Satellite and Proximal Sensing to Estimate the Yield and Quality of Table Grapes
Abstract
1. Introduction
2. Materials and Methods
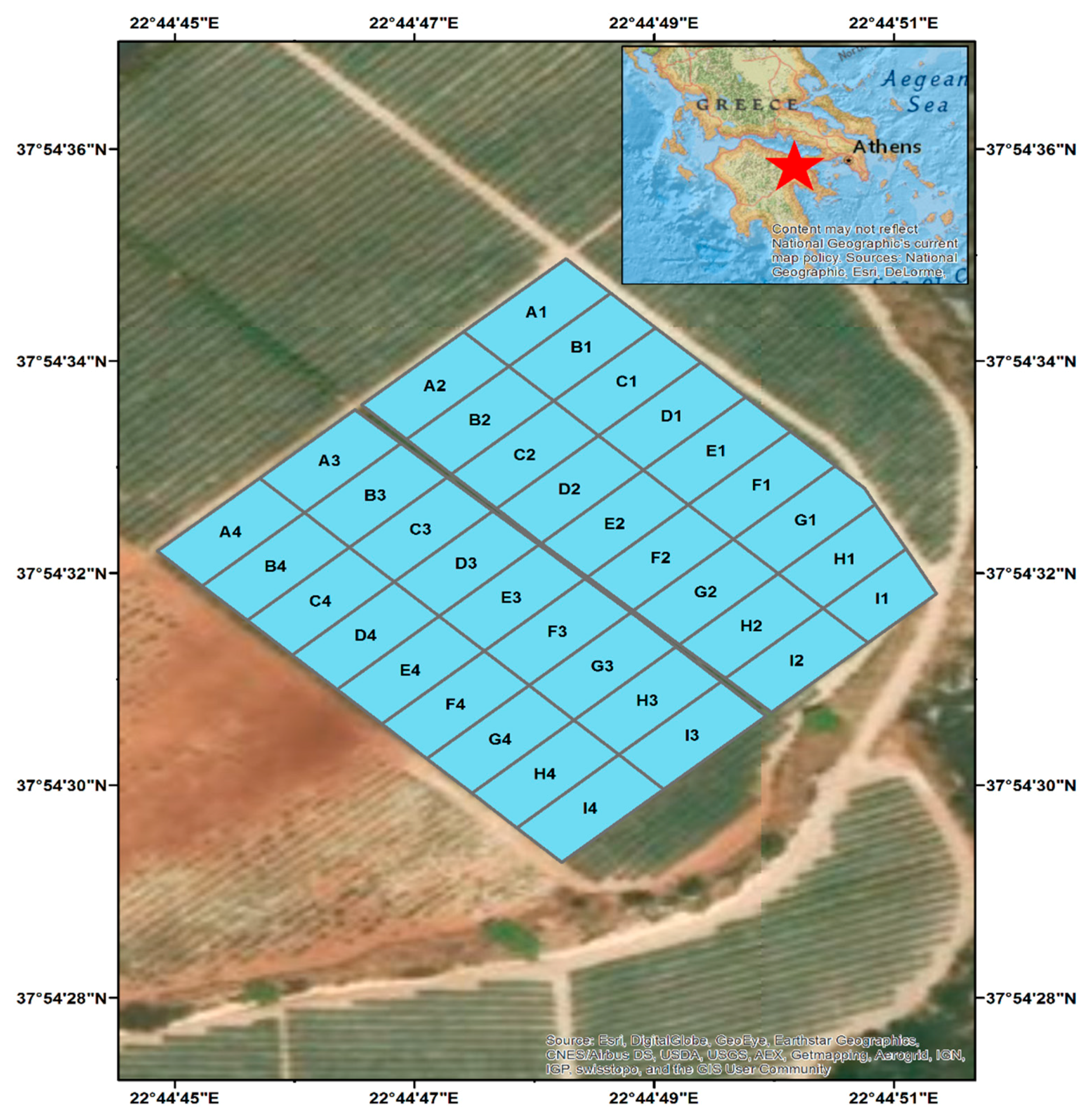
2.1. Field Site
2.2. Remote Sensing Measurements
2.3. Table Grape Measurements
2.4. Map Construction and Statistical Analysis
3. Results
3.1. Descriptive Statistics
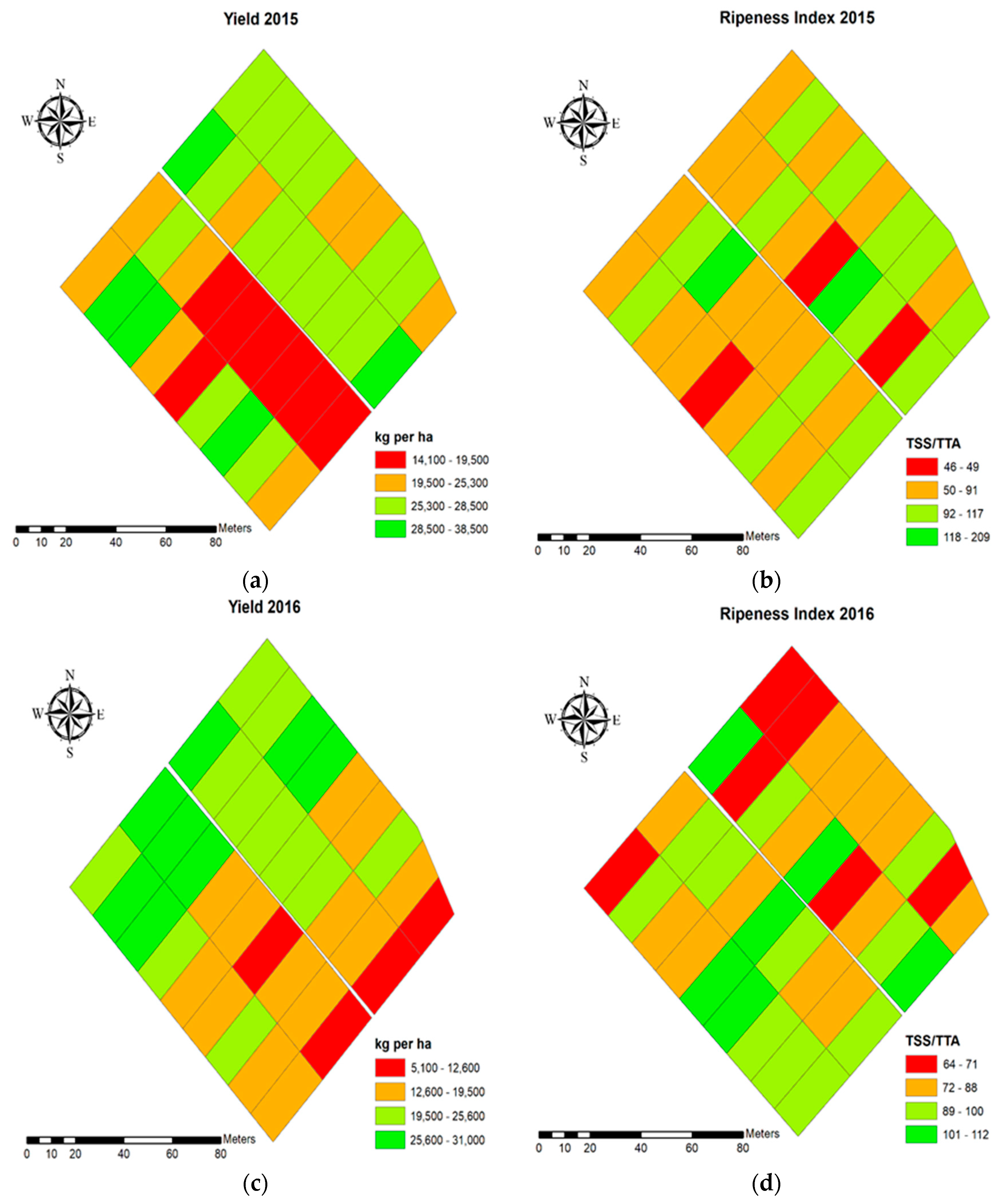
3.1.1. Table Grape Yield and Quality Characteristics
