Keeping a Clean Surface under Water: Nanoscale Nipple Array Decreases Surface Adsorption and Adhesion Forces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AFM Measurement of Surface Structure
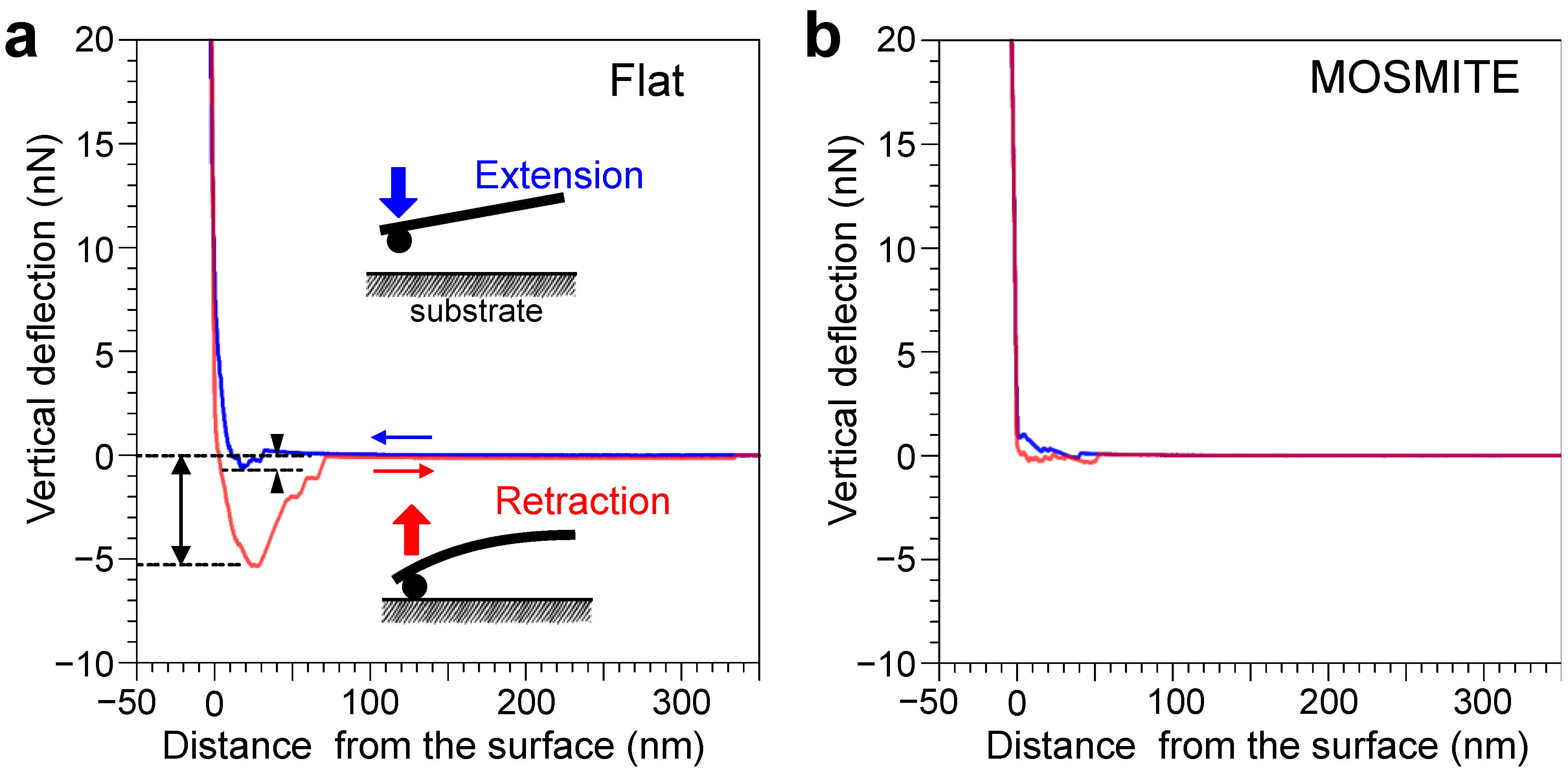
2.3. AFM Measurement of Adsorption and Adhesion Force
2.4. Statistics
3. Results
3.1. Surface Structure
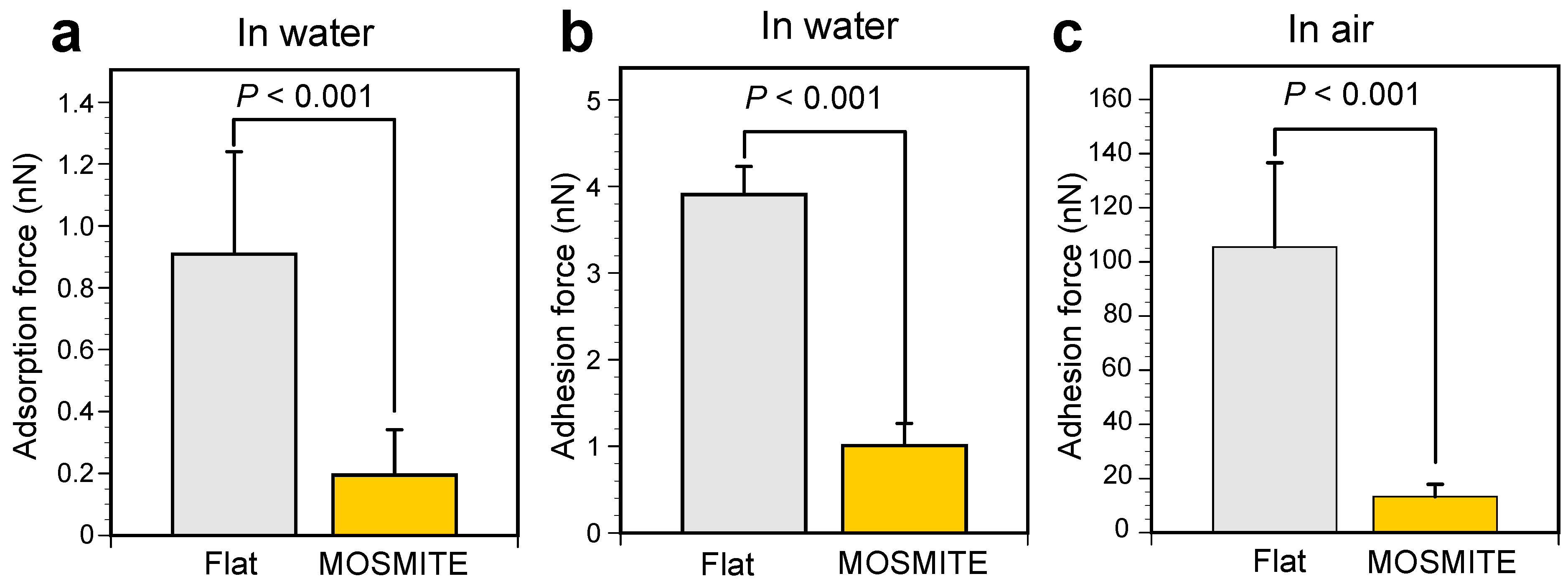
3.2. Adsorption and Adhesion Force
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Nørgaard, T.; Dacke, M. Fog-basking behaviour and water collection efficiency in Namib Desert Darkling beetles. Front. Zool. 2010, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Lauder, G.V.; Wainwright, D.K.; Domel, A.G.; Weaver, J.C.; Wen, L.; Bertoldi, K. Structure, biomimetics, and fluid dynamics of fish skin surfaces. Phys. Rev. Fluids 2016, 1, 1–18. [Google Scholar] [CrossRef]
- Uesugi, K.; Mayama, H.; Morishima, K. Proposal of a water-repellency model of water strider and its verification by considering directly measured strider leg-rowing force. J. Photopolym. Sci. Technol. 2020, 33, 185–192. [Google Scholar] [CrossRef]
- Uesugi, K. Water-repellency model of the water strider, Aquarius paludum paludum, by the curved structure of leg micro-hairs. J. Photopolym. Sci. Technol. 2021, 34, 393–399. [Google Scholar] [CrossRef]
- Bernhard, C.G. Structural and functional adaptation in a visual system. Endeavour 1967, 26, 79–84. [Google Scholar]
- Wilson, S.J.; Hutley, M.C. The optical properties of “moth eye” antireflection surfaces. Opt. Acta: Int. J. Opt. 1982, 29, 993–1009. [Google Scholar] [CrossRef]
- Watson, G.S.; Myhra, S.; Cribb, B.W.; Watson, J.A. Putative functions and functional efficiency of ordered cuticular nanoarrays on insect wings. Biophys. J. 2008, 94, 3352–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peisker, H.; Gorb, S.N. Always on the bright side of life: Anti-adhesive properties of insect ommatidia grating. J. Exp. Biol. 2010, 213, 3457–3462. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Hausen, H. Comparative structure of the epidermis in polychaetes (Annelida). Hydrobiologia 2005, 535, 25–35. [Google Scholar] [CrossRef]
