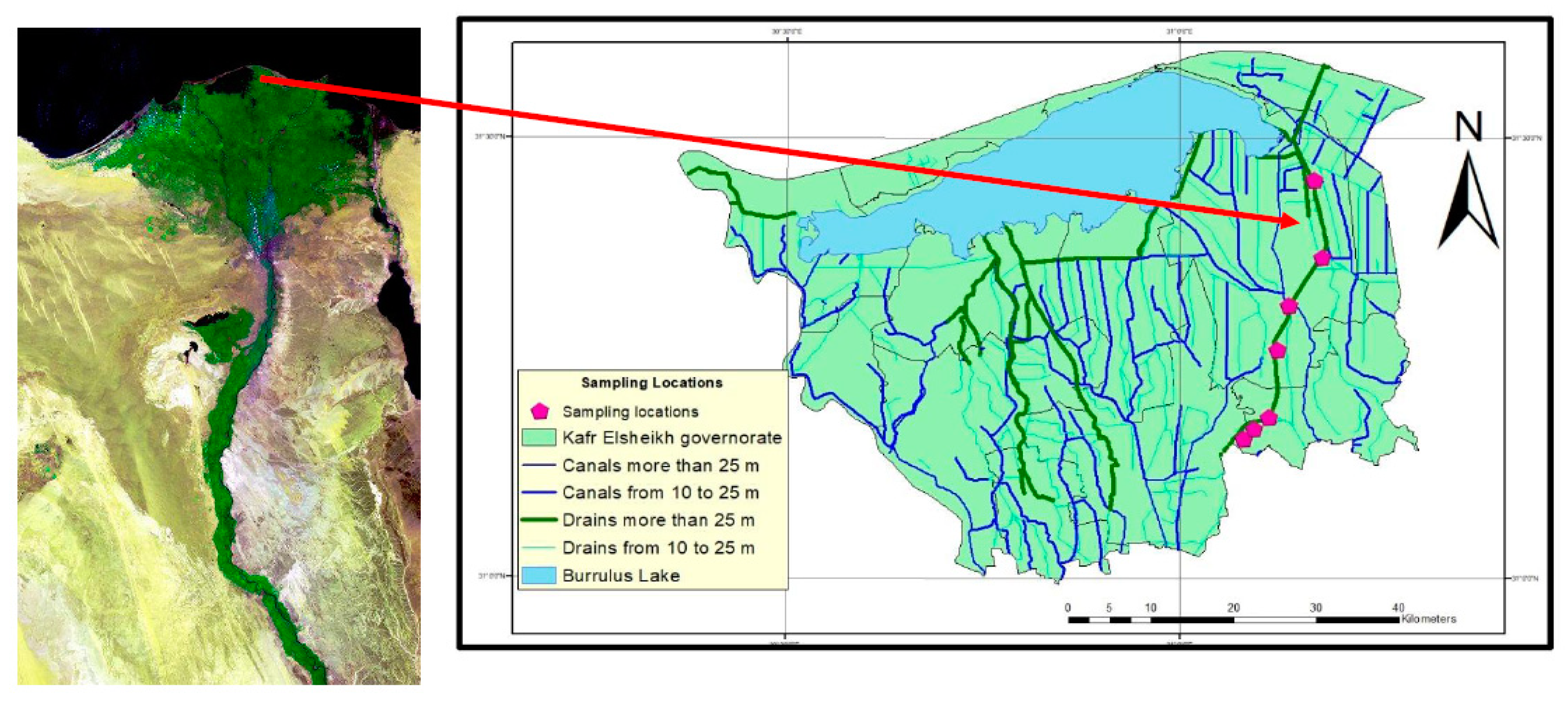
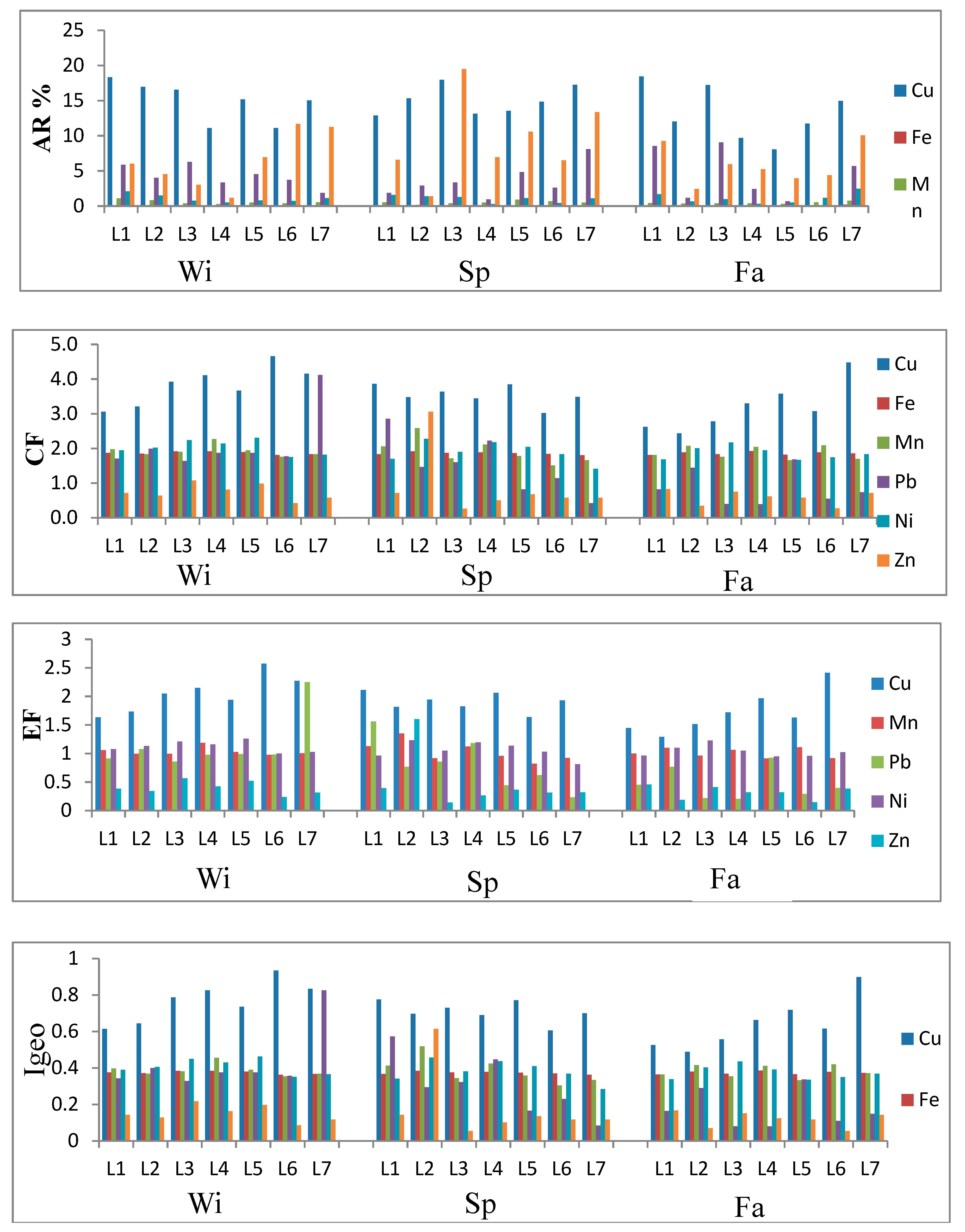
3.2. Trace Elements (TEs) Variability in Soils
Concentrations of the studied TEs were variable, either spatially or between seasons. Najamuddin et al. [
18] also identified seasonal variations in some metals such as Pb and Zn. The results of this study showed seasonal variations in Pb but not Zn; the data from this study indicated that the mean values of all studied TEs were higher in winter than the other seasons with the exception of Fe and Zn. Guo et al. [
46] reported that the increased mobilization of some elements under wetter conditions might be attributed to the absorption of these elements to Fe-(hydr)oxides in the soil. These elements will be released when the oxides are reductively dissolved. On the other hand, under oxidation conditions, Fe and Mn-oxides precipitate, and the TEs are immobilized. Rinklebe and Du [
47] also emphasized that reducing conditions in soils lead to the transformation of oxidized Fe
3+ and Mn
4+ to reduced Fe
2+ and Mn
2+, consequently raising the concentrations of soluble Fe and Mn. In the oxidized state, both elements can be immobilized by precipitation as Fe and Mn-oxyhydroxides. In addition, Kennou et al. [
42] stated that an alkaline pH increases the capacity for adsorption by oxides and manganese and iron hydroxides. Thus, metals are adsorbed or coprecipitated. This may be an important reason for the observed declines in Fe and Mn under some conditions.
