Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Samples and Experimental Design
2.2. Physico-Chemical Traits
2.3. Bioactive Compounds and Antioxidant Activity
2.4. Enzymes Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Chitosan Treatment on Weight Loss and Firmness
3.2. Bioactive Compounds
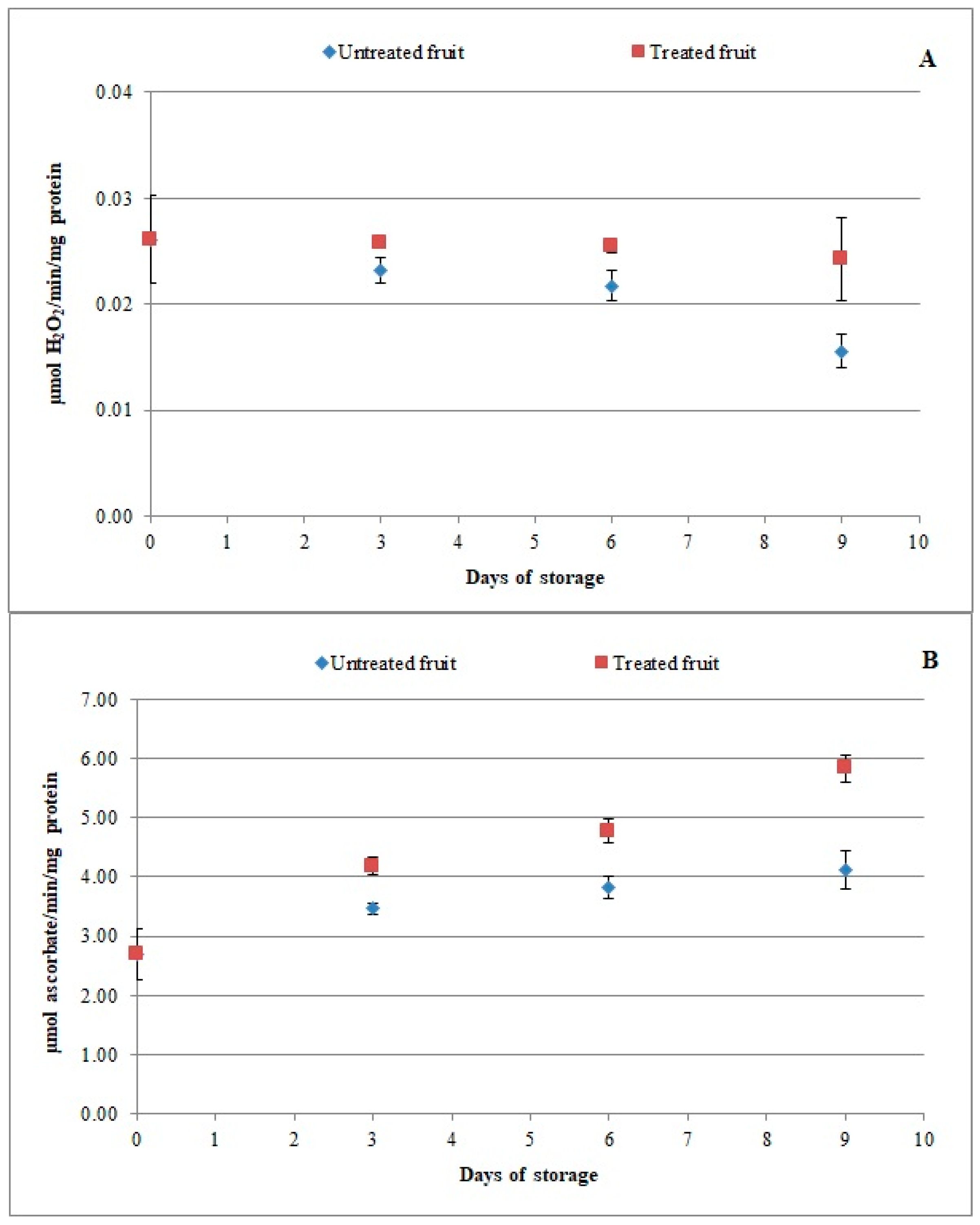