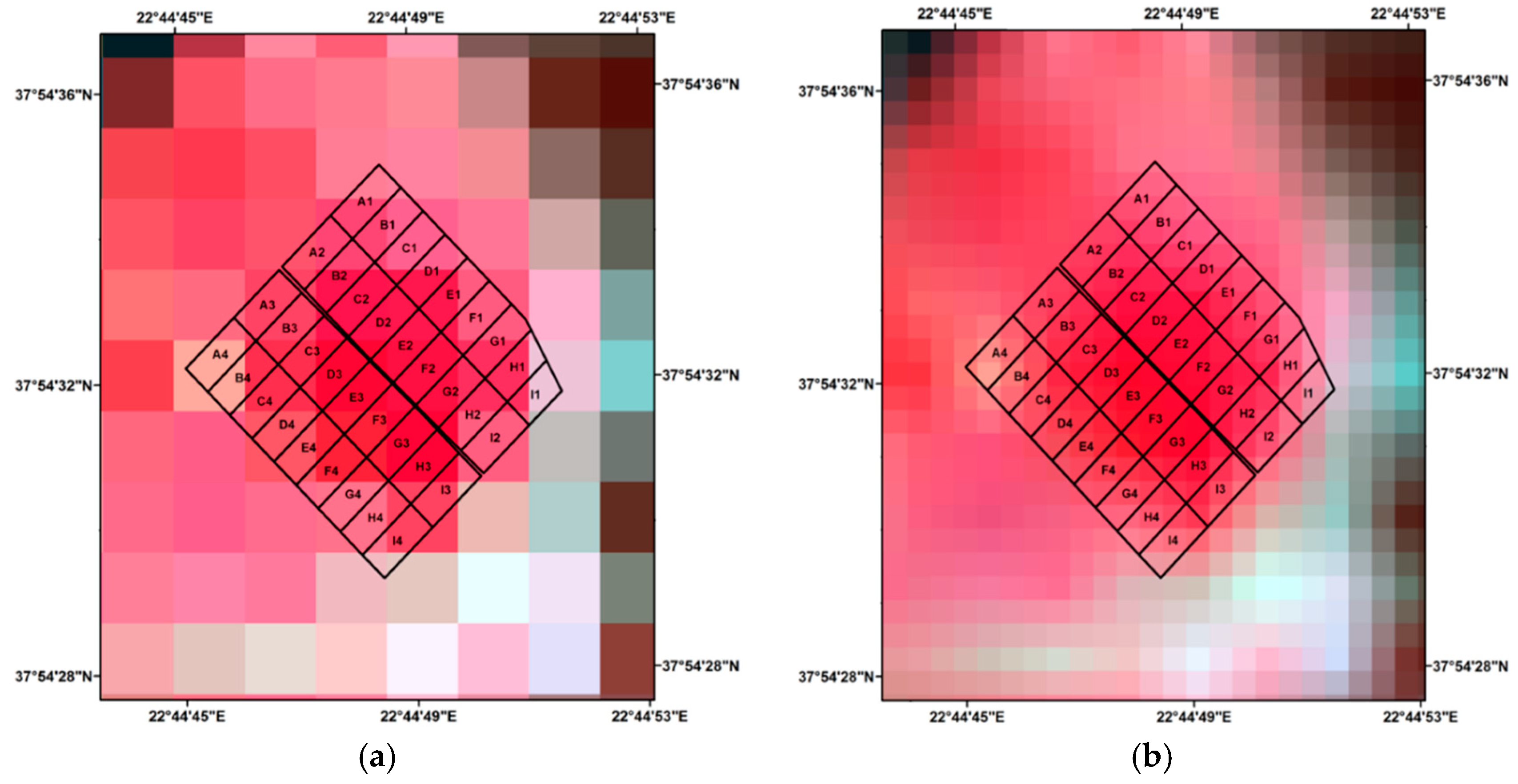
3.1.2. Satellite Based SVIs
3.1.3. Proximal Based SVIs
3.2. Pearson’s Correlation
3.2.1. Satellite SVIs x Table Grapes Characteristics
3.2.2. Proximal SVIs x Table Grapes Characteristics
3.3. Regression Analysis
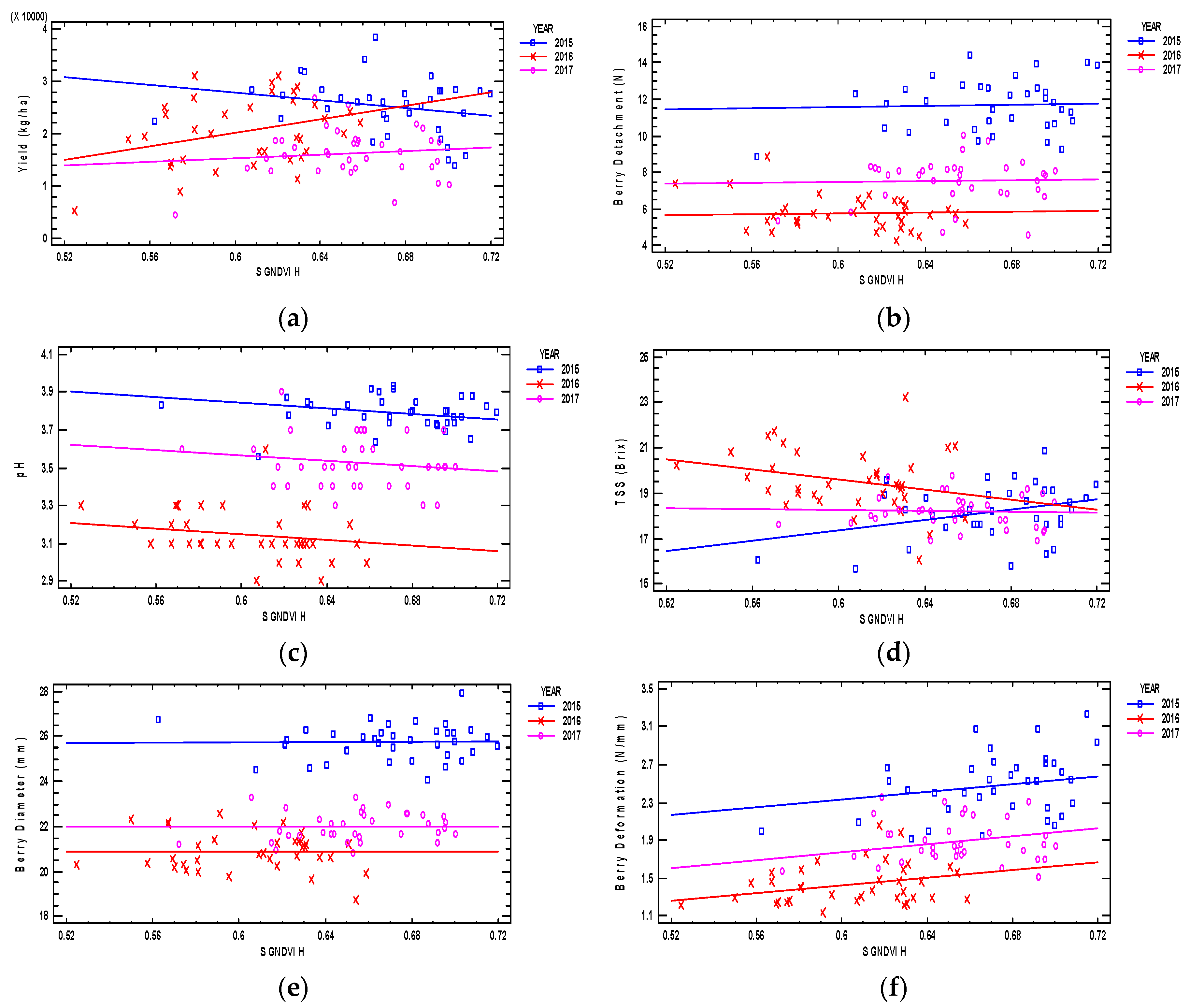
3.3.1. Satellite GNDVI H x Table Grape Characteristics
3.3.2. Proximal GRVI MV x Table Grape Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fresh Deciduous Fruit: World Markets and Trade (Apples, Grapes, & Pears). Available online: https://apps.fas.usda.gov/psdonline/circulars/fruit.pdf (accessed on 29 April 2018).
- Strik, B.C. Growing Table Grapes. 32. Available online: http://smallfarms.oregonstate.edu/sites/default/files/publications/growing_table_grapes_ec1639_may_2011.pdf (accessed on 29 April 2018).
