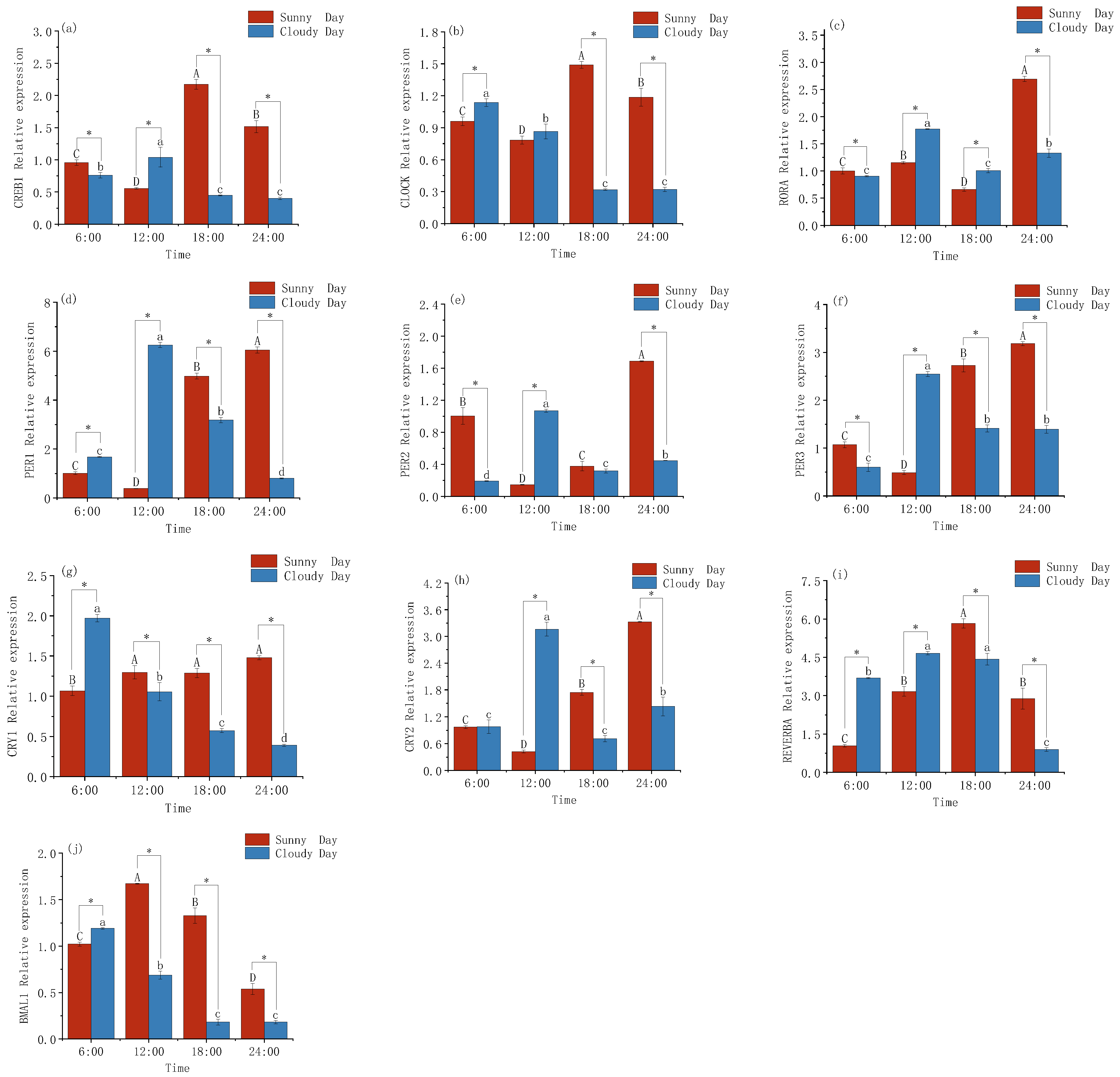
3.1. Changes in Gene Expression Levels in the Mackerel Tuna Rhythm
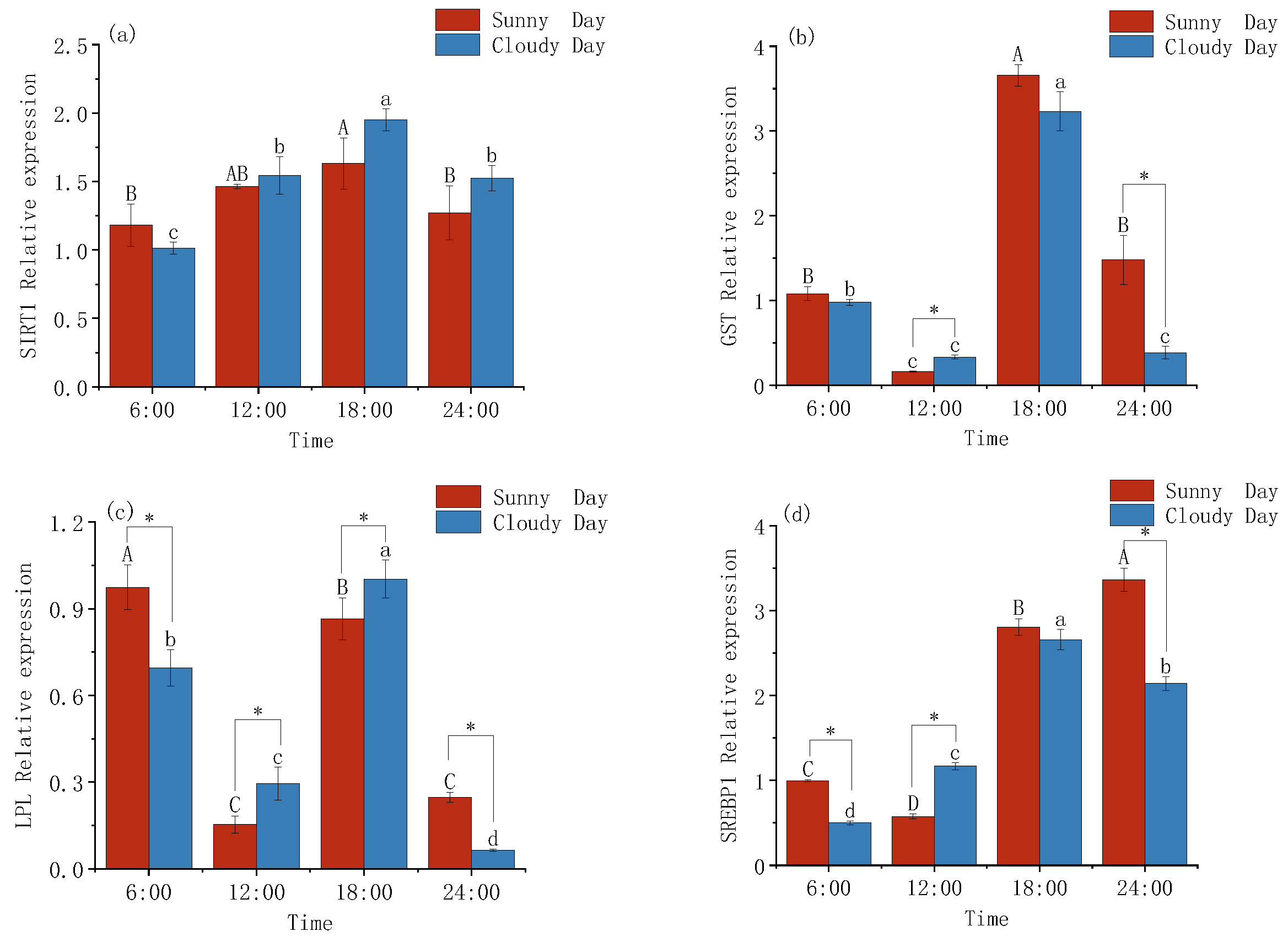
3.2. Changes in the Expression Levels of Lipid Metabolism in Mackerel Tuna
3.3. Changes in the Expression Levels of Immune Genes in Mackerel Tuna
4. Discussion
4.1. Rhythmic Gene Expression Patterns
4.2. Metabolic Gene Expression Patterns
4.3. Immune Gene Expression Patterns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
References
- Feng, N.Y.; Bass, A.H. “Singing” Fish Rely on Circadian Rhythm and Melatonin for the Timing of Nocturnal Courtship Vocalization. Curr. Biol. 2016, 19, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Hardin, P.E.; Panda, S. Circadian timekeeping and output mechanisms in animals. Curr. Opin. Neurobiol. 2013, 23, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Mahman-Averbuch, H.; King, C.D. Disentangling the roles of circadian rhythms and sleep drive in experimental pain sensitivity. Trends Neurosci. 2022, 11, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Patek, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Wakamatsu, M.; Motegi, H.; Tanaka, A.; Sutherland, K.; Ishikawa, M.; Ozaki, M.; Shirato, H.; Hamada, K.; Hamada, T. A real-time measurement system for gene expression rhythms from deep tissus of freely moving mice under light-dark conditions. Biochem. Biophys. Rep. 2022, 32, 101344. [Google Scholar]
