Concentrations and Characteristics of Polybrominated Diphenyl Ethers (PBDEs) in Marine Zooplankton from the Gaoping Waters of Southwestern Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Measurements of Chlorophyll A and Nitrate
2.3. Zooplankton Identification
2.4. Chemical Analysis of PBDEs
2.5. Statistical Analysis
3. Results and Discussion
3.1. Changes in Hydrographic and Biological Variables
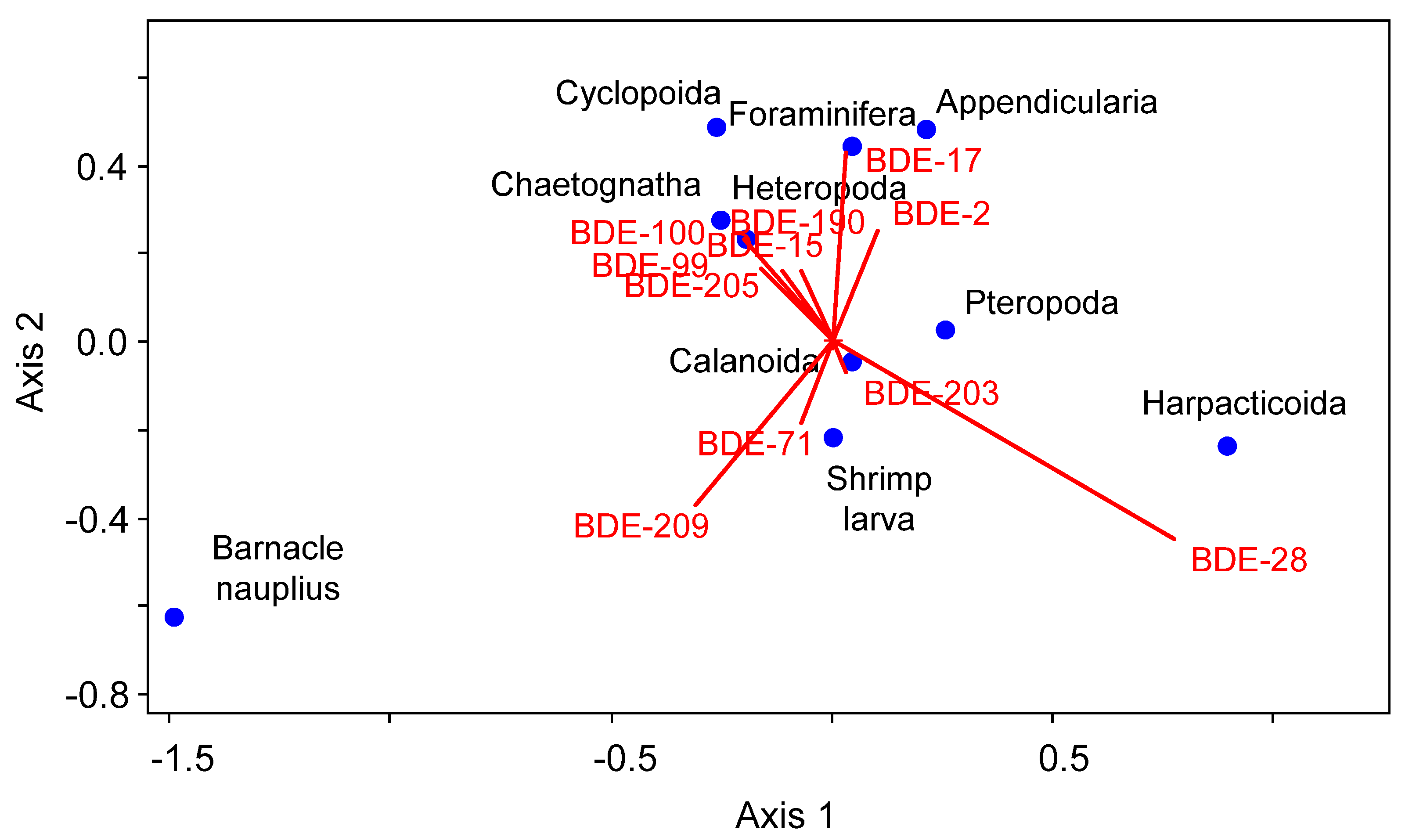
3.2. The Zooplankton Communities
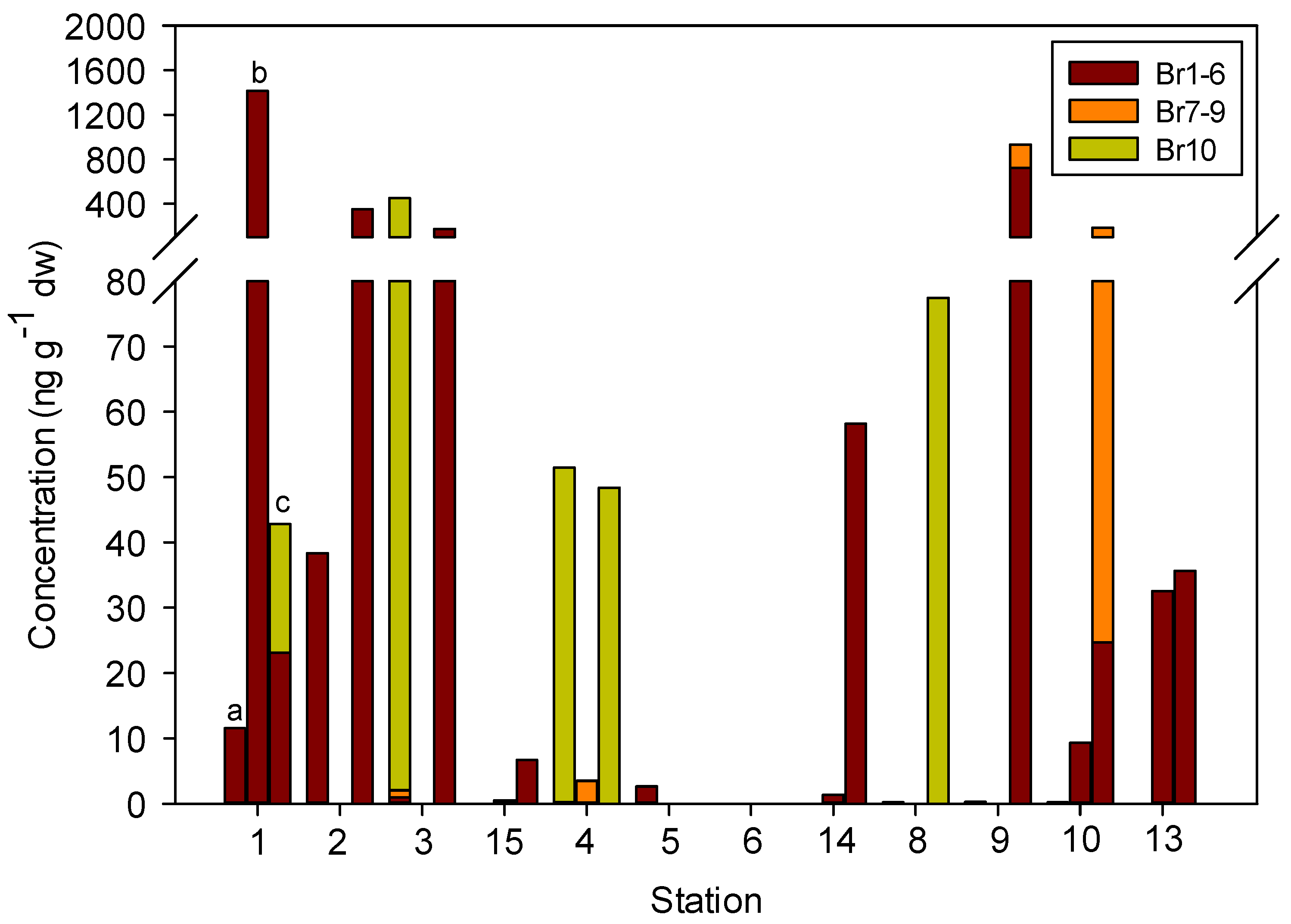
3.3. Concentrations, Compositional Profiles, and Possible Resources of PBDEs in Zooplankton
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dien, N.T.; Hirai, Y.; Miyazaki, T.; Sakai, S. Factors influencing atmospheric concentrations of polybrominated diphenyl ethers in Japan. Chemosphere 2016, 144, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O.; Eriksen, G.S.; Johannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001, 109, 49–68. [Google Scholar] [PubMed]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Kubwabo, C.; Harris, S.A. A review of the role of emerging environmental contaminants in the development of breast cancer in women. Emerg. Contam. 2016, 2, 204–219. [Google Scholar] [CrossRef]
- Meironyté, D.; Norén, K.; Bergman, A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J. Toxic. Environ. Health A 1999, 58, 329–341. [Google Scholar]
- Directive 2003/11/EC Directive 2003/11/EC of the European Parliament and of the Council of 6 February 2003 amending for the 24th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether). Official Journal of the European Union, 15 February 2003; L42/45.
- Betts, K. New flame retardants detected in indoor and outdoor environments. Environ. Sci. Technol. 2008, 42, 6778. [Google Scholar] [CrossRef][Green Version]
- Law, R.J.; Allchin, C.R.; de Boer, J.; Covaci, A.; Herzke, D.; Lepom, P.; Morris, S.; Troczynski, J.; de Wit, C.A. Levels and trends of brominated flame retardants in the European environment. Chemosphere 2006, 64, 187–208. [Google Scholar] [CrossRef]
- Frouin, H.; Dangerfield, N.; Macdonald, R.W.; Galbraith, M.; Crewe, N.; Shaw, P.; Mackas, D.; Ross, P.S. Partitioning and bioaccumulation of PCBs and PBDEs in marine plankton from the Strait of Georgia, British Columbia, Canada. Prog. Oceanogr. 2013, 115, 65–75. [Google Scholar] [CrossRef]
- Cheng, J.O.; Ko, F.C. Occurrence of PBDEs in surface sediments of metropolitan rivers: Sources, distribution pattern, and risk assessment. Sci. Total Environ. 2018, 637–638, 1578–1585. [Google Scholar] [CrossRef]
