Sea Anemone Aiptasiomorpha minuta (Verrill, 1867) as a Possible Agent to Control Biofouling in Oyster Culture and the Optimal Conditions for Its Mass Rearing under Laboratory Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.1.1. Source of Aiptasiomorpha minuta
2.1.2. Culture of Oysters in Isahaya Bay
2.1.3. Field Data Collection and Analysis
2.2. Laboratory Experiment
2.2.1. Culture of Aiptasiomorpha minuta under Laboratory Conditions
Optimization of Temperature Condition
Optimization of Diet Condition
Optimization of Salinity Condition
2.3. Statistical Analysis
3. Results
3.1. Field Experiment
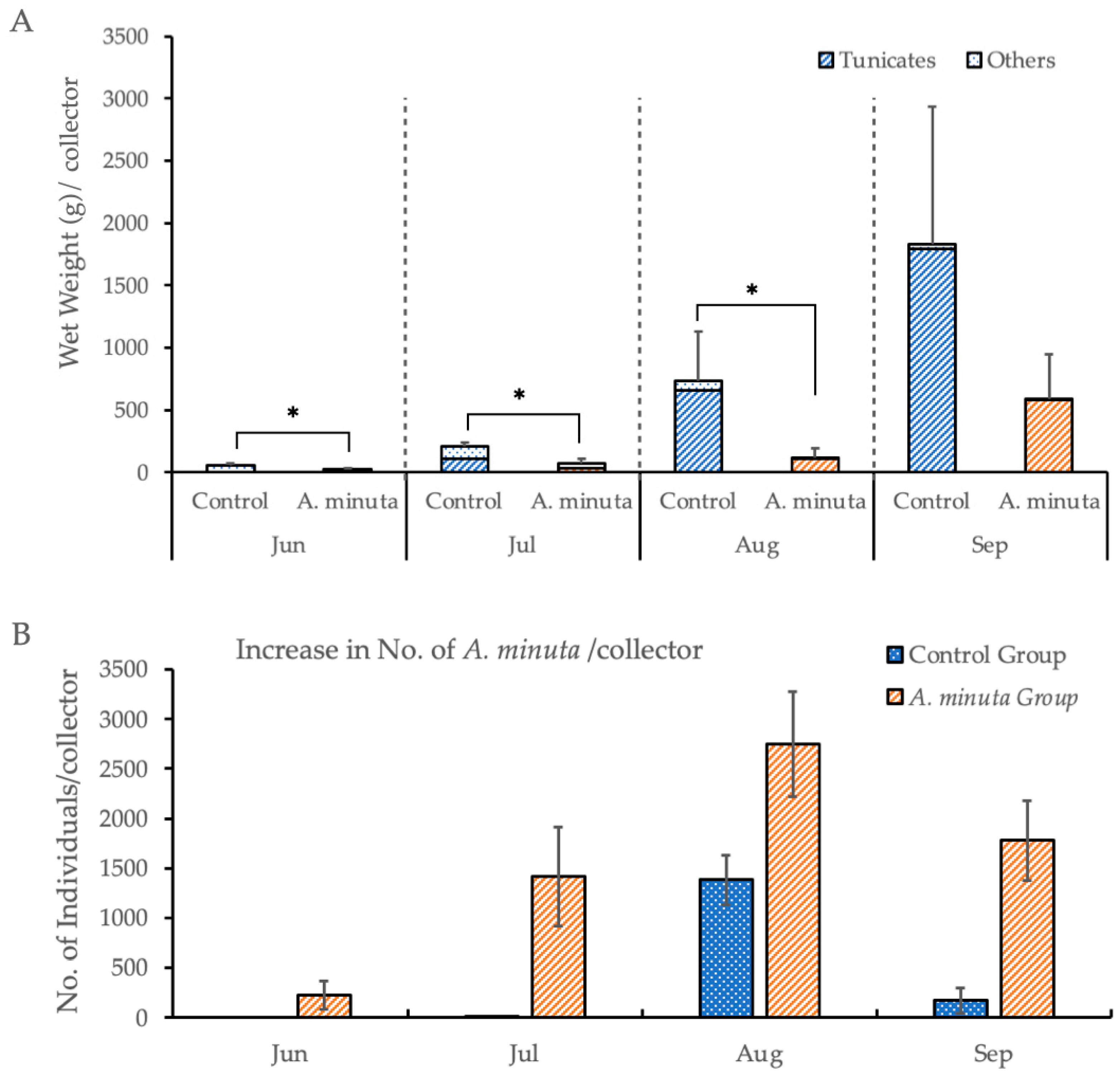
3.1.1. Effect of Aiptasiomorpha minuta on Biofouling of Oyster Collectors
