Comparative Analysis of Lipids and Fatty Acids in Beaked Redfish Sebastes mentella Travin, 1951 Collected in Wild and in Commercial Products
Abstract
:1. Introduction
2. Materials and Methods
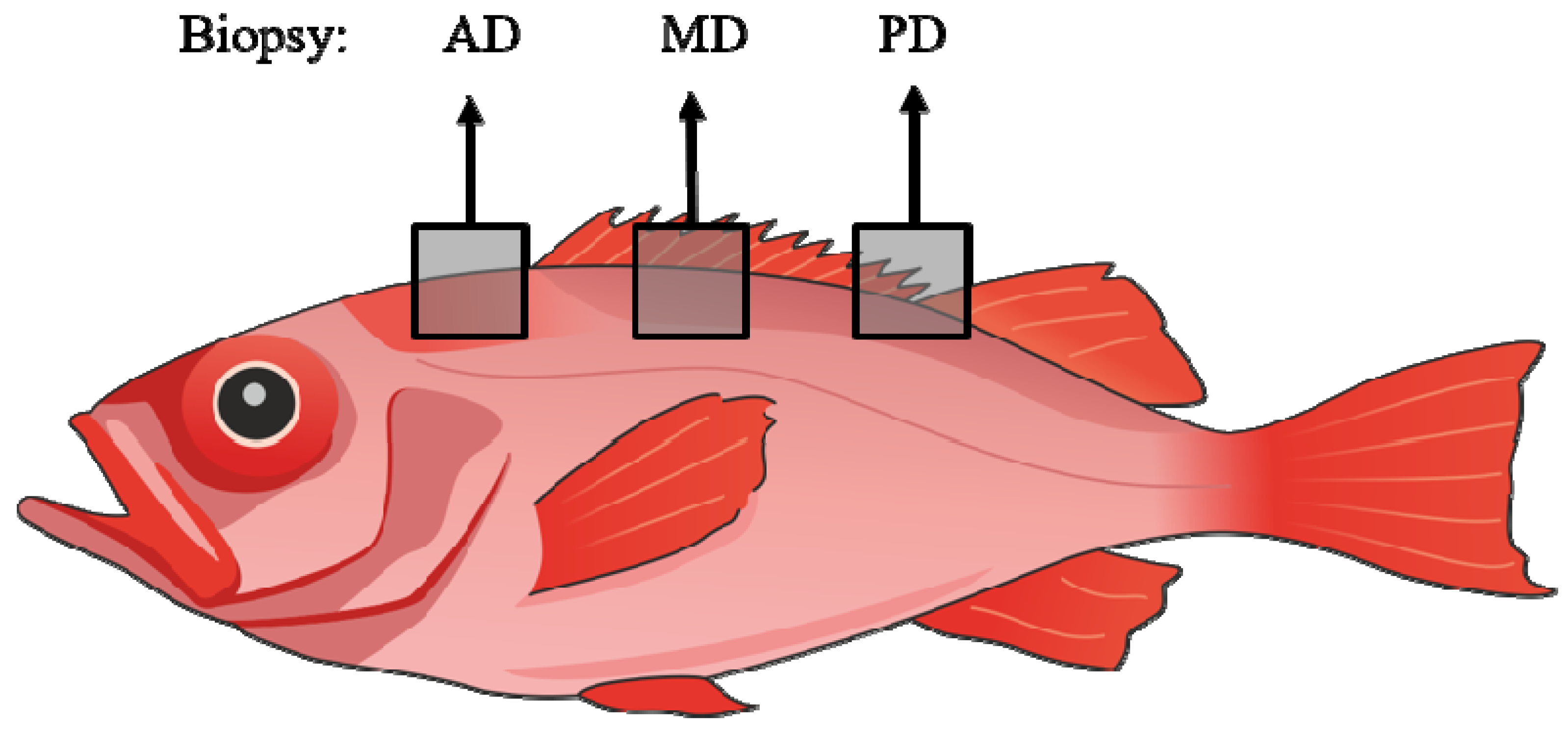
2.1. Sampling
2.2. Lipid Extraction and Analysis
2.2.1. Determination of Certain Lipid Classes by HPTLC
2.2.2. Determination of Certain Phospholipid Classes by HPLC
2.2.3. Fatty Acids Analysis by GC
2.3. The Lipid Quality, Health and Nutritional Indexes from the Fatty Acids Composition
2.4. Statistical Analysis
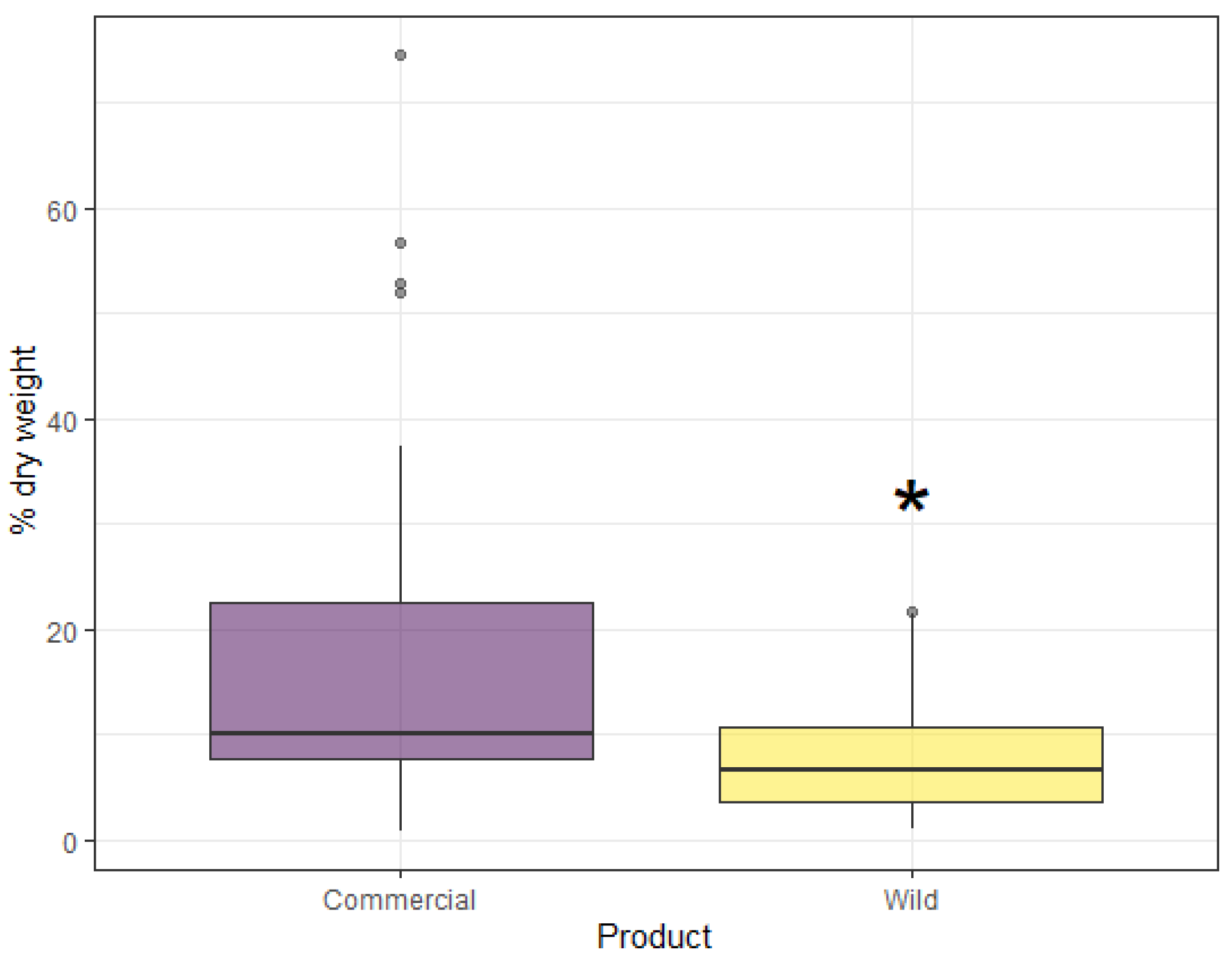
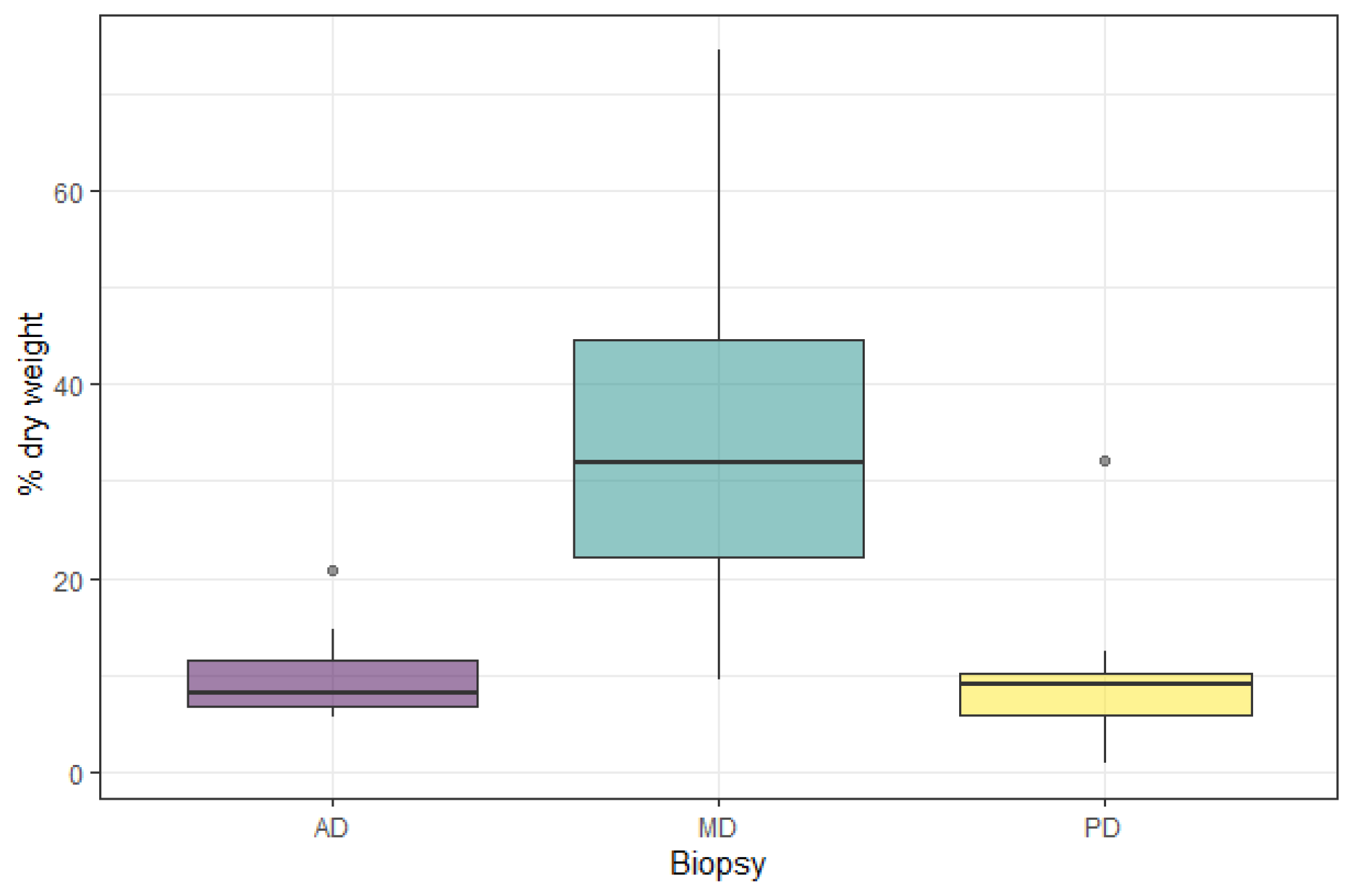
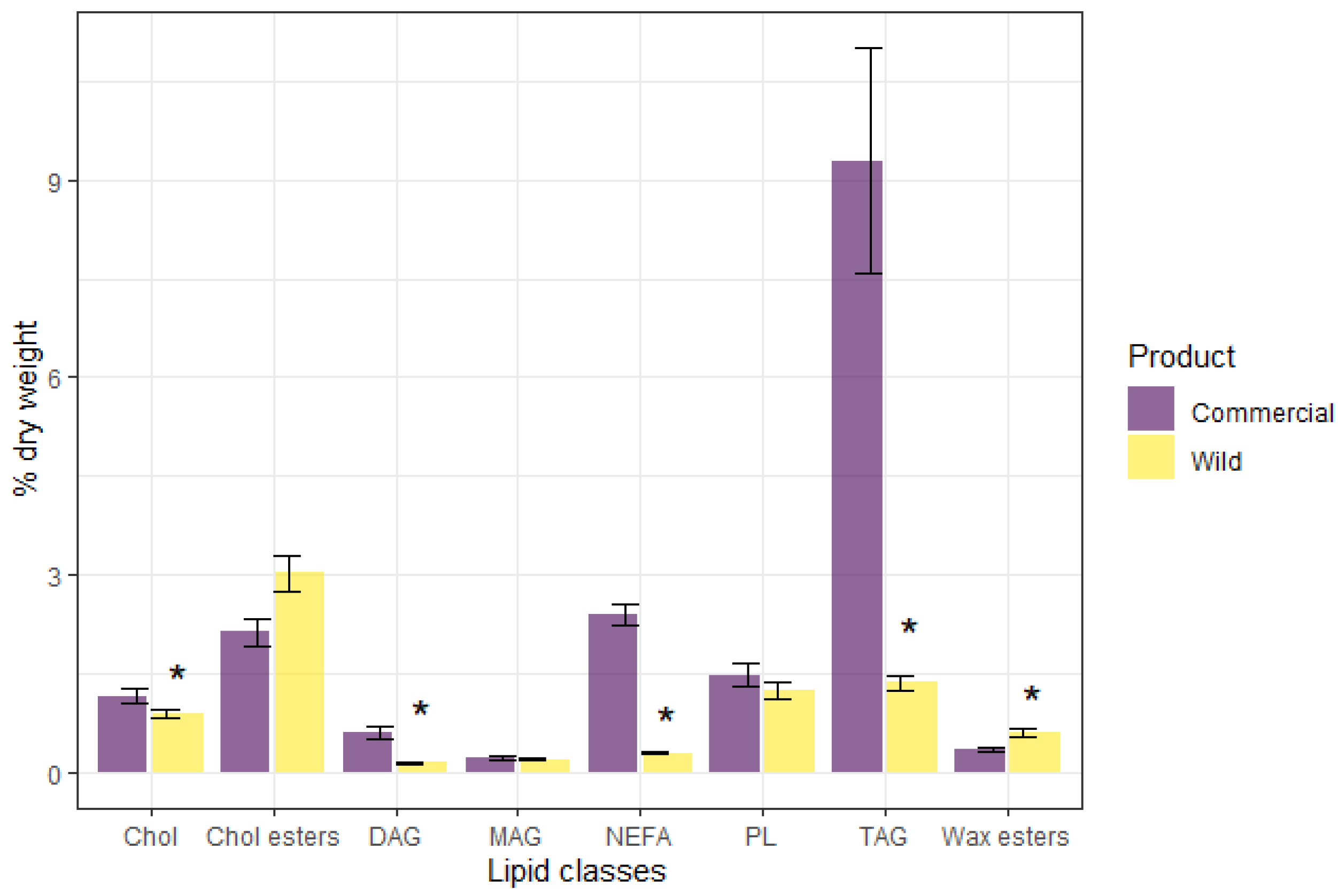
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hidalgo, M.; Browman, H.I. Developing the knowledge base needed to sustainably manage mesopelagic resources. ICES J. Mar. Sci. 2019, 76, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Ababouch, L. Impact of Fish Safety and Quality on Food Security. FAO Fish. Report (FAO). 2003. Available online: https://www.fao.org/3/y4961e/y4961e03.htm (accessed on 14 November 2021).
- Orlov, A.M.; Rabazanov, N.I. Past, Present and Future of Deep-Sea Fisheries in the Global Oceans. Mod. App. Ocean. Pet Sci. 2019, 3, 255–257. [Google Scholar] [CrossRef]
- Naito, Y.; Costa, D.P.; Adachi, T.; Robinson, P.W.; Fowler, M.; Takahashi, A. Unravelling the mysteries of a mesopelagic diet: A large apex predator specializes on small prey. Funct. Ecol. 2013, 2, 710–717. [Google Scholar] [CrossRef]
- Irigoien, X.; Klevjer, T.A.; Røstad, A.; Martinez, U.; Boyra, G.; Acuña, J.L.; Bode, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Kaartvedt, S.; et al. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 2014, 5, 3271. [Google Scholar] [CrossRef] [Green Version]
- Proud, R.; Cox, M.; Brierley, A. Biogeography of the Global Ocean’s Mesopelagic Zone. Curr. Biol. 2016, 27, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreps, E.M. Lipids of Cellular Membranes. Evolution of Brain Lipids. Adaptive Function of Lipids; Nauka: St. Petersburg, Russia, 1981; p. 339. [Google Scholar]
- Sidorov, V.S. Ecological Biochemistry of Fish; Nauka: St. Petersburg, Russia, 1983; p. 240. [Google Scholar]
- Henderson, R.J. Fatty acid metabolism in freshwater fish with particular reference to polyunsaturated fatty acid. Arch. Anim. Nutr. 1996, 49, 5–22. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bell, J.G.; Dick, J.R.; Henderson, R.J.; McGhee, F.; Michell, D.; Morris, P.C. Polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation and the effects of dietary linseed and rapeseed oils. Fish Physiol. Biochem. 2000, 23, 59–73. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: Oxford, NY, USA, 2002; p. 466. [Google Scholar]
- Arts, M.T.; Kohler, C.C. Health and conditions in fish: The influence of lipids on membrane competency and immune response. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M.J., Eds.; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2009; pp. 237–257. [Google Scholar]
