Antimicrobial Effect of Carbon Nanodots–ZnO Nanocomposite Synthesized Using Sargassum horneri
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CNDs–ZnO Nanocomposite
2.3. Preparation of “CNDs Alone” and Pure ZnO Nanoparticles
2.4. Characterization of CNDs–ZnO
2.5. Antibacterial Test
2.6. Antifungal Test
2.7. Toxicity Test in Zebrafish Embryos
3. Results and Discussion
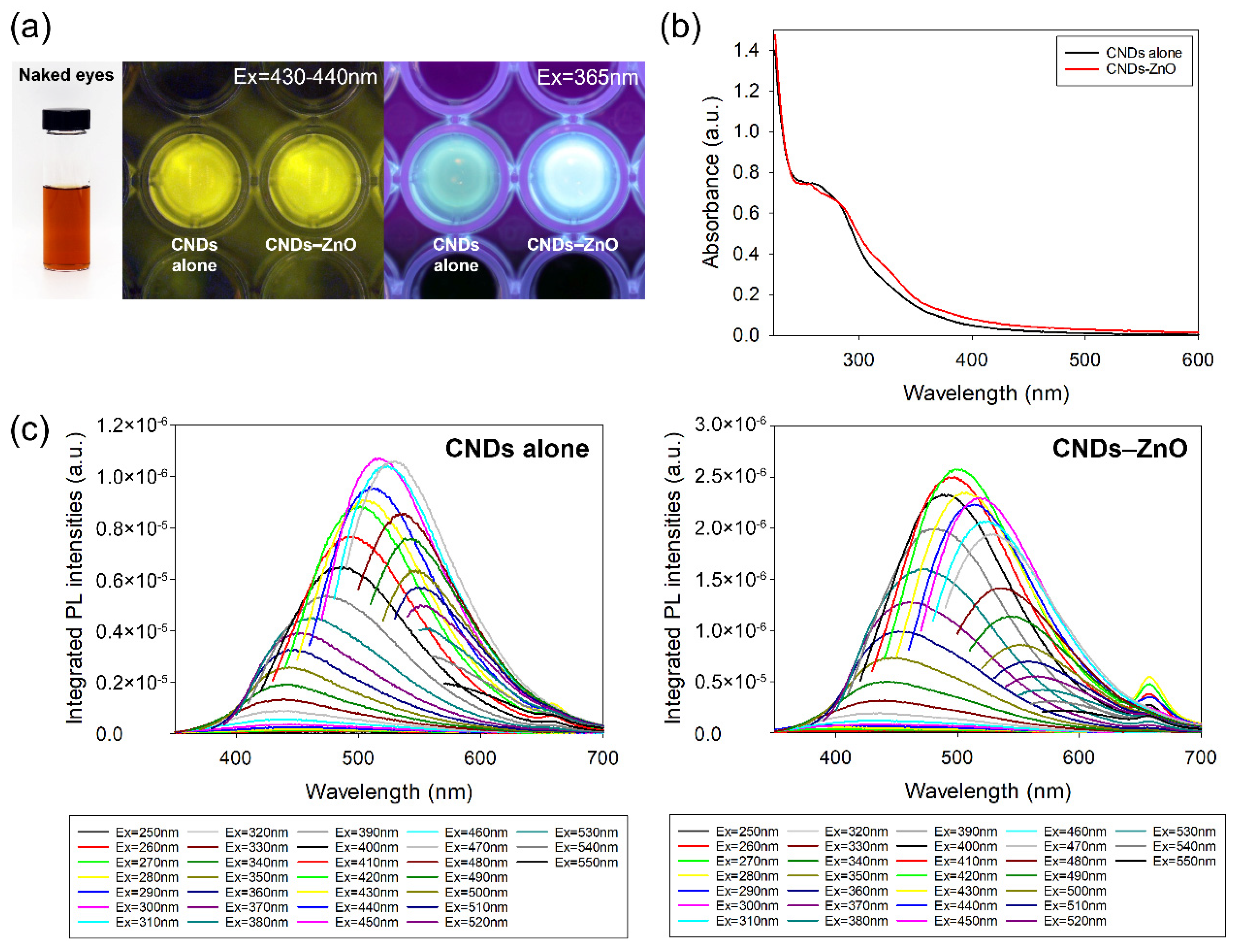
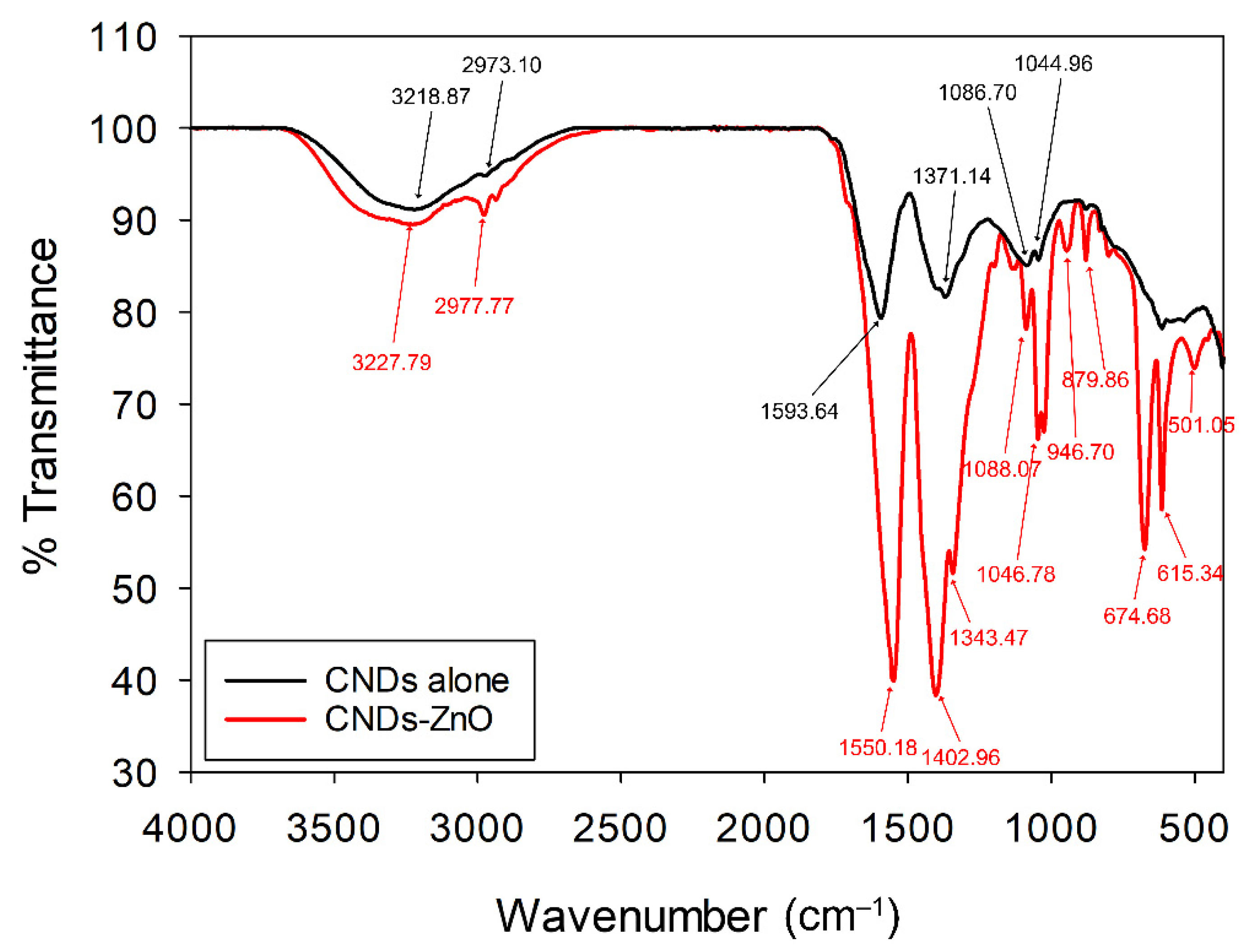
3.1. Optical Properties of the CNDs–ZnO
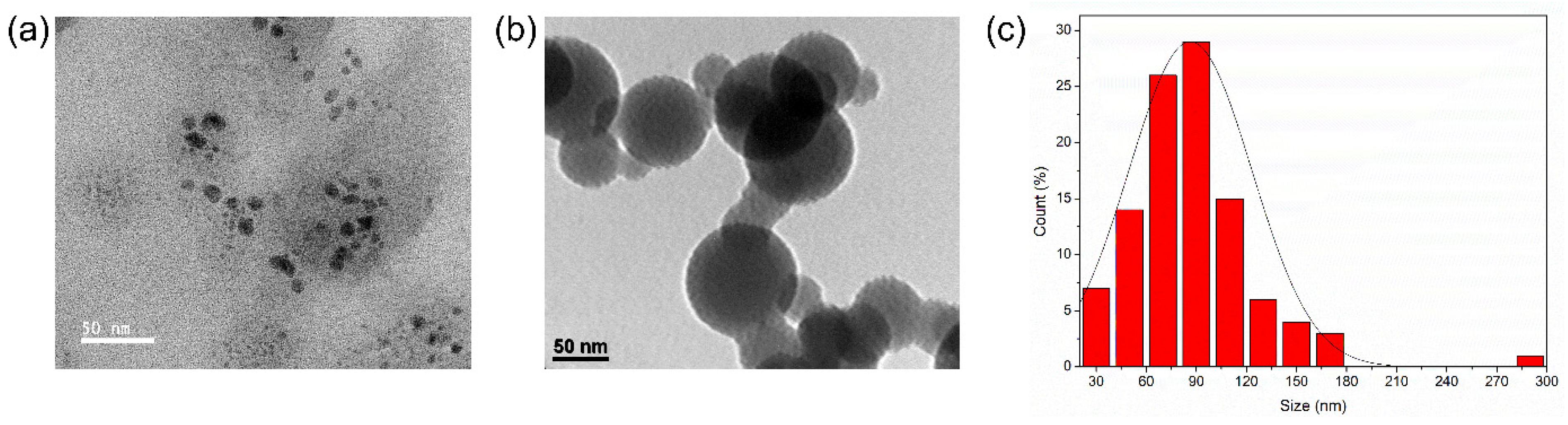
3.2. Morphological Characteristics of the CNDs–ZnO
3.3. Disk Diffusion Test for Antibacterial Activity
3.4. MIC test and Growth Curve Inhibition Analysis
3.5. Disk Diffusion Test for Antifungal Activity
3.6. Toxicity Evaluation of the CNDs–ZnO
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the antimicrobial properties of carbon nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ramos-Soriano, J.; Galan, M.C. Carbon dots as an emergent class of antimicrobial agents. Nanomaterials 2021, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. From nanobiotechnology, positively charged biomimetic dendrimers as novel antibacterial agents: A review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Miyanji, E.H.; Zhou, Y.; Pardo, J.; Hettiarachchi, S.D.; Li, S.; Blackwelder, P.L.; Skromne, I.; Leblanc, R.M. Carbon dots: Promising biomaterials for bone-specific imaging and drug delivery. Nanoscale 2017, 9, 17533–17543. [Google Scholar] [CrossRef]
- Su, W.; Wu, H.; Xu, H.; Zhang, Y.; Li, Y.; Li, X.; Fan, L. Carbon dots: A booming material for biomedical applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Puglisi, C.; Campolo, M.; Esposito, E.; Conoci, S. Carbon dots as promising tools for cancer diagnosis and therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Nanotoxicity: Prevention and Antibacterial Applications of Nanomaterials; Elsevier BV: Amsterdam, The Netherlands, 2020; Volume 14, pp. 301–315. ISBN 978-0-12-819943-5. [Google Scholar]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Wu, X.; Abbas, K.; Yang, Y.; Li, Z.; Tedesco, A.C.; Bi, H. Photodynamic Anti-Bacteria by Carbon Dots and Their Nano-Composites. Pharmaceuticals 2022, 15, 487. [Google Scholar] [CrossRef] [PubMed]