- Yeo, B.G.; Takadaa, H.; Yamashitaa, R.; Okazakia, Y.; Uchidab, K.; Tokaib, T.; Tanakaa, K.; Trenholm, N. PCBs and PBDEs in microplastic particles and zooplankton in open water in the Pacific Ocean and around the coast of Japan. Mar. Pollut. Bull. 2020, 151, 110806. [Google Scholar] [CrossRef]
- Peltonen, H.; Ruokojärvi, P.; Korhonen, M.; Kiviranta, H.; Flinkman, J.; Verta, M. PCDD/Fs, PCBs and PBDEs in zooplankton in the Baltic Sea—Spatial and temporal shifts in the congener-specific concentrations. Chemosphere 2014, 114, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Ikonomou, M.; Blair, J.; Gobas, F. Bioaccumulation behaviour of polybrominated diphenyl ethers (PBDEs) in a Canadian Arctic marine food web. Sci. Total Environ. 2008, 401, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Tao, P.; Wang, M.; Jia, H.; Li, Y.-F. Trophic magnification of polybrominated diphenyl ethers in the marine food web from coastal area of Bohai Bay, North China. Environ. Pollut. 2016, 213, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Beaugrand, G.; Brander, C.M.; Lindley, A.J.; Souissi, S.; Reid, P.C. Plankton effect on cod recruitment in the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef]
- Takahashi, K.; Ide, K. Reproduction, grazing, and development of the large subarctic calanoid Eucalanus bungii: Is the spring diatom bloom the key to controlling their recruitment? Hydrobiologia 2011, 666, 99–109. [Google Scholar] [CrossRef]
- Swackhamer, D.L.; Skoglund, R.S. Bioaccumulation of PCBs by algae: Kinetics versus equilibrium. Environ. Toxicol. Chem. 1993, 12, 831–838. [Google Scholar] [CrossRef]
- Chiuchiolo, A.L.; Dickhut, R.M.; Cochran, M.A.; Ducklow, H.W. Persistent organic pollutants at the base of the Antarctic marine food web. Environ. Sci. Technol. 2004, 38, 3551–3557. [Google Scholar] [CrossRef]
- Burreau, S.; Zebühr, Y.; Broman, D.; Ishaq, R. Biomagnification of PBDEs and PCBs in food webs from the Baltic Sea and the northern Atlantic Ocean. Sci. Total Environ. 2006, 366, 659–672. [Google Scholar] [CrossRef]
- Wan, Y.; Hu, J.; Zhang, K.; An, L. Trophodynamics of polybrominated diphenyl ethers in the marine food web of Bohai Bay, north China. Environ. Sci. Technol. 2008, 42, 1078–1083. [Google Scholar] [CrossRef]
- Burreau, S.; Zebühr, Y.; Broman, D.; Ishaq, R. Biomagnification of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) studied in pike (Esox lucius), perch (Perca fluviatilis) and roach (Rutilus rutilus) from the Baltic Sea. Chemosphere 2004, 55, 1043–1052. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, X.; Ni, X.; Ben, Y.; Guo, R.; An, L. Bioaccumulation characteristics of polybrominated diphenyl ethers in the marine food web of Bohai Bay. Chemosphere 2016, 150, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Jhong, Y.J.; Ding, W.H. Determination of polybrominated diphenyl ethers in coastal and river sediments by pressurized liquid extraction coupled with gas chromatography-mass spectrometry. J. Chin. Chem. Soc. 2008, 55, 335–344. [Google Scholar] [CrossRef]