Available Cu followed a similar pattern in winter and spring, while it was significantly different in the fall with moderate variation among the studied locations, as indicated from the CV value (
Table 2). The mean total Cu values were higher than the average for world soils (38.9 mg kg
−1) according to Kabata-Pendias [
28]. Copper values were significantly different between seasons, with their highest value in winter. Total Cu values were higher than the lower limit (60–150 mg kg
−1) of maximum allowable concentration as stated by Kabata-Pendias [
28]. Zhao et al. [
45] found that the application of river sediments led to Cu accumulation in their studied soils in a river floodplain network in southern China. Soil properties may affect total Cu as shown in
Table 3, where Cu correlated significantly (at
p = 0.05) with SOM and CaCO
3. SOM plays a significant role in the mobility of metallic cations through complexation reactions that can modify the accumulation and toxicity of trace metals [
42]. Available Cu followed a different behavioral pattern than the other studied available TEs in terms of variation among the locations. The spatial distribution of soil available heavy metals in the three seasons are shown in
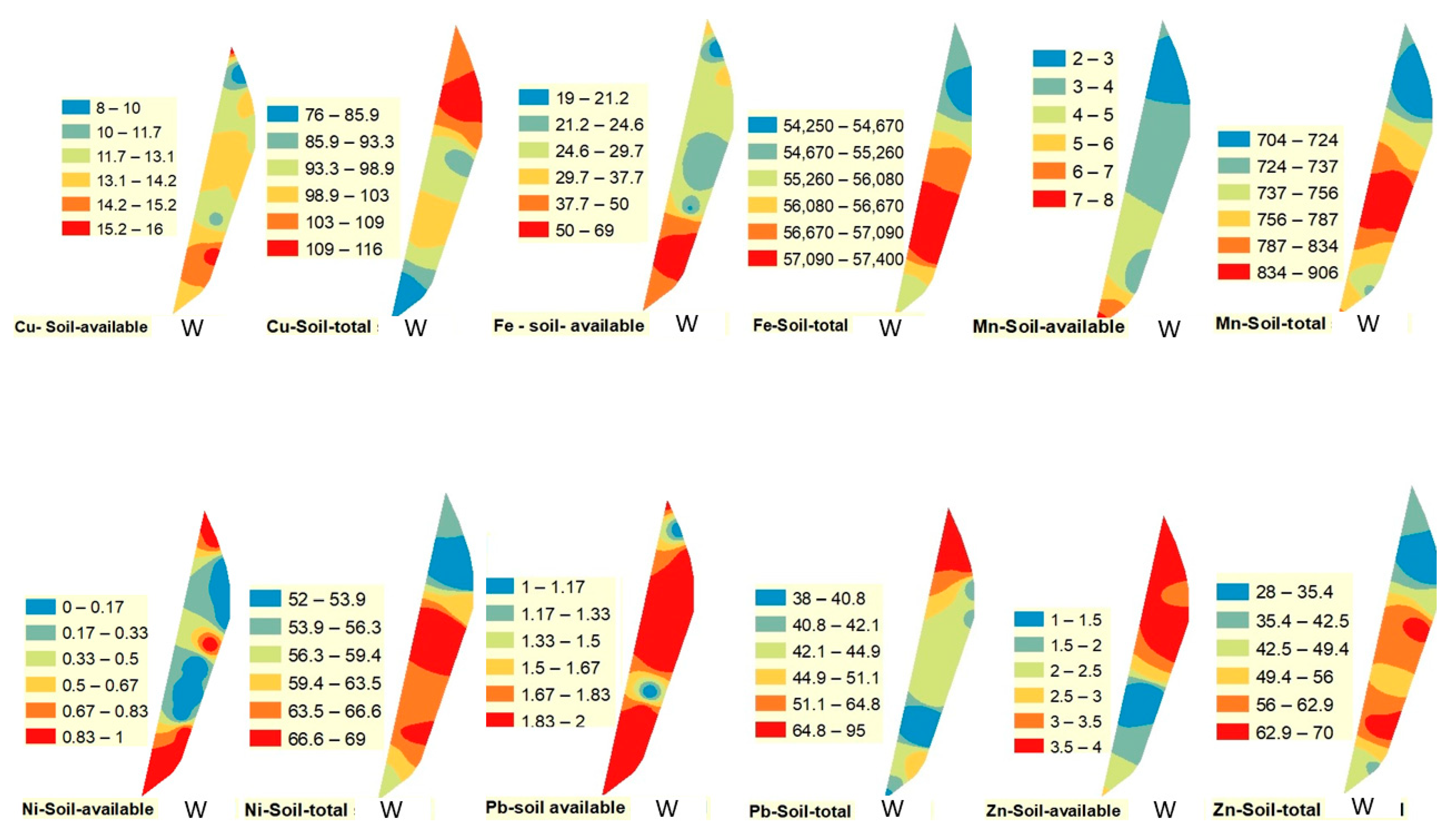
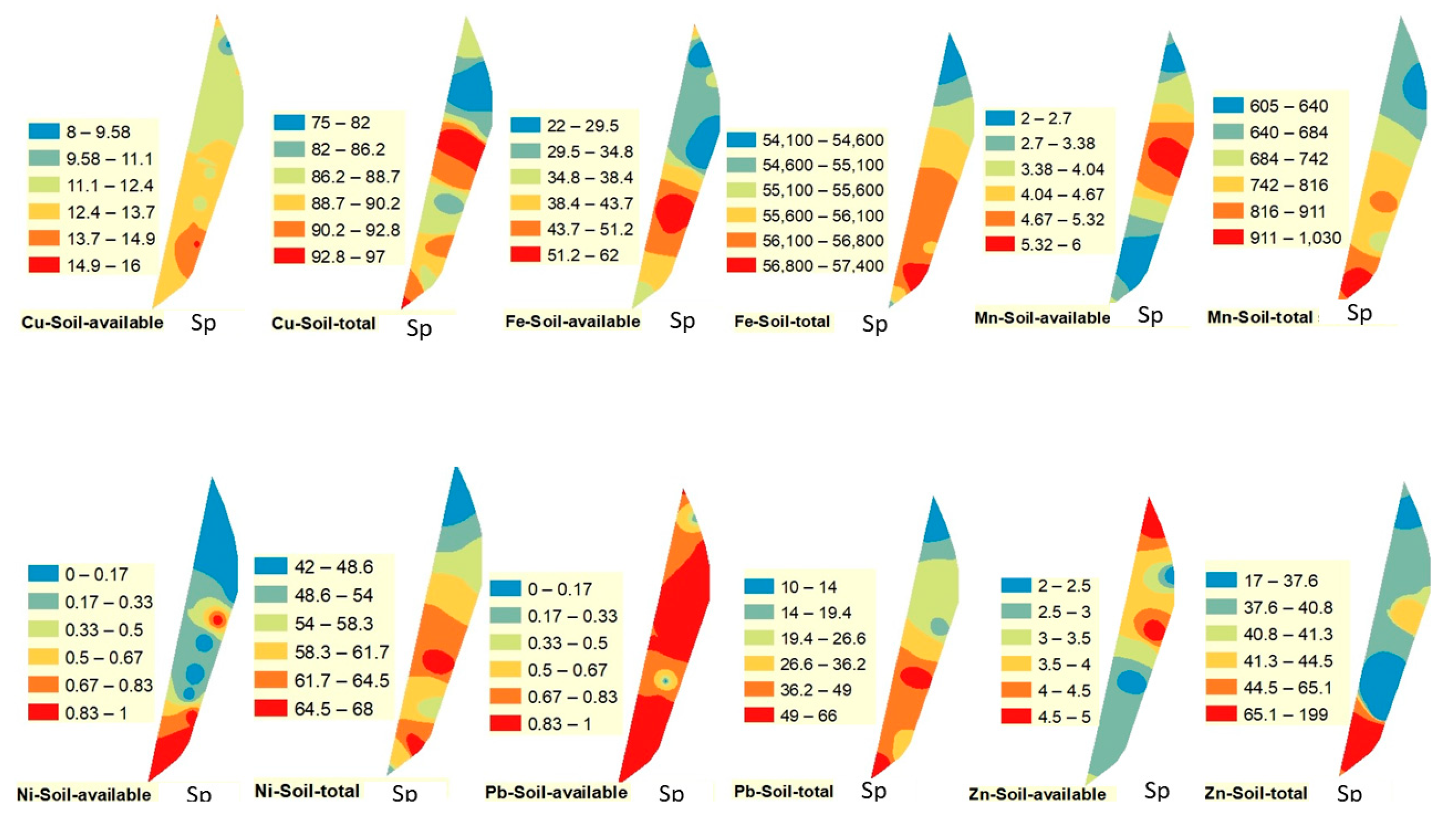
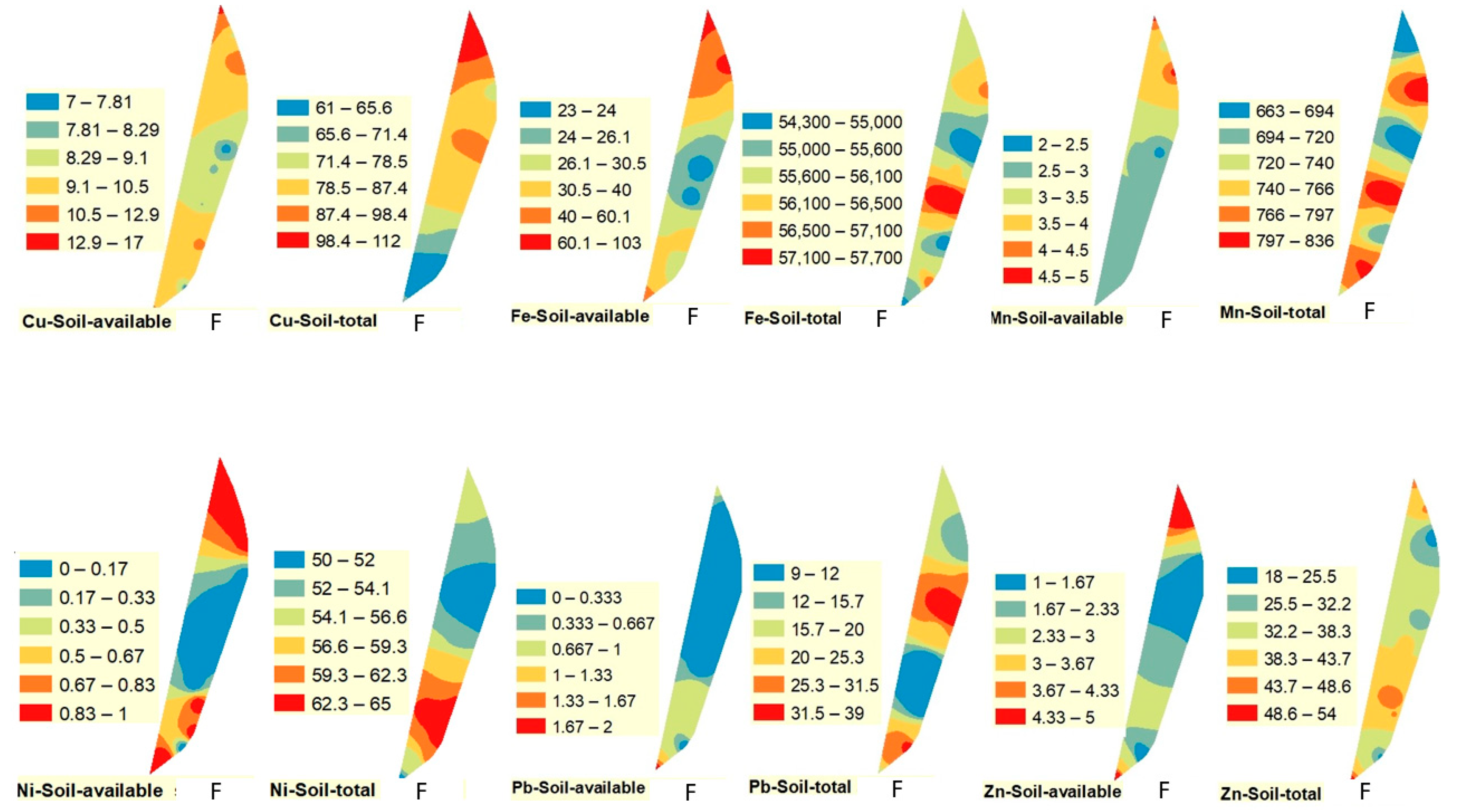
Figure 2,
Figure 3 and
Figure 4. The spatial distribution of Cu (available and total) followed a different pattern than Pb and Fe. The highest value of available Cu was observed in the southern part of the drain in winter and spring, while it was observed in the northern part of the drain in fall. On the other hand, the highest value of total Cu was recorded in the northern part of the drain in winter and fall and in the middle portion of the drain in spring.
