Salinity Tolerance and the Effect of Salinity and Algal Feed on the Demographics of Cultured Harpacticoid Copepods Tisbe holothuriae and Tigriopus sp. from the Messolonghi Lagoon (W. Greece)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Experiment on Salinity Tolerance
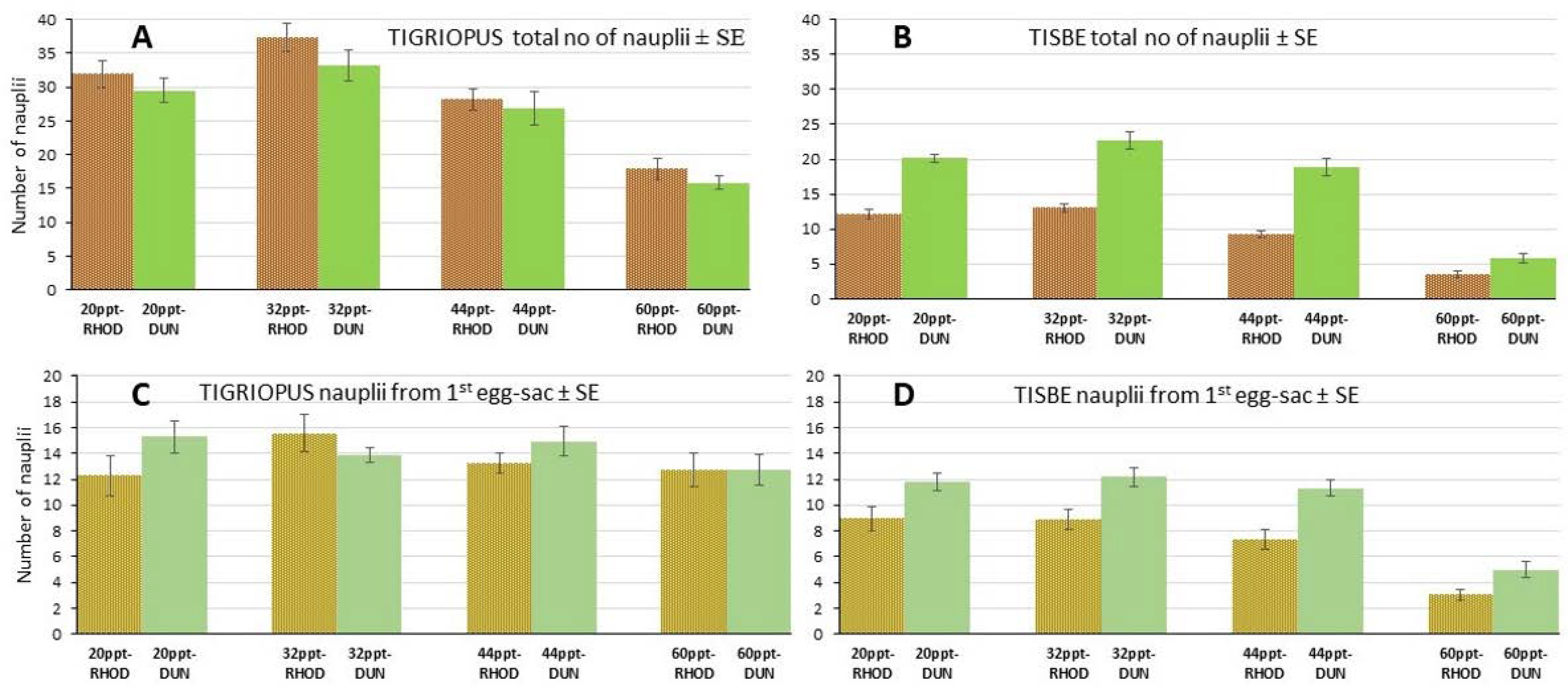
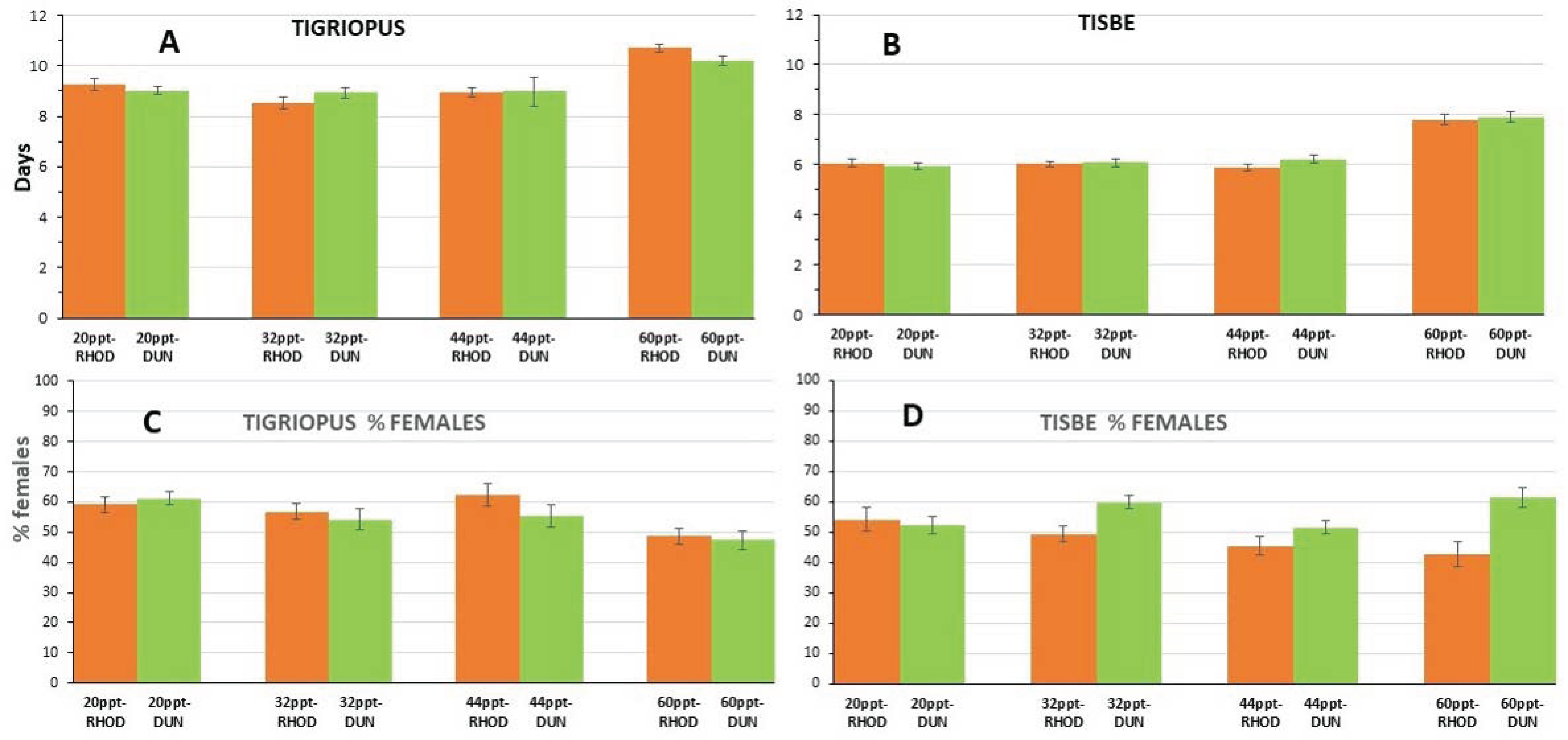
3.2. Experiment on Feed Influence
3.3. Experiment on Salinity-Feed Demographics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, M.B.; Laabir, M.; Daly Yahia, M.N. A Novel Index Based on Planktonic Copepod Reproductive Traits as a Tool for Marine Ecotoxicology Studies. Sci. Total Environ. 2020, 727, 138621. [Google Scholar] [CrossRef] [PubMed]
- Alajmi, F.; Zeng, C. Evaluation of Microalgal Diets for the Intensive Cultivation of the Tropical Calanoid Copepod, Parvocalanus crassirostris. Aquac. Res. 2015, 46, 1025–1038. [Google Scholar] [CrossRef]
- Baensch, F.; Tamaru, C.S. Spawning and Development of Larvae and Juveniles of the Rare Blue Mauritius Angelfish, Centropyge debelius (1988), in the Hatchery. J. World Aquac. Soc. 2009, 40, 425–439. [Google Scholar] [CrossRef]
- Støttrup, J.G. The Elusive Copepods: Their Production and Suitability in Marine Aquaculture. Aquac. Res. 2000, 31, 703–711. [Google Scholar] [CrossRef]
- Sun, B.; Fleeger, J.W. Sustained Mass Culture of Amphiascoides Atopus a Marine Harpacticoid Copepod in a Recirculating System. Aquaculture 1995, 136, 313–321. [Google Scholar] [CrossRef]
- Støttrup, J.G.; Norsker, N.H. Production and Use of Copepods in Marine Fish Larviculture. Aquaculture 1997, 155, 231–247. [Google Scholar] [CrossRef]
- Camus, T.; Zeng, C. Reproductive Performance, Survival and Development of Nauplii and Copepodites, Sex Ratio and Adult Life Expectancy of the Harpacticoid Copepod, Euterpina Acutifrons, Fed Different Microalgal Diets. Aquac. Res. 2012, 43, 1159–1169. [Google Scholar] [CrossRef]