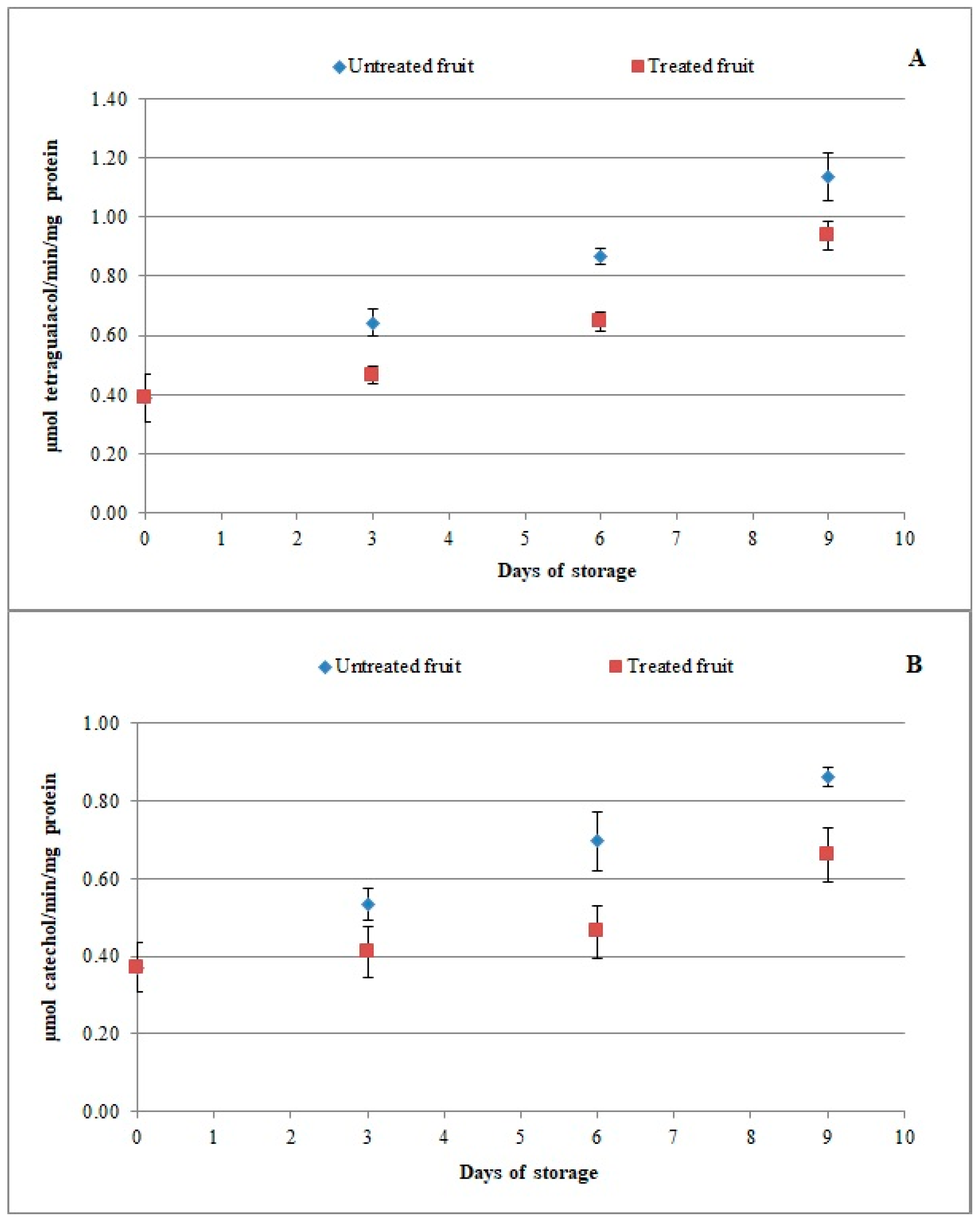
3.3. Effect of Chitosan-Based Coating on Antioxidant Enzymes and Enzymatic Browning
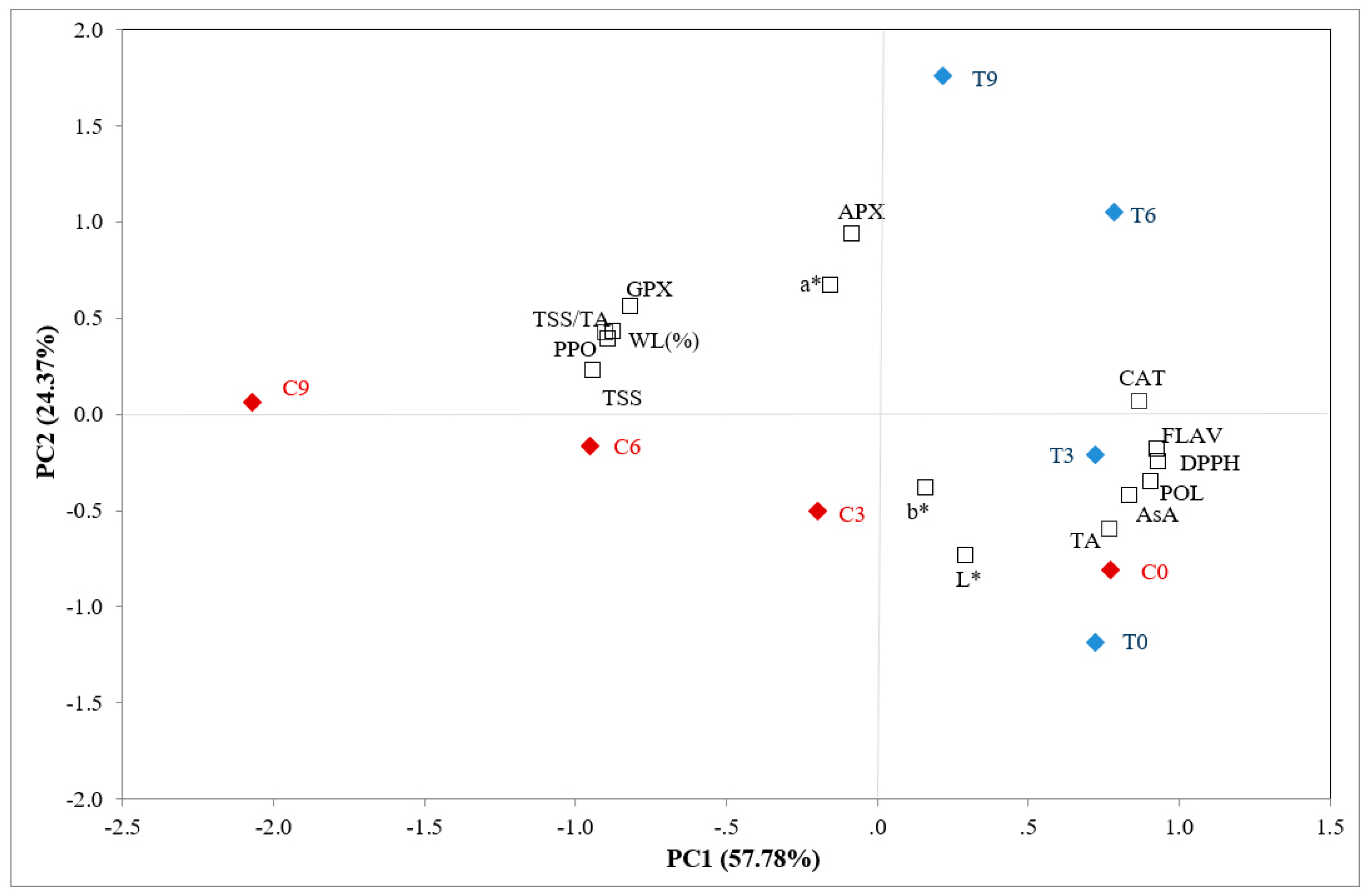
3.4. Evaluation of the Effects of Chitosan-Based Coating by PCA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kislev, M.E.; Hartmann, A.; Bar-Yosef, O. Early domesticated fig in the Jordan Valley. Science 2006, 312, 1372–1374. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef] [PubMed]
- Stover, E.W.; Aradhya, M.K.; Crisosto, C.; Ferguson, L. The fig: Overview of an ancient fruit. HortScience 2007, 42, 1083–1087. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Division. 2017. Available online: http://faostat.fao.org (accessed on 4 February 2019).