- Bertolucci, C.; Fazio, F.; Piccione, G. Daily rhythms of serum lipids in dogs: Influences of lighting and fasting cycles. Comp. Med. 2008, 58, 485–489. [Google Scholar]
- Takahashi, J.S.; Hee-Kyung, H.; Ko, C.H.; McDearmon, E.L. The genetics mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 10, 764–775. [Google Scholar] [CrossRef]
- Sato, F.; Kohsaka, A.; Bhawal, U.K.; Muragaki, Y. Potential Roles of Dec and Bmal1 Genes in Interconnecting Circadian Clock and Energy Metabolism. Int. J. Mol. Sci. 2018, 3, 781. [Google Scholar] [CrossRef]
- Lee, C.; Etchegaray, J.-P.; Cagampang, F.R.; Loudon, A.S.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [CrossRef]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian rhythms from flies to human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Lai, N.L.; Zhang, W.; Hua, J.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Acute exposure to PBDEs at an environmentally realistic concentration causes abrupt changes in the gut microbiota and host health of zebrafish. J. Environ. 2018, 240, 17–26. [Google Scholar] [CrossRef]
- Shibata, S.; Tominaga, K. Brain neuronal mechanisms of circadian rhythms in mammalians. Yakuga Zasshi 1991, 111, 270–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Welsh, D.; Takahashi, J.; Kay, S. Suprachiasmatic nucleus: Cellautonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [PubMed]