- Delbare, D.; Dhert, P.; Lavens, P. Zooplankton. In Manual on the Production and Use of Live Food for Aquaculture; Lavens, P., Sorgelos, P., Eds.; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1996; Volume 361, pp. 252–282. [Google Scholar]
- Nanton, D.A.; Castell, J.D. The Effects of Dietary Fatty Acids on the Fatty Acid Composition of the Harpacticoid Copepod, Tisbe sp., for Use as a Live Food for Marine Fish Larvae. Aquaculture 1998, 163, 251–261. [Google Scholar] [CrossRef]
- Werbrouck, E.; Bodé, S.; van Gansbeke, D.; Vanreusel, A.; de Troch, M. Fatty Acid Recovery after Starvation: Insights into the Fatty Acid Conversion Capabilities of a Benthic Copepod (Copepoda, Harpacticoida). Mar. Biol. 2017, 164, 217–226. [Google Scholar] [CrossRef]
- Punnarak, P.; Jarayabhand, P.; Piumsomboon, A. Cultivation of Harpacticoid Copepods (Families Harpacticidae and Laophontidae) under Selected Environmental Conditions. Agric. Nat. Resour. 2017, 51, 278–285. [Google Scholar] [CrossRef]
- Calliari, D.; Andersen, C.; Thor, P.; Gorokhova, E.; Tiselius, P. Salinity Modulates the Energy Balance and Reproductive Success of Co-Occurring Copepods Acartia tonsa and A. clausi in Different Ways. Mar. Ecol. Prog. Ser. 2006, 312, 177–188. [Google Scholar] [CrossRef]
- Chinnery, F.E.; Williams, J.A. The Influence of Temperature and Salinity on Acartia (Copepoda: Calanoida) Nauplii Survival. Mar. Biol. 2004, 145, 733–738. [Google Scholar] [CrossRef]
- Devreker, D.; Souissi, S.; Seuront, L. Development and Mortality of the First Naupliar Stages of Eurytemora affinis (Copepoda, Calanoida) under Different Conditions of Salinity and Temperature. J. Exp. Mar. Biol. Ecol. 2004, 303, 31–46. [Google Scholar] [CrossRef]
- Ohs, C.L.; Rhyne, A.L.; Grabe, S.W.; DiMaggio, M.A.; Stenn, E. Effects of Salinity on Reproduction and Survival of the Calanoid Copepod Pseudodiaptomus pelagicus. Aquaculture 2010, 307, 219–224. [Google Scholar] [CrossRef]