- Rather, J.A.; Wani, S.H.; Haribhushan, A.; Bhat, Z.A. Influence of girdling, thinning and GA3 on fruit quality and shelf life of grape (Vitis vinifera) cv. perlette. Elixir Agric. 2011, 41, 5731–5735. [Google Scholar]
- Sen, F.; Oksar, R.; Kesgin, M. Effects of Shading and Covering on ‘Sultana Seedless’ Grape Quality and Storability. J. Agric. Sci. Technol. 2016, 18, 245–254. [Google Scholar]
- Tehrani, M.M.; Kamgar-Haghighi, A.A.; Razzaghi, F.; Sepaskhah, A.R.; Zand-Parsa, S.; Eshghi, S. Physiological and yield responses of rainfed grapevine under different supplemental irrigation regimes in Fars province, Iran. Sci. Hortic. 2016, 202, 133–141. [Google Scholar] [CrossRef]
- Hussein, M.A.; Abd-elall, E.H. Attempts to Improve Berry Quality of flame seedless Grapevines. Egypt. J. Hortic. 2017, 44, 235–244. [Google Scholar] [CrossRef]
- Moran, M.S.; Inoue, Y.; Barnes, E.M. Opportunities and limitations for image-based remote sensing in precision crop management. Remote Sens. Environ. 1997, 61, 319–346. [Google Scholar] [CrossRef]
- Mondal, P.; Basu, M. Adoption of precision agriculture technologies in India and in some developing countries: Scope, present status and strategies. Prog. Nat. Sci. 2009, 19, 659–666. [Google Scholar] [CrossRef]
- Panda, S.S.; Ames, D.P.; Panigrahi, S. Application of Vegetation Indices for Agricultural Crop Yield Prediction Using Neural Network Techniques. Remote Sens. 2010, 2, 673–696. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyakb, M.N. Quantitative estimation of chlorophyll-u using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Pena-Barragan, J.M.; Lopez-Granados, F.; Jurado-Exposito, M.; Garcia-Torres, L. Spectral discrimination of Ridolfia segetum and sunflower as affected by phenological stage. Weed Res. 2006, 46, 10–21. [Google Scholar] [CrossRef]
- Chang, J.; Clay, D.E.; Dalsted, K.; Clay, S.; O’Neill, M. Corn (Zea mays L.) Yield Prediction Using Multispectral and Multidate Reflectance. Agron. J. 2003, 95, 1447–1453. [Google Scholar] [CrossRef]
- Ranjitha, G.; Srinivasan, M.R.; Rajesh, A. Detection and Estimation of Damage Caused by Thrips Thrips tabaci (Lind) of Cotton Using Hyperspectral Radiometer. Agrotechnology 2014, 3. [Google Scholar] [CrossRef]
- Genc, H.; Genc, L.; Turhan, H.; Smith, S.E.; Nation, J.L. Vegetation indices as indicators of damage by the sunn pest (Hemiptera: Scutelleridae) to field grown wheat. Afr. J. Biotechnol. 2008, 7, 173–180. [Google Scholar]
- Jia, L.; Yu, Z.; Li, F.; Gnyp, M.; Koppe, W.; Bareth, G.; Miao, Y.; Chen, X.; Zhang, F. Nitrogen Status Estimation of Winter Wheat by Using an IKONOS Satellite Image in the North China Plain. In Computer and Computing Technologies in Agriculture V; Li, D., Chen, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 369, pp. 174–184. ISBN 978-3-642-27277-6. [Google Scholar]
- Li, F.; Gnyp, M.L.; Jia, L.; Miao, Y.; Yu, Z.; Koppe, W.; Bareth, G.; Chen, X.; Zhang, F. Estimating N status of winter wheat using a handheld spectrometer in the North China Plain. Field Crops Res. 2008, 106, 77–85. [Google Scholar] [CrossRef]
- Glaser, J.; Casas, J.; Copenhaver, K.; Mueller, S. Development of a broad landscape monitoring system using hyperspectral imagery to detect pest infestation. In Proceedings of the IEEE2009 First Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing, Grenoble, France, 26–28 August 2009; pp. 1–4. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.; Gao, X.; Ferreira, L. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Hall, A.; Lamb, D.W.; Holzapfel, B.P.; Louis, J.P. Within-season temporal variation in correlations between vineyard canopy and winegrape composition and yield. Precis. Agric. 2011, 12, 103–117. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.P.; Goovaerts, P.; Tardaguila, J. Assessment of the spatial variability of anthocyanins in grapes using a fluorescence sensor: Relationships with vine vigour and yield. Precis. Agric. 2012, 13, 457–472. [Google Scholar] [CrossRef]
- Joshi, N.; Baumann, M.; Ehammer, A.; Fensholt, R.; Grogan, K.; Hostert, P.; Jepsen, M.R.; Kuemmerle, T.; Meyfroidt, P.; Mitchard, E.T.A.; et al. A Review of the Application of Optical and Radar Remote Sensing Data Fusion to Land Use Mapping and Monitoring. Remote Sens. 2016, 8, 70. [Google Scholar] [CrossRef]
- Hall, A.; Lamb, D.W.; Holzapfel, B.; Louis, J. Optical remote sensing applications in viticulture—A review. Aust. J. Grape Wine Res. 2002, 8, 36–47. [Google Scholar] [CrossRef]
- Gascon, F.; Bouzinac, C.; Thépaut, O.; Jung, M.; Francesconi, B.; Louis, J.; Lonjou, V.; Lafrance, B.; Massera, S.; Gaudel-Vacaresse, A.; et al. Copernicus Sentinel-2A Calibration and Products Validation Status. Remote Sens. 2017, 9, 584. [Google Scholar] [CrossRef]
- Vaudour, E.; Carey, V.A.; Gilliot, J.M. Digital zoning of South African viticultural terroirs using bootstrapped decision trees on morphometric data and multitemporal SPOT images. Remote Sens. Environ. 2010, 114, 2940–2950. [Google Scholar] [CrossRef]
- Rodríguez, J.R.; Miranda, D.; Álvarez, C.J. Application of satellite images to locate and inventory vineyards in the designation of origin “Bierzo” in Spain. Trans. ASABE 2006, 49, 277–290. [Google Scholar] [CrossRef]
- Semmens, K.A.; Anderson, M.C.; Kustas, W.P.; Gao, F.; Alfieri, J.G.; McKee, L.; Prueger, J.H.; Hain, C.R.; Cammalleri, C.; Yang, Y.; et al. Monitoring daily evapotranspiration over two California vineyards using Landsat 8 in a multi-sensor data fusion approach. Remote Sens. Environ. 2016, 185, 155–170. [Google Scholar] [CrossRef]
- Johnson, L.; Roczen, D.; Youkhana, S.; Nemani, R.; Bosch, D. Mapping vineyard leaf area with multispectral satellite imagery. Comput. Electron. Agric. 2003, 38, 33–44. [Google Scholar] [CrossRef]
- Kandylakis, Z.; Karantzalos, K. Precision viticulture from multitemporal, multispectral very high resolution satellite data. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, 41, 919–925. [Google Scholar] [CrossRef]
- Borgogno-Mondino, E.; Lessio, A.; Tarricone, L.; Novello, V.; de Palma, L. A comparison between multispectral aerial and satellite imagery in precision viticulture. Precis. Agric. 2018, 19, 195–217. [Google Scholar] [CrossRef]
- Matese, A.; Gennaro, S.F.D. Technology in Precision Viticulture: A State of the Art Review. Available online: https://www.dovepress.com/technology-in-precision-viticulture-a-state-of-the-art-review-peer-reviewed-fulltext-article-IJWR (accessed on 29 April 2018).