- Trichopoulou, A.; Vasilopoulou, E.; Georga, K.; Soukara, S.; Dilis, V. Traditional foods: Why and how to sustain them. Trends Food Sci. Technol. 2006, 17, 498–504. [Google Scholar] [CrossRef]
- Kong, M.; Lampinen, B.; Shackel, K.; Crisosto, C.H. Fruit skin side cracking and ostiole end splitting shorten postharvest life in fresh figs (Ficus carica L.), but are reduced by deficit irrigation. Postharvest Biol. Technol. 2013, 85, 154–161. [Google Scholar] [CrossRef]
- Flaishman, M.A.; Rodov, V.; Stover, E. The fig: Botany, horticulture, and breeding. Hortic. Rev. 2008, 34, 113–197. [Google Scholar]
- Chessa, I. Figure. In Postharvest Physiology and Storage of Tropical and Subtropical Fruits, 1st ed.; Mitra, S., Ed.; CAB International: Wallingford, UK, 1997; pp. 245–268. [Google Scholar]
- Venditti, T.; Molinu, M.G.; Dore, A.; Hallewin, G.D.; Fiori, P.; Tedde, M.; Agabbio, M. Treatment with gras compounds to keep fig fruit quality during cold storage. Agr. Appl. Biol. Sci. 2005, 70, 339–343. [Google Scholar]
- Doster, M.A.; Michailides, T.J. Fungal decay of first-crop and main-crop figs. Plant Dis. 2007, 91, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, H.; Ferguson, L.; Bremer, V.; Colelli, G. Fig (Ficus carica L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits, 1st ed.; Yahia Elhadi, M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; Volume 3, pp. 134–158. [Google Scholar]
- Bouzo, C.A.; Travaercicdelo, M.; Gariglio, N.F. Effect of Different Packaging Materials on Postharvest Quality of Fresh Fig Fruit. Int. J. Agric. Biol. 2012, 14, 821–825. [Google Scholar]
- Alturki, S. Utilization of modified atmosphere packaging to extend the shelf-life of fresh Figure. Biotechnology 2013, 12, 81–86. [Google Scholar]
- Polat, A.A.; Siddiq, M. Figs. In Tropical and Subtropical Fruits: Postharvest Physiology, Processing and Packaging, 1st ed.; Siddiq, M., Ed.; John Wiley & Sons, Inc.: Ames, IA, USA, 2012; pp. 154–196. [Google Scholar]
- Reyes-Avalos, M.C.; Femenia, A.; Minjares-Fuentes, R.; Contreras-Esquivel, J.C.; Aguilar-González, C.N.; Esparza-Rivera, J.R.; Meza-Velázquez, J.A. Improvement of the Quality and the Shelf Life of Figs (Ficus carica) Using an Alginate–Chitosan Edible Film. Food Bioprocess Technol. 2016, 9, 2114–2124. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on postharvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Pereira, C.; Córdoba, M.G. Synergism of defatted soybean meal extract and modified atmosphere packaging to preserve the quality of figs (Ficus carica L.). Postharvest Biol. Technol. 2016, 111, 264–273. [Google Scholar] [CrossRef]
- Adiletta, G.; Pasquariello, M.S.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Petriccione, M. Chitosan coating: A postharvest treatment to delay oxidative stress in loquat fruits during cold storage. Agronomy 2018, 8, 54. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Brasil, I.M.; Siddiqui, M. Postharvest quality of fruits and vegetables: An overview. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality, 1st ed.; Siddiqui, M.W., Ed.; Elsevier, Inc.: London, UK, 2018; pp. 1–40. [Google Scholar]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Ali, A.; Yeoh, W.K.; Forney, C.; Siddiqui, M.W. Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 58, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bussiouny, R.E.L.; Saleh, M.A. Browning inhibition mechanism by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. J. Food Sci. Technol. 2015, 52, 3651–3659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, P.; Sethia, S.; Sharmah, R.R.; Srivastav, M.; Varghese, E. Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci. Hortic. 2017, 226, 104–109. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.; Li, H.; Yuan, C.; Zhong, J.; Chen, Y. Influence of postharvest citric acid and chitosan coating treatment on ripening attributes and expression of cell wall related genes in cherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 2016, 198, 1–6. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Casariego, A.; Forbes-Hernandez, T.Y.; García, M.A. Effect of chitosan-olive oil emulsion coating on quality of tomatoes during storage at ambient conditions. J. Berry Res. 2015, 5, 207–218. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Washington, DC, USA, 1990. [Google Scholar]
- Caliskan, O.; Polat, A.A. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci. Hortic. 2011, 128, 473–478. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colourimetry of Total Phenolics with Phosphomolybdic- phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Petriccione, M.; Pagano, L.; Forniti, T.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Mencarelli, F. Postharvest treatment with chitosan affects the antioxidant metabolism and quality of wine grape during partial dehydration. Postharvest Biol. Technol. 2018, 137, 38–45. [Google Scholar] [CrossRef]
- Bradford, M.M. A dye binding assay for protein. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Q.; Cao, J.; Jiang, W. Effects of chitosan coating on postharvest quality of mango (Mangifera indica. cv. Tainong) fruits. J. Food Process. Preserv. 2008, 32, 770–784. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernandez-Lauzardo, A.N.; Velazquez-del Valle, M.G.; Hernández-López, M.; Ait Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Review: Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Kerch, G. The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases. Mar. Drugs 2015, 13, 2158–2182. [Google Scholar] [CrossRef]
- Dollahite, S.; Bremer, V.; Crisosto, G.M.; Crisosto, C.H.; Stover, E.; Ferguson, L. Effects of Delayed Cooling on Two Fresh Fig Cultivars. 2007. Available online: http://groups.ucanr.org/freshfi g/index.cfm (accessed on 4 February 2019).
