Actual Evapotranspiration and Tree Performance of Mature Micro-Irrigated Pistachio Orchards Grown on Saline-Sodic Soils in the San Joaquin Valley of California
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Southern San Joaquin Valley and of Study Orchards
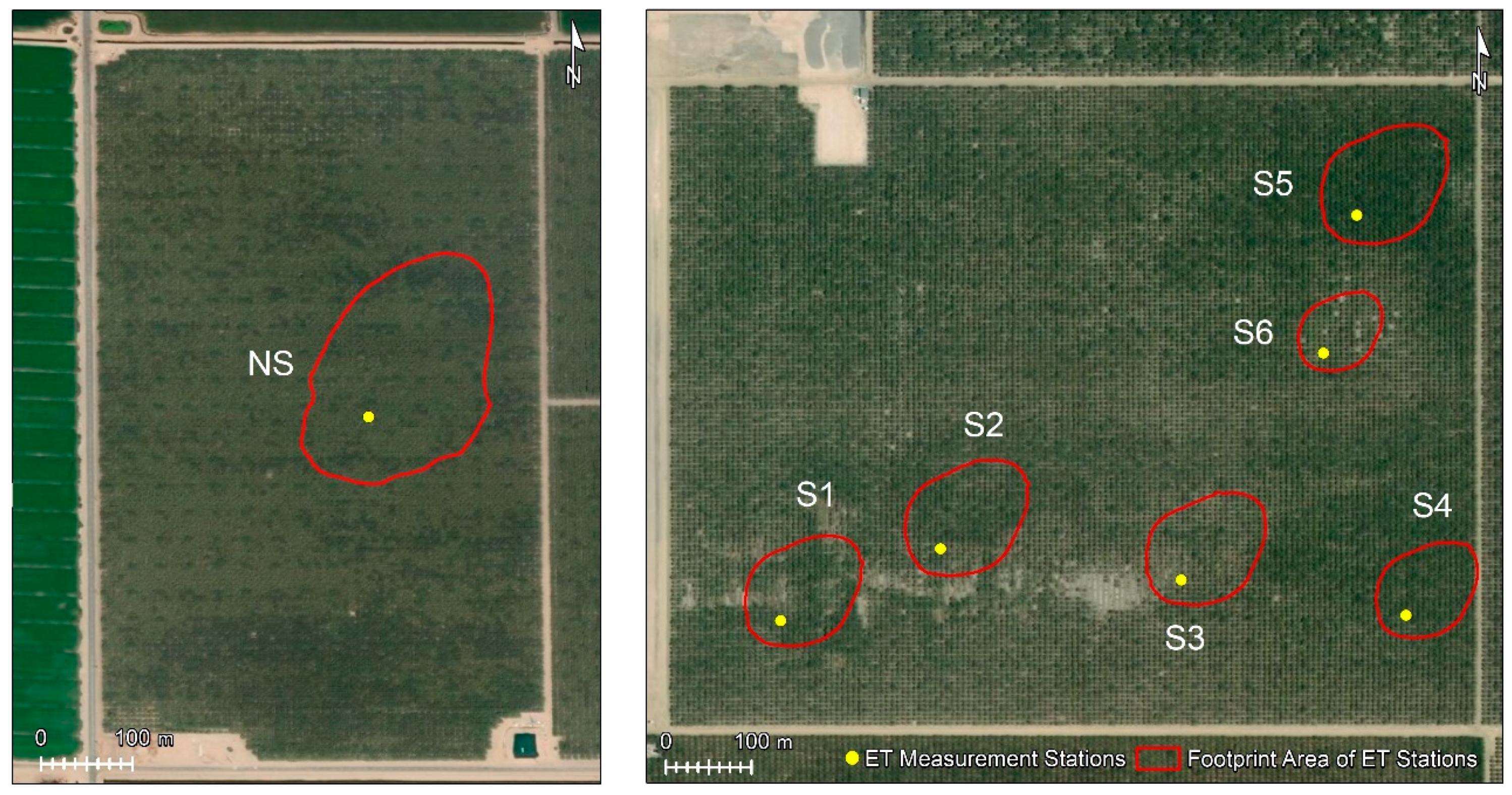
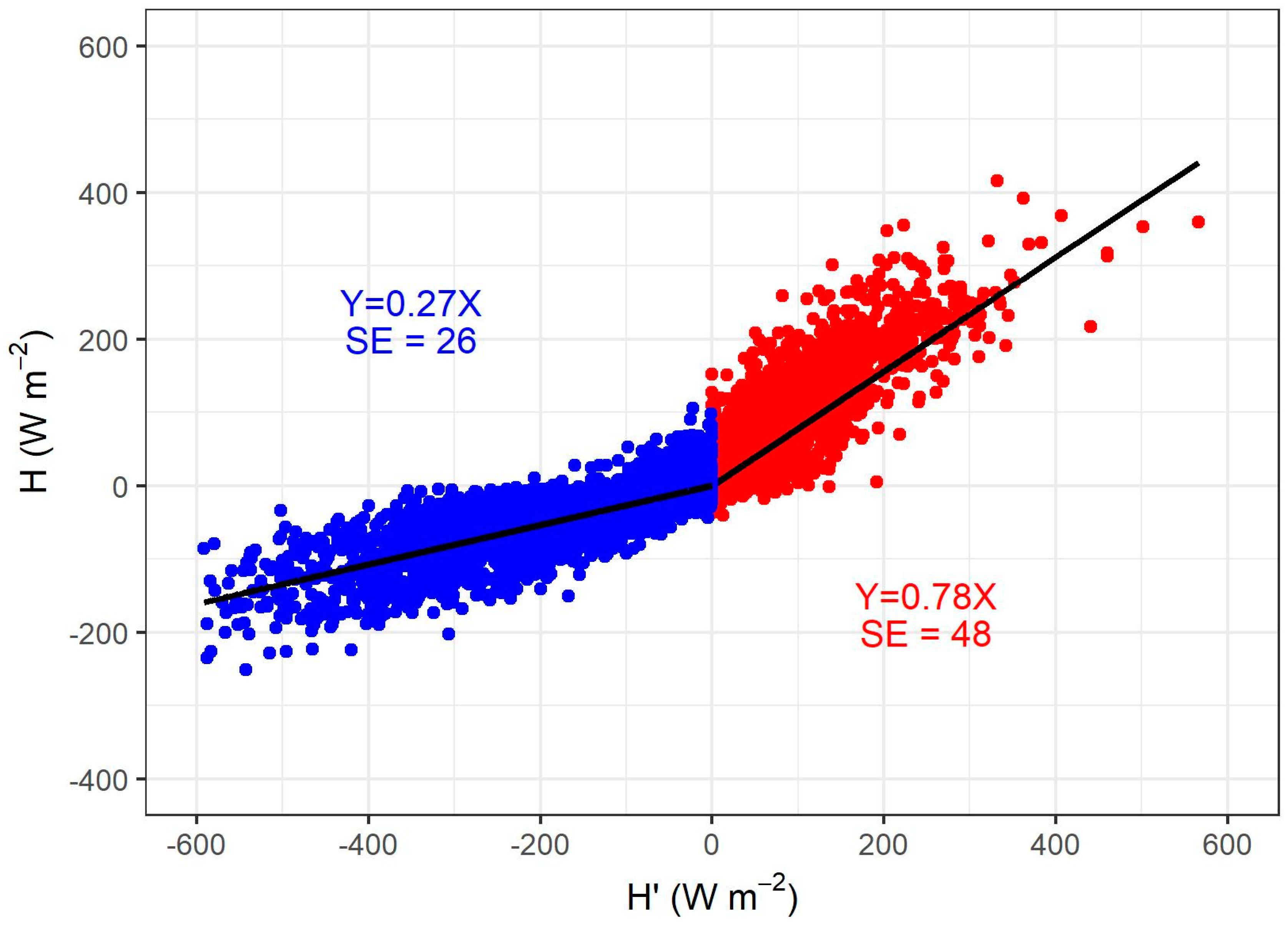
2.2. ET Measurements
2.3. PAR Light Interception by the Tree Canopy
2.4. Assessment of Soil Salinity
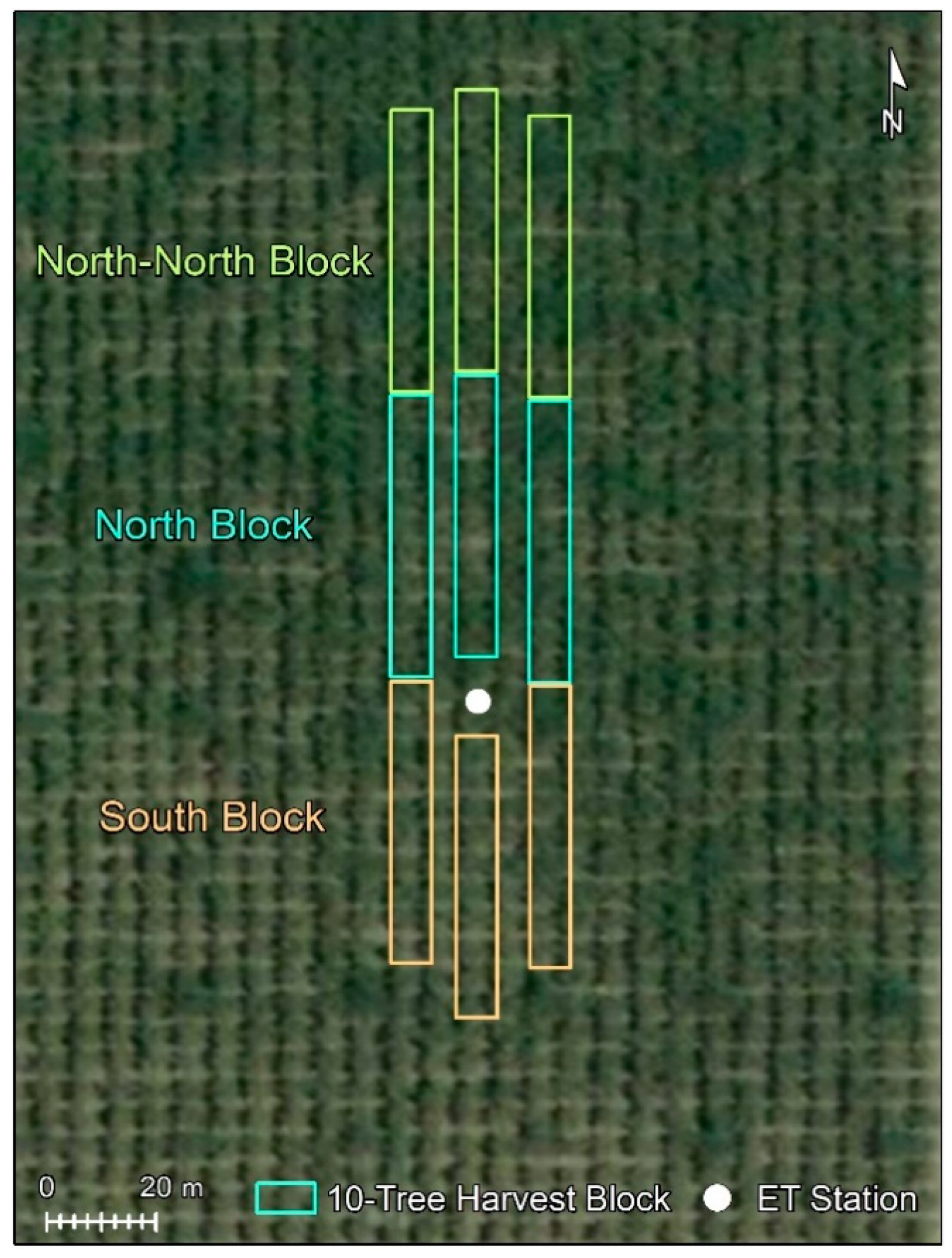
2.5. Measurement of Nut Yield and Assessment of Tree Performance
3. Results
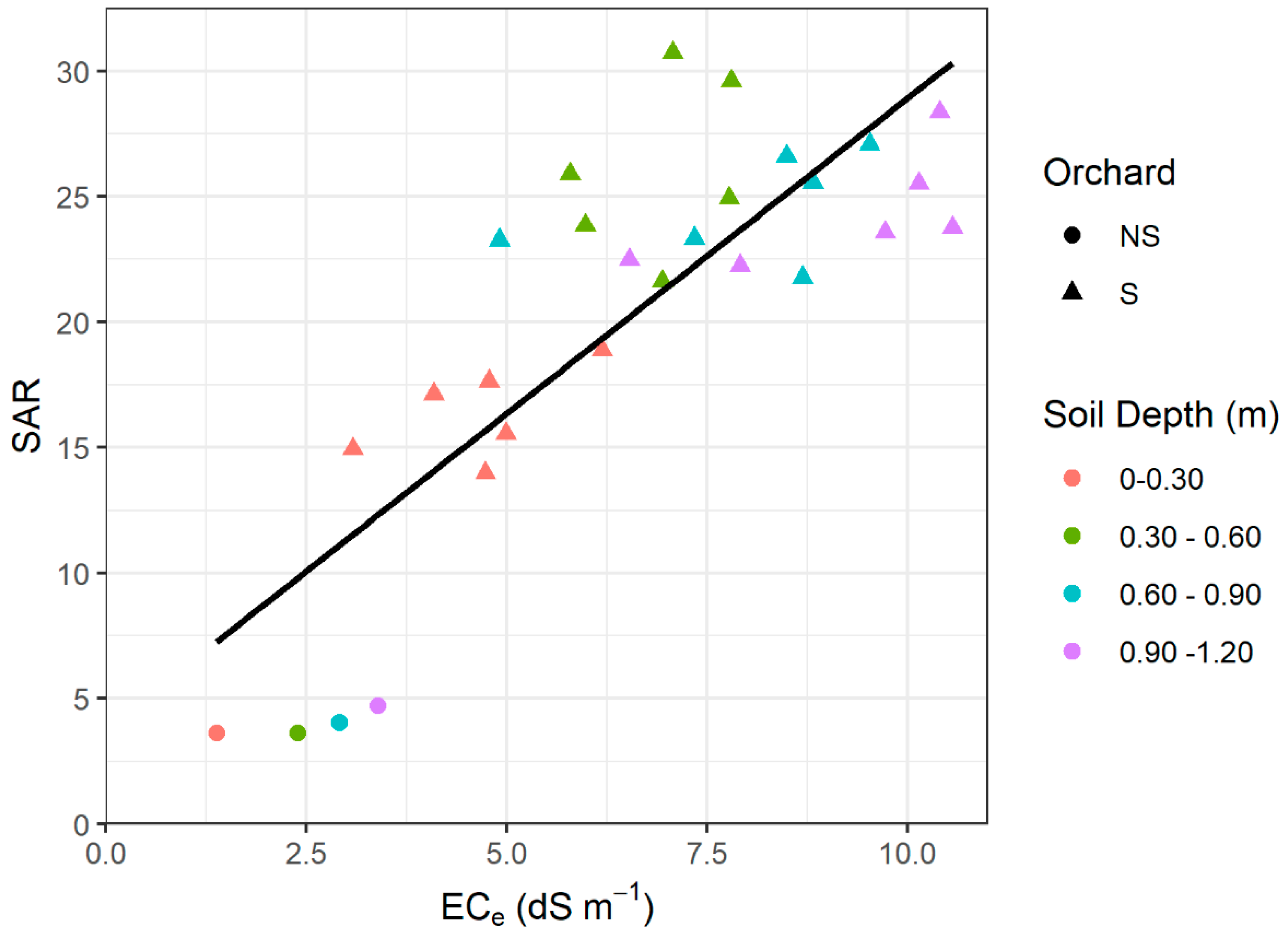
3.1. Soil Parameters
3.2. PAR Light Interception, Nut Yield, and Tree Performance
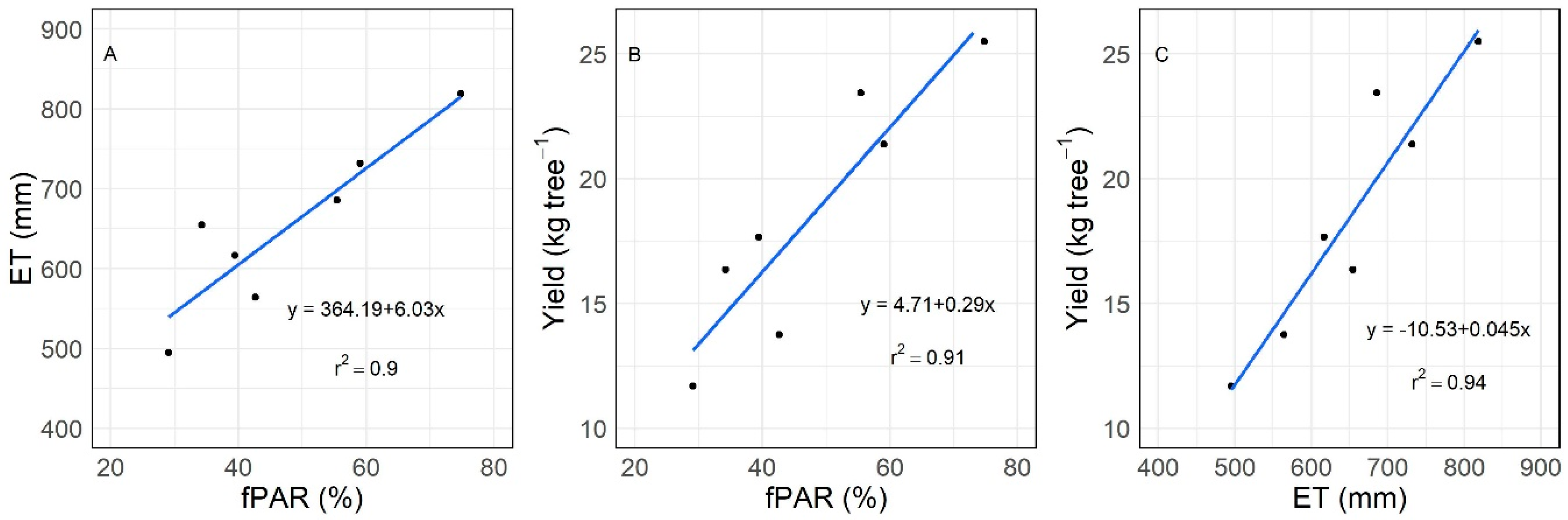
3.3. Relations between fPAR, ET, and Yield
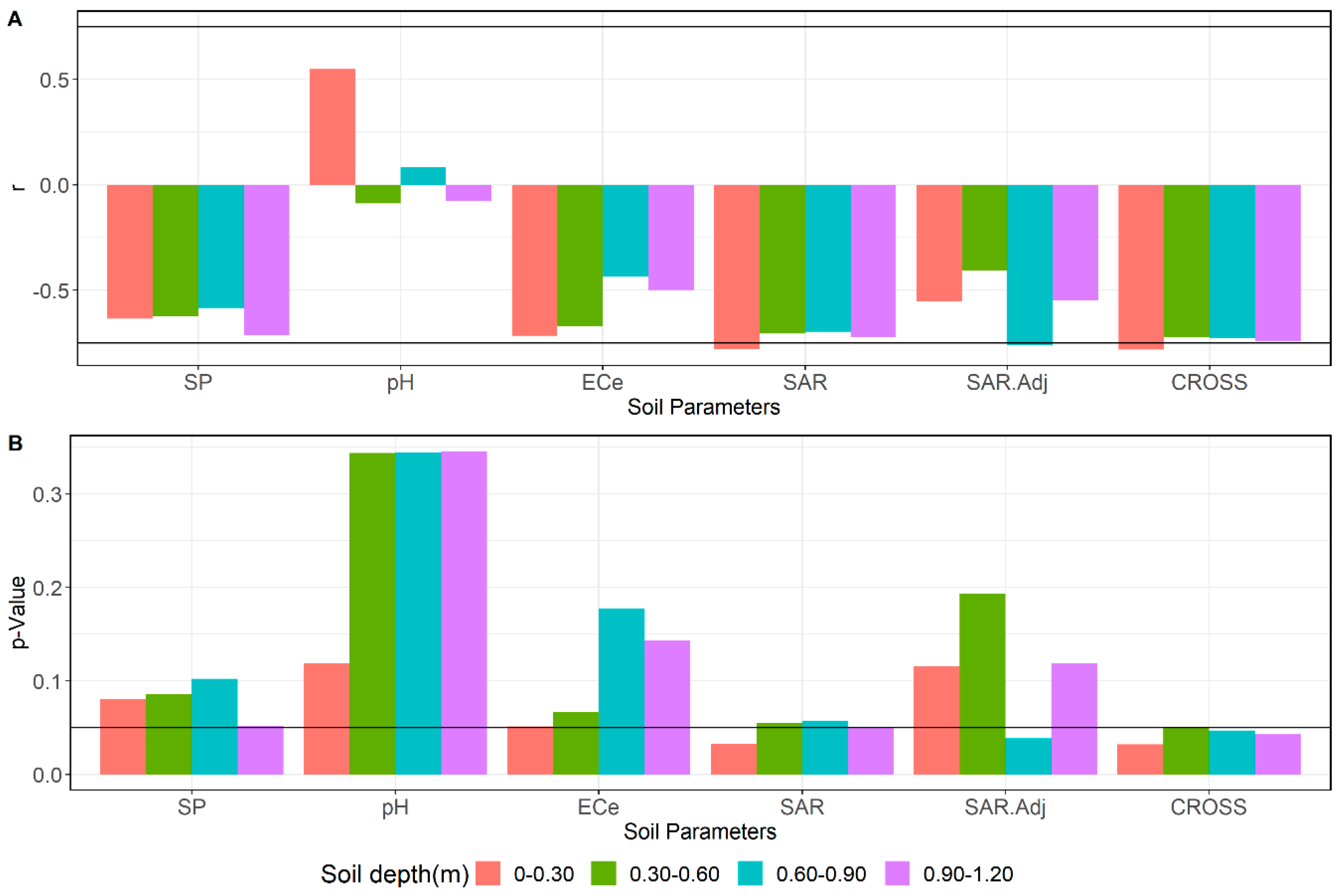
3.4. Relations between Soil Parameters and Tree Performance
4. Discussion
5. Conclusive Remarks, Practical Implications, and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Notations
| Relative crop yield | |
| Salinity threshold () | |
| Slope of yield reduction per in % | |
| CROSS | Cation Ratio of Structural Stability |
| EC | Electro Conductivity |
| ET | Evapotranspiration |
| Actual Evapotranspiration, mm | |
| Fraction of Photosynthetically Active Radiation | |
| H′ | Uncalibrated Surface Renewal Sensible Heat Flux, |
| H′ | Eddy Covariance Surface Renewal Sensible Heat Flux, Kc: Crop coefficient |
| NS | Non-saline |
| PAR | Photosynthetically Active Radiation |
| RPR | Relative Performance Reduction |
| S | Saline |
| SAR | Sodium Adsorption Ratio |
| adjusted SAR | |
| SJV | San Joaquim Valley |
| SP | Saturation percentage |
| WC | Water content |
References
- Goldhamer, D.A. Tree water requirements and regulated deficit irrigation. In Pistachio Production Manual; Ferguson, L., Ed.; Fruit and Nut Research and Information Center, University of California: Davis, CA, USA, 2005; pp. 103–116. [Google Scholar]