- Murzina, S.A.; Nefedova, Z.A.; Nemova, N.N. Effects of fatty acids (biomarkers of food sources) on the mechanisms of fish adaptation in high latitudes. Trans. KarRC RAS 2012, 2, 18–25. [Google Scholar]
- Pavlov, D.S.; Nemova, N.N.; Kirillova, E.A.; Kirillov, P.I.; Nefedova, Z.A.; Murzina, S.A. Lipid Content in the Young of the Year Sockeye Salmon Oncorhynchus nerka during Feeding Migration (the Ozernaya River, Western Kamchatka). Dokl. Biol. Sci. 2012, 445, 235–238. [Google Scholar] [CrossRef]
- Nemova, N.N.; Nefyodova, Z.A.; Murzina, S.A. Lipid patterns early in Atlantic salmon, Salmo salar L., ontogeny. Trans. KarRC RAS 2014, 5, 44–52. [Google Scholar]
- Berge, J.; Renaud, P.E.; Darnis, G.; Cottier, F.; Last, K.S.; Gabrielsen, T.M.; Johnsen, G.; Seuthe, L.; Wes1awski, J.M.; Leu, E.; et al. In the dark: A review of ecosystem processes during the Arctic polar night. Prog. Oceanogr. 2015, 139, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Murzina, S.A.; Pekkoeva, S.N.; Kondakova, E.A.; Nefedova, Z.A.; Filippova, K.A.; Nemova, N.N.; Orlov, A.M.; Berge, J.; Falk-Petersen, S. Tiny but Fatty: Lipids and Fatty Acids in the Daubed Shanny (Leptoclinus maculatus), a Small Fish in Svalbard Waters. Biomolecules 2020, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Somero, G.N. Adaptation to high hydrostatic pressure. Annu. Rev. Physiol. 1992, 54, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Gerringer, M.E.; Drazen, J.C.; Yancey, P.H. Metabolic enzyme activities of abyssal and hadal fishes: Pressure effects and a re-evaluation of depth-related changes. Deep Sea Res. Part 1 2017, 125, 135–146. [Google Scholar] [CrossRef]
- Neighbors, M.A. Triacylglycerols and wax esters in the lipids of deep midwater teleost fishes of the Southern California Bright. Mar. Biol. 1988, 98, 15–22. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nelson, M.M.; Mooney, B.D.; Nichols, P.D. Wax esters versus triacylglycerols in myctophid fishes from the Southern Ocean. Antarct. Sci. 1999, 11, 436–444. [Google Scholar] [CrossRef]
- Dobrynina, V.I. Biological Chemistry; Medicine: Moskow, Russia, 1976; p. 503. [Google Scholar]
- Osadchaya, L.M.; Galkina, O.V.; Eshchenko, N.D. Effect of Corazole on the Activity of Na+ -K+ ATP—The Basics and the Intensity of Lipid Peroxidation in Neurons and Neuroglia: Biochemical and Molecular-Biological Foundations of Physiological Functions; Publishing House of St. Petersburg State University: St. Petersburg, Russia, 2004; Volume 37, pp. 220–226. [Google Scholar]
- Berdichevets, I.N.; Tyazhelova, T.V.; Shimshilashvili, K.R.; Rogaev, E.I. Lysophosphatidic acid is a lipid mediator with wide range of biological activities. Biosynthetic pathways and mechanism of action. Biochemistry 2010, 75, 1088–1097. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Principles of Biochemistry; Worth Publishers: New York, NY, USA, 1993; p. 1010. [Google Scholar]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.H.; Crawford, M.A.; Riefen, R. Update on alpha-linolenic acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef]