- Miliou, H.; Moraitou-Apostolopoulou, M. Combined Effects of Temperature and Salinity on the Population Dynamics of Tisbe holothuriae HUMES (Copepoda: Harpacticoida). Arch. Hydrobiol. 1991, 121, 431–448. [Google Scholar] [CrossRef]
- Hotos, G.N. A Preliminary Survey on the Planktonic Biota in a Hypersaline Pond of Messolonghi Saltworks (W. Greece). Diversity 2021, 13, 270. [Google Scholar] [CrossRef]
- Wardlaw, C.A. (Ed.) Practical Statistics for Experimental Biologists; John Wiley & Sons: Plymouth, UK, 1989. [Google Scholar]
- Herbert, J. Salinity and Upper Temperature Tolerances of a Rockpool Copepod, Tigriopus Californicus (Baker, 1912); University of Oregon: Eugene, OR, USA, 1976. [Google Scholar]
- Frangoulis, C.; Christou, E.D.; Hecq, J.H. Comparison of Marine Copepod Outfluxes: Nature, Rate, Fate and Role in the Carbon and Nitrogen Cycles. Adv. Mar. Biol. 2004, 47, 253–309. [Google Scholar] [CrossRef]
- Hawkins, B.L. The Biology of the Marine Copepod, Tigriopus californicus (Baker); Humboldt State University: Arcata, CA, USA, 1962. [Google Scholar]
- Turner, J.T. The Importance of Small Planktonic Copepods and Their Roles in Pelagic Marine Food Webs. Zool. Stud. 2004, 42, 255–266. [Google Scholar]
- Allan, J.D. Life History Patterns in Zooplankton. Am. Nat. 1976, 110, 165–180. [Google Scholar] [CrossRef]
- Sanford, E.; Kelly, M.W. Local Adaptation in Marine Invertebrates. Ann. Rev. Mar. Sci. 2011, 3, 509–535. [Google Scholar] [CrossRef]
- Heath, P.; Moore, C. Rearing Dover Sole Larvae on Tisbe and Artemia Diets. Aquac. Int. 1997, 5, 29–39. [Google Scholar] [CrossRef]
- Miliou, H. The Effect of Temperature, Salinity and Diet On Final Size of Female Tisbe holothuriae (Copepoda, Harpacticoida). Crustaceana 1996, 69, 742–754. [Google Scholar] [CrossRef]
- Souza-Santos, L.P.; Pastor, J.M.O.; Ferreira, N.G.; Costa, W.M.; Araújo-Castro, C.M.; Santos, P.J.P. Developing the Harpacticoid Copepod Tisbe biminiensis Culture: Testing for Salinity Tolerance, Ration Levels, Presence of Sediment and Density Dependent Analyses. Aquac. Res. 2006, 37, 1516–1523. [Google Scholar] [CrossRef]