- Le Martelot, G.; Claudel, T.; Gatfield, D.; Schaad, O.; Kornmann, B.; Sasso, G.L.; Moschetta, A.; Schibler, U. REV-ERB alpha participates in circadian SREBP signalling and bile acid homeostasis. PLoS Biol. 2009, 7, e1000181. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Patel, V.R.; Mohney, R.P.; Vignola, K.S.; Baldi, P.; Sassone-Corsi, P. Coordination of the transcriptome and metabolome by the circadian clock. Proc. Natl. Acad. Sci. USA 2012, 109, 5541–5546. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.E.A.; Franck, K.; de la Iglesia, H.O. Slepp timing and the circadian clock in mammals: Past, present and the road ahead. Semin. Cell Dev. Biol. 2022, 126, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.F., II; Cassie, L.; Behrendt, K.; Ruhn, A.; Lee, S.; Raj, P.; Takahashi, J.S.; Hooper, L.V. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell 2021, 16, 4154–4167. [Google Scholar] [CrossRef]
- Yazawa, R.; Takeuchi, Y.; Satoh, K.; Machida, Y.; Amezawa, K.; Kabeya, N.; Shimada, Y.; Yoshizaki, G. Eastern little tuna, Euthynnus affinis (Cantor, 1849) mature and reproduce within 1 year of rearing in land-based tanks. Aquac. Res. 2016, 47, 3800–3810. [Google Scholar] [CrossRef]
- IOTC Secretariat. Assessment of Indian Ocean kawakawa (Euthynnus affinis) Using Data Poor Catch-Based Methods(2015). Available online: http://www.seafdec.org/documents/2020/02/pw-neritic-tuna_10-4.pdf (accessed on 7 November 2022).