- Al Awak, M.M.; Wang, P.; Wang, S.; Tang, Y.; Sun, Y.P.; Yang, L. Correlation of Carbon Dots’ Light-Activated Antimicrobial Activities and Fluorescence Quantum Yield. RSC Adv. 2017, 7, 30177–30184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; LeCroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L.; et al. Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Lin, X.; Xiong, M.; Zhang, J.; He, C.; Ma, X.; Zhang, H.; Kuang, Y.; Yang, M.; Huang, Q. Carbon dots based on natural resources: Synthesis and applications in sensors. Microchem. J. 2021, 160, 105604. [Google Scholar] [CrossRef]
- Bag, P.; Maurya, R.K.; Dadwal, A.; Sarkar, M.; Chawla, P.A.; Narang, R.K.; Kumar, B. Recent development in synthesis of carbon dots from natural resources and their applications in biomedicine and multi-sensing platform. ChemistrySelect. 2021, 6, 2774–2789. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of Lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Chacko, L.; Poyyakkara, A.; Kumar, V.B.S.; Aneesh, P.M. MoS2-ZnO nanocomposites as highly functional agents for anti-angiogenic and anti-cancer theranostics. J. Mater. Chem. B 2018, 6, 3048–3057. [Google Scholar] [CrossRef]
- Dar, A.; Rehman, R.; Zaheer, W.; Shafique, U.; Anwar, J. Synthesis and characterization of ZnO-nanocomposites by utilizing aloe vera leaf gel and extract of Terminalia Arjuna nuts and exploring their antibacterial potency. J. Chem. 2021, 2021, 9448894. [Google Scholar] [CrossRef]
- El-Shafai, N.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.; Eldesoukey, I.; Masoud, M. Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide nanoparticles: Fabrication, characterization, DNA interaction, and antibacterial activity. RSC Adv. 2019, 9, 3704–3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi-Kouhi, S.M.; Beyk-Khormizi, A.; Amiri, M.S.; Mashreghi, M.; Taghavizadeh Yazdi, M.E.T. Silver-zinc oxide nanocomposite: From synthesis to antimicrobial and anticancer properties. Ceram. Int. 2021, 47, 21490–21497. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Huang, H.; Liu, Y.; Li, H.; Ming, H.; Kang, Z. ZnO/carbon quantum dots nanocomposites: One-step fabrication and superior photocatalytic ability for toxic gas degradation under visible light at room temperature. New J. Chem. 2012, 36, 1031. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, P.; Lyu, B.; Li, Y.; Hou, Y.; Ma, J. Carbon quantum dots decorated on ZnO nanoparticles: An efficient visible-light responsive antibacterial agents. Appl. Organomet. Chem. 2020, 34, e5665. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, L.; Gu, H.; Li, Z.; Wang, Y.; Luo, Q.; Yang, Y.; Liu, X.; Wang, H.; Ma, C.Q. Zinc oxide coated carbon dot nanoparticles as electron transport layer for inverted polymer solar cells. ACS Appl. Energy Mater. 2020, 3, 11388–11397. [Google Scholar] [CrossRef]
- Jana, J.; Chung, J.S.; Hur, S.H. ZnO-associated carbon dot-based fluorescent assay for sensitive and selective dopamine detection. ACS Omega 2019, 4, 17031–17038. [Google Scholar] [CrossRef] [Green Version]
- Atchudan, R.; Edison, T.N.J.I.; Mani, S.; Perumal, S.; Vinodh, R.; Thirunavukkarasu, S.; Lee, Y.R. Facile synthesis of a novel nitrogen-doped carbon dot adorned zinc oxide composite for photodegradation of methylene blue. Dalton Trans. 2020, 49, 17725–17736. [Google Scholar] [CrossRef]
- Bozetine, H.; Wang, Q.; Barras, A.; Li, M.; Hadjersi, T.; Szunerits, S.; Boukherroub, R. Green chemistry approach for the synthesis of ZnO-carbon dots nanocomposites with good photocatalytic properties under visible light. J. Colloid Interface Sci. 2016, 465, 286–294. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Hybridization of carbon-dots with ZnO nanoparticles of different sizes. J. Taiwan Instig. Chem. Eng. 2018, 92, 112–117. [Google Scholar] [CrossRef]
- Halpern, B.S.; Longo, C.; Hardy, D.; McLeod, K.L.; Samhouri, J.F.; Katona, S.K.; Kleisner, K.; Lester, S.E.; O’Leary, J.; Ranelletti, M.; et al. An index to assess the health and benefits of the global ocean. Nature 2012, 488, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Lee, W.W.; Jeon, Y.J. Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish Aquat. Sci. 2018, 21, 19. [Google Scholar] [CrossRef]