3.1.2. Effect of A. minuta on the Growth and Survival of Cultured Oysters
3.2. Laboratory Experiment
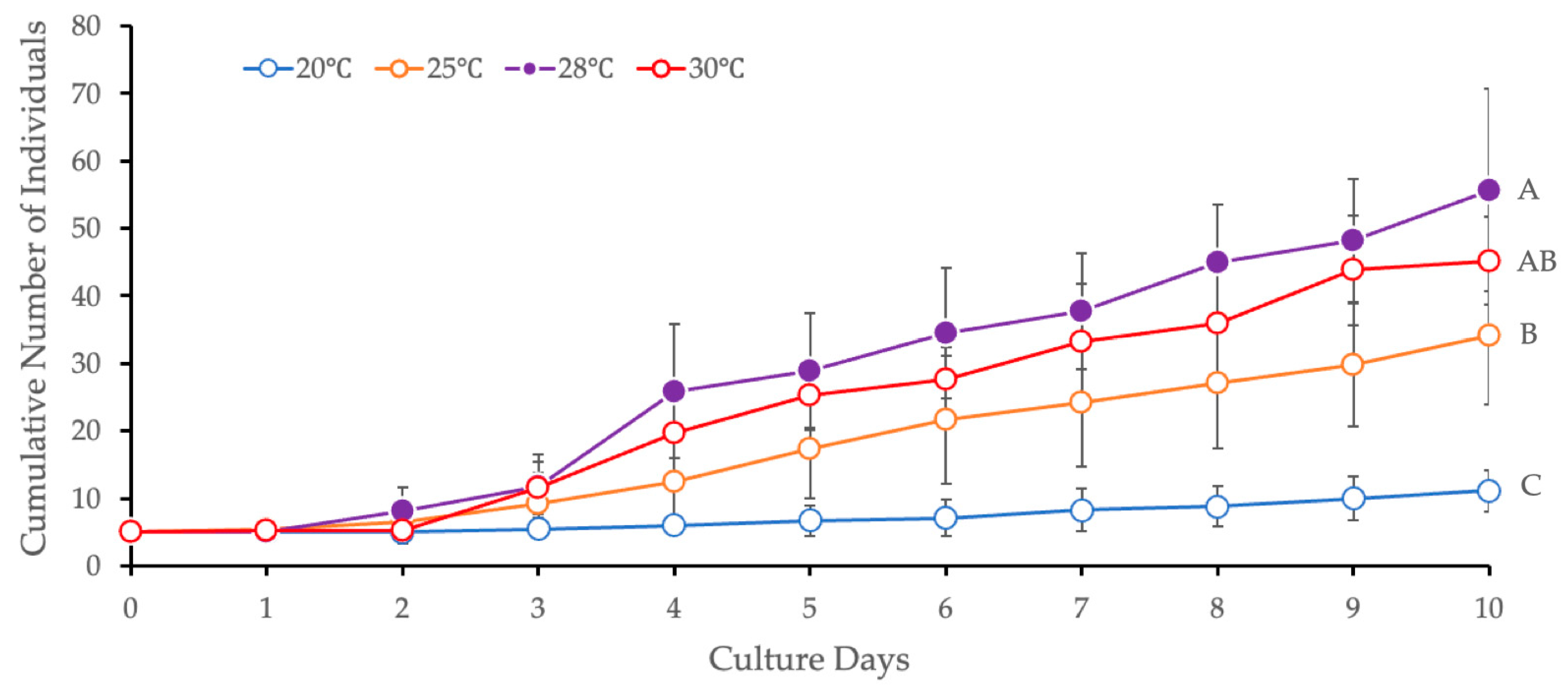
3.2.1. Temperature
3.2.2. Diet Regimen
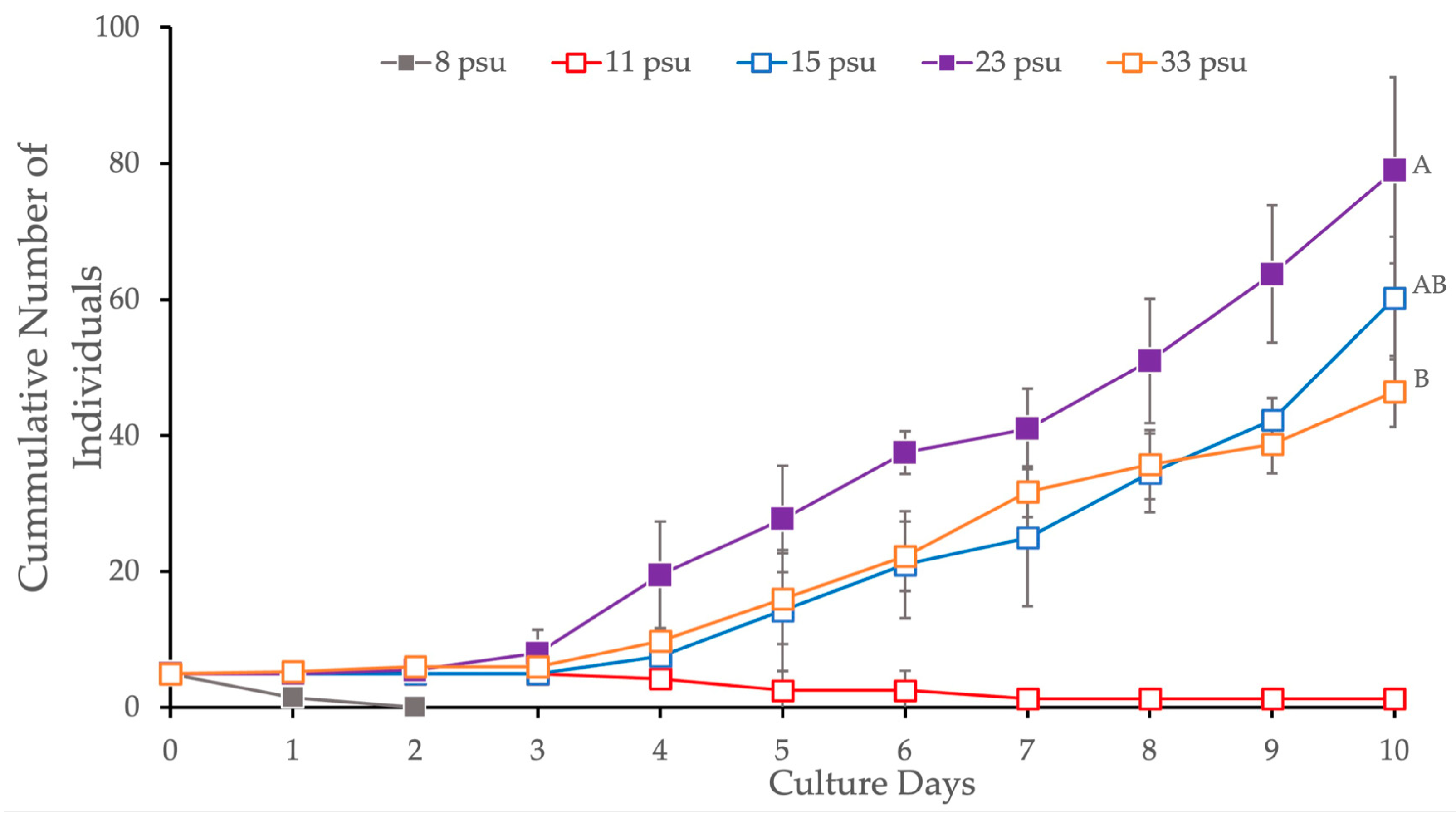
3.2.3. Salinity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Ecological Society of Japan (Ed.) Handbook OfAlien Species in Japan; Chijinshokan: Tokyo, Japan, 2002; ISBN 480520706X.