- Behboudian, M.H.; Walker, R.R.; Törökfalvy, E. Effects of water stress and salinity on photosynthesis of pistachio. Sci. Hortic. 1986, 29, 251–261. [Google Scholar] [CrossRef]
- Spiegel-Roy, P.; Nazigh, D.; Evenari, M. Response of pistachio to low soil moisture conditions. J. Am. Soc. Hortic. Sci. 1977, 102, 470–473. [Google Scholar]
- Maas, E.V.; Grattan, S.R. Crop yields as affected by salinity. In Agricultural Drainage; American Society of Agronomy: Madison, WI, USA, 1999; pp. 55–108. [Google Scholar]
- Walker, R.R.; Törökfalvy, E.; Behboudian, M.H. Uptake and distribution of chloride, sodium, and potassium ions and growth of salt-treated pistachio plants. Aust. J. Agric. Res. 1987, 38, 383–394. [Google Scholar] [CrossRef]
- Scudiero, E.; Corwin, D.L.; Anderson, R.G.; Yemoto, K.; Clary, W.; Wang, Z.L.; Skaggs, T.H. Remote sensing is a viable tool for mapping soil salinity in agricultural lands. Calif. Agric. 2017, 71, 231–238. [Google Scholar] [CrossRef]
- Letey, J. Soil salinity poses challenges for sustainable agriculture and wildlife. Calif. Agric. 2000, 54, 43–48. [Google Scholar] [CrossRef]
- Ferguson, L.; Sanden, B.; Grattan, S.; Reyes, H.C.; Wilson, C.; Cross, E. Potential for utilizing blended drainage water for irrigating west side, San Joaquin valley pistachios. Cah. Opt. Méditerr. 2001, 56, 155–167. [Google Scholar]
- Ferguson, L.; Poss, P.A.; Grattan, S.R.; Grieve, C.M.; Want, D.; Wilson, C.; Donovan, T.J.; Chao, C.T. Pistachio rootstocks influence scion growth and ion relations under salinity and boron stress. J. Am. Soc. Hortic. Sci. 2002, 127, 194–199. [Google Scholar] [CrossRef]
- Mehdi-Tounsi, H.; Chelli-Chaabouni, A.; Mahjoub-Boujnah, D.; Boukhris, M. Long-term field response of pistachio to irrigation water salinity. Agric. Water Manag. 2017, 185, 1–12. [Google Scholar] [CrossRef]
- Sanden, B.L.; Ferguson, L.; Reyes, H.C.; Grattan, S.C. Effect of salinity on evapotranspiration and yield of San Joaquin Valley pistachios. In Acta Horticulturae, Proceedings of the Fourth International Symposium on Irrigation of Horticultural Crops; ISHS: Korbeek-Lo, Belgium, 2004; Volume 664, pp. 583–589. [Google Scholar]