- Palmieri, N.; Forleo, M.B. The potential of edible seaweed within the western diet. A segmentation of Italian consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Oh, H.J.; Kim, S.; Yun, S.H.; Kang, J.H.; Park, S.R.; Lee, H.J. The origin and population genetic structure of the ‘golden tide’ seaweeds, Sargassum horneri, in Korean waters. Sci. Rep. 2019, 9, 7757. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.K.; Oh, H.J.; Yun, S.H.; Lee, H.J.; Lee, K.; Han, Y.S.; Kim, S.; Park, S.R. Population dynamics of the ‘golden tides’ seaweed, Sargassum horneri, on the Southwestern Coast of Korea: The extent and formation of golden tides. Sustainability 2020, 12, 2903. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of Green Synthesis of Metal Nanoparticles Using Algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef]
- Taherzadeh Soureshjani, P.; Shadi, A.; Mohammadsaleh, F. Algae-mediated route to biogenic cuprous oxide nanoparticles and spindle-like CaCO3: A comparative study, facile synthesis, and biological properties. RSC Adv. 2021, 11, 10599–10609. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Rajesh Babu, D.; Gengan, R.M.; Chandra, S.; Nageswara Rao, G. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Ponnuchamy, K.; Antony, J.J. Metal nanoparticles from marine seaweeds—A review. Nanotechnol. Rev. 2016, 5, 589–600. [Google Scholar] [CrossRef]
- Kim, K.W.; Choi, T.Y.; Kwon, Y.M.; Kim, J.Y.H. Simple synthesis of photoluminescent carbon dots from a marine polysaccharide found in shark cartilage. Electron. J. Biotechnol. 2020, 47, 36–42. [Google Scholar] [CrossRef]
- Kim, K.W.; Kwon, Y.M.; Kim, S.Y.; Kim, J.Y.H. One-pot synthesis of UV-protective carbon nanodots from sea cauliflower (Leathesia difformis). Electron. J. Biotechnol. 2022, 56, 22–30. [Google Scholar] [CrossRef]
- Song, W.C.; Kim, B.; Park, S.Y.; Park, G.; Oh, J.W. Biosynthesis of silver and gold nanoparticles using Sargassum horneri extract as catalyst for industrial dye degradation. Arab. J. Chem. 2022, 15, 104056. [Google Scholar] [CrossRef]
- Zeng, G.; Hong, C.; Ma, Y.; Du, M.; Zhang, Y.; Luo, H.; Chen, B.; Pan, X. Sargassum horneri-based carbon-doped TiO2 and its aquatic naphthalene photodegradation under sunlight irradiation. J. Chem. Technol. Biotechnol. 2022, 97, 1267–1274. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C.; Dahm, R. Zebrafish: A Practical Approach; Oxford University Press: Oxford, UK, 2002; ISBN 978-0-19-963809-3. [Google Scholar]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal synthesis of ZnO nanostructures with controllable morphology change. Cryst. Eng. Commun. 