- Holland, N.D.; Nealson, K.H. The fine structure of the echinoderm cuticle and the subcuticular bacteria of echinoderms. Acta Zool. 1978, 59, 169–185. [Google Scholar] [CrossRef]
- Flammang, P.; Jangoux, M. Functional morphology of coronal and peristomeal podia in Sphaerechinus granularis (Echinodermata, Echinoida) Patrick. Zoomorphology 1993, 113, 47–60. [Google Scholar] [CrossRef]
- Hirose, E.; Saito, Y.; Hashimoto, K.; Watanabe, H. Minute protrusions of the cuticle: Fine surface structures of the tunic in ascidians. J. Morphol. 1990, 204, 67–73. [Google Scholar] [CrossRef]
- Hirose, E.; Lambert, G.; Kusakabe, T.; Nishikawa, T. Tunic cuticular protrusions in ascidians (Chordata, Tunicata): A perspective of their character-state distribution. Zool. Sci. 1997, 14, 683–689. [Google Scholar] [CrossRef]
- Hirose, E.; Kimura, S.; Itoh, T.; Nishikawa, J. Tunic morphology and cellulosic components of pyrosomas, doliolids, and salps (Thaliacea, Urochordata). Biol. Bull. 1999, 196, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Uyeno, D. Histopathology of a mesoparasitic hatschekiid copepod in hospite: Does Mihbaicola sakamakii (Copepoda: Siphonostomatoida: Hatschekiidae) fast within the host fish tissue? Zool. Sci. 2014, 31, 546–552. [Google Scholar] [CrossRef]
- Hirose, E.; Uyeno, D. Regional differentiation of the cuticular surface structure in the mesoparasitic copepod Cardiodectes shini (Siphonostomatoida: Pennellidae) on a pygmy goby. Invertebr. Surviv. J. 2016, 13, 134–139. [Google Scholar] [CrossRef]
- Hirose, E.; Sakai, D.; Shibata, T.; Nishii, J.; Mayama, H.; Miyauchi, A.; Nishikawa, J. Does the tunic nipple array serve to camouflage diurnal salps? J. Mar. Biol. Assoc. UK 2015, 95, 1025–1031. [Google Scholar] [CrossRef]