Available Fe was not significantly higher in the fall, with a mean value of 41.89 mg kg
−1 and very high variation among locations (
Table 2). Differences in total Fe were not significant between seasons; however, the highest value was recorded in the winter (
Table 2). Although the total Fe values were much higher than available ones, the variation among locations was low. The mean concentrations of total Fe in the three seasons were higher than the average for world soils [
28]. Soil properties may affect total Fe, as it is significantly correlated with pH and P (
Table 3). Total Fe was also highly correlated with total Ni, whereas available Fe was highly correlated with available Cu (
Table 3). Inter-element relationships offer information on the sources of the metals and their pathways [
48]. The highest values of available and total Fe followed similar patterns in both winter and spring, when they were found in the southern part of the drain. On the other hand, the highest value of available Fe in the fall was observed in the north, while the highest value of total Fe in fall was in the middle part of the drain (
Figure 2,
Figure 3 and
Figure 4).
Available Mn values varied between the seasons, with the highest values recorded in winter (
Table 2). The available Mn was significantly different between winter and spring, however, the difference between spring and fall was not significant. There were no significant differences in the total values of Mn between seasons. Mean total Mn values were higher than the world average based on Kabata-Pendias [
28]. Total Mn was significantly correlated with the soil properties pH, SOM, BD and N (
Table 3). Total Mn was also significantly correlated with available Pb. This may indicate the same origin or same controlling factors for these metals in the analyzed soils [
48]. The variation in Mn values among the locations was moderate in winter and fall, while it was high in spring as indicated by the CV values. The highest available values of Mn were recorded in the southern, middle and northern parts of the drain in winter, spring and fall, respectively (
Figure 2,
Figure 3 and
Figure 4). On the contrary, the highest total values of Mn were observed in the middle and the southern parts of the drain in all studied seasons.
There was no significant difference in available Ni between the seasons (
Table 2), but there was high variation in available Ni among the studied sites alongside the drain. The variation in total Ni between sites was moderate. The total and available Ni values followed similar patterns between seasons. The highest available and total Ni values were recorded in winter. The total Ni values were higher than the world soil average [
28]. This could be attributed to soil use and management as suggested by Zhao et al. [
45] or proximity to roads and automobiles, which can discharge high amounts of heavy metals [
49,
50]. The total Ni concentrations in our study are in the range of the maximum allowable concentration of Ni (20–60 mg kg
−1) stated by Kabata-Pendias [
28]. Total Ni was correlated with the soil properties pH, SOM, BD and N (
Table 3). Total Ni was also significantly correlated with available Pb and total Mn, indicating the possibility of the same source for these metals. The highest value of available Ni was observed in the northern and the southern parts of the drain with a tendency to be higher in the north during fall and in the south during spring (
Figure 2,
Figure 3 and
Figure 4). Total concentration of Ni followed a similar pattern to some extent, being highest in the south in spring and fall and in the middle during winter.
Available Pb values varied significantly between seasons, with the highest level recorded in winter (
Table 2). The variation of available Pb among the study sites was moderate in winter and spring, while it was high in fall. Variation in total Pb was high in all seasons. The total concentration of Pb also varied significantly between seasons, with the highest value recorded in winter, the same as available Pb. The mean concentrations of Pb found in this study were higher than the world soil average reported by Kabata-Pendias [
28], and the mean total Pb concentrations were higher than the minimum limit (20–300 mg Kg
−1) given by Kabata-Pendias [
28]. This could be attributed to proximity of the studied soil to the drain. The Kitchener Drain is known to carry Pb and other elements from agriculture, industry and roads. The only correlations between soil properties and total Pb were with SOM and BD (
Table 3). The correlations between Pb concentrations and SOM content obtained in this study agree with the findings of Xun and Xuegang [
48], who also indicated that SOM content had an essential role in the control of Pb sorption by soils. Furthermore, Pb was significantly correlated with total Mn and total Ni in our study. The highest values of available Pb were observed along the drain in winter and spring, while in fall, the highest value was observed in the southern part of the drain. Total Pb patterns differed from those of available Pb in the three studied seasons. Total Pb was highest in the northern, southern, and the middle parts of the drain in winter, spring and fall, respectively. The source of Pb in soil is often leaded gasoline [
10]. In Kitchener Drain, this could result from running leaded gasoline in irrigation engines along the drain and in vehicles passing on nearby roads.