- Shengjie, Z.; Rui, Y.; Gang, Y.; Qiaer, W.; Zhenhua, M. Growth of Longtail Tuna Thunnus tonggol and Small Tuna Euthymmus affinis in Circulating Water Ponds. Fish. Sci. 2021, 40, 339–346. [Google Scholar]
- Ceinos, R.M.; Chivite, M.; López-Patiño, M.A.; Naderi, F.; Soengas, J.L.; Foulkes, N.S.; Míguez, J.M. Differential circadian and light-driven rhythmicity of clock gene expression and behaviour in the turbot, Scophthalmus maximus. PLoS ONE 2019, 14, e0219153. [Google Scholar] [CrossRef]
- Reeds, S.G. Sensory Systems, Perception, and Learning. Circadian Rhythms in Fish. In Encyclopedia of Fish Physiology; Academic Press: Cambridge, MA, USA, 2011; pp. 736–743. [Google Scholar]
- Xiao, L.; Yong, D.; Yan-ling, Z. Research progress of CREB in angiogenesis, anti-apoptosis and lung cancer. Tianjin Med. 2019, 47, 431–435. [Google Scholar]
- Husse, J.; Eichele, G.; Oster, H. Synchronization of the mammalian circadian timing system: Light can control peripheral clocks independently of the SCN clock: Alternate routes of entrainment optimize the alignment of the body’s circadian clock network with external time. Bioessays 2015, 37, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Betancor, M.B.; Spragu, M.; Minghetti, M.; Migaud, H.; Tocher, D.R.; Davie, A. Daily Rhythms in Expression og Genes of Hepatic Lipid Metabolism in Atlantic Salmon (Salmo salas L.). PLoS ONE 2014, 9, e106739. [Google Scholar] [CrossRef] [PubMed]
- Betancor, M.B.; Sprague, M.; Ortega, A.; de la Gándara, F.; Tocher, D.R.; Ruth, R.; Perkins, E.; Mourente, G. Central and peripheral clocks in Atlantic bluefin tuna (Thunnus thynnus, L.): Daily rhythmicity of hepatic lipid metabolism and digestive genes. Aquaculture 2020, 523, 735220. [Google Scholar] [CrossRef]
- Huang, T.S.; Ruoff, P.; Fjelldal, P.G. Effect of continuous light on daily levels of plasma melatonin and cortisol and expression of clock genes in pineal gland, brain, and liver in atlantic salmon postsmolts. Chronobiol. Int. 2010, 27, 1715–1734. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.-R.; El-Houseiny, W.; EL-Murr, A.E.; Ebraheim, L.L.M.; Ahmed, A.I.; El-Hakim, Y.M.A. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef]
- Bai, L.; Pang, W.-J.; Yang, Y.-J.; Yang, G.-S. Modulation of Sirt1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. Mol. Cell. Biochem. 2008, 307, 129–140. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Naderi, F.; Míguez, J.M.; Soengas, J.L.; López-Patiño, M.A. SIRT1 mediates the effect of stress on hypothalamic clock genes and food intake regulators in rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 235, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kaneko, G.; Takahashi, S.I.; Watabe, S.; Ushio, H. Identification and gene expression profile analysis of a major type of lipoprotein lipase in adult madaka Oryzias latipes. Fish. Sci. 2015, 81, 163–173. [Google Scholar] [CrossRef]
- Kentsis, A.; Borden, K.L. Construction Fish & Shellfish Immunology. of macromolecular assemblages in eukaryotic processes and their role in human disease: Linking EINGs together. Curr. Protein Sci. 2000, 1, 49–73. [Google Scholar]