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bin Hussein, M.Z.; Taufiq-Yap, Y.H. Hydrothermal synthesis of zinc oxide nanoparticles using rice as soft biotemplate. Chem. Cent. J. 2013, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Naik, G.G.; Alam, M.B.; Pandey, V.; Mohapatra, D.; Dubey, P.K.; Parmar, A.S.; Sahu, A.N. Multi-functional carbon dots from an ayurvedic medicinal plant for cancer cell bioimaging applications. J. Fluoresc. 2020, 30, 407–418. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial mechanisms of zinc oxide nanoparticle against bacterial food pathogens resistant to beta-lactam antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [Green Version]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nanomicrol. Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Li, W.; Chen, J.; Huang, X.; Liu, Y.; Zhang, X.; Shu, W.; Lei, B.; Zhang, H. Antibacterial activity and synergetic mechanism of carbon dots against Gram-positive and -Negative bacteria. ACS Appl. Biol. Mater. 2021, 4, 6937–6945. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Li, J.; Le, T. Zinc oxide nanoparticle as a novel class of antifungal agents: Current advances and future perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef]



| Bacteria | mm of Inhibition Zone (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| Pure ZnO (Commercial) | Pure ZnO (Synthesized) | CNDs Alone | CNDs–ZnO-1 | CNDs–ZnO-2 | 10% Clorox | ||
| Gram-negative | E. coli | 10 ± 1 | Negligible | - | 22 ± 3 | 22 ± 2 | 21 ± 2 |
| S. typhimurium | Negligible | Negligible | - | 21 ± 2 | 20 ± 2 | 25 ± 1 | |
| V. alginolyticus | 17 ± 1 | 15 ± 1 | - | 35 ± 6 | 35 ± 8 | >70 | |
| Gram-positive | B. cereus | Negligible | Negligible | - | 17 ± 2 | 16 ± 2 | 22 ± 2 |
| S. aureus | 13 | 12 ± 2 | - | 25 ± 4 | 25 ± 4 | 23 ± 3 | |
| Bacteria | mm of Inhibition Zone (Mean ± SD) | Photo-Enhanced Antibacterial Activity | ||||
|---|---|---|---|---|---|---|
| UV on | UV off (From Table 1) | |||||
| CNDs–ZnO-1 | CNDs–ZnO-2 | CNDs–ZnO-1 | CNDs–ZnO-2 | |||
| Gram-negative | E. coli | 22 ± 3 | 21 ± 2 | 22 ± 3 | 22 ± 2 | - |
| S. typhimurium | 21 ± 2 | 21 ± 2 | 21 ± 2 | 20 ± 2 | - | |