- Jiang, J.J.; Lee, C.L.; Fang, M.D.; Ko, F.C.; Baker, J.E. Polybrominated diphenyl ethers and polychlorinated biphenyls in sediments of southwest Taiwan: Regional characteristics and potential sources. Mar. Pollut. Bull. 2011, 62, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.H.; Huang, C.W.; Weng, Y.M.; Yak, H.K. Determination of polybrominated diphenyl ethers (PBDEs) in fish samples from rivers and estuaries in Taiwan. Chemoshpere 2007, 66, 1990–1997. [Google Scholar] [CrossRef]
- Ko, F.C.; We, N.Y.; Chou, L.S. Bioaccumulation of persistent organic pollutants in stranded cetaceans from Taiwan coastal waters. J. Hazard. Mater. 2014, 277, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Lee, K.T.; Shiah, G.Y. Environmental factors associated with the formation of larval anchovy fishing ground in coastal waters of southwestern Taiwan. Mar. Biol. 1995, 121, 621–625. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chen, P.Y.; Chen, C.P.; Lee, M.A.; Shiah, G.Y.; Lee, K.T. Fluctuation in abundance of larval anchovy and environmental conditions in coastal waters off southwestern Taiwan as associated with the El Niño-Southern Oscillation. Fish. Oceanogr. 1997, 6, 238–249. [Google Scholar] [CrossRef]
- Yang, L. Review of marine outfall systems in Taiwan. Water Sci. Technol. 1995, 32, 257–264. [Google Scholar]
- Gobas, F.A.P.C. A model for predicting the bioaccumulation of hydrophobic organic chemicals in aquatic food webs: Application to Lake Ontario. Ecol. Model. 1993, 69, 1–17. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ. Toxicol. Chem. 2004, 23, 2343–2355. [Google Scholar] [CrossRef]
- Lobban, C.S.; Chapman, D.J.; Kremer, B.P. (Eds.) Experimental Phycology: A Laboratory Manual; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Welschmeyer, N.A. Fluormetric analysis of chlorophyll a on the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Garside, C. A chemiluminescent technique for the determination of nanomolar concentrations of nitrate and nitrite in seawater. Mar. Chem. 1982, 11, 159–167. [Google Scholar] [CrossRef]
- Omori, M.; Ikeda, T. Methods in Marine Zooplankton Ecology; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Ko, F.C.; Baker, J.E. Partitioning of hydrophobic organic contaminants to resuspended sediment and plankton in the Chesapeake Bay. Mar. Chem. 1995, 49, 171–188. [Google Scholar] [CrossRef]