Available Zn varied significantly between winter and spring, as well as between spring and fall (
Table 2). However, the mean values of total Zn did not show significant differences between seasons. The highest available and total Zn concentrations were recorded in the spring. Unlike most of the other TEs investigated in this study, Zn was lower than the world soil average and the maximum allowable Zn concentrations reported by Kabata-Pendias [
28]. Total Zn did not correlate with any of the soil properties (
Table 3). This is contrary to the findings of Xun and Xuegang [
48], who found that Zn was negatively correlated with pH. Khaledian et al. [
43] found a positive correlation between exchangeable Zn and both pH and total organic carbon, and a positive correlation between total Zn and total organic carbon. In this study, total Zn was significantly correlated with available Ni and available Zn. This implied that they may have the same source. The highest value of available Zn was observed in the northern part of the drain in all seasons, while the highest value of total Zn was observed toward the southern part of the drain in all seasons. The measured TEs exhibited wide differences from one site to another. This may be related to anthropogenic sources within the heavily inhabited and industrialized areas surrounding the study area.
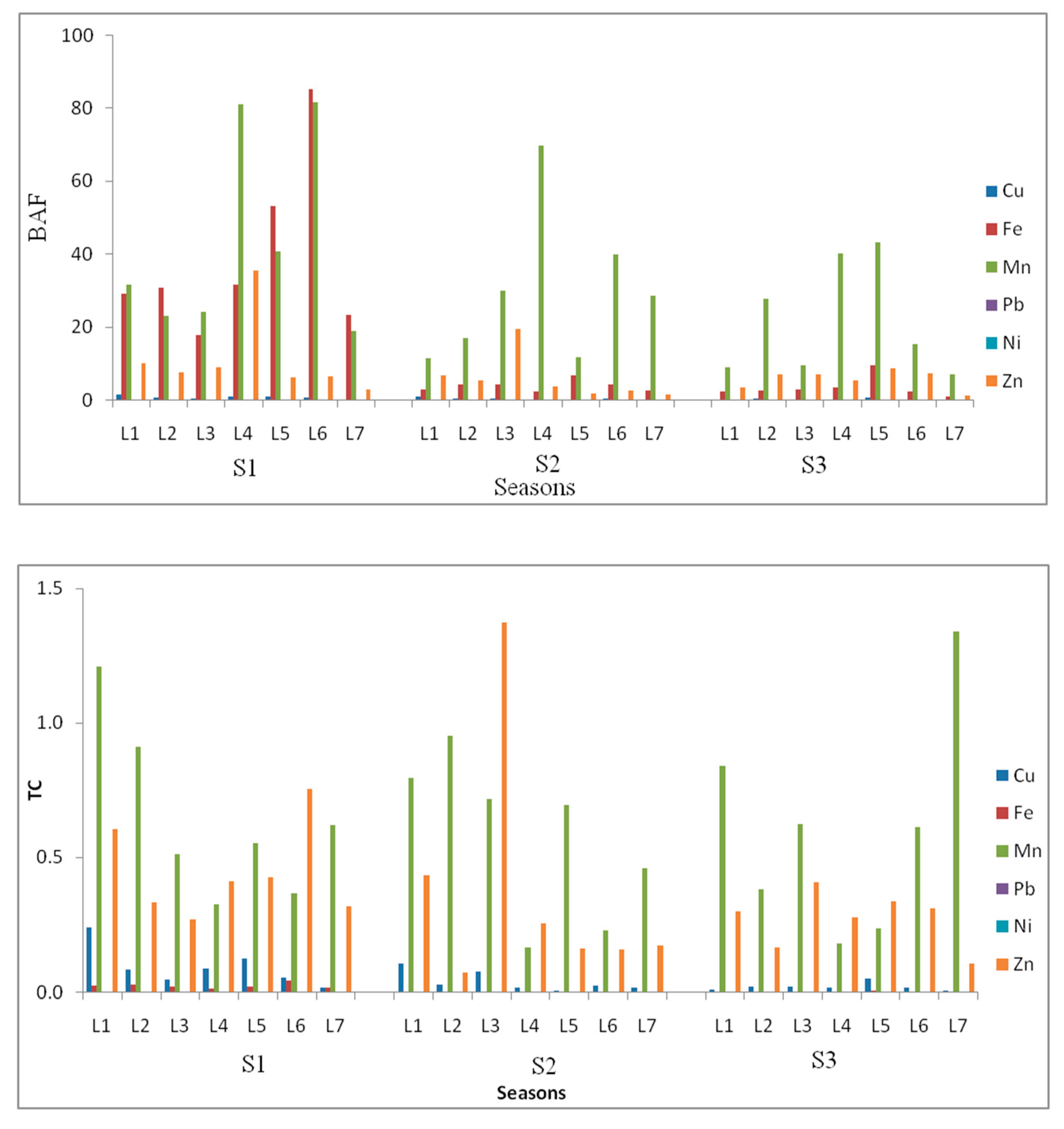
Generally, total Fe, Mn, Ni, and Zn followed similar patterns in the winter. Total Fe, Mn, Ni, and Pb followed similar patterns in the spring, while total Ni and Mn only followed the same pattern in the fall. This indicated that Ni and Mn may have the same source in all seasons, while they share mutual sources with Fe and Zn in winter and with Fe and P in fall, which indicates that the season has a role in changing the variability of these elements. The spatial and seasonal distribution of elements in the study area clearly illustrate that some TEs increase toward the northern part of the drain that is located close to the Mediterranean Sea, such as total Cu, total and available Pb and available Zn in winter; available Pb and available Zn in spring; and total and available Cu, available Fe, available Ni, and available Zn in fall. Nethaji et al. [
5] found metals increased toward the coast in their study area. They attributed this enrichment in TEs to anthropogenic activities. Najamuddin et al. [
18] found that the seasons effect environmental physico-chemical characteristics differentially. They also confirmed that changes in environmental parameters such as temperature, pH, and redox potential (Eh) could influence elemental release from the solid soil phase to the liquid phase. This converted the sediments from an element sink to an elemental source for the overlying waters. The Kitchener Drain flows through highly fertile and densely populated areas (Garbia