- Massey, K.A.; Nicolaou, A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem. Soc. Trans. 2011, 39, 1240–1246. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Wang, Q.; You, Q.L.; Li, Z.L.; Hu, N.Y.; Wang, Y.; Jin, Z.; Li, S.; Li, X.; Gao, T.M.; et al. Acute EPA-induced learning and memory impairment in mice is prevented by DHA. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Ponomarenco, A.I.; Tyrtyshnaia, A.A.; Pislyagin, E.A.; Dyuizen, I.V.; Sultanov, R.M.; Manzhulo, I.V. N-docosahexaenoylethanolamine reduces neuroinflammation and cognitive impairment after mild traumatic brain injury in rats. Sci. Rep. 2021, 11, 756. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Salem, N., Jr. International society for the study of fatty acids and lipids 2018 Symposium: Arachidonic and docosahexaenoic acids in infant development. Annu. Nutr. Metab. 2019, 74, 83–91. [Google Scholar] [CrossRef]
- Sergeeva, M.G.; Varfolomeeva, A.T. Arachidonic Acid Cascade; Public Education: Moscow, Russia, 2006; p. 256. [Google Scholar]
- Zaporozhskaya, L.I.; Gammel, I.V. Characteristics and biological role of essential polyunsaturated fatty acids. Med. Advice 2012, 12, 134–136. [Google Scholar]
- Alvheim, A.R.; Kjellevold, M.; Strand, E.; Sanden, M.; Wiech, M. Mesopelagic species and their potential contribution to food and feed security—A case study from Norway. Foods 2020, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.H.; Meng, M.; Fang, M.; Song, W.; Chen, J.; Tay, F.; Niu, L.N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Opstvedt, J. Fish lipids: More than n-3 fatty acids? Med. Hypotheses 1997, 48, 481–483. [Google Scholar] [CrossRef]
- Østbye, T.K.K.; Berge, G.M.; Nilsson, A.; Romarheim, O.H.; Bou, M.; Ruyter, B. The long-chain monounsaturated cetoleic acid improves the efficiency of the n-3 fatty acid metabolic pathway in Atlantic salmon and human HepG2 cells. Br. J. Nutr. 2019, 122, 755–768. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.H.; Emma-Okon, B.; Remaley, A.T. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: A mini review. Lipids Health Dis. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldo, E.; Grimsmo, L.; Alvarez, P.; Herrmann, B.; Møen Tveit, G.; Tiller, R.; Slizyte, R.; Aldanondo, N.; Guldberg, T.; Selnes, M.; et al. Investigating the potential for a commercial fishery in the Northeast Atlantic utilizing mesopelagic species. ICES J. Mar. Sci. 2020, 77, 2541–2556. [Google Scholar] [CrossRef]
- Gjøsæter, J.; Kawaguchi, K. A Review of the World Resources of Mesopelagic Fish. Fish. Aquac. Tech. Pap. 2002, 193, 151. [Google Scholar]