- Ko, F.C.; Baker, J.E. Loading of hydrophobic organic contaminants from Susquehanna River to Chesapeake Bay. Mar. Pollut. Bull. 2004, 48, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.O.; Ko, F.C.; Li, J.J.; Chen, T.H.; Cheng, Y.M.; Lee, C.L. Concentrations of polycyclic aromatic hydrocarbon in the surface sediments from inter-tidal areas of Kenting coast, Taiwan. Environ. Monit. Assess. 2012, 184, 3481–3490. [Google Scholar] [CrossRef]
- Dunn, O.J.; Clark, V.A. Applied Statistics: Analysis of Variance and Regression; John Wiley: New York, NY, USA, 1974. [Google Scholar]
- ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Meng, P.J.; Chang, Y.C.; Lo, W.T. Temporal and spatial occurrence of mesopelagic fish larvae during epipelagic drift associated with hydrographic features in the Gaoping coastal waters off southwestern Taiwan. Mar. Coast. Fish. 2017, 9, 244–259. [Google Scholar] [CrossRef]
- Liu, J.T.; Hsu, R.T.; Hung, J.J.; Chang, Y.P.; Wang, Y.H.; Rendle-Bühring, R.H.; Lee, C.L.; Huh, C.A.; Yang, R.J. From the highest to the deepest: The Gaoping River-Gaoping Submarine Canyon dispersal system. Earth-Sci. Rev. 2016, 153, 274–300. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lo, W.T.; Liao, C.C.; Meng, P.J. Shifts in the assemblage of summer mesopelagic fish larvae in the Gaoping waters of southwestern Taiwan: A comparison between El Niño events and regular years. J. Mar. Sci. Eng. 2021, 9, 1065. [Google Scholar] [CrossRef]
- García-Comas, C.; Stemmann, L.; Ibanez, F.; Berline, L.; Mazzocchi, M.G.; Gasparini, S.; Picheral, M.; Gorsky, G. Zooplankton long-term changes in the NW Mediterranean Sea: Decadal periodicity forced by winter hydrographic conditions related to large-scale atmospheric changes? J. Mar. Syst. 2011, 87, 216–226. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hirota, Y. Seasonal changes of primary productivity in Tosa Bay. J. Oceanogr. Soc. Japan 2004, 13, 259–269. [Google Scholar]
- Sassa, C.; Hirota, Y. Seasonal occurrence of mesopelagic fish larvae on the onshore side of the Kuroshio off southern Japan. Deep-Sea Res. Part I 2013, 81, 49–61. [Google Scholar] [CrossRef]
- Hunt, B.P.V.; Pakhomov, E.A.; Siegel, V.; Strass, V.; Cisewski, B.; Bathmann, U. The seasonal cycle of the Lazarev Sea macrozooplankton community and a potential shift to top-down trophic control in winter. Deep-Sea Res. II 2011, 58, 1662–1676. [Google Scholar] [CrossRef]
- Tommasi, D.; Hunt, B.P.V.; Pakhomov, E.A.; Mackas, D.L. Mesozooplankton community seasonal succession and its drivers: Insights from a British Columbia, Canada, fjord. J. Mar. Syst. 2013, 115, 10–32. [Google Scholar] [CrossRef]