- Uchida, H.; Soyama, I. Sea Anemones in Japanese Waters; TBS Britannica Co., Ltd.: Tokyo, Japan, 2001; ISBN 4484014076. [Google Scholar]
- Bocharova, E. Reproduction of Sea Anemones and Other Hexacorals. In The Cnidaria, Past, Present and Future; Goffredo, S., Dubinsky, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 239–248. ISBN 978-3-319-31303-0. [Google Scholar]
- Gashout, S.E.; Ormond, R.F.G. Evidence for Parthenogenetic Reproduction in the Sea Anemone Actinia equina L. J. Mar. Biol. Assoc. UK 1979, 59, 975–987. [Google Scholar] [CrossRef]
- Grawunder, D.; Hambleton, E.A.; Bucher, M.; Wolfowicz, I.; Bechtoldt, N.; Guse, A. Induction of Gametogenesis in the Cnidarian Endosymbiosis Model Aiptasia sp. Sci. Rep. 2015, 5, 15677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, N.; Onitsuka, T.; Takeyama, S.; Maekawa, K. Oyster Culture in Hokkaido, Japan. Bull. Fish. Res. Agency 2015, 173–177. [Google Scholar]
- Higano, J.; Hirano, K.; Kitahara, S.; Matsuda, M.; Mizuta, K.; Fujii, A.; Shinagawa, A. Manila Clam and Pacific Oyster Culture in Isahaya Bay. Bull. Fish. Res. Agency 2010, 29, 39–47. [Google Scholar]
- Satuito, C.G.; Yamada, H.; Ohashi, S.; Kitamura, H. Occurrence and Variation in Abundance of Fouling Organisms in an Oyster Farm in Isahaya Bay, Nagasaki Prefecture. Sess. Org. 2013, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, K. Prevention and Removal of Fouling on Cultured Oysters: A Handbook for Growers (Translated into English by R. B. Gillmor). Maine Sea Grant Tech. Rep. 1973, 56, 1–38. [Google Scholar]
- Takahashi, T.; Umehara, A.; Tsutsumi, H. Diffusion of Microcystins (Cyanobacteria hepatotoxins) from the Reservoir of Isahaya Bay, Japan, into the Marine and Surrounding Ecosystems as a Result of Large-Scale Drainage. Mar. Pollut. Bull. 2014, 89, 250–258. [Google Scholar] [CrossRef]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for Unifying the Terminology in Biological Control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Baker, K.F.; Cook., R.J. Biological Control of Plant Pathogens; W.H. Freeman and Company: San Francisco, CA, USA, 1974; ISBN 9780716705895. [Google Scholar]
- Cigarría, J.; Fernández, J.; Magadán, L.P. Feasibility of Biological Control of Algal Fouling in Intertidal Oyster Culture Using Periwinkles. J. Shellfish Res. 1998, 17, 1167–1169. [Google Scholar]
- Ross, K.A.; Thorpe, J.P.; Brand, A.R. Biological Control of Fouling in Suspended Scallop Cultivation. Aquaculture 2004, 229, 99–116. [Google Scholar] [CrossRef]
- Lodeiros, C.; García, N. The Use of Sea Urchins to Control Fouling during Suspended Culture of Bivalves. Aquaculture 2004, 231, 293–298. [Google Scholar] [CrossRef]
- Zhanhui, Q.; Jun, W.; Yuze, M.; Jihong, Z.; Zengjie, J.; Jianguang, F. Use of the Sea Urchin Hemicentrotus pulcherrimus for Biological Control of Fouling in Suspended Scallop Cultivation in Northern China. Aquaculture 2014, 420–421, 270–274. [Google Scholar] [CrossRef]
- Sterling, A.M.; Cross, S.F.; Pearce, C.M. Co-Culturing Green Sea Urchins (Strongylocentrotus droebachiensis) with Mussels (Mytilus Spp.) to Control Biofouling at an Integrated Multi-Trophic Aquaculture Site. Aquaculture 2016, 464, 253–261. [Google Scholar] [CrossRef]
- Atalah, J.; Hopkins, G.A.; Forrest, B.M. Augmentative Biocontrol in Natural Marine Habitats: Persistence, Spread and Non-Target Effects of the Sea Urchin Evechinus chloroticus. PLoS ONE 2013, 8, e80365. [Google Scholar] [CrossRef] [PubMed]
- Atalah, J.; Bennett, H.; Hopkins, G.A.; Forrest, B.M. Evaluation of the Sea Anemone Anthothoe albocincta as an Augmentative Biocontrol Agent for Biofouling on Artificial Structures. Biofouling 2013, 29, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-I.; Donaghy, L.; Kang, H.-S.; Hong, H.-K.; Kim, Y.-O.; Choi, K.-S. Assessment of Immune Parameters of Manila Clam Ruditapes philippinarum in Different Physiological Conditions Using Flow Cytometry. Ocean Sci. J. 2012, 47, 19–26. [Google Scholar] [CrossRef]
- SAS Institute Inc. JMP®. Pro 16.0. Cary, NC, USA 1989–2021. Available online: https://www.jmp.com/en_us/software/predictive-analytics-software.html (accessed on 13 September 2022).