- Proud, R.; Handegard, N.; Kloser, R.; Cox, M.; Brierley, A. From siphonophores to deep scattering layers: Uncertainty ranges for the estimation of global mesopelagic fish biomass. ICES J. Mar. Sci. 2019, 76, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Gnaiger, E.; Bitterlich, G. Proximate biochemical composition and caloric content calculated from elemental CHN analysis: A stoichiometric concept. Oecologia 1984, 62, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, T.; Jonsson, G.; Pálsson, J. Deep scattering layer over Reykjanes Ridge and in the Irminger Sea. ICES CM 2002, 1000, 9. [Google Scholar]
- Anderson, C.; Brierley, A.; Armstrong, F. Spatio-temporal variability in the distribution of epi- and mesopelagic acoustic backscatter in the Irminger Sea, North Atlantic, with implications for predation on Calanus finmarchicus. Mar. Biol. 2005, 146, 1177–1188. [Google Scholar] [CrossRef]
- Berntssen, M.H.; Thoresen, L.; Albrektsen, S.; Grimaldo, E.; Grimsmo, L.; Whitaker, R.D.; Sele, V.; Wiech, M. Processing Mixed Mesopelagic Biomass from the North-East Atlantic into Aquafeed Resources; Implication for Food Safety. Foods 2021, 10, 1265. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Fish, whales, crustaceans, mollusks. Food Chem. 2009, 13, 617–639. [Google Scholar]
- Joensen, H.; Grahl-Nielsen, O. Stock structure of Sebastes mentella in the North Atlantic revealed by chemometry of the fatty acid profile in heart tissue. ICES J. Mar. Sci. 2004, 61, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Bakay, Y.I.; Melnikov, S.P. Biological and ecological characteristics of deepwater redfish Sebastes mentella (Scorpaenidae) at different depths in the pelagial of the Irminger Sea. J. Ichthyol. 2008, 48, 68–80. [Google Scholar] [CrossRef]
- Krovnin, A.S.; Melnikov, S.P.; Kivva, K.K.; Artemenkov, D.V.; Moury, G.P. Influence of variability of oceanological conditions on redfish in the North Atlantic pelagial. Trudy VNIRO 2017, 169, 51–63. [Google Scholar]
- Voronin, V.P.; Nemova, N.N.; Ruokolainen, T.R.; Artemenkov, D.V.; Rolskii, A.Y.; Orlov, A.M.; Murzina, S.A. Into the Deep: New Data on the Lipid and Fatty Acid Profile of Redfish Sebastes mentella Inhabiting Different Depths in the Irminger Sea. Biomolecules 2021, 11, 704. [Google Scholar] [CrossRef]
- Petursdottir, H.; Gislason, A.; Falk-Petersen, S. Lipid classes and fatty acid composition of muscle, liver and skull oil in deep-sea redfish Sebastes mentella over the Reykjanes Ridge. J. Fish Biol. 2008, 73, 2485–2496. [Google Scholar] [CrossRef]
- Petursdottir, H.; Gislason, A.; Falk-Petersen, S.; Hop, H.; Svavarsson, J. Trophic interaction of the pelagic ecosystem over the Reykjanes Ridge as evaluated by fatty acid and stable isotope analyses. Deep Sea Res. Part II 2008, 55, 83–93. [Google Scholar] [CrossRef] [Green Version]
- RussianGost. Available online: https://www.russiangost.com/p-72251-gost-32366-2013.aspx (accessed on 15 November 2021).