- Webber, M.K.; Roff, J.C. Annual structure of the copepod community and its associated pelagic environment off Discovery Bay, Jamaica. Mar. Biol. 1995, 123, 467–479. [Google Scholar] [CrossRef]
- Cornils, A.; Schnack-Schiel, S.B.; Al-Najjar, T.; Badran, M.I.; Rasheed, M.; Manasreh, R.; Richter, C. The seasonal cycle of the epipelagic mesozooplankton in the northern Gulf of Aqaba (Red Sea). J. Mar. Syst. 2007, 68, 278–292. [Google Scholar] [CrossRef]
- Sherr, E.; Sherr, B. Understanding Roles of Microbes in Marine Pelagic Food Webs: A Brief History. In Microbial Ecology of the Oceans; Kirchman, D.L., Ed.; Wiley: New York, NY, USA, 2009; pp. 27–44. [Google Scholar]
- La Guardia, M.J.; Hale, R.C.; Harvey, E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ. Sci. Technol. 2006, 40, 6247–6254. [Google Scholar] [CrossRef]
- Sellström, U.; Jansson, B.; Kierkegaard, A.; de Wit, C.A.; Odsjö, T.; Olsson, M. Polybrominated diphenyl ethers (PBDE) in biological samples from the Swedish environment. Chemosphere 1993, 26, 1703–1718. [Google Scholar] [CrossRef]
- Lacorte, S.; Guillamón, M.; Martínez, E.; Viana, P.; Barceló, D. Occurrence and specific congener profile of 40 polybrominated diphenyl ethers in river and coastal sediments from Portugal. Environ. Sci. Technol. 2003, 37, 892–898. [Google Scholar] [CrossRef]
- Domínguez, A.A.; Law, R.J.; Herzke, D.; Boer, J. Bioaccumulation of brominated flame retardants. In Brominated Flame Retardants, the Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 141–185. [Google Scholar]
- Eljarrat, E.; Feo, M.L.; Barceló, D. Degradation of brominated flame retardants. In Brominated Flame Retardants, the Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2011; pp. 187–202. [Google Scholar]
- Hu, G.-C.; Dai, J.-Y.; Xu, Z.-C.; Luo, X.-J.; Cao, H.; Wang, J.-S.; Mai, B.-X.; Xu, M.-Q. Bioaccumulation behavior of polybrominated diphenyl ethers (PBDEs) in the freshwater food chain of Baiyangdian Lake, North China. Environ. Int. 2010, 36, 309–315. [Google Scholar] [CrossRef]
- Poma, G.; Volta, P.; Roscioli, C.; Bettinetti, R.; Guzzella, L. Concentrations and trophic interactions of novel brominated flame retardants, HBCD, and PBDEs in zooplankton and fish from Lake Maggiore (Northern Italy). Sci. Total Environ. 2014, 481, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mackas, D.L.; Batten, S.; Trudel, M. Effects on zooplankton of a warmer ocean: Recent evidence from the northeast Pacific. Prog. Oceanogr. 2007, 75, 223–252. [Google Scholar] [CrossRef]
- Francis, T.; Scheuerell, M.; Brodeur, R.; Levin, P.S.; Ruzicka, J.; Tolimieri, N.; Peterson, W.T. Climate shifts the interaction web of a marine plankton community. Glob. Chang. Biol. 2012, 18, 2498–2508. [Google Scholar] [CrossRef]