- Goldhamer, D.A.; Kjelgren, R.; Beede, R.; Williams, L.; Moore, M.J.; Lan, J.; Weinberger, G.; Menezes, J. Water Use Requirements of Pistachio Trees and Response to Water Stress; Annual Report of the California Pistachio Commission; California Pistachio Commission: Fresno, CA, USA, 1985. [Google Scholar]
- Goldhamer, D.A.; Beede, R.; Moore, J.M.; Weinberger, G.; Menezes, J., Jr. Water Use Requirements and Physiological Response to Water Stress in Pistachio; Annual Report of the California Pistachio Commission, Crop Year; California Pistachio Commission: Fresno, CA, USA, 1983; pp. 53–57. [Google Scholar]
- Kanber, R.; Yazar, A.; Önder, S.; Köksal, H. Irrigation response of pistachio (Pistaciavera L.). Irrig. Sci. 1993, 14, 7–14. [Google Scholar] [CrossRef]
- Goldhamer, D.A. Irrigation Management. In Pistachio Production Manual; Ferguson, L., Ed.; Fruit and Nut Research and Information Center, University of California: Davis, CA, USA, 1995; pp. 71–81. [Google Scholar]
- Iniesta, F.; Testi, L.; Goldhamer, D.A.; Fereres, E. Quantifying reductions in consumptive water use under regulated deficit irrigation in pistachio (Pistacia vera L.). Agric. Water Manag. 2008, 95, 877–886. [Google Scholar] [CrossRef]
- Marino, G.; Di Martino, S.; Roxas, A.A.; Caruso, T.; Ferguson, L.; Barone, E.; Marra, F.P. Sustainability of pistachio production (Pistacia vera L.) under supplemental irrigation in a Mediterranean climate. Sci. Hortic. 2018, 241, 260–266. [Google Scholar] [CrossRef]
- Meiri, A.; Kamburov, J.; Shalhevet, J. Transpiration effects on leaching fractions. Agron. J. 1977, 69, 779–782. [Google Scholar] [CrossRef]
- Letey, J.; Dinar, A. Simulated crop-water production functions for several crops when irrigated with saline waters. Hilgardia 1986, 54, 1–32. [Google Scholar] [CrossRef]