| V. alginolyticus | 36 ± 9 | 37 ± 10 | 35 ± 6 | 35 ± 8 | Yes | |
| Gram-positive | B. cereus | 18 ± 4 | 20 ± 2 | 17 ± 2 | 16 ± 2 | Yes |
| S. aureus | 26 ± 4 | 27 ± 3 | 25 ± 4 | 25 ± 4 | Yes | |
| Conc. | Reaction | Bacteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | B. cereus | S. typhimurium | S. aureus | V. alginolyticus | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 1 | No treatment | 1.695 | 0.054 | 1.205 | 0.110 | 1.582 | 0.012 | 1.178 | 0.033 | 1.389 | 0.009 |
| with CNDs–ZnO | 0.375 | 0.019 | 0.399 | 0.098 | 0.371 | 0.050 | 0.418 | 0.056 | 0.112 | 0.006 | |
| 2 | No treatment | 1.570 | 0.021 | 1.176 | 0.061 | 1.555 | 0.056 | 1.096 | 0.012 | 1.610 | 0.213 |
| with CNDs–ZnO | 0.243 | 0.057 | 0.229 | 0.041 | 0.285 | 0.053 | 0.250 | 0.010 | 0.113 | 0.004 | |
| 3 | No treatment | 1.531 | 0.053 | 1.207 | 0.105 | 1.565 | 0.051 | 1.112 | 0.006 | 1.628 | 0.268 |
| with CNDs–ZnO | 0.124 | 0.005 | 0.123 | 0.005 | 0.125 | 0.016 | 0.111 | 0.005 | 0.119 | 0.024 | |
| 4 | No treatment | 1.536 | 0.126 | 1.198 | 0.089 | 1.520 | 0.009 | 1.119 | 0.012 | 1.685 | 0.213 |
| with CNDs–ZnO | 0.111 | 0.012 | 0.105 | 0.006 | 0.107 | 0.006 | 0.091 | 0.034 | 0.114 | 0.017 | |
| 5 | No treatment | 1.517 | 0.051 | 1.217 | 0.063 | 1.505 | 0.016 | 1.114 | 0.036 | 1.582 | 0.199 |
| with CNDs–ZnO | 0.062 | 0.013 | 0.384 | 0.109 | 0.056 | 0.005 | 0.064 | 0.018 | 0.146 | 0.037 | |
| 6 | No treatment | 1.425 | 0.117 | 1.191 | 0.116 | 1.472 | 0.012 | 1.180 | 0.044 | 1.594 | 0.208 |
| with CNDs–ZnO | 1.135 | 0.154 | 0.940 | 0.126 | 1.162 | 0.164 | 0.047 | 0.001 | 0.150 | 0.040 | |
| 7 | No treatment | 1.477 | 0.108 | 1.197 | 0.062 | 1.498 | 0.013 | 1.126 | 0.001 | 1.578 | 0.221 |
| with CNDs–ZnO | 1.388 | 0.055 | 1.061 | 0.116 | 1.317 | 0.057 | 0.044 | 0.003 | 0.276 | 0.079 | |
| 8 | No treatment | 1.478 | 0.101 | 1.206 | 0.061 | 1.486 | 0.014 | 1.149 | 0.024 | 1.568 | 0.143 |
| with CNDs–ZnO | 1.485 | 0.069 | 1.175 | 0.084 | 1.386 | 0.057 | 0.802 | 0.051 | 0.268 | 0.070 | |
| 9 | No treatment | 1.456 | 0.109 | 1.289 | 0.101 | 1.462 | 0.002 | 1.131 | 0.025 | 1.522 | 0.132 |
| with CNDs–ZnO | 1.383 | 0.106 | 1.182 | 0.064 | 1.447 | 0.001 | 1.118 | 0.004 | 0.952 | 0.042 | |
| 10 | No treatment | 1.419 | 0.092 | 1.225 | 0.059 | 1.449 | 0.002 | 1.138 | 0.052 | 1.485 | 0.140 |