- Dang, H.T.T.; Gudjonsdóttir, M.; Karlsdóttir, M.G.; Van Nguyen, M.; Tómasson, T.; Arason, S. Stability of Golden redfish (Sebastes marinus) during frozen storage as affected by raw material freshness and season of capture. Food Sci. Nutr. 2018, 6, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Giannini, D.; Parin, M.A.; Gadaleta, L.; Carrizo, D.; Zugarramurdi, A. Influence of raw material quality of quality of Iced and frozen white fish products. J. Food Qual. 2001, 24, 527–538. [Google Scholar] [CrossRef]
- Lauder, J.T.; MacCallum, W.A.; Idler, D.R. Keeping time of frozen redfish (Sebastes marinus mentella) fillets in relation to handling of the raw material and storage temperatures after processing and freezing. J. Fish. Res. Board Can. 1970, 27, 1589–1905. [Google Scholar] [CrossRef]
- Dang, H.T.T.; Gudjónsdóttir, M.; Karlsdóttir, M.G.; Nguyen, M.V.; Romotowska, P.E.; Tómasson, T.; Arason, S. Influence of temperature stress on lipid stability of Atlantic herring (Clupea harengus) muscle during frozen storage. J. Am. Oil Chem. Soc. 2017, 94, 1439–1449. [Google Scholar] [CrossRef]
- Nazarova, M.A.; Vasilyeva, O.B.; Ripatti, P.O.; Nemova, N.N. The concentration of lipid components in the muscles of rainbow trout Parasalmo mykiss (Walbaum, 1972) depending on the pre-fixation period. Trans. KarRC RAS 2014, 5, 157–162. [Google Scholar]
- European Commission. Available online: https://fish-commercial-names.ec.europa.eu/fish-names/species_en?sn=33081 (accessed on 15 November 2021).
- ESIMO. Available online: http://esimo.oceanography.ru/esp1/index.php?menu_code=3622&sea_code=5§ion=17 (accessed on 15 November 2021).
- Manual for the International Deep Pelagic Ecosystem Survey in the Irminger Sea and Adjacent Waters: Series of ICES Survey Protocol SISP 11—IDEEPS VI; ICES: Copenhagen, Denmark, 2015; p. 49.
- Folch, J.; Lees, M.; Sloan-Syanley, G.H. A simple method for the isolation and purification of total lipids from animal tissue (for brain, liver and muscle). J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Olsen, R.E.; Henderson, R.J. The rapid analysis of neutral and polar marine lipids using double development HPTLC and scanning densitometry. J. Exp. Mar. Biol. Ecol. 1989, 129, 189–197. [Google Scholar] [CrossRef]