- Erena, M.; Montesinos, S.; Portillo, D.; Alvarez, J.; Marin, C.; Fernandez, L.; Henarejos, J.M.; Ruiz, L.A. Configuration and specifications of an unmanned aerial vehicle for precision agriculture. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, XLI-B1, 809–816. [Google Scholar] [CrossRef]
- Marçal, A.; Gonçalves, J.; Cunha, M. Analysis of the temporal signature of vineyards in Portugal using VEGETATION. In Proceedings of the 26th EARSeL Symposium, New Developments and Challenges in Remote Sensing, Warsaw, Poland, 29 May–2 June 2006; Millpress: Rotterdam, The Netherlands, 2007; pp. 377–384. [Google Scholar]
- Cerovic, Z.G.; Moise, N.; Agati, G.; Latouche, G.; Ben Ghozlen, N.; Meyer, S. New portable optical sensors for the assessment of winegrape phenolic maturity based on berry fluorescence. J. Food Compos. Anal. 2008, 21, 650–654. [Google Scholar] [CrossRef]
- Llorens, J.; Gil, E.; Llop, J.; Escolà, A. Ultrasonic and LIDAR Sensors for Electronic Canopy Characterization in Vineyards: Advances to Improve Pesticide Application Methods. Sensors 2011, 11, 2177–2194. [Google Scholar] [CrossRef] [PubMed]
- Del-Moral-Martínez, I.; Rosell-Polo, J.R.; Company, J.; Sanz, R.; Escolà, A.; Masip, J.; Martínez-Casasnovas, J.A.; Arnó, J. Mapping Vineyard Leaf Area Using Mobile Terrestrial Laser Scanners: Should Rows be Scanned On-the-Go or Discontinuously Sampled? Sensors 2016, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Dosso, P.; Maurino, M.; Merli, M.C.; Bernizzoni, F.; José Pirez, F.; Platè, B.; Bertuzzi, G.C.; Poni, S. MECS-VINE®: A New Proximal Sensor for Segmented Mapping of Vigor and Yield Parameters on Vineyard Rows. Sensors 2016, 16, 2009. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, M.; Glémas, P.; Rousseau, J.; Tisseyre, B. Temporal stability of within-field patterns of NDVI in non irrigated Mediterranean vineyards. OENO One 2011, 45, 61. [Google Scholar] [CrossRef]
- Fountas, S.; Anastasiou, E.; Balafoutis, A. The influence of vine variety and vineyard management on the effectiveness of canopy sensors to predict winegrape yield and quality. In Proceedings of the International Conference of Agricultural Engineering, Zurich, Switzerland, 6–10 July 2014; p. 8. [Google Scholar]
- Tagarakis, A.; Liakos, V.; Fountas, S.; Koundouras, S.; Gemtos, T.A. Management zones delineation using fuzzy clustering techniques in grapevines. Precis. Agric. 2013, 14, 18–39. [Google Scholar] [CrossRef]
- Farid, H.U.; Bakhsh, A.; Ahmad, N.; Ahmad, A.; Mahmood-Khan, Z. Delineating site-specific management zones for precision agriculture. J. Agric. Sci. 2016, 154, 273–286. [Google Scholar] [CrossRef]
- Sonnekus, N. Development and Change that Occurs in Table Grape Berry Composition during Growth. Ph.D. Thesis, Stellenosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Huete, A. A comparison of vegetation indices over a global set of TM images for EOS-MODIS. Remote Sens. Environ. 1997, 59, 440–451. [Google Scholar] [CrossRef]
- Vescovo, L.; Wohlfahrt, G.; Balzarolo, M.; Pilloni, S.; Sottocornola, M.; Rodeghiero, M.; Gianelle, D. New spectral vegetation indices based on the near-infrared shoulder wavelengths for remote detection of grassland phytomass. Int. J. Remote Sens. 2012, 33, 2178–2195. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.K.; Roy, D.P.; Yan, L.; Li, Z.; Huang, H.; Vermote, E.; Skakun, S.; Roger, J.-C. Characterization of Sentinel-2A and Landsat-8 top of atmosphere, surface, and nadir BRDF adjusted reflectance and NDVI differences. Remote Sens. Environ. 2018. [Google Scholar] [CrossRef]
