Possibilities of Ammonia as Both Fuel and NOx Reductant in Marine Engines: A Numerical Study
Abstract
:1. Introduction
2. Materials and Methods
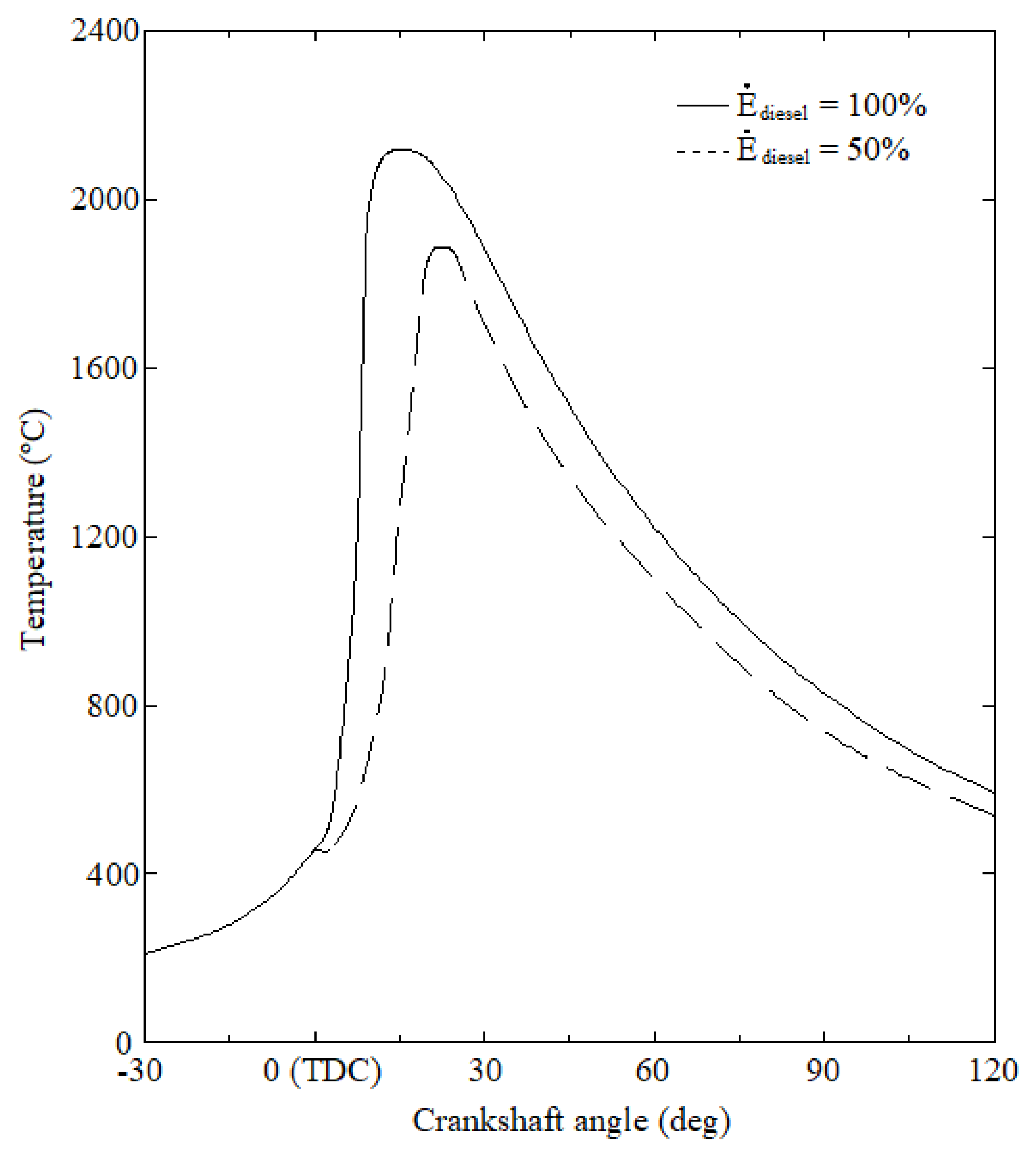
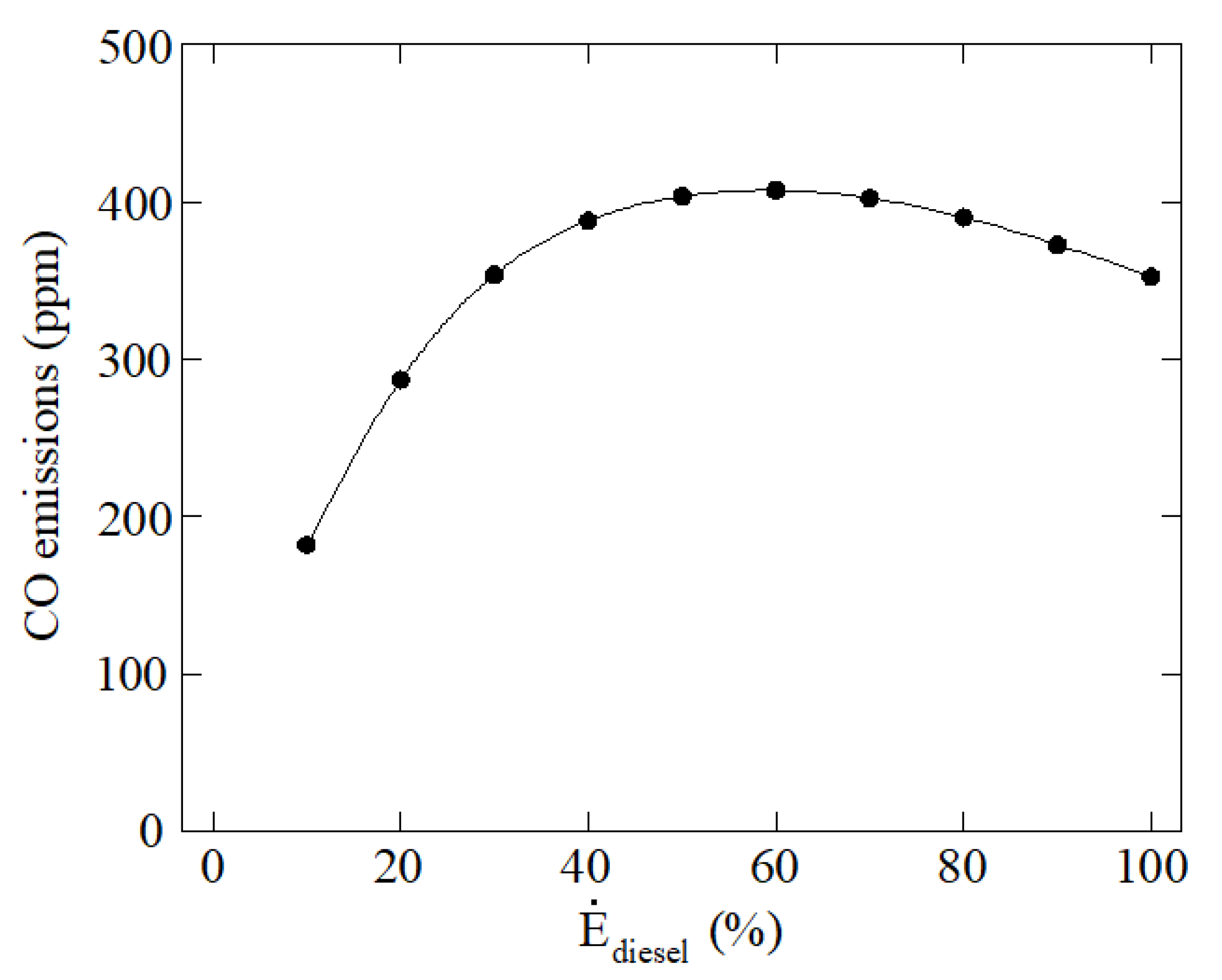
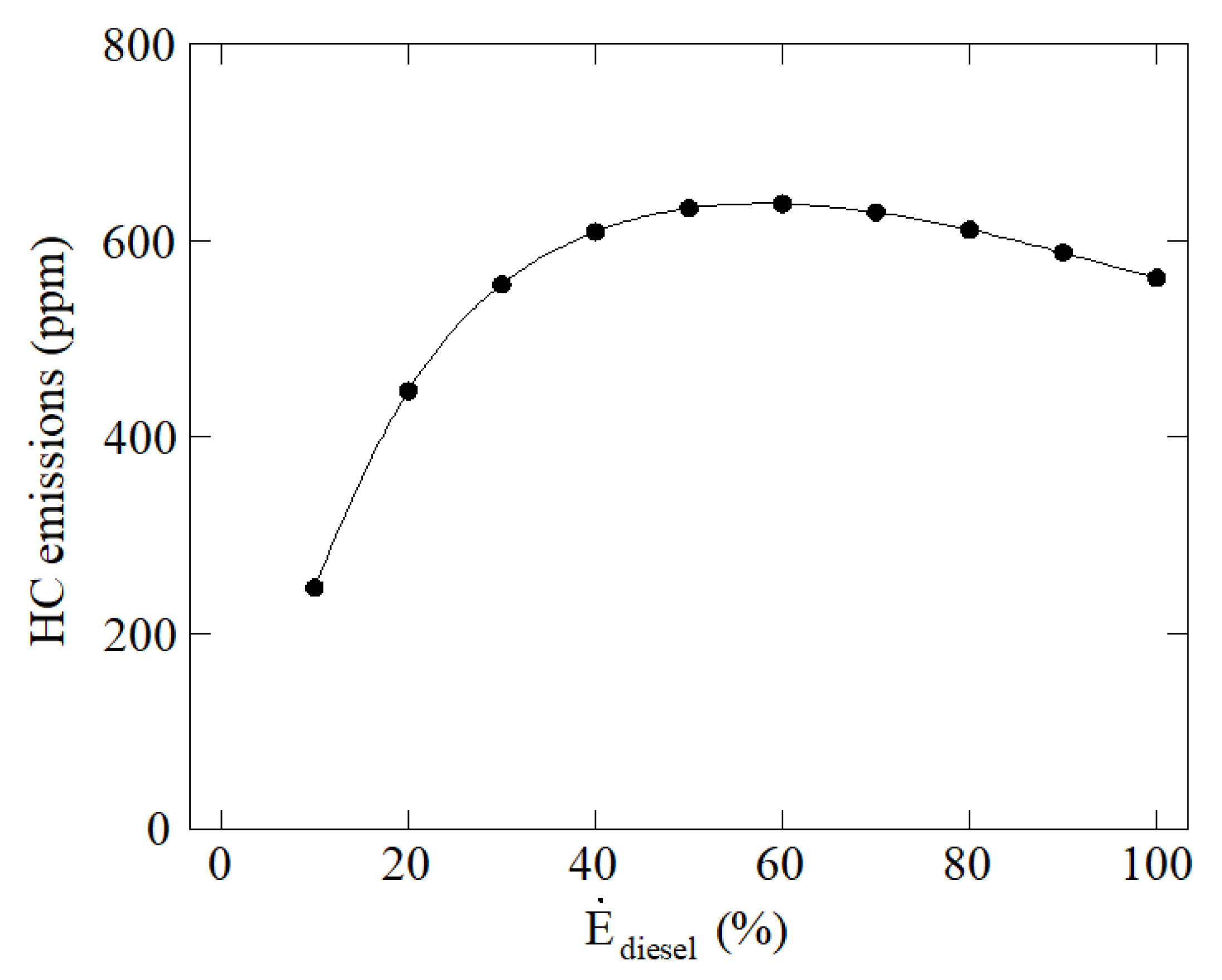
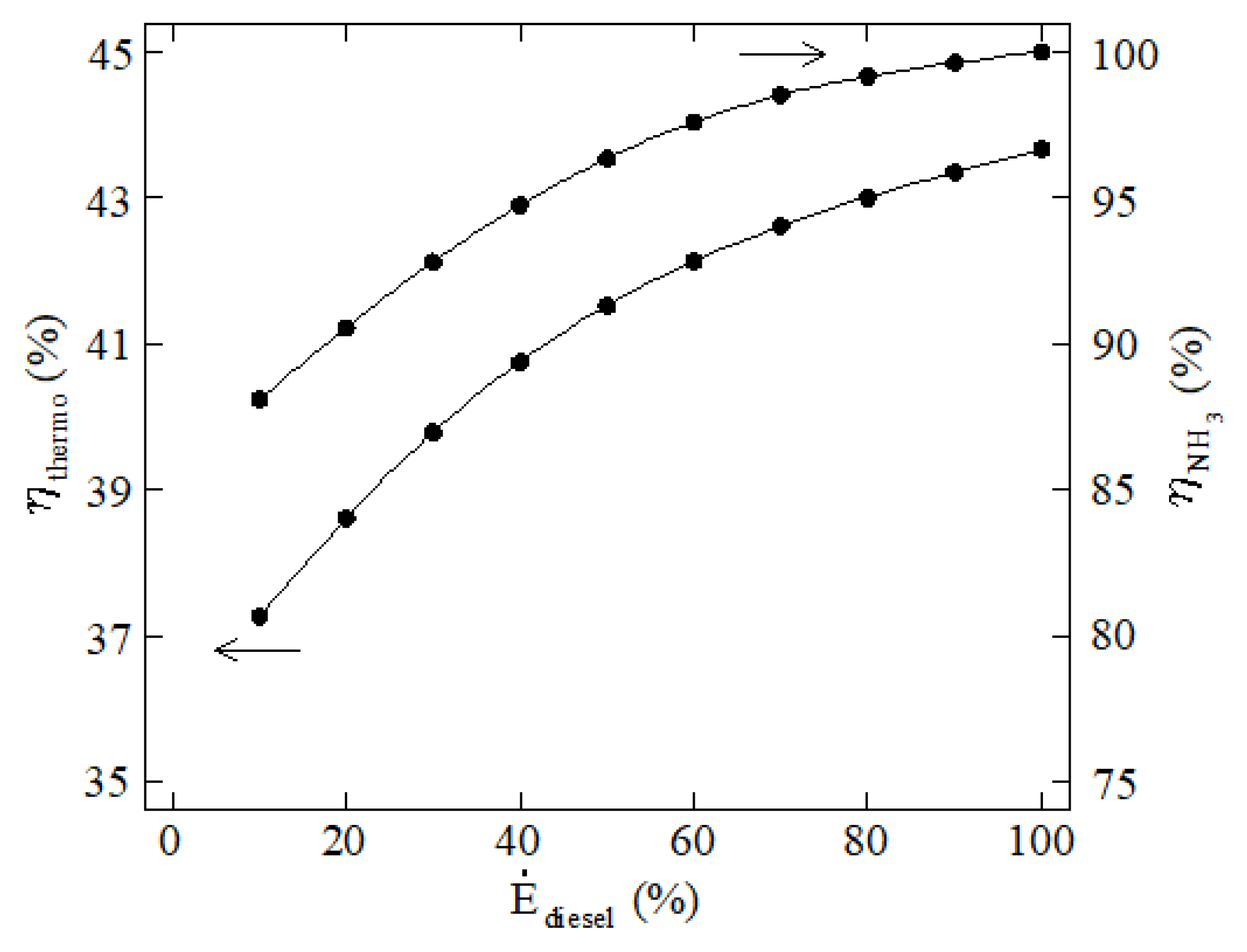
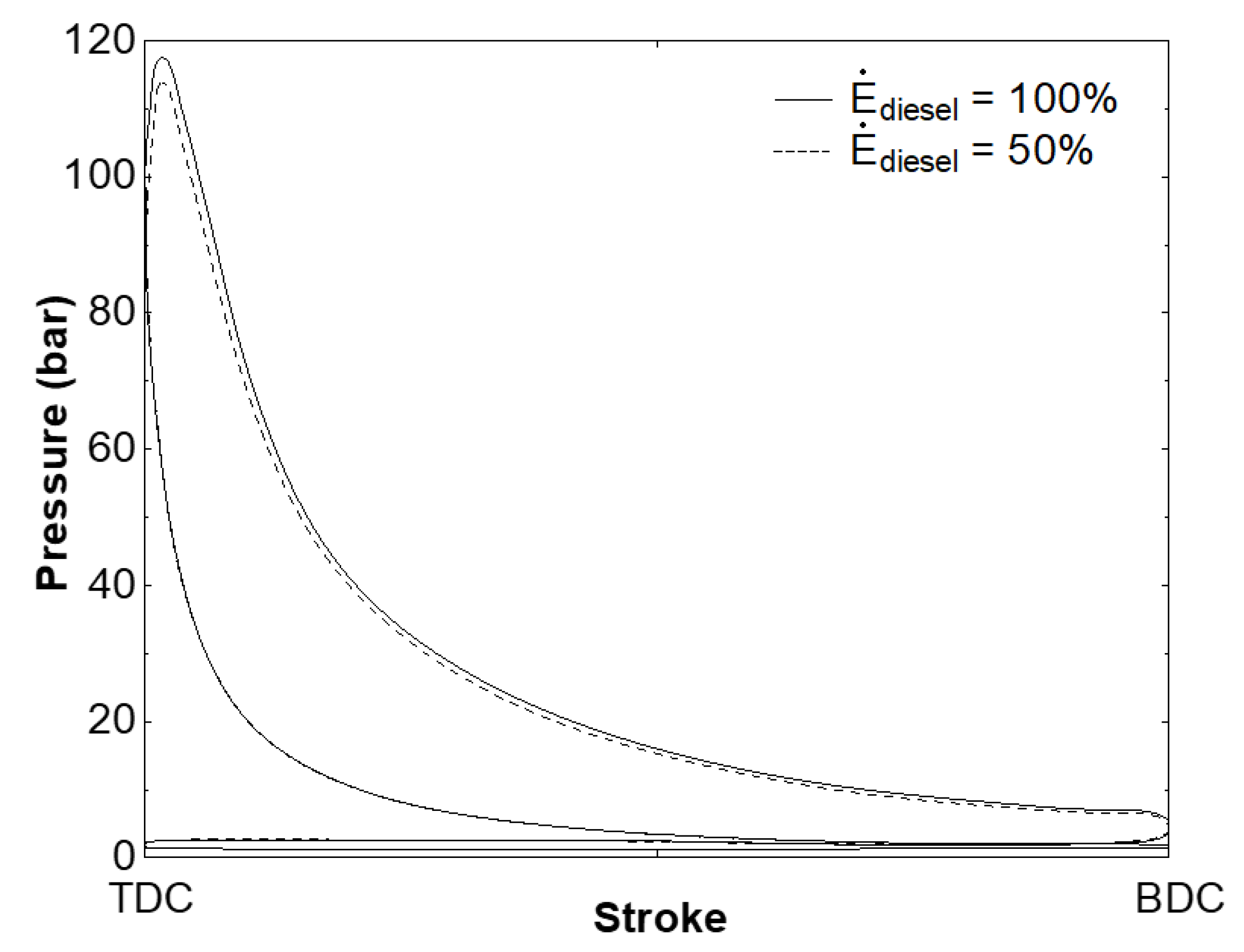
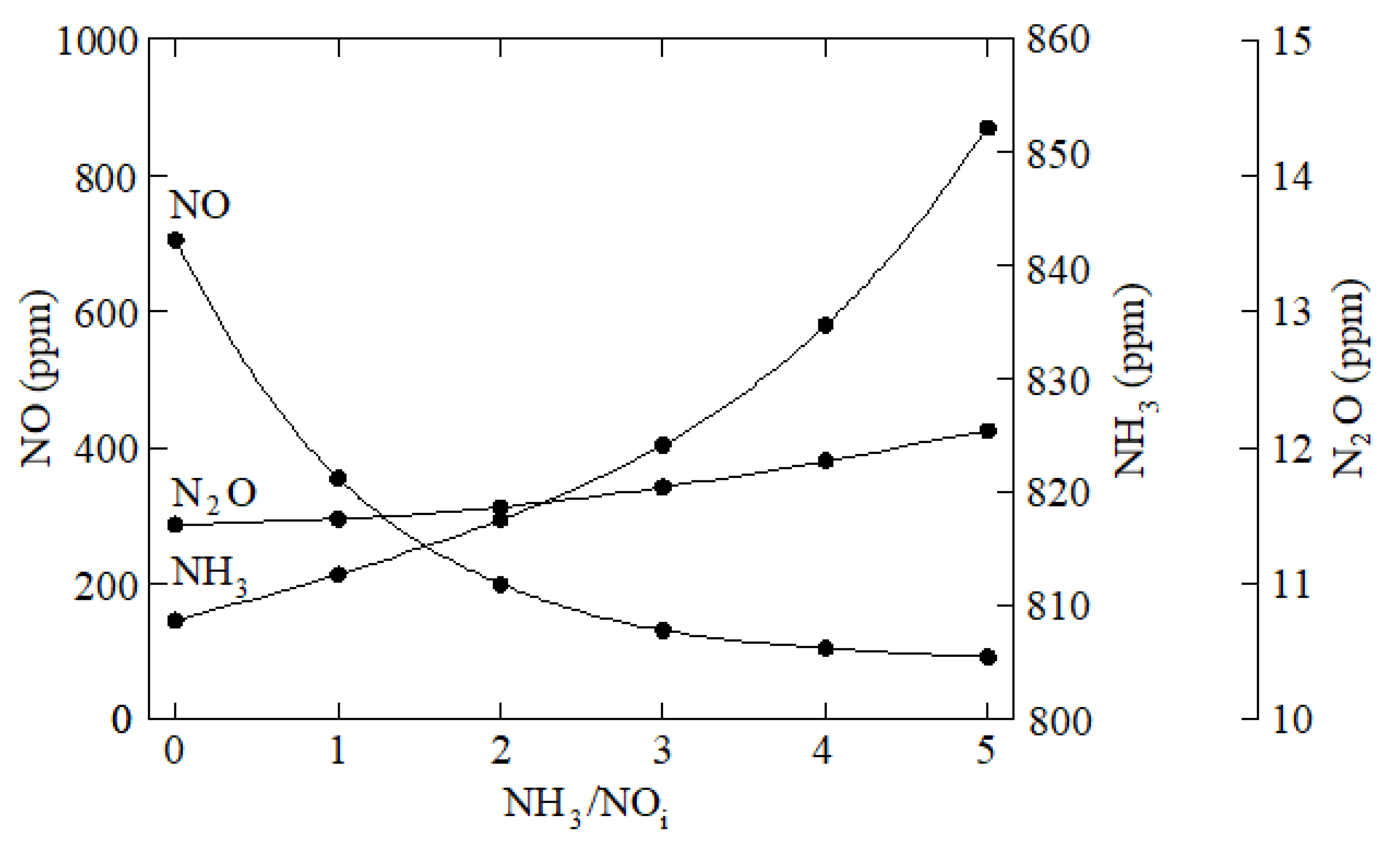
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Deng, J.; Wang, X.; Wei, Z.; Wang, L.; Wang, C.; Chen, Z. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total Environ. 2021, 766, 144319. [Google Scholar] [CrossRef] [PubMed]
- Zincir, B. A short review of ammonia as an alternative marine fuel for decarbonised maritime transportation. In Proceedings of the ICEESEN2020, Kayseri, Turkey, 19–21 November 2020. [Google Scholar]
- European Energy Agency (EEA). Emission of Air Pollutants from Transport. Available online: https://www.eea.europa.eu/data-and-maps/indicators/transport-emissions-of-air-pollutants-8/transport-emissions-of-air-pollutants-6#tab-related-briefings (accessed on 26 November 2021).