| with CNDs–ZnO | 1.401 | 0.080 | 1.172 | 0.054 | 1.470 | 0.011 | 1.150 | 0.021 | 1.429 | 0.051 | |
| 11 | No treatment | 1.439 | 0.082 | 1.199 | 0.061 | 1.457 | 0.002 | 1.128 | 0.060 | 1.428 | 0.106 |
| with CNDs–ZnO | 1.413 | 0.075 | 1.200 | 0.068 | 1.453 | 0.018 | 1.180 | 0.009 | 1.546 | 0.063 | |
| Fungi | mm of Inhibition Zone (Mean ± SD) | |||
|---|---|---|---|---|
| Pure ZnO (Commercial) | CNDs–ZnO-1 | CNDs–ZnO-2 | ||
| Mold | A. flavus | - | 14 ± 1 | 15 ± 1 |
| A. niger | Negligible | 22 ± 2 | 23 ± 2 | |
| A. terreus | - | 12 ± 1 | 13 ± 1 | |
| Yeast | R. mucilaginosa | Negligible | 24 ± 1 | 28 ± 2 |
| S. cerevisiae | - | 12 ± 1 | 16 ± 1 | |
| Time | Developmental Rate of Zebrafish Embryos (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorine Bleach | Pure ZnO | Dilution Ratio of CNDs–ZnO | ||||||||||
| CNDs–ZnO-1 | CNDs–ZnO-2 | |||||||||||
| 0 | 1:10 | 1:100 | 1:1000 | 1:10,000 | 0 | 1:10 | 1:100 | 1:1000 | 1:10,000 | |||
| 0 h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 0.5 h | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 24 h | 0 | 0 | 0 | 80 | 100 | 100 | 100 | 0 | 80 | 60 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.W.; Chung, D.; Jung, S.-H.; Kwon, Y.M.; Kim, J.Y.H.; Baek, K. Antimicrobial Effect of Carbon Nanodots–ZnO Nanocomposite Synthesized Using Sargassum horneri. J. Mar. Sci. Eng. 2022, 10, 1546. https://doi.org/10.3390/jmse10101546
Kim KW, Chung D, Jung S-H, Kwon YM, Kim JYH, Baek K. Antimicrobial Effect of Carbon Nanodots–ZnO Nanocomposite Synthesized Using Sargassum horneri. Journal of Marine Science and Engineering. 2022; 10(10):1546. https://doi.org/10.3390/jmse10101546
Chicago/Turabian StyleKim, Kyung Woo, Dawoon Chung, Seung-Hyun Jung, Yong Min Kwon, Jawoon Young Hwan Kim, and Kyunghwa Baek. 2022. "Antimicrobial Effect of Carbon Nanodots–ZnO Nanocomposite Synthesized Using Sargassum horneri" Journal of Marine Science and Engineering 10, no. 10: 1546. https://doi.org/10.3390/jmse10101546
APA StyleKim, K. W., Chung, D., Jung, S.-H., Kwon, Y. M., Kim, J. Y. H., & Baek, K. (2022). Antimicrobial Effect of Carbon Nanodots–ZnO Nanocomposite Synthesized Using Sargassum horneri. Journal of Marine Science and Engineering, 10(10), 1546. https://doi.org/10.3390/jmse10101546