- Neto, F.J.D.; Tecchio, M.A.; Junior, A.P.; Vedoato, B.T.F.; Lima, G.P.P.; Roberto, S.R. Effect of ABA on colour of berries, anthocyanin accumulation and total phenolic compounds of ‘Rubi’ table grape (Vitis vinifera). Aust. J. Crop Sci. 2017, 11, 199–205. [Google Scholar] [CrossRef]
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Academic Press: London, UK, 2002; ISBN 0-12-119062-5. [Google Scholar]
- Muñoz-Robredo, P.; Robledo, P.; Manríquez, D.; Molina, R.; Defilippi, B.G. Characterization of Sugars and Organic Acids in Commercial Varieties of Table Grapes. Chil. J. Agric. Res. 2011, 71, 452–458. [Google Scholar] [CrossRef]
- Escalona, J.M.; Flexas, J.; Bota, J.; Medrano, H. Distribution of leaf photosynthesis and transpiration within grapevine canopies under different drought conditions. Vitis 2003, 42, 57–64. [Google Scholar]
- Bertamini, M.; Nedunchezhian, N. Photosynthetic functioning of individual grapevine leaves (Vitis vinifera L. cv. Pinot noir) during ontogeny in the field. Vitis 2003, 42, 13–17. [Google Scholar]
- Martinez-Casasnovas, J.A.; Agelet-Fernandez, J.; Arno, J.; Ramos, M.C. Analysis of vineyard differential management zones and relation to vine development, grape maturity and quality. Span. J. Agric. Res. 2012, 10, 326. [Google Scholar] [CrossRef]
- Esgici, R.; Özdemir, G.; Pekitkan, G.; Eliçin, K.; Öztürk, F.; Sessiz, A. Engineering Properties of the Şire Grape (Vitis vinifera L. cv.). Scientific Papers. Ser. B Horticult. 2017, LXI, 195–204, Print ISSN 2285-5653. [Google Scholar]
- Giacosa, S.; Torchio, F.; Rio Segade, S.; Giust, M.; Tomasi, D.; Gerbi, V.; Rolle, L. Selection of a Mechanical Property for Flesh Firmness of Table Grapes in Accordance with an OIV Ampelographic Descriptor. Am. J. Enol. Vitic. 2014, 65, 206–214. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Cobb, F.; Tracana, S.; Costa, G.J.; Valente, I.; Goulao, L.F.; Amâncio, S. Relating Water Deficiency to Berry Texture, Skin Cell Wall Composition, and Expression of Remodeling Genes in Two Vitis vinifera L. Varieties. J. Agric. Food Chem. 2015, 63, 3951–3961. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, F.; Anderson, M.; Kustas, W.; Alsina, M.; Sanchez, L.; Sams, B.; McKee, L.; Dulaney, W.; White, W.; et al. Daily Mapping of 30 m LAI and NDVI for Grape Yield Prediction in California Vineyards. Remote Sens. 2017, 9, 317. [Google Scholar] [CrossRef]
- García-Estévez, I.; Quijada-Morín, N.; Rivas-Gonzalo, J.C.; Martínez-Fernández, J.; Sánchez, N.; Herrero-Jiménez, C.M.; Escribano-Bailón, M.T. Relationship between hyperspectral indices, agronomic parameters and phenolic composition of Vitis vinifera cv. Tempranillo grapes: Hyperspectral indices, agronomic parameters and phenolic composition of V. vinifera. J. Sci. Food Agric. 2017, 97, 4066–4074. [Google Scholar] [CrossRef] [PubMed]
- Tattaris, M.; Reynolds, M.P.; Chapman, S.C. A Direct Comparison of Remote Sensing Approaches for High-Throughput Phenotyping in Plant Breeding. Front. Plant Sci. 2016, 7, 1131. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Everitt, J.H.; Du, Q.; Luo, B.; Chanussot, J. Using High-Resolution Airborne and Satellite Imagery to Assess Crop Growth and Yield Variability for Precision Agriculture. Proc. IEEE 2013, 101, 582–592. [Google Scholar] [CrossRef]
- Hall, A.; Wilson, M.A. Object-based analysis of grapevine canopy relationships with winegrape composition and yield in two contrasting vineyards using multitemporal high spatial resolution optical remote sensing. Int. J. Remote Sens. 2013, 34, 1772–1797. [Google Scholar] [CrossRef]