- Rajsbaum, R.; Garcia-Sastre, A.; Versteeg, G.A. TRIM munity: The roles of the TRIM E3-ubiquitin ligase family in innate antiviral immunity. Mol. Biol. 2014, 426, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Borden, K.L. RING fingers and B-boxes: Zinc-binding protein-protein interaction domains. Biochem. Cell Biol. 1998, 76, 351–358. [Google Scholar] [CrossRef]
- Kong, Y.; Gao, C.; Du, X.; Zhao, J.; Li, M.; Shan, X.; Wang, G. Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on growth, immune response and disease resistance of snakehead fish (Channa argus). Fish Shellfish Immunol. 2020, 102, 412–421. [Google Scholar] [CrossRef]
- Xiao, X.; Zhu, W.T.; Zhang, Y.Q.; Liao, Z.; Wu, C.; Yang, C.; Zhang, Y.; Xiao, S.; Su, J. Broad-spectrum robust direct bactericidal activity of fish IFN ϕ1 reveals an antimicrobial peptide-like function for type I IFNs in vertebrates. J. Immunol. 2021, 206, 1337–1347. [Google Scholar] [CrossRef]
- Gale, M., Jr. Interference with virus infection. J. Immunol. 2015, 195, 1909–1910. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef]
- Langevin, C.; Levraud, J.; Boudinot, P. Fish antiviral tripartite motif (TRIM35) proteins. Fish Shellfish Immunol. 2019, 86, 724–733. [Google Scholar] [CrossRef]
- Zheng, J.; Zhi, L.; Wang, W.; Ni, N.; Huang, Y.; Qin, Q.; Huang, X. Fish TRIM21 exhibits antiviral activity against grouper iridovirus and nodavirus infection. Fish Shellfish Immunol. 2022, 127, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, S. Genomic structure of DAA gene and polymorphism within MHC -DAA alleles in apanese flounder (Paralichthys olivaceus). Hereditas 2009, 31, 1020–1028. [Google Scholar] [PubMed]
- Zhang, Y.; Chen, S. Full length cDNA cloning and tissus expression of major histocompatibility complex (MHC)IIa from turbot Scophthalmus maximus. Oceanol. Limnol. Sin. 2007, 38, 221–226. [Google Scholar]
- Glamann, J. Complete coding sequence of rainbow trout Mhc II beta chain. Scand. Immunol. 1995, 41, 365–372. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Huang, J.; Li, X.; Guan, L.; Wang, Q.; Yang, M.; Qin, Q. Functional analysis of a novel MHC-Iα genotype in orange-spotted grouper: Effects on Singapore grouper iridovirus (SGIV) replication and apoptosis. Fish Shellfish Immunol. 2022, 121, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. Cell. Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Y.; Liu, Z.; Tao, Q.; Huang, J.; Yang, W. Hepatic pathology of biliary atresia: A new comprehensive evaluation method using liver biopsy. Turk. Gastroenterol. 2016, 27, 257–263. [Google Scholar] [CrossRef]
- Yang, H.; Magilnick, N.; Lee, C.; Kalmaz, D.; Ou, X.; Chan, J.Y.; Lu, S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol. Cell. Biol. 2005, 25, 5933–5946. [Google Scholar] [CrossRef]

| 6:00 | 12:00 | 18:00 | 24:00 | ||
|---|---|---|---|---|---|
| Sunny day | Water temperature (°C) | 31.8 ± 0.13 | 33 ± 0.21 | 33.6 ± 0.17 | 32.6 ± 0.22 |
| Light intensity (Lx) | 2.7 ± 0.02 | 1116 ± 0.12 | 913 ± 0.04 | 1 ± 0.01 | |
| DO (mg·L) | 7.76 ± 0.24 | 7.59 ± 0.19 | 7.6 ± 0.2 | 7.61 ± 0.25 | |
| Cloudy day | Water temperature (°C) | 31.6 ± 0.11 | 33 ± 0.16 | 32.9 ± 0.2 | 32.6 ± 0.16 |
| Light intensity (Lx) | 1.9 ± 0.01 | 698 ± 0.03 | 192 ± 0.05 | 1.4 ± 0.01 | |
| DO (mg·L) | 7.62 ± 0.17 | 7.54 ± 0.11 | 7.55 ± 0.13 | 7.58 ± 0.09 |