- Belmonte, G.; Moscatello, S.; Batogova, E.A.; Pavlovskaya, T.; Shadrin, N.V.; Litvinchuk, L.F. Fauna of Hypersaline Lakes of the Crimea (Ukraine). Thalass. Salentina 2012, 34, 11–24. [Google Scholar]
- El-Tohamy, W.; Qin, J.; Abdel-Aziz, N.; El-Ghobashy, A.; Dorgham, M. Suitable Algal Species and Density for the Culture of Copepod Gladioferens imparipes as a Potential Live Food for Fish Larvae. Aquac. Int. 2021, 29, 105–125. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Souissi, S.; Souissi, A.; Wu, C.-H.; Cheng, S.-H.; Hwang, J.-S. Dietary Effects on Egg Production, Egg-Hatching Rate and Female Life Span of the Tropical Calanoid Copepod Acartia bilobata. Aquac. Res. 2014, 45, 1659–1671. [Google Scholar] [CrossRef]
- Lee, M.-C.; Choi, H.; Park, J.C.; Yoon, D.-S.; Lee, Y.; Hagiwara, A.; Park, H.G.; Shin, K.-H.; Lee, J.-S. A Comparative Study of Food Selectivity of the Benthic Copepod Tigriopus japonicus and the Pelagic Copepod Paracyclopina nana: A Genome-Wide Identification of Fatty Acid Conversion Genes and Nitrogen Isotope Investigation. Aquaculture 2020, 521, 734930. [Google Scholar] [CrossRef]
- Hong, H.; Wang, J.; Shi, D. Effects of Salinity on the Chronic Toxicity of 4-Methylbenzylidene Camphor (4-MBC) in the Marine Copepod Tigriopus japonicus. Aquat. Toxicol. 2021, 232, 105742. [Google Scholar] [CrossRef]
- Raisuddin, S.; Kwok, K.W.H.; Leung, K.M.Y.; Schlenk, D.; Lee, J.-S. The Copepod Tigriopus: A Promising Marine Model Organism for Ecotoxicology and Environmental Genomics. Aquat. Toxicol. 2007, 83, 161–173. [Google Scholar] [CrossRef]
- Kwok, K.W.H.; Leung, K.M.Y. Toxicity of Antifouling Biocides to the Intertidal Harpacticoid Copepod Tigriopus japonicus (Crustacea, Copepoda): Effects of Temperature and Salinity. Mar. Pollut. Bull. 2005, 51, 830–837. [Google Scholar] [CrossRef]
- Paiva, F.; Pauli, N.; Briski, E. Are Juveniles as Tolerant to Salinity Stress as Adults? A Case Study of Northern European, Ponto-Caspian and North American Species. Divers. Distrib. 2020, 26, 1627–1641. [Google Scholar] [CrossRef]