- Junges, A.H.; Fontana, D.C.; Anzanello, R.; Bremm, C. Normalized difference vegetation index obtained by ground-based remote sensing to characterize vine cycle in Rio Grande do Sul, Brazil. Ciênc. E Agrotecnol. 2017, 41, 543–553. [Google Scholar] [CrossRef]
- Er-Raki, S.; Rodriguez, J.C.; Garatuza-Payan, J.; Watts, C.J.; Chehbouni, A. Determination of crop evapotranspiration of table grapes in a semi-arid region of Northwest Mexico using multi-spectral vegetation index. Agric. Water Manag. 2013, 122, 12–19. [Google Scholar] [CrossRef]

| Spectral Vegetation Index | Equation | Bibliography |
|---|---|---|
| Normalized Difference Vegetation Index | [43] | |
| Green Normalized Difference Vegetation Index | [13] |
| Year | Satellite Imagery Dates | Proximal Sensing Dates |
|---|---|---|
| 2015 | 16 July 2015 1 August 2015 2 September 2015 | 18 July 2015 30 July 2015 1 September 2015 |
| 2016 | 18 July 2016 3 August 2016 19 August 2016 | 16 July 2016 2 August 2016 17 August 2016 |
| 2017 | 14 July 2017 30 July 2017 15 August 2017 | 9 July 2017 26 July 2017 16 August 2017 |
| Table Grape Parameters | Min | Max | Mean | SD | CV (%) |
|---|---|---|---|---|---|
| Yield (kg/ha) | 4518 | 38432 | 20612 | 6575 | 32% |
| Berry Detachment (N) | 4.265 | 14.373 | 8.332 | 2.752 | 33% |
| pH | 2.90 | 3.93 | 3.49 | 0.29 | 8% |
| Total Soluble Solids (TSS, °Brix) | 15.7 | 23.2 | 18.6 | 1.3 | 7% |
| Total Titratable Acidity (TTA, %) | 0.10 | 0.39 | 0.22 | 0.05 | 21% |
| Berry Diameter (mm) | 19 | 28 | 23 | 2.215 | 10% |
| Berry Deformation (N/mm) | 1.131 | 3.227 | 1.934 | 0.501 | 26% |
| TSS/TTA | 45 | 209 | 87 | 21 | 24% |
| Satellite Sensing SVIs | Min | Max | Mean | SD | CV (%) |
|---|---|---|---|---|---|
| S NDVI SV | 0.505 | 0.749 | 0.658 | 0.060 | 9% |
| S GNDVI SV | 0.544 | 0.701 | 0.648 | 0.032 | 5% |
| S NDVI MV | 0.509 | 0.769 | 0.649 | 0.066 | 10% |
| S GNDVI MV | 0.544 | 0.710 | 0.641 | 0.038 | 6% |
| S NDVI H | 0.493 | 0.762 | 0.649 | 0.068 | 10% |
| S GNDVI H | 0.525 | 0.720 | 0.643 | 0.043 | 7% |
| Proximal Sensing SVIs | Min | Max | Mean | SD | CV (%) |
|---|---|---|---|---|---|
| P NDVI SV | 0.187 | 0.924 | 0.631 | 0.166 | 26% |
| P GNDVI SV | 0.336 | 0.691 | 0.573 | 0.091 | 16% |
| P NDVI MV | 0.717 | 0.824 | 0.793 | 0.022 | 3% |
| P GNDVI MV | 0.339 | 0.718 | 0.615 | 0.068 | 11% |
| P NDVI H | 0.623 | 0.836 | 0.775 | 0.027 | 3% |
| P GNDVI H | 0.390 | 0.771 | 0.615 | 0.059 | 10% |
| Satellite Sensing SVIs | Yield | Berry Detachment | pH | TSS | TTA | Berry Diameter | Berry Deformation | TSS/TTA |
|---|---|---|---|---|---|---|---|---|
| S NDVI SV | −0.016 | 0.140 | 0.155 | −0.307 ** | −0.043 | 0.131 | 0.222 * | −0.011 |
| S GNDVI SV | 0.083 | 0.153 | 0.161 | −0.281 * | −0.056 | 0.172 | 0.244 * | −0.013 |
| S NDVI MV | −0.136 | 0.182 | 0.254 ** | −0.362 ** | 0.020 | 0.128 | 0.278 ** | −0.085 |
| S GNDVI MV | −0.069 | 0.225 * | 0.306 ** | −0.373 ** | 0.033 | 0.181 | 0.338 ** | −0.097 |
| S NDVI H | 0.048 | 0.459 ** | 0.471 ** | −0.335 ** | 0.008 | 0.424 ** | 0.551 ** | −0.022 |
| S GNDVI H | 0.150 | 0.536 ** | 0.537 ** | −0.322 ** | 0.006 | 0.522 ** | 0.629 ** | −0.009 |
| Proximal Sensing SVIs | Yield | Berry Detachment | pH | TSS | TTA | Berry Diameter | Berry Deformation | TSS/TTA |
|---|---|---|---|---|---|---|---|---|
| P NDVI SV | 0.259 ** | −0.037 | −0.163 | 0.241 * | −0.173 | 0.014 | −0.009 | 0.208 * |
| P GNDVI SV | 0.085 | −0.400 ** | −0.627 ** | 0.497 ** | −0.066 | −0.395 ** | −0.444 ** | 0.160 |
| P NDVI MV | 0.218 * | 0.488 ** | 0.524 ** | −0.255 ** | −0.041 | 0.465 ** | 0.549 ** | 0.037 |