- Dudley, L.M.; Ben-Gal, A.; Lazarovitch, N. Drainage water reuse: Biological, physical, and technological considerations for system management. J. Environ. Qual. 2008, 37 (Suppl. 5), S25–S35. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Ann. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Stavarek, S.J.; Rains, D.W. Effect of salinity on growth and maintenance costs of plant cells. In Cellular and Molecular Biology of Plant Stress; Key, J.L., Kosuge, T., Alan, R., Eds.; Liss, Inc.: New York, NY, USA, 1985; pp. 129–143. [Google Scholar]
- Yeo, A.R. Salinity resistance: Physiology and prices. Physiol. Plant. 1983, 58, 214–222. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance. In ASCE Manual and Reportson Engineering Practice No. 71 Agricultural Salinity Assessment and Management, 2nd ed.; Wallender, W.W., Tanji, K.K., Eds.; ASCE: Reston, VA, USA, 2012; Chapter 13; pp. 405–459. [Google Scholar]
- Suarez, D.L. Irrigation water quality assessments. In Agricultural Salinity Assessment and Management, 2nd ed.; Wallender, W.W., Tanji, K.K., Eds.; ASCE: Reston, VA, USA, 2012; pp. 343–370. [Google Scholar]
- Tripler, E.; Shani, U.; Mualem, Y.; Ben-Gal, A. Long-term growth, water consumption and yield of date palm as a function of salinity. Agric. Water Manag. 2011, 99, 128–134. [Google Scholar] [CrossRef]
- Skaggs, T.H.; Poss, J.A.; Shouse, P.J.; Grieve, C.M. Irrigating forage crops with saline waters: 1. Volumetric lysimeter studies. Vadose Zone J. 2006, 5, 815–823. [Google Scholar] [CrossRef]
- Shani, U.; Dudley, L.M. Field studies of crop response to water and salt stress. Soil Sci. Soc. Am. J. 2001, 65, 1522–1528. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Karlberg, L.; Janson, P.K.; Shani, U. Temporal robustness of linear relationships between production and transpiration. Plant Soil 2003, 251, 211–218. [Google Scholar] [CrossRef]
- Shani, U.; Ben-Gal, A.; Tripler, E.; Dudley, L.M. Plant response to the soil environment: An analytical model integrating yield, water, soil type, and salinity. Water Resour. Res. 2007, 43, 1–12. [Google Scholar] [CrossRef]