- Tanasichuk, R.W. Implications of interannual variability in euphausiid population biology for fish production along the south-west coast of Vancouver Island: A synthesis. Fish. Oceanogr. 2002, 11, 18–30. [Google Scholar] [CrossRef]
- Kiørboe, T.; Nielsen, T.G. Regulation of zooplankton biomass and production in a temperate coastal ecosystem. 1. Copepods. Limnol. Oceanogr. 1994, 39, 493–507. [Google Scholar]
- Longhurst, A. Seasonal cycles of pelagic production and consumption. Prog. Oceanogr 1995, 36, 77–167. [Google Scholar] [CrossRef]
- Hirche, H.J.; Meyer, U.; Niehoff, B. Egg production of Calanus finmarchicus: Effect of temperature, food and season. Mar. Biol. 1997, 127, 609–620. [Google Scholar] [CrossRef]
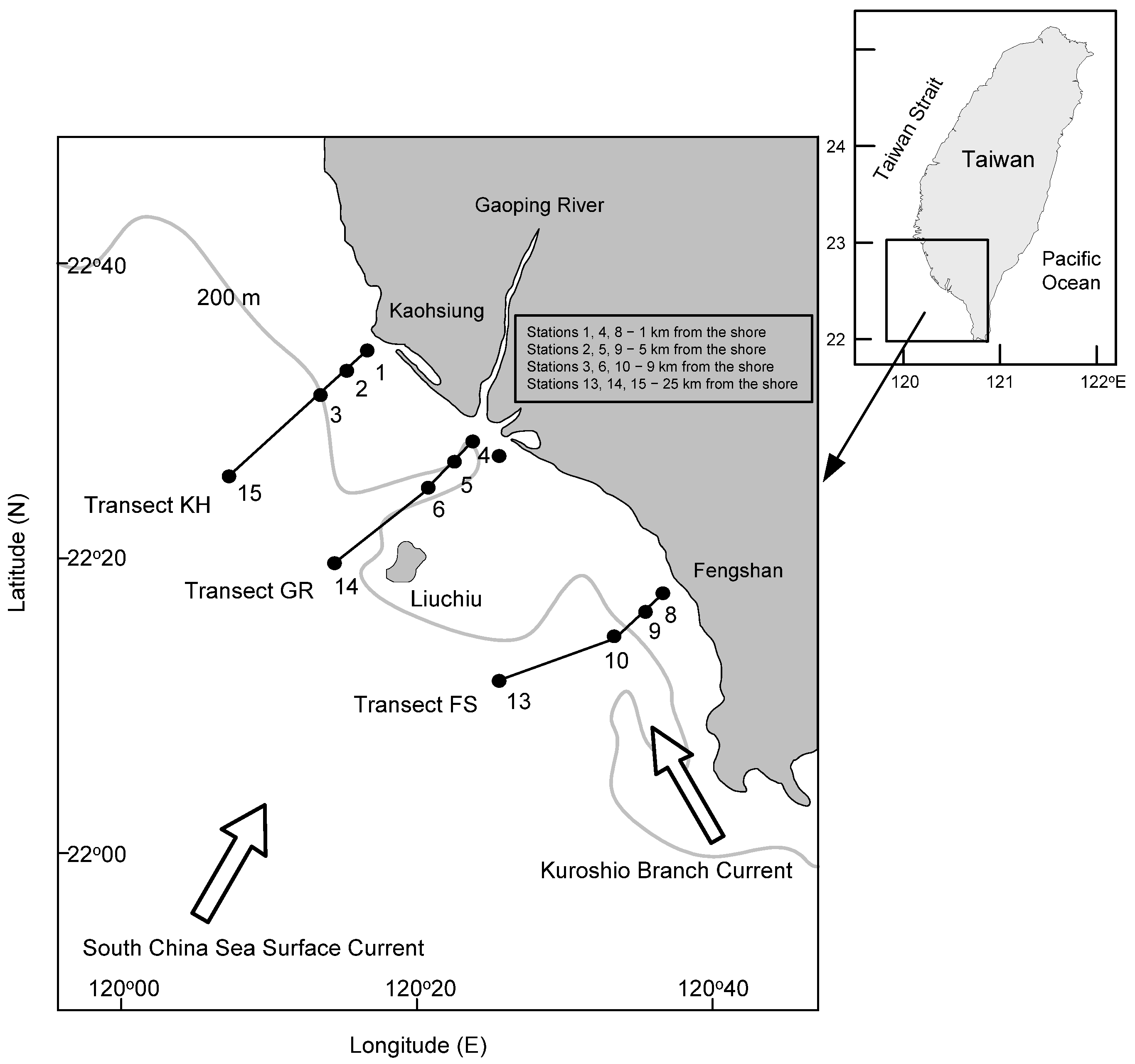
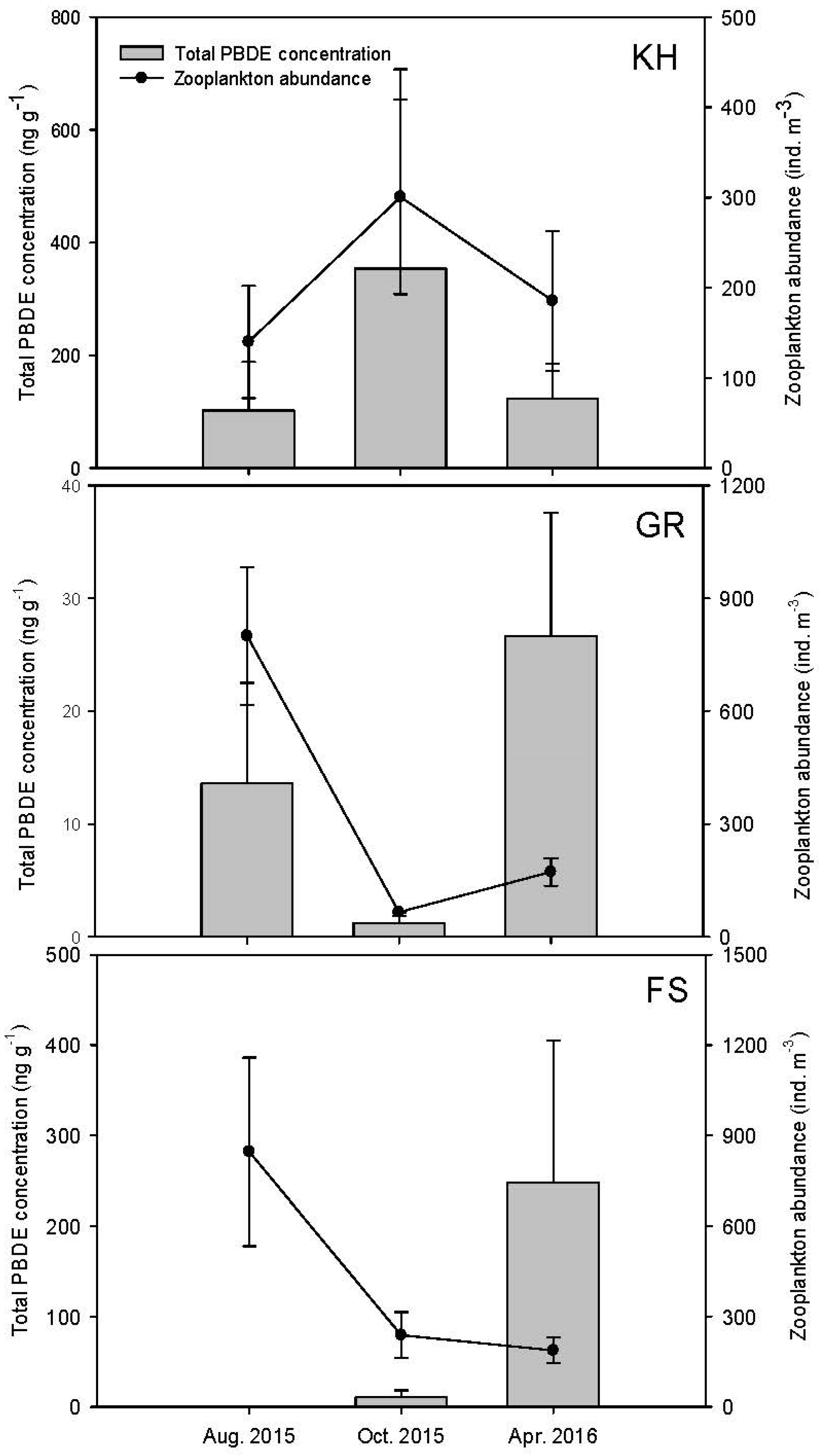
| Transect | Station | Depth (m) | August 2015 | October 2015 | April 2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Salinity | NO3− (mg L−1) | Chl. a (ug L−1) | Temperature (°C) | Salinity | NO3− (mg L−1) | Chl. a (ug L−1) | Temperature (°C) | Salinity | NO3− (mg L−1) | Chl. a (ug L−1) | |||
| KH | 1 | 12 | 27.73 | 33.18 | 0.010 | 1.92 | 29.39 | 32.00 | 0.027 | 1.03 | 26.18 | 34.49 | 0.006 | 3.26 |
| 2 | 58 | 28.29 | 33.55 | 0.004 | 1.86 | 29.07 | 33.42 | 0.006 | 0.54 | 26.36 | 34.54 | 0.009 | 0.60 | |
| 3 | 188 | 28.90 | 33.48 | 0.004 | 1.26 | 29.02 | 33.63 | 0.004 | 0.34 | 26.54 | 34.41 | 0.012 | 0.26 | |
| 15 | 582 | 29.13 | 33.59 | 0.031 | 0.80 | 28.94 | 34.07 | 0.008 | 0.44 | 28.05 | 34.53 | 0.032 | 0.22 | |
| GR | 4 | 33 | 28.06 | 33.71 | 0.009 | 0.66 | 27.99 | 25.63 | 0.019 | 0.03 | 25.10 | 33.64 | 0.061 | 5.50 |
| 5 | 294 | 28.08 | 33.42 | 0.005 | 0.46 | 28.16 | 33.20 | 0.011 | 0.52 | 25.16 | 34.38 | 0.014 | 3.24 | |
| 6 | 310 | 28.50 | 32.83 | 0.006 | 0.57 | 28.28 | 32.92 | 0.016 | 0.52 | 25.95 | 34.48 | 0.015 | 2.18 | |
| 14 | 545 | 28.60 | 34.02 | 0.021 | 0.32 | 28.95 | 33.83 | 0.028 | 0.10 | 27.67 | 34.45 | 0.024 | 0.24 | |
| FS | 8 | 29 | 29.02 | 33.40 | 0.004 | 0.31 | 29.16 | 33.87 | 0.005 | 0.54 | 26.01 | 34.49 | 0.008 | 0.43 |
| 9 | 138 | 29.30 | 34.05 | 0.004 | 0.26 | 29.02 | 33.90 | 0.004 | 0.26 | 25.79 | 34.43 | 0.011 | 2.52 | |
| 10 | 557 | 28.80 | 33.65 | 0.013 | 0.63 | 29.12 | 33.98 | 0.007 | 0.40 | 26.05 | 34.42 | 0.016 | 0.83 | |
| 13 | 423 | 29.10 | 33.74 | 0.034 | 0.22 | 29.41 | 34.59 | 0.016 | – | 27.17 | 34.49 | 0.031 | 0.22 | |
| Taxa | August 2015 | October 2015 | April 2016 | RA |
|---|---|---|---|---|
| Foraminifera | 9.13 | 5.47 | 1.32 | 1.63 |
| Radiolaria | 0.13 | 0.36 | – | 0.05 |
| Polyp | – | – | – | – |