| P GNDVI MV | −0.423 ** | −0.572 ** | −0.493 ** | 0.321 ** | 0.169 | −0.682 ** | −0.565 ** | −0.121 |
| P NDVI H | −0.115 | −0.108 | −0.146 | 0.102 | 0.002 | −0.196 * | −0.156 | 0.014 |
| P GNDVI H | −0.077 | 0.108 | 0.153 | −0.039 | −0.086 | 0.110 | 0.039 | 0.142 |
| Regression Model | Year | Adjusted R2 | RMSE |
|---|---|---|---|
| Yield = 50389 – 37530 × S GNDVI H | 2015 | 33% | 5382 kg/ha |
| Yield = −18,473 + 64276 × S GNDVI H | 2016 | ||
| Yield = 5379 + 16583 × S GNDVI H | 2017 | ||
| Berry Detachment = −2.14 + 20.95 × S GNDVI H | 2015 | 83% | 1.13 N |
| Berry Detachment = 13.86 − 13.77 × S GNDVI H | 2016 | ||
| Berry Detachment = 0.24 + 11.52 × S GNDVI H | 2017 | ||
| pH = 4.31 − 0.79 × S GNDVI H | 2015 | 83% | 0.12 |
| pH = 3.61 − 0.79 × S GNDVI H | 2016 | ||
| pH = 4.03 − 0.79 × S GNDVI H | 2017 | ||
| TSS = 6.12 + 18.27 × S GNDVI H | 2015 | 28% | 1.10 °Brix |
| TSS = 25.15 − 9.55 × S GNDVI H | 2016 | ||
| TSS = 16.71 + 2.34 × S GNDVI H | 2017 | ||
| Berry Diameter = 24.7 + 1.5 × S GNDVI H | 2015 | 88% | 0.8 mm |
| Berry Diameter = 20 + 1.5 × S GNDVI H | 2016 | ||
| Berry Diameter = 21 + 1.5 × S GNDVI H | 2017 | ||
| Berry Deformation = 0.21 + 3.44 × S GNDVI H | 2015 | 77% | 0.24 N/mm |
| Berry Deformation = −0.58 + 3.44 × S GNDVI H | 2016 | ||
| Berry Deformation = −0.3 + 3.44 × S GNDVI H | 2017 |
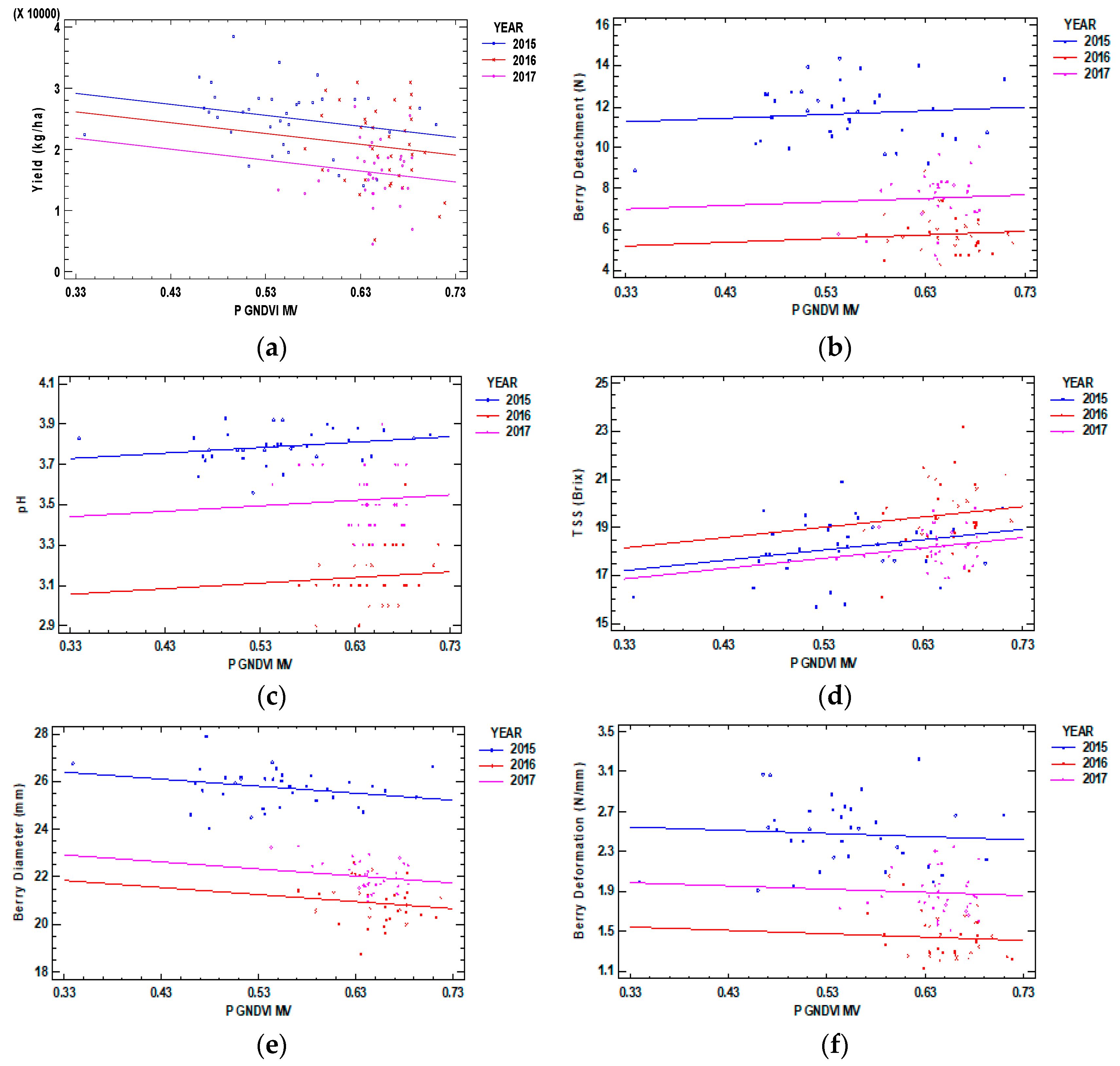
| Regression Model | Year | Adjusted R2 | RMSE |
|---|---|---|---|
| Yield = 34965 − 17742 × P GNDVI MV | 2015 | 31% | 5450 kg/ha |
| Yield = 31970 − 17742 × P GNDVI MV | 2016 | ||
| Yield = 27654 - 17742 × P GNDVI MV | 2017 | ||
| Berry Detachment = 10.68 + 1.78 × P GNDVI MV | 2015 | 81% | 1.21 N |
| Berry Detachment = 4.61 + 1.78 × P GNDVI MV | 2016 | ||
| Berry Detachment = 6.41 + 1.78 × P GNDVI MV | 2017 | ||
| pH = 3.64 + 0.27 × P GNDVI MV | 2015 | 83% | 0.12 |
| pH = 2.97 + 0.27 × P GNDVI MV | 2016 | ||
| pH = 3.34 + 0.27 × P GNDVI MV | 2017 | ||
| TSS = 15.78 + 4.31 × P GNDVI MV | 2015 | 26% | 1.12 °Brix |
| TSS = 16.74 + 4.31 × P GNDVI MV | 2016 | ||
| TSS = 15.43 + 4.31 × P GNDVI MV | 2017 | ||
| Berry Diameter = 27.4 − 3 × P GNDVI MV | 2015 | 89% | 0.7 mm |
| Berry Diameter = 22.8 − 3 × P GNDVI MV | 2016 | ||
| Berry Diameter = 23.9 − 3 × P GNDVI MV | 2017 | ||
| Berry Deformation = 2.66 − 0.33 × P GNDVI MV | 2015 | 72% | 0.21 N/mm |
| Berry Deformation = 1.65 − 0.33 × P GNDVI MV | 2016 | ||
| Berry Deformation = 2.1 − 0.33 × P GNDVI MV | 2017 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiou, E.; Balafoutis, A.; Darra, N.; Psiroukis, V.; Biniari, A.; Xanthopoulos, G.; Fountas, S. Satellite and Proximal Sensing to Estimate the Yield and Quality of Table Grapes. Agriculture 2018, 8, 94. https://doi.org/10.3390/agriculture8070094
Anastasiou E, Balafoutis A, Darra N, Psiroukis V, Biniari A, Xanthopoulos G, Fountas S. Satellite and Proximal Sensing to Estimate the Yield and Quality of Table Grapes. Agriculture. 2018; 8(7):94. https://doi.org/10.3390/agriculture8070094
Chicago/Turabian StyleAnastasiou, Evangelos, Athanasios Balafoutis, Nikoleta Darra, Vasileios Psiroukis, Aikaterini Biniari, George Xanthopoulos, and Spyros Fountas. 2018. "Satellite and Proximal Sensing to Estimate the Yield and Quality of Table Grapes" Agriculture 8, no. 7: 94. https://doi.org/10.3390/agriculture8070094
APA StyleAnastasiou, E., Balafoutis, A., Darra, N., Psiroukis, V., Biniari, A., Xanthopoulos, G., & Fountas, S. (2018). Satellite and Proximal Sensing to Estimate the Yield and Quality of Table Grapes. Agriculture, 8(7), 94. https://doi.org/10.3390/agriculture8070094