- Mauchline, J. The Biology of Calanoid Copepods. In Advances in Marine Biology; Mauchline, J., Ed.; Academic Press: Cambridge, MA, USA, 1998; Volume 33, pp. 71–81. [Google Scholar]
- Cutts, C.J. Culture of Harpacticoid Copepods: Potential as Live Feed for Rearing Marine Fish. Adv. Mar. Biol. 2003, 44, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Fava, G.; Crotti, E. Effect of Crowding on Nauplii Production during Mating Time in Tisbe clodiensis and T. holothuriae (Copepoda, Harpacticoida). Helgoländer Wiss. Meeresunters. 1979, 32, 466–475. [Google Scholar] [CrossRef]
- Polis, G.A. The Evolution and Dynamics of Intraspecific Predation. Annu. Rev. Ecol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Luppi, T.A.; Spivak, E.D.; Anger, K. Experimental Studies on Predation and Cannibalism of the Settlers of Chasmagnathus granulate and Cyrtograpsus angulatus (Brachyura: Grapsidae). J. Exp. Mar. Biol. Ecol. 2001, 265, 29–48. [Google Scholar] [CrossRef][Green Version]
- Moksnes, P.-O. The Relative Importance of Habitat-Specific Settlement, Predation and Juvenile Dispersal for Distribution and Abundance of Young Juvenile Shore Crabs Carcinus maenas L. J. Exp. Mar. Biol. Ecol. 2002, 271, 41–73. [Google Scholar] [CrossRef]
- Landry, M.R. Switching between Herbivory and Carnivory by the Planktonic Marine Copepod Calanus pacificus. Mar. Biol. 1981, 65, 77–82. [Google Scholar] [CrossRef]
- Conley, W.J.; Turner, J.T. Omnivory by the Coastal Marine Copepods Centropages hamatus and Labidocera aestiva. Mar. Ecol. Prog. Ser. 1985, 21, 113–120. [Google Scholar] [CrossRef]
- Lazzaretto, I.; Salvato, B. Cannibalistic Behaviour in the Harpacticoid Copepod Tigriopus fulvus. Mar. Biol. 1992, 113, 579–582. [Google Scholar] [CrossRef]
- Gallucci, F.; Ólafsson, E. Cannibalistic Behaviour of Rock-Pool Copepods: An Experimental Approach for Space, Food and Kinship. J. Exp. Mar. Biol. Ecol. 2007, 342, 325–331. [Google Scholar] [CrossRef]