- Tripler, E.; Ben-Gal, A.; Shani, U. Consequence of salinity and excess boron on growth, evapotranspiration and ion uptake in date palm (Phoenix dactylifera L., cv. Medjool). Plant Soil 2007, 297, 147–155. [Google Scholar] [CrossRef]
- Bhantana, P.; Lazarovitch, N. Evapotranspiration, crop coefficient and growth of two young pomegranate (Punica granatum L.) varieties under salt stress. Agric. Water Manag. 2010, 97, 715–722. [Google Scholar] [CrossRef]
- Burt, C.M. Rapid field evaluation of drip and microspray distribution uniformity. Irrig. Drain. Syst. 2004, 18, 275–297. [Google Scholar] [CrossRef]
- Marino, G.; Zaccaria, D. (University of California, Davis, CA, USA). Unpublished work. 2019.
- Hanson, B.; Orloff, S.; Sanden, B. Monitoring Soil Moisture for Irrigation Water Management; Publication Number 21635; Agriculture and Natural Resources, University of California: Davis, CA, USA, 2007. [Google Scholar]
- Hanson, B.R.; Schwankl, L.J.; Fulton, A.E. Scheduling Irrigations: When and How Much Water to Apply; Publication Number 3396; Agriculture and Natural Resources, University of California: Davis, CA, USA, 1999. [Google Scholar]
- Hart, Q.J.; Brugnach, M.; Temesgen, B.; Rueda, C.; Ustin, S.L.; Frame, K. Daily reference evapotranspiration for California using satellite imagery and weather station measurement interpolation. Civ. Eng. Environ. Syst. 2009, 26, 19–33. [Google Scholar] [CrossRef]
- Kochendorfer, J.; Meyers, T.P.; Frank, J.; Massman, W.J.; Heuer, M.W. How well can we measure the vertical wind speed? Implications for fluxes of energy and mass. Bound. Layer Meteorol. 2012, 145, 383–398. [Google Scholar] [CrossRef]
- Shapland, T.M.; McElrone, A.J.; Khaw, T.P.U.; Snyder, R.L. A turnkey data logger program for field-scale energy flux density measurements using eddy covariance and surface renewal. Ital. J. Agrometeorol. 2013, 18, 5–16. [Google Scholar]
- De Vries, D.A. Thermal properties of soil. In Physics of Plant Environment; van Wijk, W.R., Ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1963; pp. 210–234. [Google Scholar]