- Puškár, M.; Kopas, M.; Sabadka, D.; Kliment, M.; Šoltésová, M. Reduction of the gaseous emissions in the marine diesel engine using biodiesel mixtures. J. Mar. Sci. Eng. 2020, 8, 330. [Google Scholar] [CrossRef]
- Seddiek, I.S.; Elgohary, M.M.; Ammar, N.R. The hydrogen-fuelled internal combustion engines for marine applications with a case study. Brodogradnja 2015, 66, 23–38. [Google Scholar]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrog. Energy. 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Lee, D.; Song, H.H. Development of combustion strategy for the internal combustion engine fueled by ammonia and its operating characteristics. J. Mech. Sci. Technol. 2018, 32, 1905–1925. [Google Scholar] [CrossRef]
- Reiter, A.J.; Jong, S.C. Demonstration of compression-ignition engine combustion using ammonia in reducing greenhouse gas emissions. Energy Fuels. 2008, 22, 2963–2971. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Niki, Y.; Nitta, Y.; Sekiguchi, H.; Hirata, K. Diesel fuel multiple injection effects on emission characteristics of diesel engine mixed ammonia gas into intake air. J. Eng. Gas Turbines Power 2019, 141, 061020. [Google Scholar] [CrossRef]
- Gill, S.S.; Chatha, G.S.; Tsolakis, A.; Golunski, S.E.; York, A.P.E. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. Int. J. Hydrog. Energy 2012, 37, 6074–6083. [Google Scholar] [CrossRef]
- Niki, Y.; Nitta, Y.; Sekiguchi, H.; Hirata, K. Emission and combustion characteristics of diesel engine fumigated with ammonia. In Proceedings of the ASME 2018 Internal Combustion Engine Division Fall Technical Conference, San Diego, CA, USA, 7 November 2018. [Google Scholar] [CrossRef]
- Tay, K.L.; Wang, W.M.; Li, J.; Zhou, D. Numerical investigation on the combustion and emissions of a kerosene-diesel fueled compression ignition engine assisted by ammonia fumigation. Appl. Energy 2017, 204, 1476–1488. [Google Scholar] [CrossRef]