- Hellwig, J. Defining Parameters for A Reproducible TLC-Separation of Phospholipids Using ADC 2. Ph.D. Thesis, University of Applied Sciences Northwestern Switzerland (FHNW), Windisch, Switzerland, 2005. [Google Scholar]
- Kabakoff, R. R in Action: Data Analysis and Graphics with R; Volkova, P.A., Translator; DMK Press: Moscow, Russia, 2014; p. 588. [Google Scholar]
- Bruce, A.; Bruce, P. Practical Statistic for Data Scientists; Bruce, P.; Bruce, A., Translators; BHV-Peterburg: St. Peterburg, Russia, 2018; p. 304. [Google Scholar]
- Orlov, A.M.; Tokranov, A.M. New data on distribution and biology of grey, angry, and northern rockfishes from the Northwestern Pacific. In Biology, Assessment and Management of North Pacific Rockfishes; Heifetz, J., Di Cosimo, J., Gharrett, A.J., Love, M.S., O’Connell, V.M., Stanley, R.D., Eds.; Alaska Sea Grant College Program: Fairbanks, Alaska, 2007; pp. 59–85. [Google Scholar]
- Orlov, A.M. Ocean current patterns and aspects of life history of some northwestern Pacific scorpaenids. In Spatial Processes and Management of Marine Populations: Pub. No. AK-SG-01-02; University Alaska Sea Grant College Program: Fairbanks, AK, USA, 2001; pp. 161–184. [Google Scholar]
- Romotowska, P.E.; Karlsdóttir, M.G.; Gudjónsdóttir, M.; Kristinsson, H.G.; Arason, S. Influence of feeding state and frozen storage temperature on the lipid stability of Atlantic mackerel (Scomber scombrus). Int. J. Food Sci. Technol. 2016, 51, 1711–1720. [Google Scholar] [CrossRef]
- Erickson, M.C. Lipid oxidation: Flavor and nutritional quality deterioration in frozen foods. In Quality in Frozen Food; Springer: Boston, MA, USA, 1997; pp. 141–173. [Google Scholar]
- Nazemroaya, S.; Sahari, M.A.; Rezaei, M. Effect of frozen storage on fatty acid composition and changes in lipid content of Scomberomorus commersoni and Carcharhinus dussumieri. J. Appl. Ichthyol. 2009, 25, 91–95. [Google Scholar] [CrossRef]
- Anokhina, O.N. Preservation of the quality of muscle tissue lipids during refrigerated storage of fish. KSTU News 2010, 18, 61–67. [Google Scholar]
- Pacheco-Aguilar, R.; Lugo-Sa′nchez, M.E.; Robles-Burgueň, M.R. Postmortem biochemical and functional characteristic of monterey sardine muscle stored at 0 °C. J. Food Sci. 2000, 65, 40–47. [Google Scholar] [CrossRef]