- Khaw, T.P.U.; Qiu, J.; Su, H.B.; Watanabe, T.; Brunet, Y. Surface renewal analysis: A new method to obtain scalar fluxes without velocity data. Agric. For. Meteorol. 1995, 74, 119–137. [Google Scholar]
- Snyder, R.L.; Spano, D.; Khaw, T.P.U. Surface renewal analysis for sensible and latent heat flux density. Bound. Layer Meteorol. 1996, 77, 249–266. [Google Scholar] [CrossRef]
- Shapland, T.M.; Snyder, R.L.; Smart, D.R.; Williams, L.E. Estimation of actual evapotranspiration in winegrape vineyards located on hillside terrain using surface renewal analysis. Irrig. Sci. 2012, 30, 471–484. [Google Scholar] [CrossRef]
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A simple two-dimensional parameterisation for Flux Footprint Prediction (FFP). Geosci. Model Dev. 2015, 8, 3695–3713. [Google Scholar] [CrossRef]
- Montazar, A.; Rejmanek, H.; Tindula, G.; Little, C.; Shapland, T.; Anderson, F.; Inglese, G.; Mutters, R.; Linquist, B.; Greer, C.A.; et al. Crop coefficient curve for paddy rice from residual energy balance calculations. J. Irrig. Drain. Eng. 2017, 143, 04016076. [Google Scholar] [CrossRef]
- Simmons, L.J.; Wang, J.; Sammis, T.W.; Miller, D.R. An evaluation of two inexpensive energy-balance techniques for measuring water use in flood-irrigated pecans (Carya illinoinensis). Agric. Water Manag. 2007, 88, 181–191. [Google Scholar] [CrossRef]
- Zaccaria, D.; Marino, G.; Shapiro, K.; Snyder, R.L. (University of California, Davis, CA, USA). Unpublished work. 2019.
- Zarate, J.; Metcalf, S.; Stewart, W.; Ustin, S.L.; Lampinen, B. Estimating light interception in tree crops with digital images of canopy shadow. Precis. Agric. 2015, 16, 425–440. [Google Scholar] [CrossRef]
- Lampinen, B.D.; Udompetaikul, V.; Browne, G.T.; Metcalf1, S.G.; Stewart, W.L.; Contador, L.; Negron, C.; Upadhyaya, S.K. A Mobile Platform for Measuring Canopy Photosynthetically Active Radiation Interception in Orchard Systems. Hortechnology 2012, 22, 237–244. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 29 November 2017).