- Tay, K.L.; Yang, W.; Chou, S.K.; Zhou, D.; Li, J.; Yu, W.; Zhao, F.; Mohan, B. Effects of injection timing and pilot fuel on the combustion of a kerosene-diesel/ammonia dual fuel engine: A numerical study. Energy Procedia 2017, 105, 4621–4626. [Google Scholar] [CrossRef]
- Kim, K.; Roh, G.; Kim, W.; Chun, K. A preliminary study on an alternative ship propulsion system fueled by ammonia: Environmental and economic assessments. J. Mar. Sci. Eng. 2020, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Ra, Y.; Reitz, R.D. A reduced chemical kinetic model for IC engine combustion simulations with primary reference fuels. Combust. Flame 2008, 155, 713–738. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.A.; Glarborg, P. Modeling the Formation of N2O and NO2 in the Thermal DeNOx Process; Springer Series in Chemical Physic; Springer: Berlin, Germany, 1996; pp. 318–333. [Google Scholar]
- Dukowicz, J.K. A particle-fluid numerical model for liquid sprays. J. Comput. Phys. 1980, 35, 229–253. [Google Scholar] [CrossRef]
- Ricart, L.M.; Xin, J.; Bower, G.R.; Reitz, R.D. In-Cylinder Measurement and Modeling of Liquid Fuel Spray Penetration in a Heavy-Duty Diesel Engine. SAE Technical Paper 971591; SAE International: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Lamas Galdo, M.I.; Rodriguez García, J.D.; Rodriguez Vidal, C.G. Modelo de mecánica de fluidos computacional para el estudio de la combustión en un motor diésel de cuatro tiempos. DYNA Ing. E Ind. 2013, 88, 91–98. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodriguez, C.G. Numerical model to analyze NOx reduction by ammonia injection in diesel-hydrogen engines. Int. J. Hydrog. Energy. 2017, 42, 26132–26141. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodriguez, C.G. NOx reduction in diesel-hydrogen engines using different strategies of ammonia injection. Energies 2019, 12, 1255. [Google Scholar] [CrossRef] [Green Version]