- Lewis, A.G.; Chatters, L.; Raudsepp, M. Feeding Structures and Their Functions in Adult and Preadult Tigriopus californicus (Copepoda: Harpacticoida). J. Mar. Biol. Assoc. United Kingd. 1998, 78, 451–466. [Google Scholar] [CrossRef]
- Puello-Cruz, A.C.; Mezo-Villalobos, S.; González-Rodríguez, B.; Voltolina, D. Culture of the Calanoid Copepod Pseudodiaptomus euryhalinus (Johnson 1939) with Different Microalgal Diets. Aquaculture 2009, 290, 317–319. [Google Scholar] [CrossRef]
- Gaudy, R.; Guerin, J.P.; Moraitou-Apostolopoulou, M. Effect of Temperature and Salinity on the Population Dynamics of Tisbe holothuriae Humes (Copepoda: Harpacticoida) Fed on Two Different Diets. J. Exp. Mar. Biol. Ecol. 1982, 57, 257–271. [Google Scholar] [CrossRef]
- Edmands, S. Phylogeography of the Intertidal Copepod Tigriopus californicus Reveals Substantially Reduced Population Differentiation at Northern Latitudes. Mol. Ecol. 2001, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Hassett, R.P.; Landry, M.R. Effects of Diet and Starvation on Digestive Enzyme Activity and Feeding Behavior of the Marine Copepod Calanus pacificus. J. Plankton Res. 1990, 12, 991–1010. [Google Scholar] [CrossRef]
- Tsuda, A. Starvation Tolerance of a Planktonic Marine Copepod Pseudocalanus newmani Frost. J. Exp. Mar. Biol. Ecol. 1994, 181, 81–89. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Støttrup, J.G. The Importance of Dietary HUFAs for Fecundity and HUFA Content in the Harpacticoid, Tisbe holothuriae Humes. Aquaculture 1994, 125, 155–166. [Google Scholar] [CrossRef]
- Miles, D.; Auchterlonie, N.; Cutts, C.; de Quero, C.M. Rearing of the Harpacticoid Copepod Tisbe holothuriae and Its Application for the Hatchery Production of Atlantic Halibut Hippoglossus hippoglossus; Link Project FIN 23; SEAFISH Development Aquaculture: Edinburgh, UK, 2001. [Google Scholar]