- Polvi, S.M.; Ackman, R.G.; Lall, S.P.; Saunders, R.L. Stability of lipids and omega-3-fatty-acids during frozen storage of Atlantic salmon. J. Food Process. Preserv. 1991, 15, 167–181. [Google Scholar] [CrossRef]
- Panov, V.P.; Safonova, S.S.; Orlov, A.M.; Rolskii, A.Y.; Artemenkov, D.V. Development of axial locomotor musculation of beaked redfish Sebastes mentella (Sebastidae). Neurosci. Behav. Physiol. 2021, 107, 203–220. [Google Scholar]
- Shulman, G.E.; Yuneva, T.V. Role of docosahexaenoic acid in adaptations fishes (review). Hydrobiol. J. 1990, 26, 43–51. [Google Scholar]
- Kanazawa, A. Effects of docosahexaenoic acid and phospholipids on stress tolerance of fish. Aquaculture 1997, 155, 129–134. [Google Scholar] [CrossRef]
- Abrami, G.; Natiello, F.; Bronzi, P.; McKenzie, D.; Bolis, L.; Aggrade, E. A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla anguilla). Comp. Biochem. Physiol. 1992, 1/2, 71–82. [Google Scholar] [CrossRef]
- Senso, L.; Suárez, M.D.; Ruiz-Cara, T.; García-Gallego, M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- Turan, H.; Sönmez, G.; Kaya, Y. Fatty acid profile and proximate composition of the thorn backray (Raja clavata L. 1758) from the Sinop coast in the Black Sea. J. Fisheries Sci. 2007, 1, 97–103. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Veira, C.P.; Alvares, T.S.; Gomes, L.S.; Torres, A.G.; Paschoalin, V.M.F.; Conte-Junior, C.A. Kefir grains change fatty acid profile of milk during fermentation and storage. PLoS ONE 2015, 10, e0139910. [Google Scholar]
- Ohlsson, L. Dairy products and plasma cholesterol levels. Food Nutr. Res. 2010, 54, 5124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, P.S.; Inada, A.C.; Marcelino, G.; Cardozo, C.M.L.; De Cássia Freitas, K.; De Cássia Avellaneda Guimarães, R.; De Castro, A.P.; Do Nascimento, V.A.; Hiane, P.A. Fatty acids consumption: The role metabolic aspects involved in obesity and its associated disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Hu, F.B. Dietary fat and risk of cardiovascular disease: Recent controversies and advances. Ann. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Muller, C.J.C.; Sasanti, B. Effect of alternating total mixed ration and pasture feeding on the fatty acid content and health indices of Jersey and Fleckvieh × Jersey milk. S. Afr. J. Anim. Sci. 2019, 49, 432–440. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