| Medusa | – | – | 0.03 | 0.003 |
| Obelia | – | – | – | – |
| Siphonophore | 5.83 | 1.85 | 1.00 | 0.89 |
| Ctenophora | 0.19 | 0.03 | – | 0.02 |
| Polychaeta | 2.02 | 0.19 | 0.37 | 0.26 |
| Pteropoda | 13.58 | 2.82 | 2.36 | 1.92 |
| Heteropoda | 8.88 | 5.90 | 4.57 | 1.98 |
| Amphipoda | 0.36 | 0.47 | 0.32 | 0.12 |
| Crab zoea | 1.06 | 1.25 | 2.15 | 0.46 |
| Crab megalopa | 0.15 | 0.11 | 0.01 | 0.03 |
| Lucifera | 3.44 | 2.94 | 0.75 | 0.73 |
| Sergestidae | 0.05 | – | 0.24 | 0.03 |
| Other Decapoda | – | – | – | – |
| Cladocera | 0.75 | 0.72 | 0.56 | 0.21 |
| Ostracoda | 2.53 | 0.80 | 0.61 | 0.40 |
| Copepoda nauplius | – | – | – | – |
| Calanoida | 451.19 | 129.42 | 119.83 | 71.61 |
| Cyclopoida | 6.29 | 6.12 | 7.40 | 2.03 |
| Harpacticoida | 10.19 | 0.42 | 0.16 | 1.10 |
| Shrimp larva | 18.42 | 6.08 | 3.86 | 2.90 |
| Mysidacea | – | – | – | – |
| Euphausiacea | 4.78 | 0.68 | 0.28 | 0.59 |
| Barnacle nauplius | 2.12 | 0.07 | 9.53 | 1.20 |
| Echinodermata larva | 0.26 | – | 0.01 | 0.03 |
| Chaetognatha | 35.75 | 28.91 | 15.74 | 8.22 |
| Appendicullaria | 10.09 | 1.96 | 3.21 | 1.56 |
| Thaliacea | 3.97 | 2.71 | 1.44 | 0.83 |
| Fish eggs | 1.94 | 1.36 | 4.45 | 0.79 |
| Fish larva | 1.91 | 0.64 | 1.21 | 0.38 |
| Insect larva | – | 0.03 | 0.01 | 0.004 |
| Others | 0.29 | 0.02 | 0.15 | 0.05 |
| Compound | Transect KH | Transect GR | Transect FS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 15 | 4 | 5 | 6 | 14 | 8 | 9 | 10 | 13 | |
| BDE-2 | 4.66 (0.00–13.97) a | 54.25 (0.00–133.79) | 25.90 (0.00–77.70) | ND | ND | 0.50 (0.00–1.49) | ND | ND | ND | ND | ND | ND |
| BDE-15 | 424.54 (0.00–1273.61) | 3.13 (0.00–9.39) | ND | 0.56 (0.00–1.18) | ND | 0.31 (0.00–0.94) | ND | 19.84 (0.00–58.16) | ND | 32.45 (0.00–97.36) | 1.40 (0.00–2.30) | 20.03 (0.00–31.23) |
| BDE-17 | 50.33 (0.00–141.88) | 50.67 (0.00–152.00) | 25.86 (0.00–77.59) | 1.84 (0.00–5.52) | ND | ND | ND | ND | ND | 0.78 (0.00–2.34) | 9.95 (0.00–22.40) | 2.70 (0.00–4.41) |
| BDE-28 | ND | ND | ND | ND | 0.07 (0.00–0.21) | 0.08 (0.00–0.24) | 0.05 (0.00–0.16) | ND | 0.07 (0.00–0.20) | 0.09 (0.00–0.28) | 0.07 (0.00–0.22) | ND |
| BDE-71 | 3.85 (0.00–11.54) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| BDE-99 | ND | ND | 0.33 (0.00–0.99) | ND | ND | ND | ND | ND | ND | 151.63 (0.00–454.88) | ND | ND |
| BDE-100 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.62 (0.00–4.85) | ND | ND |
| BDE-190 | ND | ND | 0.36 (0.00–1.07) | ND | ND | ND | ND | ND | ND | 51.30 (0.00–153.89) | 43.66 (0.00–130.97) | ND |
| BDE-203 | ND | ND | ND | ND | 1.17 (0.00–3.52) | ND | ND | ND | ND | ND | ND | ND |
| BDE-205 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.35 (0.00–7.05) | ND |