- Chomsky, O.; Kamenir, Y.; Hyams, M.; Dubinsky, Z.; Chadwick-Furman, N.E. Effects of Temperature on Growth Rate and Body Size in the Mediterranean Sea Anemone Actinia equina. J. Exp. Mar. Biol. Ecol. 2004, 313, 63–73. [Google Scholar] [CrossRef]
- Clayton, W.S.; Lasker, H.R. Individual and Population Growth in the Asexually Reproducing Anemone Aiptasia pallida perrill. J. Exp. Mar. Biol. Ecol. 1985, 90, 249–258. [Google Scholar] [CrossRef]
- Glon, H.; Haruka, Y.; Daly, M.; Nakaoka, M. Temperature and Salinity Survival Limits of the Fluffy Sea Anemone, Metridium senile (L.), in Japan. Hydrobiologia 2019, 830, 303–315. [Google Scholar] [CrossRef]





| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| (Temperature) | (Diet) | (Salinity) | ||||
| Container | 1 L beaker | 1 L beaker | 1 L beaker | |||
| Water exchange | once/day | once/day | once/day | |||
| Light intensity and cycle | 3.2 W/m2, 12 h light:12 h dark | 3.2 W/m2, 12 h light:12 h dark | 3.2 W/m2, 12 h light:12 h dark | |||
| Aeration | yes | yes | yes | |||
| Temperature (°C) | 20, 25, 28, 30 | 28 | 28 | |||
| Salinity (psu) | 33 to 34 | 33 to 34 | 8, 11, 15, 23, 33 | |||
| Diet | Artemia salina nauplii (ind./day) | 250 | unfed | N/A | A. salina nauplii (ind./day) | 1000 |
| A. salina nauplii (ind./day) | 250, 500, 1000 | |||||
| Fistulobalanus kondakovi nauplii (ind./day) | 1000 | |||||
| Culture days | 10 | 14 | 10 | |||
| Submerged Period | |||||||||
| May 25 to Jun 22 | May 25 to Jul 18 | May 25 to Aug 17 | May 25 to Sep 21 | ||||||
| Water Temperature (C°) | 23.5 | 24.2 | 27 | 29.3 | 26.5 | ||||
| Salinity (psu) | 33 | 34 | 33 | 32 | 30 | ||||
| Phylum/Class | Organism | Occurrence | |||||||
| Control | A. minuta | Control | A. minuta | Control | A. minuta | Control | A. minuta | ||
| Rhodophyta | Ceremiales | ± | + | ± | ± | ||||
| Chlorophyta | Ulvales | ± | ± | ||||||
| Porifera | Halichondria japonica (Kadota, 1922) | ± | |||||||
| H. okadai (Kadota, 1922) | ± | ||||||||
| unidentified sponges | ± | ± | ± | ||||||
| Cnidaria | Tubulariidae | ⧻ | ± | ± | ± | ||||
| Ectoprocta | Bugula californica Robertson, 1905 | + | ± | ± | ± | ± | ± | ± | |
| Bugula neritina (Linnaeus, 1758) | ⧻ | + | + | + | ± | ± | ± | ± | |
| Bugulidae | ± | ± | |||||||
| Membraniporidae | ± | ± | ± | ± | ± | ||||
| Mollusca/Bivalvia | Modiolus nipponicus (Oyama, 1950) | ± | ± | ± | ± | ||||
| Musculita senhousia (Benson, 1842) | ± | ± | ± | ± | |||||
| Mytilus galloprovincialis Lamarck, 1819 | ± | ± | |||||||
| other bivalves | ± | ± | ± | ± | |||||
| Arthropoda | Fistulobalanus kondakovi (Tarasov and Zevina, 1957) | ± | ± | ||||||
| Small barnacles (AD < 4 mm) | ± | ± | ± | ± | |||||
| Chordata | Ciona intestinalis (Linnaeus, 1767) | ± | ± | ± | ± | ± | |||
| Styela plicata (Lesueur, 1823) | + | + | ⧻ | ⧻ | ⧻ | ⧻ | |||
| Solitary ascidians (BL < 5 mm) | ± | ± | |||||||