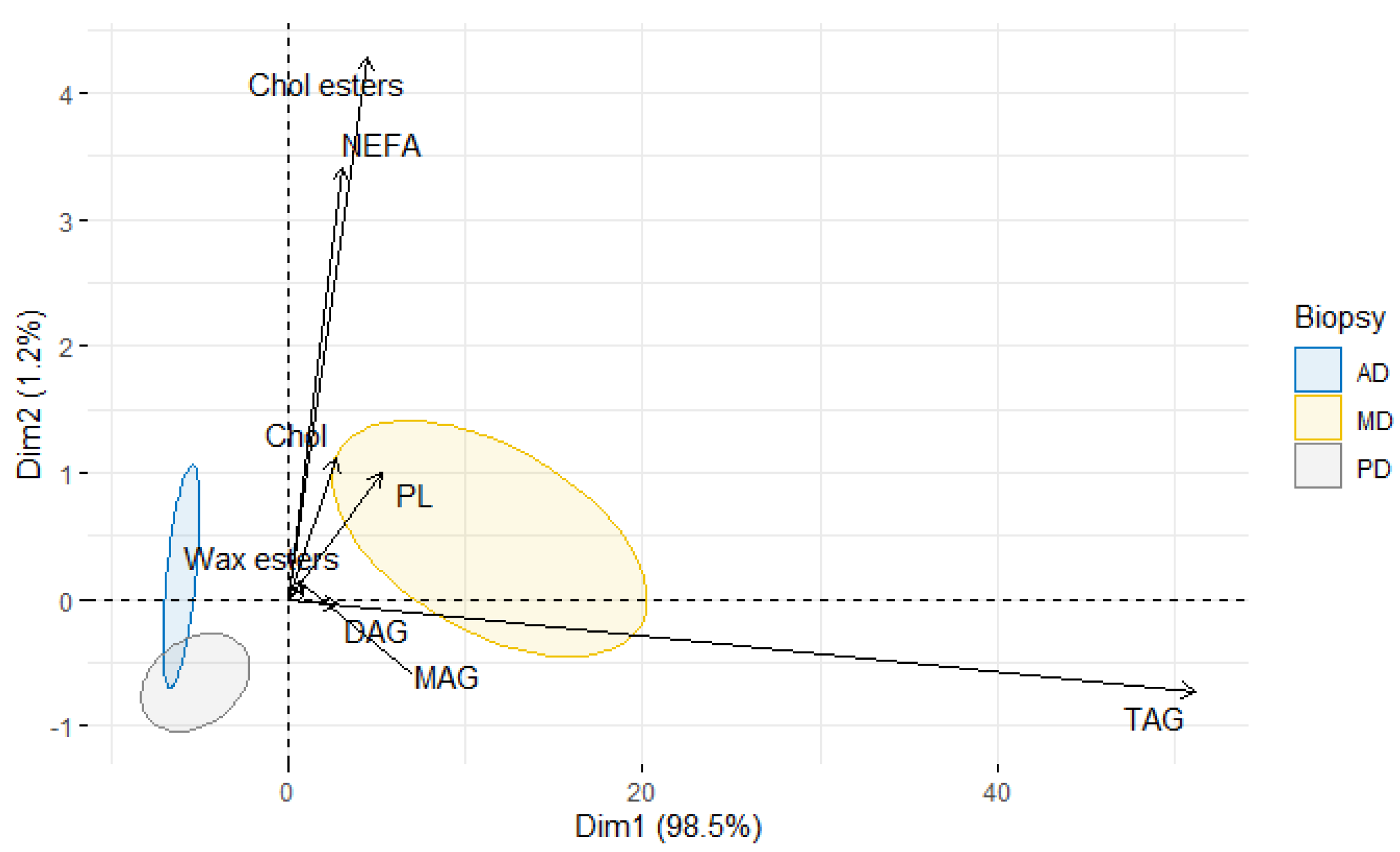
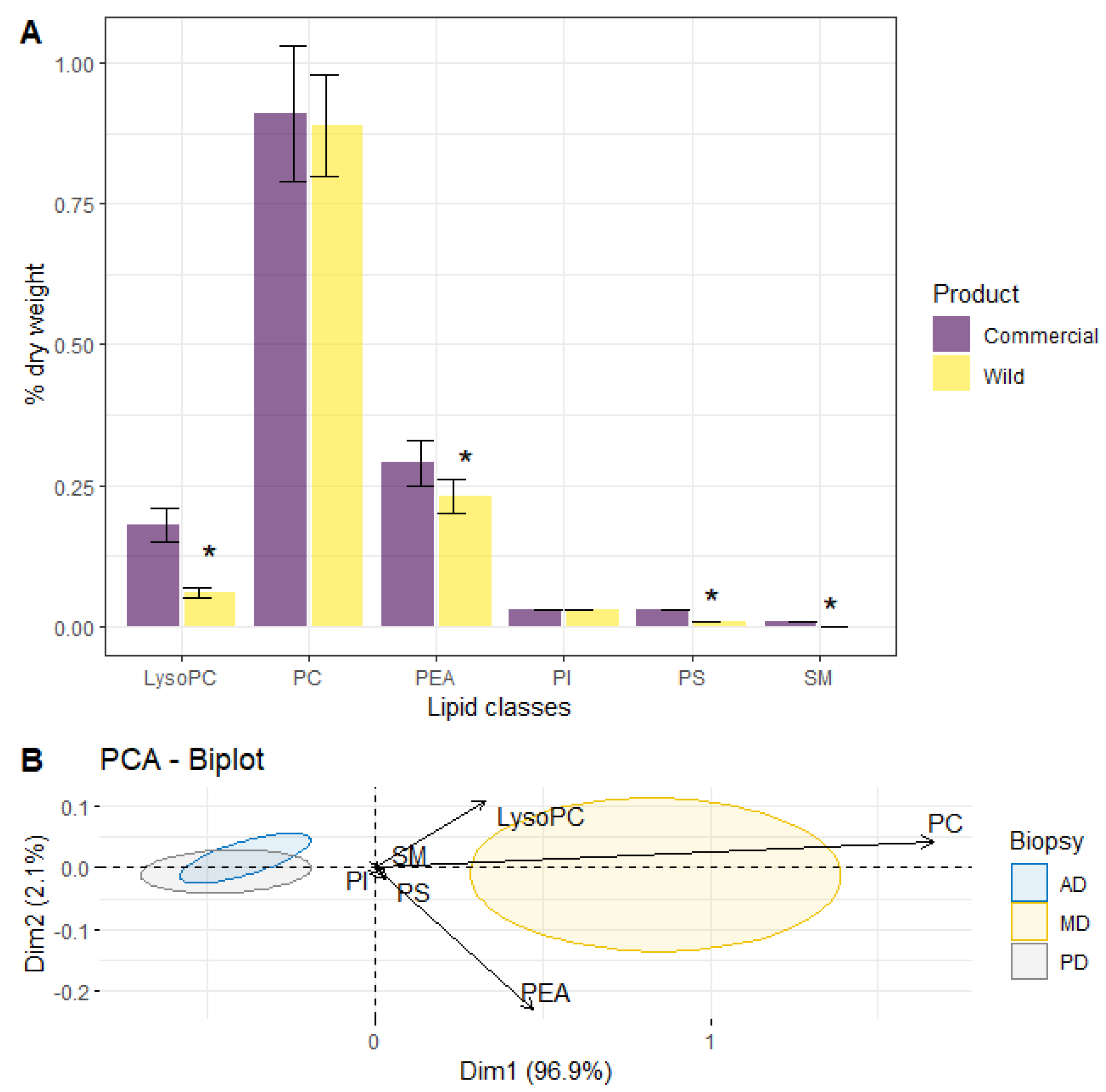
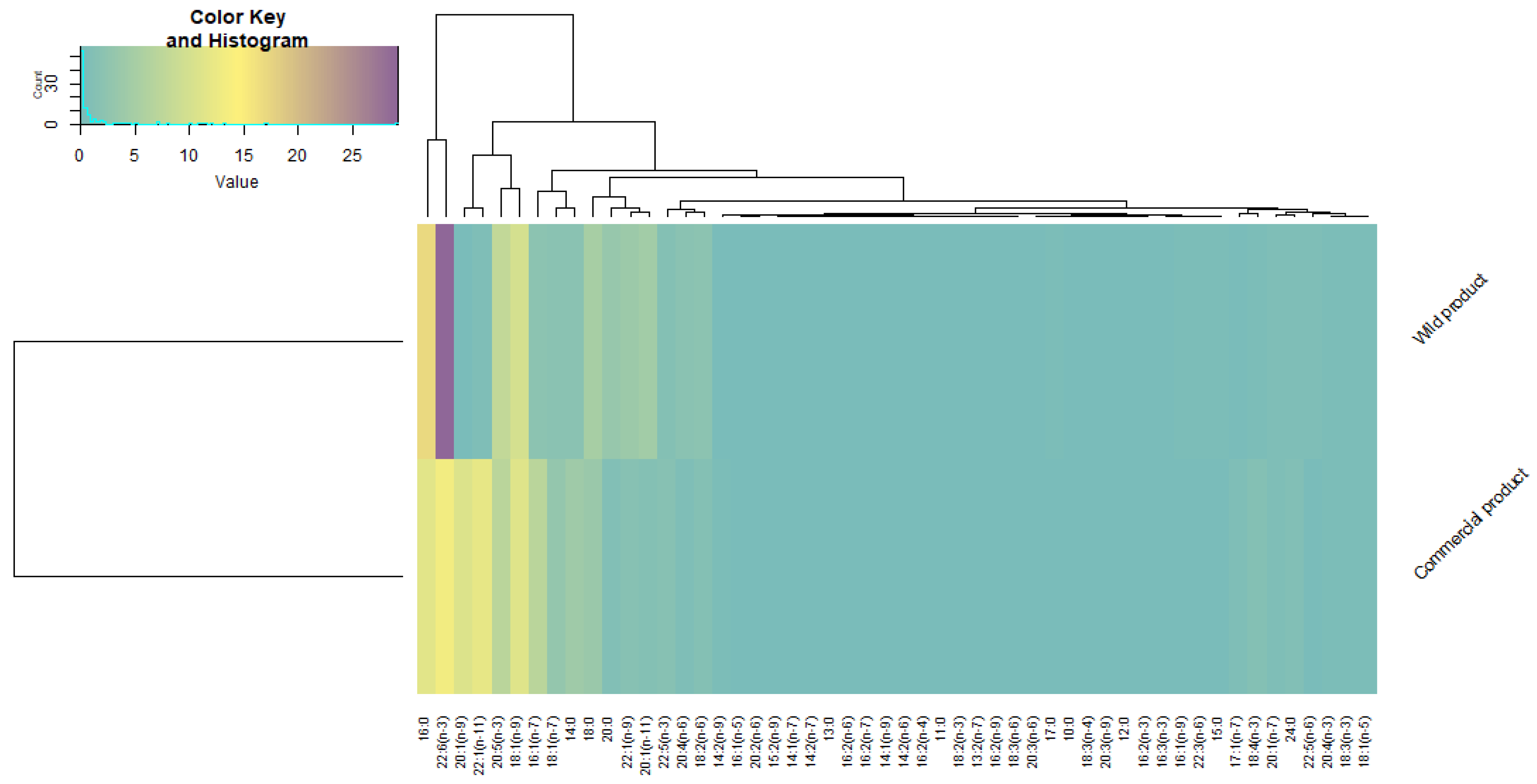
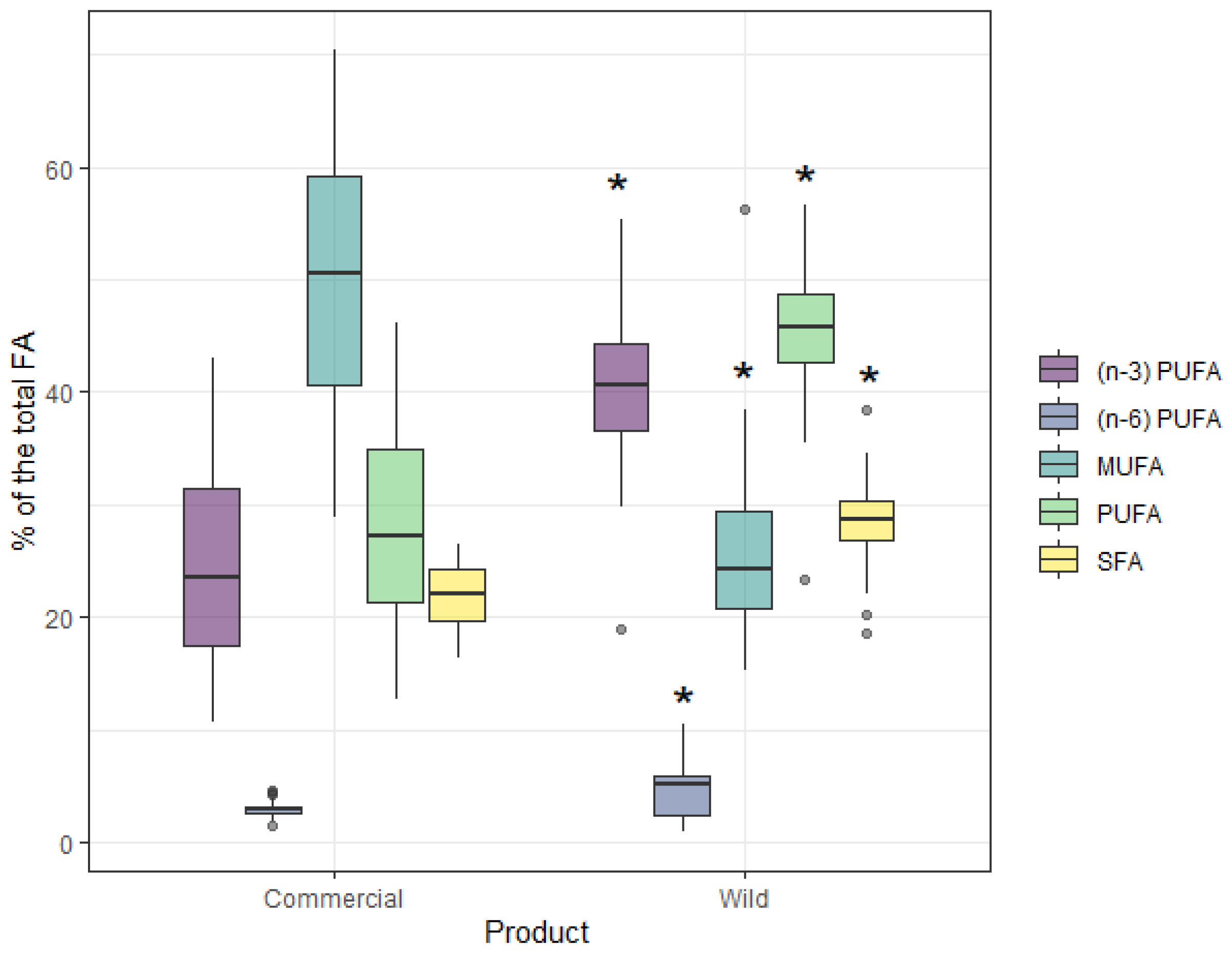
| Index | Commercial | Wild |
|---|---|---|
| (n-3)/(n-6) | 8.35 ± 0.32 | 13.72 ± 1.42 |
| 16:0/18:1(n-9) | 1.18 ± 0.08 | 1.79 ± 0.06 * |
| 18:1(n-9)/18:0 | 3.36 ± 0.24 | 1.98 ± 0.06 * |
| 18:3(n-3)/18:2(n-6) | 0.32 ± 0.01 | 0.56 ± 0.38 * |
| 20:4(n-6)/18:2(n-6) | 0.52 ± 0.04 | 1.14 ± 0.22 * |
| 20:5(n-3)/18:3(n-3) | 18.71 ± 1.46 | 20.82 ± 0.68 |
| 22:6(n-3)/20:5(n-3) | 2.14 ± 0.38 | 3.85 ± 0.15 * |
| MUFA/SFA | 2.36 ± 0.12 | 0.93 ± 0.04 * |
| MUFA/PUFA | 2.14 ± 0.19 | 0.59 ± 0.03 * |
| IA | 0.37 ± 0.01 | 0.35 ± 0 |
| IT | 0.2 ± 0.01 | 0.16 ± 0 * |
| h/H | 2.71 ± 0.08 | 3.06 ± 0.04 * |
| HPI | 2.78 ± 0.07 | 2.91 ± 0.04 |
| FLQ | 20.41 ± 1.26 | 37 ± 0.68 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzina, S.A.; Voronin, V.P.; Ruokolainen, T.R.; Artemenkov, D.V.; Orlov, A.M. Comparative Analysis of Lipids and Fatty Acids in Beaked Redfish Sebastes mentella Travin, 1951 Collected in Wild and in Commercial Products. J. Mar. Sci. Eng. 2022, 10, 59. https://doi.org/10.3390/jmse10010059
Murzina SA, Voronin VP, Ruokolainen TR, Artemenkov DV, Orlov AM. Comparative Analysis of Lipids and Fatty Acids in Beaked Redfish Sebastes mentella Travin, 1951 Collected in Wild and in Commercial Products. Journal of Marine Science and Engineering. 2022; 10(1):59. https://doi.org/10.3390/jmse10010059
Chicago/Turabian StyleMurzina, Svetlana A., Viktor P. Voronin, Tatjana R. Ruokolainen, Dmitrii V. Artemenkov, and Alexei M. Orlov. 2022. "Comparative Analysis of Lipids and Fatty Acids in Beaked Redfish Sebastes mentella Travin, 1951 Collected in Wild and in Commercial Products" Journal of Marine Science and Engineering 10, no. 1: 59. https://doi.org/10.3390/jmse10010059
APA StyleMurzina, S. A., Voronin, V. P., Ruokolainen, T. R., Artemenkov, D. V., & Orlov, A. M. (2022). Comparative Analysis of Lipids and Fatty Acids in Beaked Redfish Sebastes mentella Travin, 1951 Collected in Wild and in Commercial Products. Journal of Marine Science and Engineering, 10(1), 59. https://doi.org/10.3390/jmse10010059