- Corwin, D.L.; Lesch, S.M. Application of soil electrical conductivity to precision agriculture: Theory, principles, and guidelines. Agron. J. 2003, 95, 455–471. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Characterizing soil spatial variability with apparent soil electrical conductivity: I. Survey protocols. Comput. Electron. Agric. 2005, 46, 103–133. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Apparent soil electrical conductivity measurements in agriculture. Comput. Electron. Agric. 2005, 46, 11–43. [Google Scholar] [CrossRef]
- Corwin, D.L.; Scudiero, E. Field-scale apparent soil electrical conductivity. In Methods of Soil Analysis; Logsdon, S., Ed.; Soil Science Society of America: Madison, WI, USA, 2016; Volume 1. [Google Scholar]
- Lesch, S.M. Sensor-directed response surface sampling designs for characterizing spatial variation in soil properties. Comput. Electron. Agric. 2005, 46, 153–179. [Google Scholar] [CrossRef]
- Lesch, S.M.; Rhoades, J.D.; Corwin, D.L. ESAP-95 Version 2.01 R. User Manual and Tutorial Guide; Research Report No. 146; U.S. Salinity Laboratory: Riverside, CA, USA, 2000; p. 161.
- Corwin, D.L.; Yemoto, K. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis; Logsdon, S., Ed.; Soil Science Society of America: Madison, WI, USA, 2017; Volume 2. [Google Scholar]
- Sparks, D.L. Methods of Soil Analysis. Part 3-Chemical Methods; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Dane, J.H.; Topp, G.C. Methods of Soil Analysis: Part 4-Physical Methods; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA, 2002. [Google Scholar]
- Lesch, S.M.; Suarez, D.L. A short note on calculating the adjusted SAR index. Trans. ASABE 2009, 52, 493–496. [Google Scholar] [CrossRef]
- Rengasamy, P.; Marchuk, A. Cation ratio of soils structural stability (CROSS). Soil Res. 2011, 49, 280–285. [Google Scholar] [CrossRef]
- Smith, C.J.; Oster, J.D.; Sposito, G. Potassium and magnesium in irrigation water quality assessment. Agric. Water Manag. 2015, 152, 59–64. [Google Scholar] [CrossRef]
- Sposito, G.; Oster, J.D.; Smith, C.J.; Assouline, S. Assessing soil permeability impacts from irrigation with marginal-quality waters. CAB Rev. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Scudiero, E.; Lesch, S.M.; Corwin, D.L. Validation of sensor-directed spatial simulated annealing soil sampling strategy. J. Environ. Qual. 2016, 45, 1226–1233. [Google Scholar] [CrossRef]
- Rummel, R.J. Understanding Correlation; Department of Political Science, University of Hawaii: Honolulu, HI, USA, 1976. [Google Scholar]
- Zarate-Valdez, J.L.; Whiting, M.L.; Lampinen, B.D.; Metcalf, S.; Ustin, S.L.; Brown, P.H. Prediction of leaf area index in almonds by vegetation indexes. Comput. Electron. Agric. 2012, 85, 24–32. [Google Scholar] [CrossRef]
- Fereres, E.; Martinich, D.A.; Aldrich, T.M.; Castel, J.R.; Holzapfel, E.; Schulbach, H. Drip irrigation saves money in young almond orchards. Calif. Agric. 1982, 36, 12–13. [Google Scholar]
- Abbaspour, H.; Afshari, H.; Abdel-Wahhab, M.A. Influence of salt stress on growth, pigments, soluble sugars and ion accumulation in three pistachio cultivars. J. Med. Plants Res. 2012, 6, 2468–2473. [Google Scholar] [CrossRef]
- Chelli Chaabouni, M.; Hammami, M.; Gouia, K.; Gargouri, R.; Gargouri, K.; Drira, N. Effect of salt stress on Pistacia atlantica seedlings at nursery conditions. Options Méditerranéennes. Série A Séminaires Méditerranéens 2010, 94, 135–140. [Google Scholar]
- Epstein, E. Responses of plants to saline environments. In Genetic Engineering of Osmoregulation: Impact on Plant Productivity for Food, Chemicals, and Energy; Rains, D.W., Valentine, R.C., Hollaender, A., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 7–21. [Google Scholar]
- Läuchli, A.; Grattan, S.R. Soil pH extremes. In Plant Stress Physiology; CAB International: Wallingford, UK, 2012; p. 194. [Google Scholar]
- Raven, J.A. Regulation of pH and generation of osmolarity in vascular plants: A cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol. 1985, 101, 25–77. [Google Scholar] [CrossRef]
- Picchioni, G.A.; Miyamoto, S.; Storey, J.B. Salt effects on growth and ion uptake of pistachio rootstock seedlings. J. Am. Soc. Hortic. Sci. 1990, 115, 647–653. [Google Scholar] [CrossRef]
- Grattan, S.R.; Zeng, L.; Shannon, M.C.; Roberts, S.R. Rice is more sensitive to salinity than previously thought. Calif. Agric. 2002, 56, 189–195. [Google Scholar] [CrossRef]
- Chelli-Chaabouni, A.; Ben Mosbah, A.; Maalej, M.; Gargouri, K.; Gargouri-Bouzid, R.; Drira, N. In vitro salinity tolerance of two pistachio rootstocks: Pistacia vera L. and Pistacia atlantica Desf. Environ. Exp. Bot. 2010, 69, 302–312. [Google Scholar] [CrossRef]