- Kakiuchida, H.; Sakai, D.; Nishikawa, J.; Hirose, E. Measurement of refractive indices of tunicates’ tunics: Light reflection of the transparent integuments in an ascidian Rhopalaea sp. and a salp Thetys vagina. Zool. Lett. 2017, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Hirose, E.; Mayama, H.; Miyauchi, A. Does the aquatic invertebrate nipple array prevent bubble adhesion? An experiment using nanopillar sheets. Biol. Lett. 2013, 9, 20130552. [Google Scholar] [CrossRef] [Green Version]
- Ballarin, L.; Franchi, N.; Gasparini, F.; Caicci, F.; Miyauchi, A.; Hirose, E. Suppression of cell-spreading and phagocytic activity on nano-pillared surface: In vitro experiment using hemocytes of the colonial ascidian Botryllus schlosseri. Invertebr. Surviv. J. 2015, 12, 82–88. [Google Scholar]
- Hirose, E.; Sensui, N. Does a nano-scale nipple array (moth-eye structure) suppress the settlement of ascidian larvae? J. Mar. Biol. Assoc. UK 2019, 99, 1393–1397. [Google Scholar] [CrossRef]
- Nagayama, K. Biomechanical analysis of the mechanical environment of the cell nucleus in serum starvation-induced vascular smooth muscle cell differentiation. J. Biomech. Sci. Eng. 2019, 14, 19-00364. [Google Scholar] [CrossRef] [Green Version]
- Terzaghi, K.; Peck, R.B.; Mesri, G. Soil Mechanics in Engineering Practice, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1996; ISBN 978-0-471-08658-1. [Google Scholar]
- Murray, H.H. Chapter 2 Structure and composition of the clay minerals and their physical and chemical properties. In Applied Clay Mineralogy; Murray, H.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 7–31. ISBN 9780444517012. [Google Scholar]
- Borges Cabrera, M.; Satomi, T.; Takahashi, H. Temperature: A key parameter on soil content reduction simulation in recycled asphalt aggregate. J. Clean. Prod. 2020, 261, 121236. [Google Scholar] [CrossRef]
- Zhang, W.; Stack, A.G.; Chen, Y. Interaction force measurement between E. coli cells and nanoparticles immobilized surfaces by using AFM. Colloids Surf. B Biointerfaces 2011, 82, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Spalding, A.; Shanks, K.; Bennie, J.; Potter, U.; Ffrench-Constant, R. Optical modelling and phylogenetic analysis provide in butterflies and moths. Insects 2019, 10, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagodatski, A.; Sergeev, A.; Kryuchkov, M.; Lopatina, Y.; Katanaev, V.L. Diverse set of Turing nanopatterns coat corneae across insect lineages. Proc. Natl. Acad. Sci. USA 2015, 112, 10750–10755. [Google Scholar] [CrossRef] [Green Version]