- Heiwood, H.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill: New York, NY, USA, 1988; ISBN 007028637X. [Google Scholar]
- Reusser, C.A.; Pérez Osses, J.R. Challenges for zero-emissions ship. J. Mar. Sci. Eng. 2021, 9, 1042. [Google Scholar] [CrossRef]
- Mallouppas, G.; Yfantis, E.A. Decarbonization in shipping industry: A review of research, technology development, and innovation proposals. J. Mar. Sci. Eng. 2021, 9, 415. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodriguez, C.G.; Aas, H.P. Computational fluid dynamics analysis of NOx and other pollutants in the MAN B&W 7S50MC marine engine and effect of EGR and water addition. Trans. RINA Int. J. Marit. Eng. 2013, 155, 81–88. [Google Scholar]
- Lamas, M.I.; Rodriguez, C.G.; Rodriguez, J.D.; Telmo, J. Computational fluid dynamics analysis of NOx reduction by ammonia injection in the MAN B&W 7S50MC marine engine. Trans. RINA Int. J. Marit. Eng. 2014, 156, 213–220. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodríguez, C.G.; Rodriguez, J.D.; Telmo, J. Internal modifications to reduce pollutant emissions from marine engines. A numerical approach. Int. J. Nav. Archit. Ocean Eng. 2013, 5, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.G.; Lamas, M.I.; Rodriguez, J.D.; Caccia, C. Analysis of the pre-injection configuration in a marine engine through several MCDM techniques. Brodogradnja 2021, 72, 1–17. [Google Scholar] [CrossRef]
- Rodriguez, C.G.; Lamas, M.I.; Rodriguez, J.D.; Abbas, A. Analysis of the pre-injection system of a marine diesel engine through multiple-criteria decision making and artificial neural networks. Polish Marit. Res. 2021, 28, 88–96. [Google Scholar] [CrossRef]
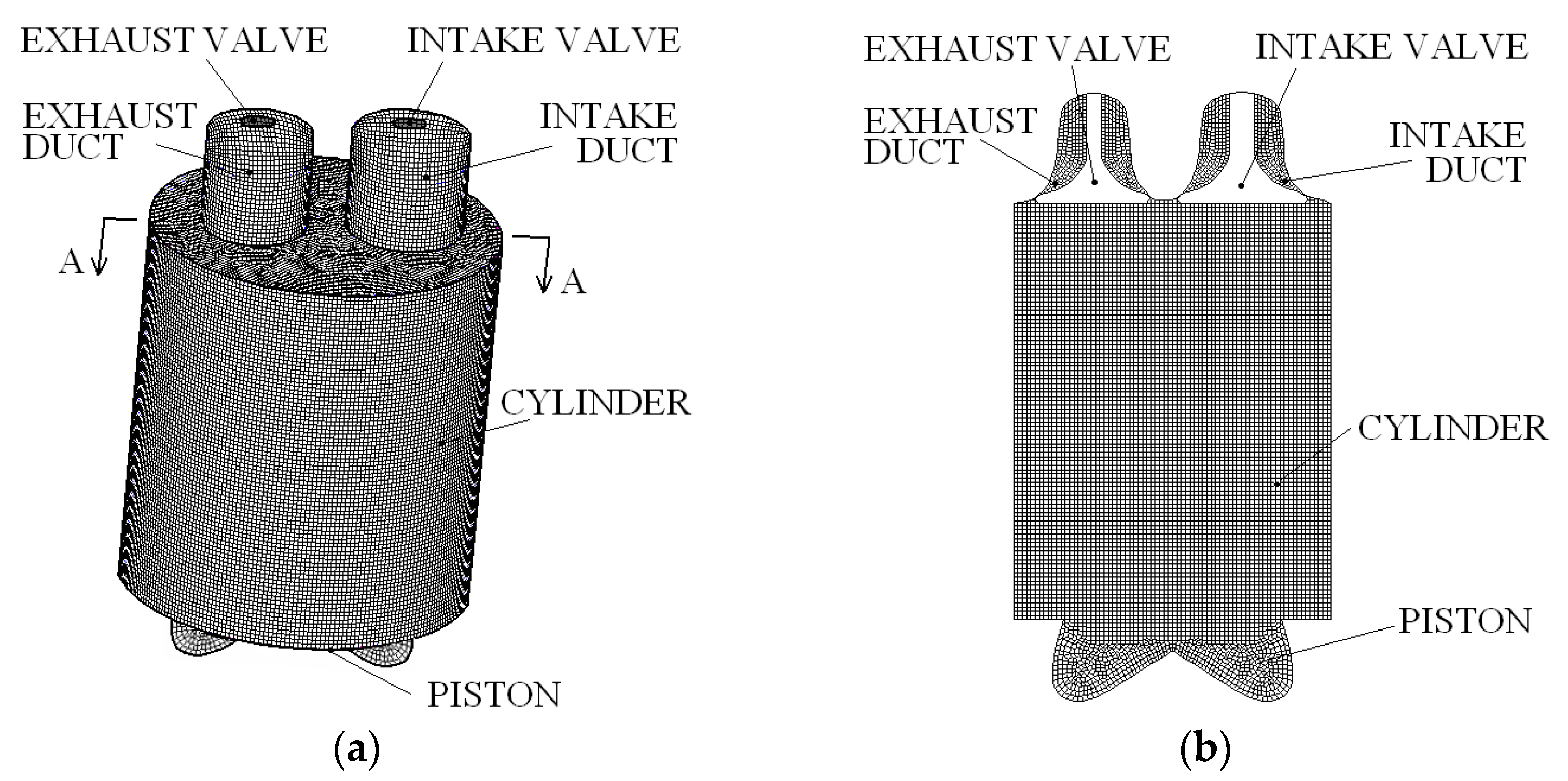




| Parameter | Value |
|---|---|
| Power (kW) | 320 |
| Speed (rpm) | 1500 |
| Compression ratio | 13.5:1 |