- Hajiboland, R.; Noroozi, F.; Poschenrieder, C. Growth physiological, biochemical and ionic responses of pistachio seedlings to mild and high salinity. Trees 2014, 28, 1065–1078. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Ann. Rev. Plant Physiol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–685. [Google Scholar] [CrossRef]
- Karimi, H.R.; Zamani, Z.; Ebadi, A.; Fatahi, R. Effects of Water Salinity on Growth Indices and Physiological Parameters in Some Wild Pistachio. Int. J. Nuts Relat. Sci. 2012, 3, 41–48. [Google Scholar]
- Marino, G.; La Mantia, M.; Caruso, T.; Marra, F.P. Seasonal dynamics of photosynthesis and total carbon gain in bearing and nonbearing pistachio (Pistacia vera L.) shoots. Photosynthetica 2018, 56, 932–941. [Google Scholar] [CrossRef]
| Year | Orchard | Pre-Season Irrigation (mm) | In-Season Irrigation (mm) | Total Irrigation (mm) | ETo (mm) |
|---|---|---|---|---|---|
| 2016 | Non-saline | 254 | 965 | 1219 | 946 |
| Saline | 262 | 960 | 1222 | 941 | |
| 2017 | Non-saline | 329 | 823 | 1152 | 930 |
| Saline | 406 | 719 | 1125 | 939 |
| Orchard | Station ID | Type of ET Stations | Method for Estimating H |
|---|---|---|---|
| Non-Saline | NS | Full | EC and SR |
| Saline | S1 | Lite | SR |
| S2 | Full | EC and SR | |
| S2 | Lite | SR | |
| S4 | Lite | SR | |
| S5 | Lite | SR | |
| S6 | Lite | SR |
| Depth | 0–0.30 m | 0.30–0.60 m | 0.60–0.90 m | 0.90–1.20 m |
|---|---|---|---|---|
| WC | ||||
| ECa EMh | 0.66 | 0.63 | 0.52 | 0.63 |
| ECa EMv | 0.63 | 0.62 | 0.51 | 0.64 |
| SP | ||||
| ECa EMh | 0.60 | 0.71 | 0.65 | 0.56 |
| ECa EMv | 0.58 | 0.70 | 0.67 | 0.62 |
| PH | ||||
| ECa EMh | −0.18 | 0.13 | −0.07 | −0.06 |
| ECa EMv | −0.14 | 0.18 | −0.01 | 0.01 |
| ECe | ||||
| ECa EMh | 0.45 | 0.21 | 0.45 | 0.58 |
| ECa EMv | 0.40 | 0.22 | 0.44 | 0.60 |
| SAR | ||||
| ECa EMh | 0.47 | 0.68 | 0.71 | 0.74 |
| ECa EMv | 0.45 | 0.67 | 0.73 | 0.76 |
| Orchard | Value | SAR | SP (%) | pH | CROSS | |||
|---|---|---|---|---|---|---|---|---|
| NS | Ave | 4.3 | 2.7 | 0.1 | 0.3 | 7.6 | 4.9 | 2.9 |
| Min | 1.1 | 0.58 | 0.04 | 0.2 | 6.5 | 2.1 | 1.8 | |
| Max | 11.9 | 6.4 | 0.16 | 0.42 | 8.4 | 11.2 | 4.3 | |
| S | Ave | 22.6 | 7.1 | 0.19 | 0.61 | 7.6 | 25.2 | 17.1 |
| Min | 7.4 | 1.65 | 0.09 | 0.27 | 6.5 | 22.4 | 15.3 | |
| Max | 44.8 | 14.04 | 0.31 | 1.17 | 8.8 | 27.1 | 15.3 |
| Orchard | Site | CETa (mm) | Nut Yield (kg tree−1) | RPR | ||||
|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |||
| Non saline | NS | 75 a | 75 a | 771 | 867 | 10 c | 41 a | 0.00 |
| Saline | S1 | 45 c | 40 c,d | 571 | 557 | 12 c | 16 b,c | 0.40 |
| S2 | 35 d | 44 c | 611 | 622 | 20 a,b | 15 b,c | 0.34 | |
| S3 | 27 e | 32 e | 525 | 465 | 14 b,c | 10 c,d | 0.52 | |
| S4 | 51 b,c | 60 b | 721 | 650 | 23 a | 24 b | 0.17 | |
| S5 | 56 b | 62 | 739 | 724 | 20 a,b | 22 b | 0.16 | |
| S6 | 33 b,e | 36 d,e | 671 | 638 | 14 b,c | 19 b | 0.37 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, G.; Zaccaria, D.; Snyder, R.L.; Lagos, O.; Lampinen, B.D.; Ferguson, L.; Grattan, S.R.; Little, C.; Shapiro, K.; Maskey, M.L.; et al. Actual Evapotranspiration and Tree Performance of Mature Micro-Irrigated Pistachio Orchards Grown on Saline-Sodic Soils in the San Joaquin Valley of California. Agriculture 2019, 9, 76. https://doi.org/10.3390/agriculture9040076
Marino G, Zaccaria D, Snyder RL, Lagos O, Lampinen BD, Ferguson L, Grattan SR, Little C, Shapiro K, Maskey ML, et al. Actual Evapotranspiration and Tree Performance of Mature Micro-Irrigated Pistachio Orchards Grown on Saline-Sodic Soils in the San Joaquin Valley of California. Agriculture. 2019; 9(4):76. https://doi.org/10.3390/agriculture9040076
Chicago/Turabian StyleMarino, Giulia, Daniele Zaccaria, Richard L. Snyder, Octavio Lagos, Bruce D. Lampinen, Louise Ferguson, Stephen R. Grattan, Cayle Little, Kristen Shapiro, Mahesh Lal Maskey, and et al. 2019. "Actual Evapotranspiration and Tree Performance of Mature Micro-Irrigated Pistachio Orchards Grown on Saline-Sodic Soils in the San Joaquin Valley of California" Agriculture 9, no. 4: 76. https://doi.org/10.3390/agriculture9040076
APA StyleMarino, G., Zaccaria, D., Snyder, R. L., Lagos, O., Lampinen, B. D., Ferguson, L., Grattan, S. R., Little, C., Shapiro, K., Maskey, M. L., Corwin, D. L., Scudiero, E., & Sanden, B. L. (2019). Actual Evapotranspiration and Tree Performance of Mature Micro-Irrigated Pistachio Orchards Grown on Saline-Sodic Soils in the San Joaquin Valley of California. Agriculture, 9(4), 76. https://doi.org/10.3390/agriculture9040076