- Ersoy, N.; Gözlekçi, S.; Kaynak, L. Changes in sugar contents of fig fruit (Ficus carica l. Cv. Bursa Siyahı) during development. Süleyman Demirel Üniversitesi Ziraat Fakültesi Dergisi 2007, 2, 22–26. [Google Scholar]
- Veberic, R.; Mikulic-Petkovsek, M. Phytochemical composition of common figs (Ficus carica L.) cultivars. In Nutrional Composition of Fruit Cultivars, 1st ed.; Simmonds, M.S.J., Preedy, V.R., Eds.; American Press: London, UK, 2016; pp. 235–255. [Google Scholar]
- Silva, W.B.; Cosme Silva, G.M.; Bortolini Santana, D.; Rodrigues Salvador, A.; Barbosa Medeiros, D.; Belghith, I.; Martins da Silva, N.; Menezes Cordeiro, M.H.; Polete Misobutsi, G. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Kittur, F.; Saroja, N.; Habibunnisa; Tharanathan, R. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of Chitosan Coating on Postharvestlife and Quality of Guava (Psidium guajava L.) Fruit during Cold Storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Trad, M.; Ginies, C.; Gaaliche, B.; Renard, C.M.G.C.; Mars, M. Does pollination affect aroma development in ripened fig [Ficus carica L.] fruit? Sci. Hortic. 2012, 134, 93–99. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate Coatings Preserve Fruit Quality and Bioactive Compounds during Storage of Sweet Cherry Fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Changes in hydrophilic and lipophilic antioxidant activity and related bioactive compounds during postharvest storage of yellow and purple plum cultivars. Postharvest Biol. Technol. 2009, 51, 354–363. [Google Scholar] [CrossRef]
- Hernandez-Munoz, P.; Almenar, E.; Del Valle, V.; Velez, D.; Gavara, R. Effect of Chitosan Coating Combined with Postharvest Calcium Treatment on Strawberry (Fragaria ananassa) Quality during Refrigerated Storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, T. Effect of chitosan on incidence of brown rot, quality and physiological attributes of postharvest peach fruit. J. Sci. Food Agric. 2001, 81, 269–274. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Effect of chitosan and other polyions on chitin deacetylase in Rhizopus stolonifer. Exp. Mycol. 1992, 16, 173–177. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effect of chitosan coating on quality and shelf-life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Petriccione, M.; Pasquariello, M.S.; Mastrobuoni, F.; Zampella, L.; Di Patre, D.; Scortichini, M. Influence of a chitosan coating on the quality and nutraceutical traits of loquat fruit during postharvest life. Sci. Hortic. 2015, 197, 287–296. [Google Scholar] [CrossRef]
- Irfan, P.K.; Vanjakshic, V.; Keshava Prakasha, M.N.; Ravie, R.; Kudachikara, V.B. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Marpudi, L.S.; Ramachandran, P.; Srividya, N. Aloe vera gel coating for postharvest quality maintenance of fresh fig fruits. Res. Pharm. Biol. Chem. Sci. 2013, 4, 878–887. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Del Caro, A.; Piga, A. Polyphenol composition of peel and pulp of two Italian fresh fig fruits cultivars (Ficus carica L.). Eur. Food Res. Technol. 2008, 226, 715–719. [Google Scholar] [CrossRef]