| Biological Parameter | Month | p-Value | Statistical Test |
|---|---|---|---|
| Total weight of fouling organisms | JUN | 0.0304 * | Wilcoxon matched pairs test |
| JUL | 0.0304 * | ||
| AUG | 0.0304 * | ||
| SEP | 0.1124 | ||
| Shell Growth | JUN | 0.4893 | Wilcoxon matched pairs test |
| JUL | 0.0724 | ||
| AUG | 0.0258 * | ||
| SEP | 0.0012 * | ||
| Condition Index | JUN | 0.1872 | Wilcoxon matched pairs test |
| JUL | 0.0004 * | ||
| AUG | 0.0207 * | ||
| SEP | <0.0001 * | ||
| Oyster Survival | JUN | 0.1587 | Student’s t-test |
| JUL | 0.1473 | ||
| AUG | 0.2247 | ||
| SEP | 0.003 * |
| Experiment | Groups Compared | p-Value | Statistical Test |
|---|---|---|---|
| 1 (Temperature) | 20 vs. 25 | <0.0001 * | Steel-Dwass multiple comparison test |
| 20 vs. 28 | 0.0007 * | ||
| 20 vs. 30 | 0.0115 * | ||
| 25 vs. 28 | 0.0025 * | ||
| 25 vs. 30 | 0.1221 | ||
| 28 vs. 30 | 0.6875 | ||
| 2 (Diet) | not fed vs. A. salina 250 | 0.5457 | Tukey-HSD |
| not fed vs. A. salina 500 | 0.1525 | ||
| not fed vs. A. salina 1000 | 0.0273 * | ||
| not fed vs. F. albicostatus 1000 | 0.2728 | ||
| F. albicostatus 1000 vs. A. salina 250 | 0.9752 | ||
| F. albicostatus 1000 vs. A. salina 500 | 0.993 | ||
| F. albicostatus 1000 vs. A. salina 1000 | 0.5665 | ||
| A. salina 250 vs. A. salina 500 | 0.8539 | ||
| A. salina 250 vs. A. salina 1000 | 0.2868 | ||
| A. salina 500 vs. A. salina 1000 | 0.7925 | ||
| 3 (Salinity) | 15 vs. 23 | 0.0605 | Tukey-HSD |
| 15 vs. 33 | 0.1785 | ||
| 23 vs. 33 | 0.0032 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedanza, M.G.; Kim, H.-J.; Satuito, C.G. Sea Anemone Aiptasiomorpha minuta (Verrill, 1867) as a Possible Agent to Control Biofouling in Oyster Culture and the Optimal Conditions for Its Mass Rearing under Laboratory Conditions. J. Mar. Sci. Eng. 2022, 10, 1490. https://doi.org/10.3390/jmse10101490
Sedanza MG, Kim H-J, Satuito CG. Sea Anemone Aiptasiomorpha minuta (Verrill, 1867) as a Possible Agent to Control Biofouling in Oyster Culture and the Optimal Conditions for Its Mass Rearing under Laboratory Conditions. Journal of Marine Science and Engineering. 2022; 10(10):1490. https://doi.org/10.3390/jmse10101490
Chicago/Turabian StyleSedanza, Mary Grace, Hee-Jin Kim, and Cyril Glenn Satuito. 2022. "Sea Anemone Aiptasiomorpha minuta (Verrill, 1867) as a Possible Agent to Control Biofouling in Oyster Culture and the Optimal Conditions for Its Mass Rearing under Laboratory Conditions" Journal of Marine Science and Engineering 10, no. 10: 1490. https://doi.org/10.3390/jmse10101490
APA StyleSedanza, M. G., Kim, H.-J., & Satuito, C. G. (2022). Sea Anemone Aiptasiomorpha minuta (Verrill, 1867) as a Possible Agent to Control Biofouling in Oyster Culture and the Optimal Conditions for Its Mass Rearing under Laboratory Conditions. Journal of Marine Science and Engineering, 10(10), 1490. https://doi.org/10.3390/jmse10101490