- de Troch, M.; Chepurnov, V.; Gheerardyn, H.; Vanreusel, A.; Ólafsson, E. Is Diatom Size Selection by Harpacticoid Copepods Related to Grazer Body Size? J. Exp. Mar. Biol. Ecol. 2006, 332, 1–11. [Google Scholar] [CrossRef][Green Version]
- Kawecki, T.J.; Ebert, D. Conceptual Issues in Local Adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
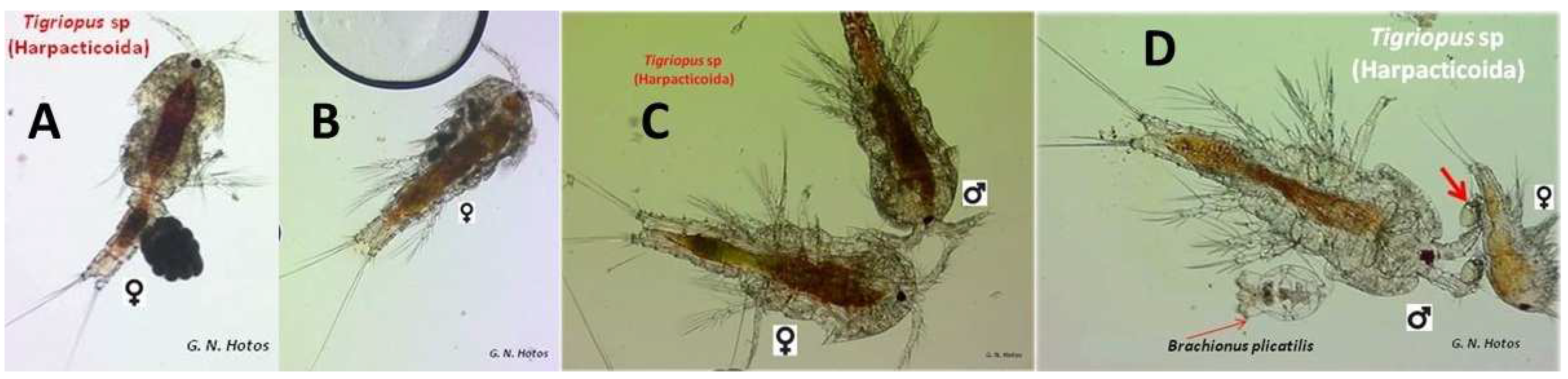
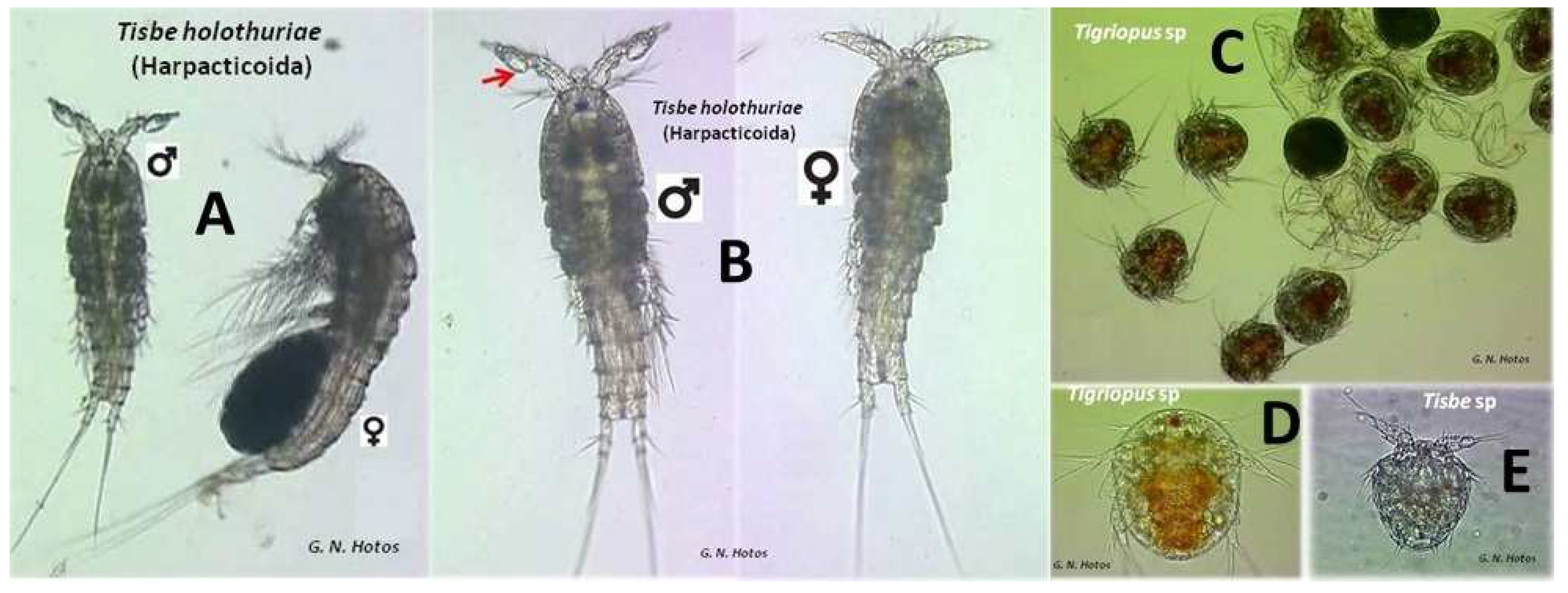

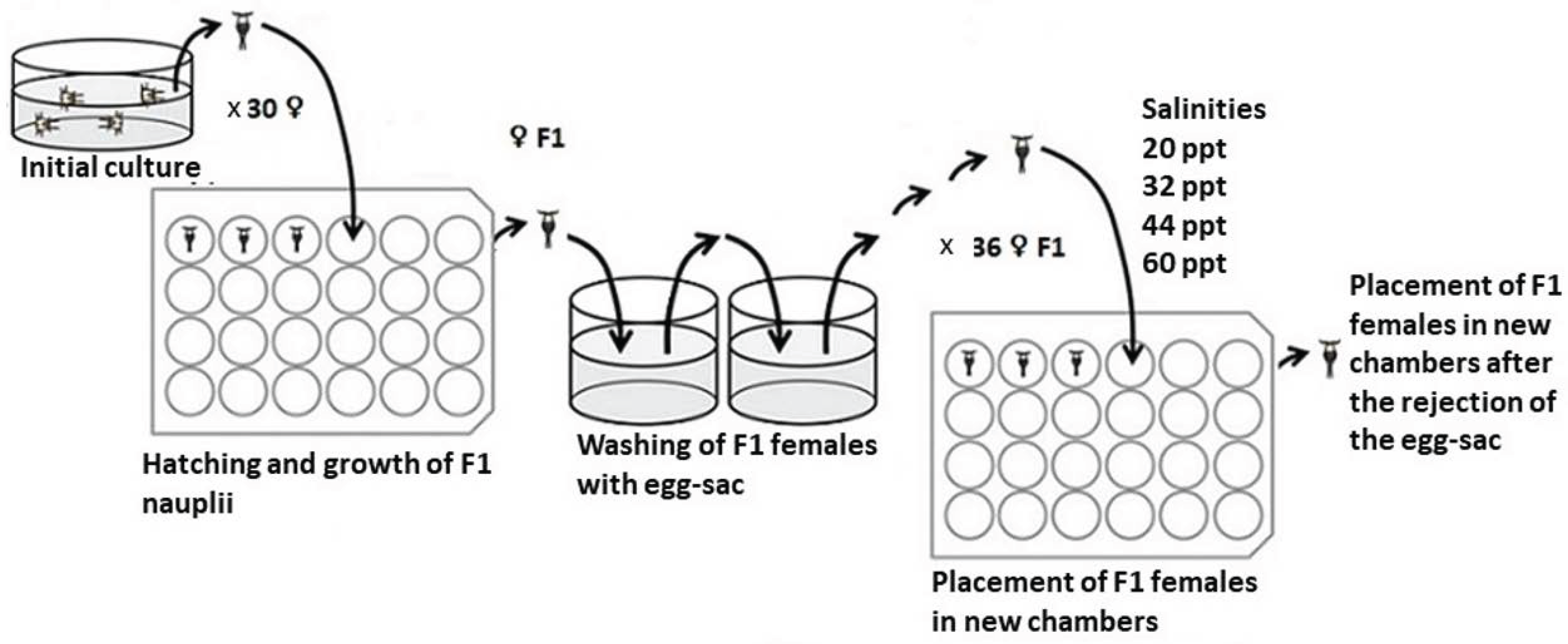
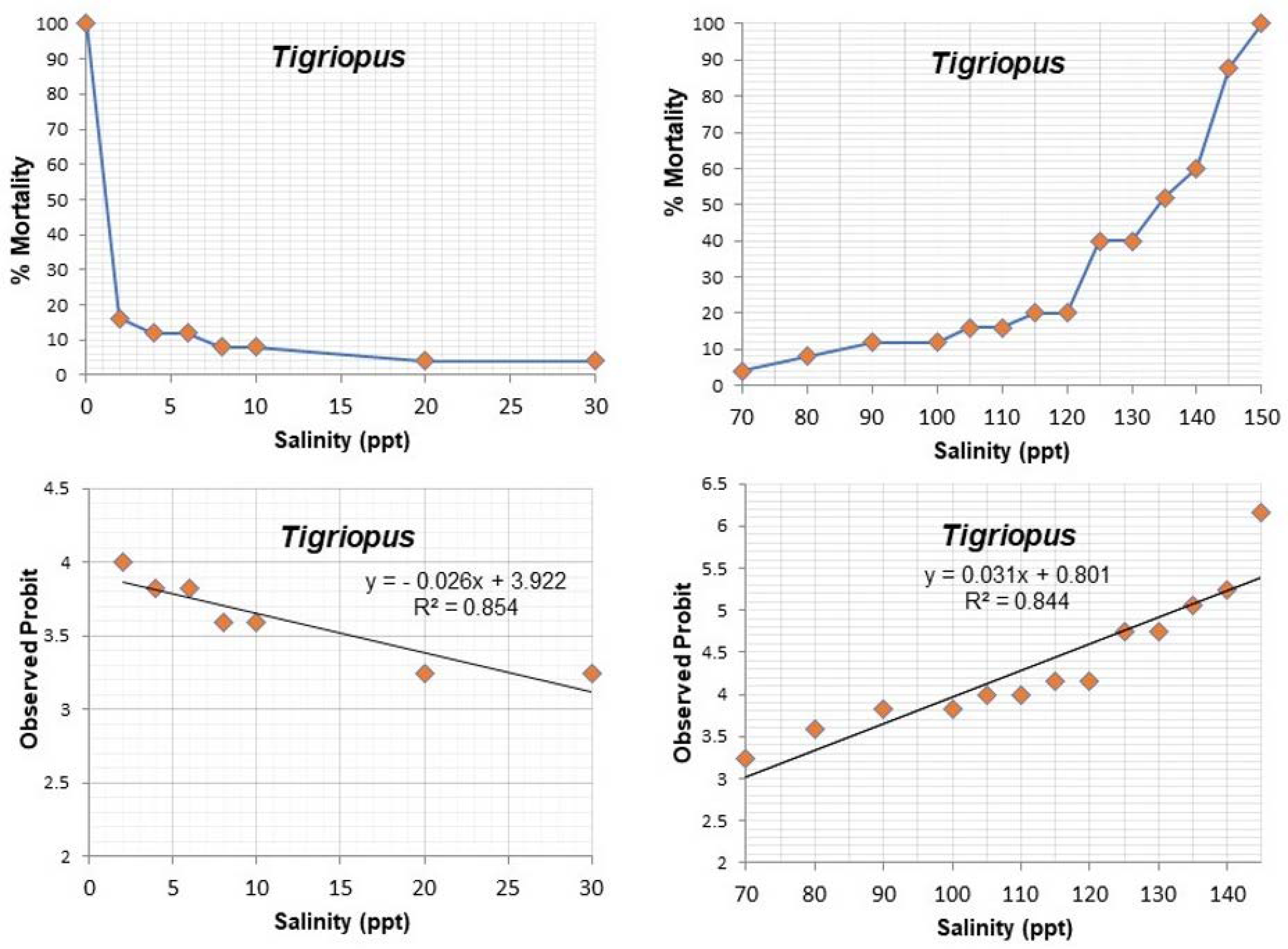
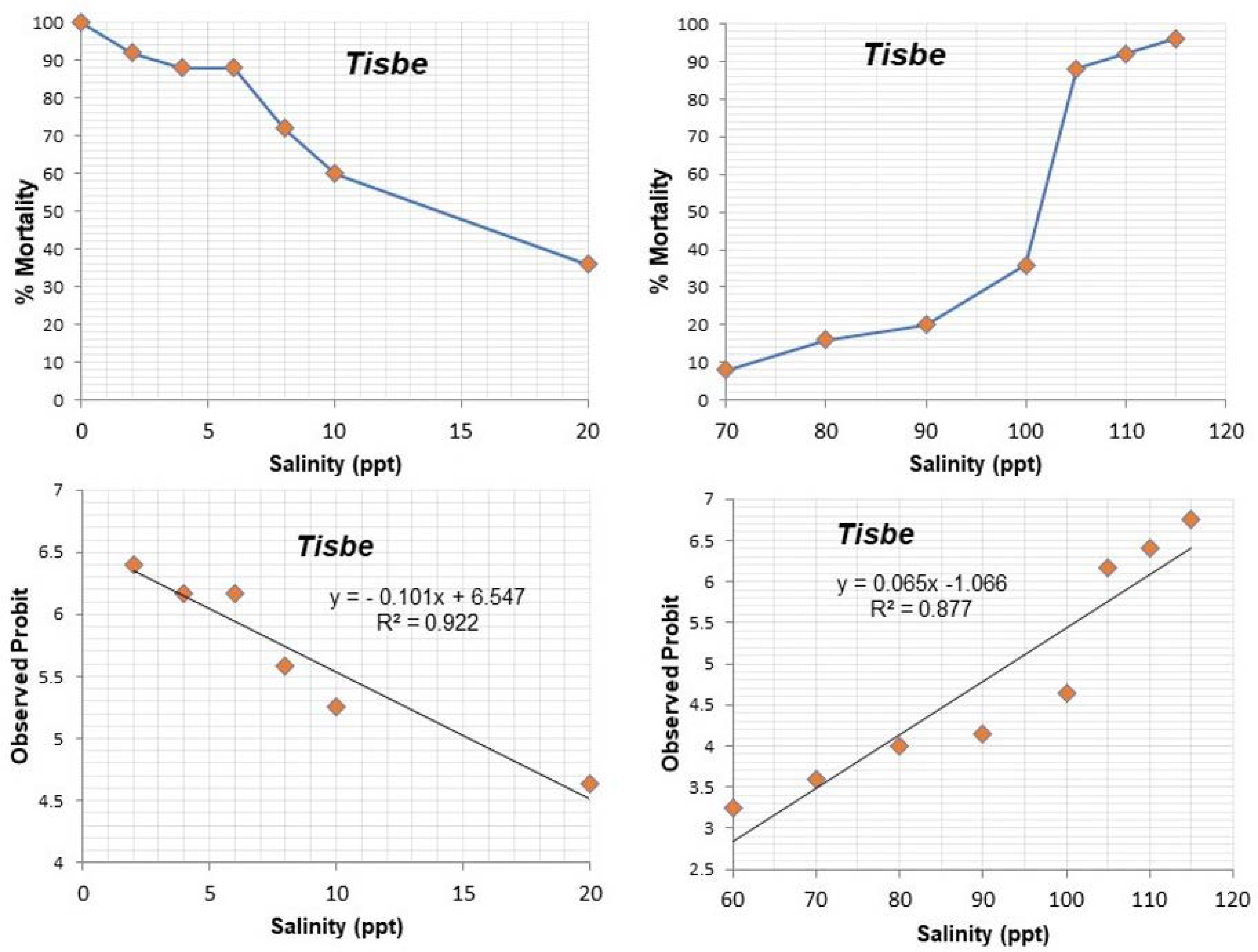


| Salinities (ppt) | Mortality Tisbe | Mortality Tigriopus | % Mortality Tisbe | % Mortality Tigriopus | Observed Probit Tisbe | Observed Probit Tigriopus | Expected Probit Tisbe | Expected Probit Tigriopus | Weight Tisbe | Weight Tigriopus |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | (25/25) | (25/25) | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | (23/25) | (4/25) | 92 | 16 | 6.4 | 4 | 6.34 | 3.88 | 0.336 | 0.405 |
| 4 | (22/25) | (3/25) | 88 | 12 | 6.17 | 3.82 | 6.15 | 3.82 | 0.37 | 0.37 |
| 6 | (22/25) | (3/25) | 88 | 12 | 6.17 | 3.82 | 5.95 | 3.77 | 0.439 | 0.37 |
| 8 | (18/25) | (2/25) | 72 | 8 | 5.58 | 3.59 | 5.73 | 3.7 | 0.532 | 0.336 |
| 10 | (15/25) | (2/25) | 60 | 8 | 5.25 | 3.59 | 5.53 | 3.65 | 0.581 | 0.336 |
| 20 | (9/25) | (1/25) | 36 | 4 | 4.64 | 3.24 | 4.52 | 3.38 | 0.581 | 0.237 |
| 30 | 0 | (1/25) | 0 | 4 | 0 | 3.24 | 0 | 3.12 | 0 | 0.154 |
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 60 | (1/25) | 0 | 4 | 0 | 3.24 | 0 | 2.83 | 0 | 0.092 | 0 |
| 70 | (2/25) | (1/25) | 8 | 4 | 3.59 | 3.24 | 3.48 | 3.1 | 0.269 | 0.154 |
| 80 | (4/25) | (2/25) | 16 | 8 | 4 | 3.59 | 4.13 | 3.33 | 0.471 | 0.208 |
| 90 | (5/25) | (3/25) | 20 | 12 | 4.15 | 3.82 | 4.78 | 3.64 | 0.627 | 0.302 |
| 100 | (9/25) | (3/25) | 36 | 12 | 4.64 | 3.82 | 5.43 | 3.95 | 0.601 | 0.439 |
| 105 | (22/25) | (4/25) | 88 | 16 | 6.17 | 4 | 5.76 | 4.12 | 0.503 | 0.471 |
| 110 | (23/25) | (4/25) | 92 | 16 | 6.4 | 4 | 6.08 | 4.28 | 0.401 | 0.532 |