- Ercisli, S.; Tosun, M.; Karlidag, H.; Dzubur, A.; Hadziabulic, S.; Aliman, Y. Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from northeastern turkey. Plant Food Hum. Nutr. 2012, 67, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Bal, E. Postharvest application of chitosan and low temperature storage affect respiration rate and quality of plum fruits. JAST 2013, 15, 1219–1230. [Google Scholar]
- Pande, G.; Akoh, C.C. Organic acids, antioxidant capacity, phenolic content and lipid characterisation of Georgia-grown underutilized fruit crops. Food Chem. 2010, 120, 1067–1075. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- De Gara, L.; Paciolla, C.; De Tullio, M.C.; Motto, M.; Arrigoni, O. Ascorbate-dependent hydrogen peroxide detoxification and ascorbate regeneration during germination of a highly productive maize hybrid: Evidence of an improved detoxification mechanism against reactive oxygen species. Physiol. Plant 2000, 109, 7–13. [Google Scholar] [CrossRef]
| Samples | Days | WL | TSS | TA | TSS/TA | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|
| C | 0 | - | 14.87 ± 0.21 a | 5.67 ± 0.20 e | 2.63 ± 0.13 a | 61.49 ± 5.83 bc | −6.78 ± 3.10 c | 44.71 ± 1.41 ab |
| 3 | 7.82 ± 0.70 b | 16.20 ± 0.43 bc | 4.56 ± 0.26 c | 3.56 ± 0.26 c | 58.17 ± 2.42 ab | −6.06 ± 1.57 c | 44.50 ± 0.35 ab | |
| 6 | 14.74 ± 1.04 d | 16.90 ± 0.43 c | 3.91 ± 0.12 b | 4.32 ± 0.23 d | 54.50 ± 4.80 a | −3.82 ± 1.85 b | 43.85 ± 1.39 ab | |
| 9 | 22.23 ± 0.21 e | 18.17 ± 0.25 d | 2.94 ± 0.06 a | 6.18 ± 0.22 e | 52.54 ± 3.86 a | −2.41 ± 3.27 a | 40.69 ± 2.15 a | |
| T | 0 | - | 14.77 ± 0.32 a | 5.59 ± 0.16 e | 2.64 ± 0.08 a | 68.11 ± 7.15 d | −6.99 ± 2.54 c | 45.13 ± 1.22 b |
| 3 | 4.02 ± 0.13 a | 15.07 ± 0.25 a | 5.02 ± 0.08 d | 3.00 ± 0.08 ab | 64.92 ± 0.91 cd | −6.06 ± 1.59 c | 44.67 ± 0.35 ab | |
| 6 | 6.6 ± 50.97 b | 15.20 ± 0.26 a | 4.45 ± 0.30 c | 3.42 ± 0.21 bc | 56.01 ± 3.35 ab | −5.48 ± 2.08 bc | 43.86 ± 1.56 ab | |
| 9 | 12.30 ± 1.67 c | 16.03 ± 0.57 b | 3.57 ± 0.32 b | 4.52 ± 0.51 d | 55.26 ± 3.98 ab | −4.25 ± 5.28 b | 40.56 ± 3.06 a |
| Samples | Days | POL | FLAV | AsA | Antioxidant Activity |
|---|---|---|---|---|---|
| C | 0 | 47.95 ± 0.83 f | 23.25 ± 0.33 de | 16.14 ± 0.84 de | 8.71 ± 0.13 de |
| 3 | 40.61 ± 2.36 c | 19.73 ± 0.40 b | 15.20 ± 0.26 cd | 7.67 ± 0.28 c | |
| 6 | 37.46 ± 0.74 b | 18.41 ± 0.6 b | 12.10 ± 0.33 b | 6.24 ± 0.27 b | |
| 9 | 30.94 ± 1.10 a | 15.41 ± 0.77 a | 10.75 ± 0.63 a | 5.67 ± 0.36 a | |
| T | 0 | 47.72 ± 0.98 ef | 24.52 ± 1.36 e | 16.63 ± 0.74 e | 8,98 ± 0.13 e |
| 3 | 45.35 ± 0.73 de | 22.39 ± 0.27 cd | 16.00 ± 0.10 de | 8.78 ± 0.09 de | |
| 6 | 44.23 ± 0.90 d | 22.02 ± 0.63 cd | 14.57 ± 0.29 c | 8.33 ± 0.48 d | |
| 9 | 38.70 ± 1.47 bc | 21.42 ± 1.17 c | 13.18 ± 1.24 b | 7.49 ± 0.27 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.). Agriculture 2019, 9, 84. https://doi.org/10.3390/agriculture9040084
Adiletta G, Zampella L, Coletta C, Petriccione M. Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.). Agriculture. 2019; 9(4):84. https://doi.org/10.3390/agriculture9040084
Chicago/Turabian StyleAdiletta, Giuseppina, Luigi Zampella, Caterina Coletta, and Milena Petriccione. 2019. "Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.)" Agriculture 9, no. 4: 84. https://doi.org/10.3390/agriculture9040084
APA StyleAdiletta, G., Zampella, L., Coletta, C., & Petriccione, M. (2019). Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.). Agriculture, 9(4), 84. https://doi.org/10.3390/agriculture9040084