- Kryuchkov, M.; Bilousov, O.; Lehmann, J.; Fiebig, M.; Katanaev, V.L. Reverse and forward engineering of Drosophila corneal nanocoatings. Nature 2020, 585, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Oeffner, J.; Lauder, G.V. The hydrodynamic function of shark skin and two biomimetic applications. J. Exp. Biol. 2012, 215, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihm, J.W.; Banta, W.C.; Loeb, G.I. Effects of adsorbed organic and primary fouling films on bryozoan settlement. J. Exp. Mar. Biol. Ecol. 1981, 54, 167–179. [Google Scholar] [CrossRef]
- Flemming, H.C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Hirose, E.; Sensui, N. Substrate selection of ascidian larva: Wettability and nano-structures. J. Mar. Sci. Eng. 2021, 9, 634. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uesugi, K.; Nagayama, K.; Hirose, E. Keeping a Clean Surface under Water: Nanoscale Nipple Array Decreases Surface Adsorption and Adhesion Forces. J. Mar. Sci. Eng. 2022, 10, 81. https://doi.org/10.3390/jmse10010081
Uesugi K, Nagayama K, Hirose E. Keeping a Clean Surface under Water: Nanoscale Nipple Array Decreases Surface Adsorption and Adhesion Forces. Journal of Marine Science and Engineering. 2022; 10(1):81. https://doi.org/10.3390/jmse10010081
Chicago/Turabian StyleUesugi, Kaoru, Kazuaki Nagayama, and Euichi Hirose. 2022. "Keeping a Clean Surface under Water: Nanoscale Nipple Array Decreases Surface Adsorption and Adhesion Forces" Journal of Marine Science and Engineering 10, no. 1: 81. https://doi.org/10.3390/jmse10010081
APA StyleUesugi, K., Nagayama, K., & Hirose, E. (2022). Keeping a Clean Surface under Water: Nanoscale Nipple Array Decreases Surface Adsorption and Adhesion Forces. Journal of Marine Science and Engineering, 10(1), 81. https://doi.org/10.3390/jmse10010081