and Kafr El-Sheikh governorates) in the Nile Delta, Egypt. It carries fertilizers from agricultural activities conducted beside the drain, urban and sewage effluents, and industrial effluents from textiles industries in Mahala Al-kobra, Garbia. The drain is cleared in the winter, and the dredged sediment is moved onto the land on either side of the drain. The people there work the sediment gradually into their soils because they think this will be beneficial to their soils. However, this creates a high risk of heavy metals’ contamination in their soils. This management of dredged materials is probably the main reason for high average TEs contents and high variations in TEs between seasons. Zhao et al. [
45] explained that the application of sediments into soil has played a very important role in many agricultural civilizations such as China because the dredged sediments contain high levels of SOM and can improve soil quality and crop production. However, trace metals may accumulate to toxic levels in agricultural soils over time through the application of polluted sediments. In addition, many workers have studied elemental pollution in settings such as marine, fluvial, drains, and estuarine environments and found variation in the distribution of heavy metals. Ali et al. [
8] reported accumulation of TEs in soil that resulted from continuous irrigation with polluted water. The relatively low concentration of TEs at some locations in the study area is probably due to the soil characteristics [
51] and tidal effects in the Kitchener Drain, which flush the finer particles into deeper regions [
5].
Our results were generally similar to those of Chen et al. [
52], who reported that Cu, Fe, Mn, Ni, Pb, and Zn concentrations decreased with distance from the drainage canal in three short sediment cores sampled from Burullus Lake. Our results for total and available TE concentration were higher than those from some other studies in the Nile Delta, such as Elkady et al. [
16], who studied TEs in sediment samples collected from Lake Manzala, Egypt. Our TE concentrations were also higher than background values recorded by Melegy et al. [
53] in 24 soil samples from the El-Tabbin region (Great Cairo, Egypt). Elbana et al. [
54], when studying agricultural soils near Cairo, Egypt, found available TE concentrations (AB-DTPA) in soils for Cu (0.9–42.3 mg kg
−1), Ni (0.2–4.1 mg kg
−1), and Pb (0.5–20.0 mg kg
−1) that tended to be higher than the levels found in this study. However, Elbana et al. [
54] reported total TEs concentrations for Cu (1.6–120.5 mg kg
−1), Ni (0.2–31.3 mg kg
−1), and Pb (7.0–50.0 mg kg
−1) that tended to be lower than the total concentrations found in this study, as did Khalil et al. [
55] who reported total TEs as Cu (23–57.8 mg kg
−1), Ni (12.3–53.3 mg kg
−1), and Pb (8–36 mg kg
−1) in sediments from Burullus Lagoon, Egypt.