| 115 | (24/25) | (5/25) | 96 | 20 | 6.75 | 4.15 | 6.41 | 4.43 | 0.302 | 0.558 |
| 120 | (25/25) | (5/25) | 100 | 20 | 0 | 4.15 | 0 | 4.59 | 0 | 0.601 |
| 125 | (10/25) | 40 | 4.74 | 4.75 | 0.627 | |||||
| 130 | (10/25) | 40 | 4.74 | 4.9 | 0.634 | |||||
| 135 | (13/25) | 52 | 5.05 | 5.06 | 0.634 | |||||
| 140 | (15/25) | 60 | 5.25 | 5.22 | 0.627 | |||||
| 145 | (22/25) | 88 | 6.17 | 5.38 | 0.601 | |||||
| 150 | (25/25) | 100 | 0 | 0 | 0 | |||||
| SUM Weight Tisbe | 6.105 | |||||||||
| MEAN Weight Tisbe | 0.436 | |||||||||
| SUM Weight Tigriopus | 8.596 | |||||||||
| MEAN Weight Tigriopus | 0.429 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotos, G.N.; Kourelea, E.; Fotodimas, I. Salinity Tolerance and the Effect of Salinity and Algal Feed on the Demographics of Cultured Harpacticoid Copepods Tisbe holothuriae and Tigriopus sp. from the Messolonghi Lagoon (W. Greece). J. Mar. Sci. Eng. 2022, 10, 1663. https://doi.org/10.3390/jmse10111663
Hotos GN, Kourelea E, Fotodimas I. Salinity Tolerance and the Effect of Salinity and Algal Feed on the Demographics of Cultured Harpacticoid Copepods Tisbe holothuriae and Tigriopus sp. from the Messolonghi Lagoon (W. Greece). Journal of Marine Science and Engineering. 2022; 10(11):1663. https://doi.org/10.3390/jmse10111663
Chicago/Turabian StyleHotos, George N., Evi Kourelea, and Ioannis Fotodimas. 2022. "Salinity Tolerance and the Effect of Salinity and Algal Feed on the Demographics of Cultured Harpacticoid Copepods Tisbe holothuriae and Tigriopus sp. from the Messolonghi Lagoon (W. Greece)" Journal of Marine Science and Engineering 10, no. 11: 1663. https://doi.org/10.3390/jmse10111663
APA StyleHotos, G. N., Kourelea, E., & Fotodimas, I. (2022). Salinity Tolerance and the Effect of Salinity and Algal Feed on the Demographics of Cultured Harpacticoid Copepods Tisbe holothuriae and Tigriopus sp. from the Messolonghi Lagoon (W. Greece). Journal of Marine Science and Engineering, 10(11), 1663. https://doi.org/10.3390/jmse10111663