| Gene | Full Names of Target Genes | Primer Sequence (5′-3′) | Amplicon Size/bp |
|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | F: ACACTCACTCCTCCATCTTTG | 100 |
| R: TTGCTGTAGCCGAACTCAT | |||
| TRIM35 | Tripartite motif-containing 35 | F: GTCTGAAGAGCTGGTGGA | 85 |
| R: TTACGAGGTGGTTTGTCC | |||
| NF-kB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | F: CCCAAAGACTCCAGCATCA | 117 |
| R: GCAGTTGTATCCCATCCTCAA | |||
| MHC-I | Major histocompatibility complex 1 | F: GCCCTCCTGCTCCTTCTT | 83 |
| R: GGTTTGCCTCCTCCAATT | |||
| ALT | Alanine aminotransferase | F: CAGGCTTACGGAGCAAAT | 100 |
| R: TCGTGGTGGGATGAAGAT | |||
| IFNA3 | Interferon a3 | F: GGTCTGCGTCCCTGTATT | 102 |
| R: AGCACTGTGACCCATTCG | |||
| ISY1 | Splicing factor ISY1 homolog | F: GTTCGGATCAAAGAGTTGGG | 90 |
| R: CAGACTGGCTGGTGTATAATGG | |||
| ARHGEF13 | A-kinase anchor protein 13 | F: CCGTCAACTTCTACAAGGA | 85 |
| R: CGCACAATGCTGCTACTC | |||
| SIRT1 | Sirtuin 1 | F: AGAAGAGGCTGCGGAAGT | 99 |
| R: AGGCGTTTGCTGATTGGA | |||
| GCLM | Glutamate cysteine ligase modifier subunit | F: CTGAGCGACTGGTCTTCC | 93 |
| R: CGTGATAGCGTCTGTTGG | |||
| GCLC | Glutamate-cysteine ligase catalytic subunit | F: CTGTTGAGAAGGGAGTGTC | 87 |
| R: TGTTTCTGGTAAGGGTGC | |||
| GST | Glutathione s-transferase | F: CGCCAAGAAGAACAACAT | 117 |
| R: TCTCGAAGAGCAGGGACT | |||
| LPL | Lipoprotein lipase | F: AGGATGCGACATACAGAACA | 112 |
| R: GAAGAGGTGGATGGAACG | |||
| SREBP1 | Sterol regulatory element binding transcription factor 1 | F: GACTGACTTGACCGTGTTC | 112 |
| R: CTCCTCCTCTTGTTCATCCT | |||
| CREB1 | cAMP responsive element binding protein 1 | F: TGCCCACTCCCATCTATC | 93 |
| R: CTCCATCTGTGCCGTTATT | |||
| CLOCK | Clock circadian regulator | F: TGTGGACGACCTGGAGAC | 84 |
| R: AGGAAACGGTAGTAGCAAG | |||
| PER1 | Period 1 | F: CCAAAGGCGGTTCAGTTA | 144 |
| R: GAGGCTTCTTGTCTCCCAC | |||
| PER2 | Period 2 | F: TCTAATGGAGTCGTCAGGGAG | 119 |
| R: AGCCGCTGGTTGAAGGAT | |||
| PER3 | Period 3 | F: TCATCGGACGGCATAAAG | 85 |
| R: TGGGTGACTGGGAAATACTC | |||
| RORA | Homo sapiens RAR-related orphan receptor A | F: CTGGATAGGGTGGGTGGAA | 84 |
| R: CGTTGGCCCGGATTAGAG | |||
| REV-ERBA | Nuclear receptor subfamily 1 group D member 1 | F: CCTACAACCATCCCACAG | 88 |
| R: ACCTTACATAGAAGCACCATA | |||
| CRY1 | Cryptochrome 1 | F: GTGGGCAGCCTCCTCTTA | 145 |
| R: CCGTACTTGTCTCCGTGGTC | |||
| CRY2 | Cryptochrome 2 | F: CTACATGAAGCTCCGTAAGC | 108 |
| R: CGGTCAAAGTTTGGGTTG | |||
| BMAL1 | Brain and muscle Arnt-like 1 | F: CGTCCAGTGGTAATGTCA | 176 |
| R: CATGAGTGCTTCTCCTCC |
| Gene | Weather | Acro (p-Value) | Acrophase |
|---|---|---|---|
| CREB1 | Sunny day | n.s. | |
| Cloudy day | <0.01 | 12 ± 1.16 | |
| CLOCK | Sunny day | n.s. | |
| Cloudy day | <0.005 | 6 ± 0.92 | |
| PER1 | Sunny day | n.s. | |
| Cloudy day | <0.001 | 12 ± 0.79 | |
| PER2 | Sunny day | <0.001 | 0 ± 0.61 |
| Cloudy day | n.s. | ||
| PER3 | Sunny day | n.s. | |
| Cloudy day | <0.05 | 0 ± 1.89 | |
| RORA | Sunny day | n.s. | |
| Cloudy day | n.s. | ||
| REVERBA | Sunny day | <0.001 | 18 ± 0.43 |
| Cloudy day | <0.001 | 12 ± 0.85 | |
| CRY1 | Sunny day | n.s. | |
| Cloudy day | n.s. | ||
| CRY2 | Sunny day | <0.001 | 0 ± 0.83 |
| Cloudy day | n.s. | ||
| BMAL1 | Sunny day | <0.001 | 12 ± 0.57 |
| Cloudy day | <0.005 | 6 ± 0.81 |
| p-Value | |||
|---|---|---|---|