| BDE-209 | 6.57 (0.00–19.71) | ND | 119.10 (0.00–357.30) | ND | 33.21 (0.00–51.26) | ND | ND | ND | 25.82 (0.00–77.45) | ND | ND | ND |
| ΣPBDE | 489.94 (11.54–1415.48) | 108.05 (0.00–285.79) | 171.55 (0.00–359.37) | 2.40 (0.00–6.71) | 34.46 (3.52–51.47) | 0.89 (0.00–2.68) | 0.05 (0.00–0.16) | 19.84 (0.00–58.16) | 25.88 (0.00–77.45) | 237.87 (0.00–713.32) | 57.43 (0.22–162.72) | 22.73 (0.00–35.64) |
| Location | PBDE Numbers | PBDEs (ng g−1 dw) | Reference |
|---|---|---|---|
| Marine | |||
| Gaoping waters (Taiwan) | Current study | ||
| —transect KH | 19 | 0–1415.48 | |
| —transect GR | 19 | 0–58.16 | |
| —transect FS | 19 | 0–713.32 | |
| Bohai Bay (China) | 13 | 0.15–32.8 (ng g−1 lw) | Wan et al. (2008) [20] |
| Baltic Sea (in 2002) | 14 | 2.36–4.87 (ng g−1 lw) | Peltonen et al. (2014) [12] |
| Baltic Sea (in 2010) | 14 | 2.76–10.69 (ng g−1 lw) | Peltonen et al. (2014) [12] |
| Bohai Bay (China) | 13 | 12 ± 1 (ng g−1 ww) | Shao et al. (2016) [14] |
| Bohai Bay (China) | 6 | 0.75–7.29 | Zheng et al. (2016) [22] |
| Tokyo Bay (Japan) | 45 | 0–23.24 | Yeo et al. (2020) [11] |
| Sagami Bay (Japan) | 45 | 7.18–27.09 | Yeo et al. (2020) [11] |
| Freshwater | |||
| Baiyangdian Lake (China) | 9 | 14.3 | Hu et al. (2010) [57] |
| Lago Maggiore (Italy) | 15 | 379–2094 (ng g−1 lw) | Poma et al. (2014) [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, H.-Y.; Huang, K.-C.; Cheng, J.-O.; Ko, F.-C.; Meng, P.-J. Concentrations and Characteristics of Polybrominated Diphenyl Ethers (PBDEs) in Marine Zooplankton from the Gaoping Waters of Southwestern Taiwan. J. Mar. Sci. Eng. 2022, 10, 1943. https://doi.org/10.3390/jmse10121943
Hsieh H-Y, Huang K-C, Cheng J-O, Ko F-C, Meng P-J. Concentrations and Characteristics of Polybrominated Diphenyl Ethers (PBDEs) in Marine Zooplankton from the Gaoping Waters of Southwestern Taiwan. Journal of Marine Science and Engineering. 2022; 10(12):1943. https://doi.org/10.3390/jmse10121943
Chicago/Turabian StyleHsieh, Hung-Yen, Kuang-Ching Huang, Jing-O Cheng, Fung-Chi Ko, and Pei-Jie Meng. 2022. "Concentrations and Characteristics of Polybrominated Diphenyl Ethers (PBDEs) in Marine Zooplankton from the Gaoping Waters of Southwestern Taiwan" Journal of Marine Science and Engineering 10, no. 12: 1943. https://doi.org/10.3390/jmse10121943
APA StyleHsieh, H.-Y., Huang, K.-C., Cheng, J.-O., Ko, F.-C., & Meng, P.-J. (2022). Concentrations and Characteristics of Polybrominated Diphenyl Ethers (PBDEs) in Marine Zooplankton from the Gaoping Waters of Southwestern Taiwan. Journal of Marine Science and Engineering, 10(12), 1943. https://doi.org/10.3390/jmse10121943