| Injection pressure (bar) | 220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, C.G.; Lamas, M.I.; Rodríguez, J.d.D.; Abbas, A. Possibilities of Ammonia as Both Fuel and NOx Reductant in Marine Engines: A Numerical Study. J. Mar. Sci. Eng. 2022, 10, 43. https://doi.org/10.3390/jmse10010043
Rodríguez CG, Lamas MI, Rodríguez JdD, Abbas A. Possibilities of Ammonia as Both Fuel and NOx Reductant in Marine Engines: A Numerical Study. Journal of Marine Science and Engineering. 2022; 10(1):43. https://doi.org/10.3390/jmse10010043
Chicago/Turabian StyleRodríguez, Carlos Gervasio, María Isabel Lamas, Juan de Dios Rodríguez, and Amr Abbas. 2022. "Possibilities of Ammonia as Both Fuel and NOx Reductant in Marine Engines: A Numerical Study" Journal of Marine Science and Engineering 10, no. 1: 43. https://doi.org/10.3390/jmse10010043
APA StyleRodríguez, C. G., Lamas, M. I., Rodríguez, J. d. D., & Abbas, A. (2022). Possibilities of Ammonia as Both Fuel and NOx Reductant in Marine Engines: A Numerical Study. Journal of Marine Science and Engineering, 10(1), 43. https://doi.org/10.3390/jmse10010043