| Genes | Time | Weather | Interactions |
| CREB1 | <0.001 | <0.001 | <0.001 |
| CLOCK | <0.001 | <0.001 | <0.001 |
| RORA | <0.001 | <0.001 | <0.001 |
| PER1 | <0.001 | <0.001 | <0.001 |
| PER2 | <0.001 | <0.001 | <0.001 |
| PER3 | <0.001 | <0.001 | <0.001 |
| CRY1 | <0.001 | <0.001 | <0.001 |
| CRY2 | <0.001 | 0.339 | <0.001 |
| REVERBA | <0.001 | <0.001 | <0.001 |
| BMAL1 | <0.001 | 0.026 | <0.001 |
| Gene | Weather | Acro (p-Value) | Acrophase |
|---|---|---|---|
| SIRT1 | Sunny day | n.s. | |
| Cloudy day | <0.001 | 18 ± 0.59 | |
| GST | Sunny day | n.s. | |
| Cloudy day | n.s. | ||
| LPL | Sunny day | n.s. | |
| Cloudy day | n.s. | ||
| SREBP1 | Sunny day | <0.05 | 0 ± 1.29 |
| Cloudy day | <0.001 | 18 ± 0.77 |
| p-Value | |||
|---|---|---|---|
| Genes | Time | Weather | Interactions |
| SIRT1 | <0.001 | 0.035 | 0.022 |
| GST | <0.001 | <0.001 | <0.001 |
| LPL | <0.001 | 0.007 | <0.001 |
| SREBP1 | <0.001 | <0.001 | <0.001 |
| Gene | Weather | Acro (p-value) | Acrophase |
|---|---|---|---|
| TRIM35 | Sunny day | n.s. | |
| Cloudy day | n.s. | ||
| NF-KB1 | Sunny day | <0.001 | 18 ± 0.5 |
| Cloudy day | <0.01 | 12 ± 1.13 | |
| MHC-I | Sunny day | <0.005 | 18 ± 1.03 |
| Cloudy day | n.s. | ||
| ALT | Sunny day | <0.005 | 18 ± 1.07 |
| Cloudy day | <0.005 | 12 ± 1.03 | |
| IFNA3 | Sunny day | <0.001 | 18 ± 0.39 |
| Cloudy day | n.s. | ||
| ISY1 | Sunny day | <0.001 | 18 ± 0.79 |
| Cloudy day | <0.001 | 18 ± 0.64 | |
| ARHGEF13 | Sunny day | <0.05 | 18 ± 1.43 |
| Cloudy day | <0.005 | 12 ± 1.03 | |
| GCLM | Sunny day | <0.05 | 12 ± 1.26 |
| Cloudy day | <0.001 | 12 ± 0.75 | |
| GCLC | Sunny day | n.s. | |
| Cloudy day | <0.05 | 0 ± 1.36 |
| p-Value | |||
|---|---|---|---|
| Genes | Time | Weather | Interactions |
| TRIM35 | <0.001 | <0.001 | <0.001 |
| NF-kB1 | <0.001 | <0.001 | <0.001 |
| MHC-I | <0.001 | <0.001 | <0.001 |
| ALT | <0.001 | <0.001 | <0.001 |
| IFNA3 | <0.001 | 0.022 | <0.001 |
| ISY1 | <0.001 | <0.001 | <0.001 |
| ARHGEF13 | <0.001 | <0.001 | <0.001 |
| GCLM | <0.001 | <0.001 | <0.001 |
| GCLC | <0.001 | 0.205 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hu, J.; Fu, Z.; Yu, G.; Ma, Z. Daily Rhythmicity of Hepatic Rhythm, Lipid Metabolism and Immune Gene Expression of Mackerel Tuna (Euthynnus affinis) under Different Weather. J. Mar. Sci. Eng. 2022, 10, 2028. https://doi.org/10.3390/jmse10122028
Wang W, Hu J, Fu Z, Yu G, Ma Z. Daily Rhythmicity of Hepatic Rhythm, Lipid Metabolism and Immune Gene Expression of Mackerel Tuna (Euthynnus affinis) under Different Weather. Journal of Marine Science and Engineering. 2022; 10(12):2028. https://doi.org/10.3390/jmse10122028
Chicago/Turabian StyleWang, Wenwen, Jing Hu, Zhengyi Fu, Gang Yu, and Zhenhua Ma. 2022. "Daily Rhythmicity of Hepatic Rhythm, Lipid Metabolism and Immune Gene Expression of Mackerel Tuna (Euthynnus affinis) under Different Weather" Journal of Marine Science and Engineering 10, no. 12: 2028. https://doi.org/10.3390/jmse10122028
APA StyleWang, W., Hu, J., Fu, Z., Yu, G., & Ma, Z. (2022). Daily Rhythmicity of Hepatic Rhythm, Lipid Metabolism and Immune Gene Expression of Mackerel Tuna (Euthynnus affinis) under Different Weather. Journal of Marine Science and Engineering, 10(12), 2028. https://doi.org/10.3390/jmse10122028







