Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials and Treatment
2.3. Drought Screening Procedure
2.4. Extraction of Phenolic Acids
2.5. Determination of Total Phenolic Contents
2.6. Determination of Total Flavonoid Contents
2.7. Antioxidant Assay Using the DPPH Radical Scavenging System
2.8. Antioxidant Test Using β-Carotene Bleaching Method
2.9. Identification of Phenolic Compounds
2.10. Statistical Analysis
3. Results
3.1. Influence of Drought Stress on Rice Leaves
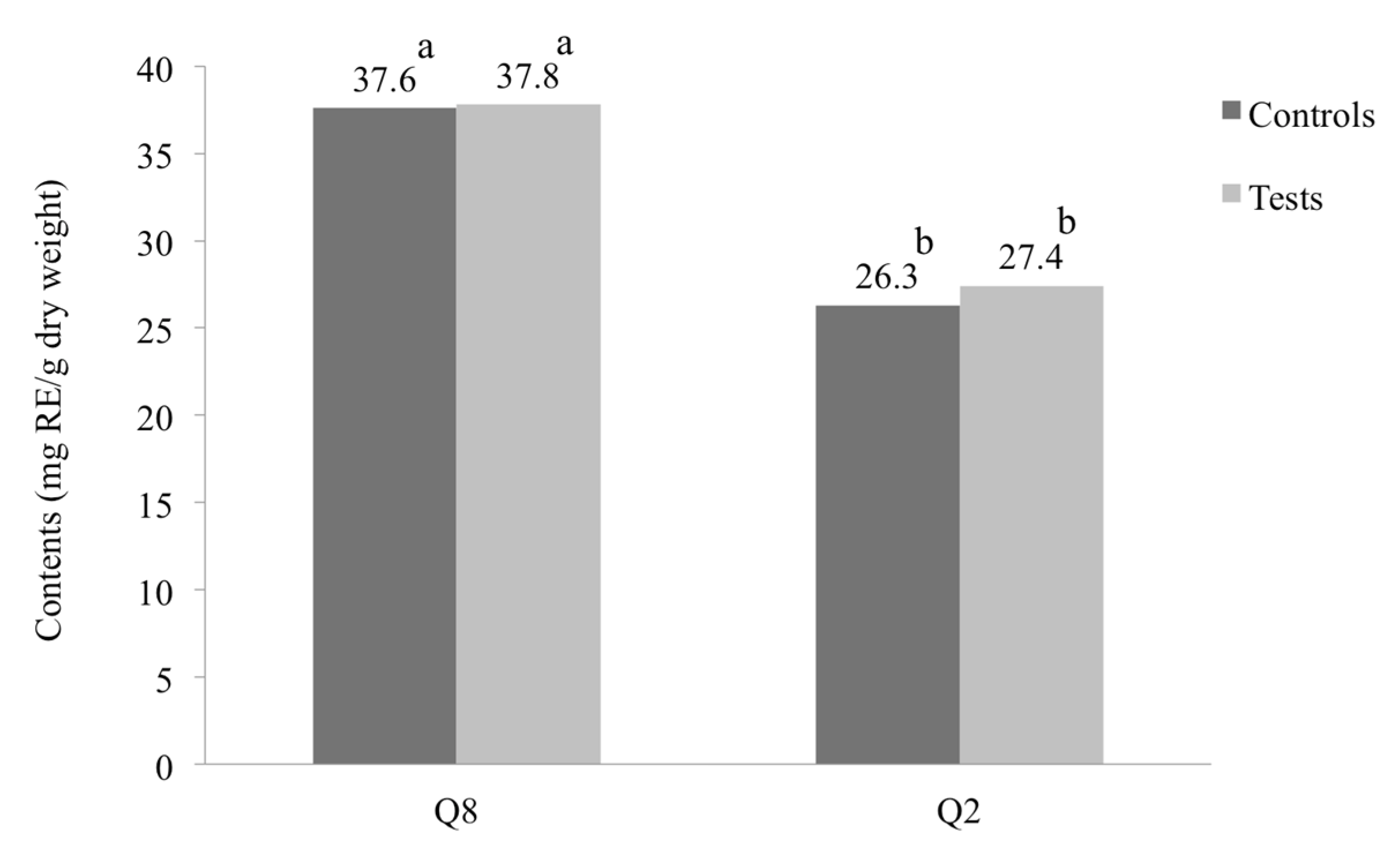
3.2. Effect of Water Deficit Stress on Total Phenolic and Flavonoid Contents
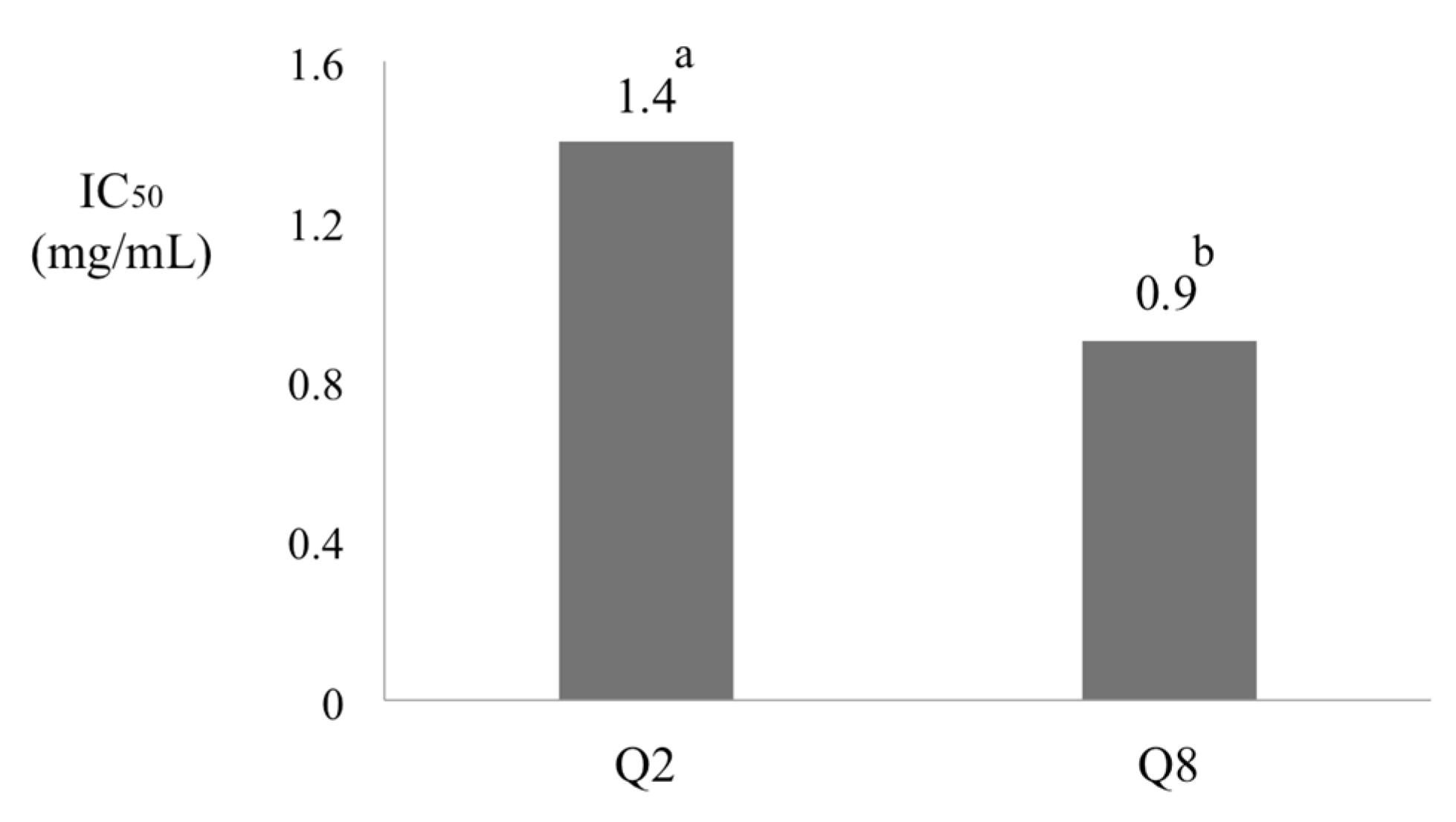
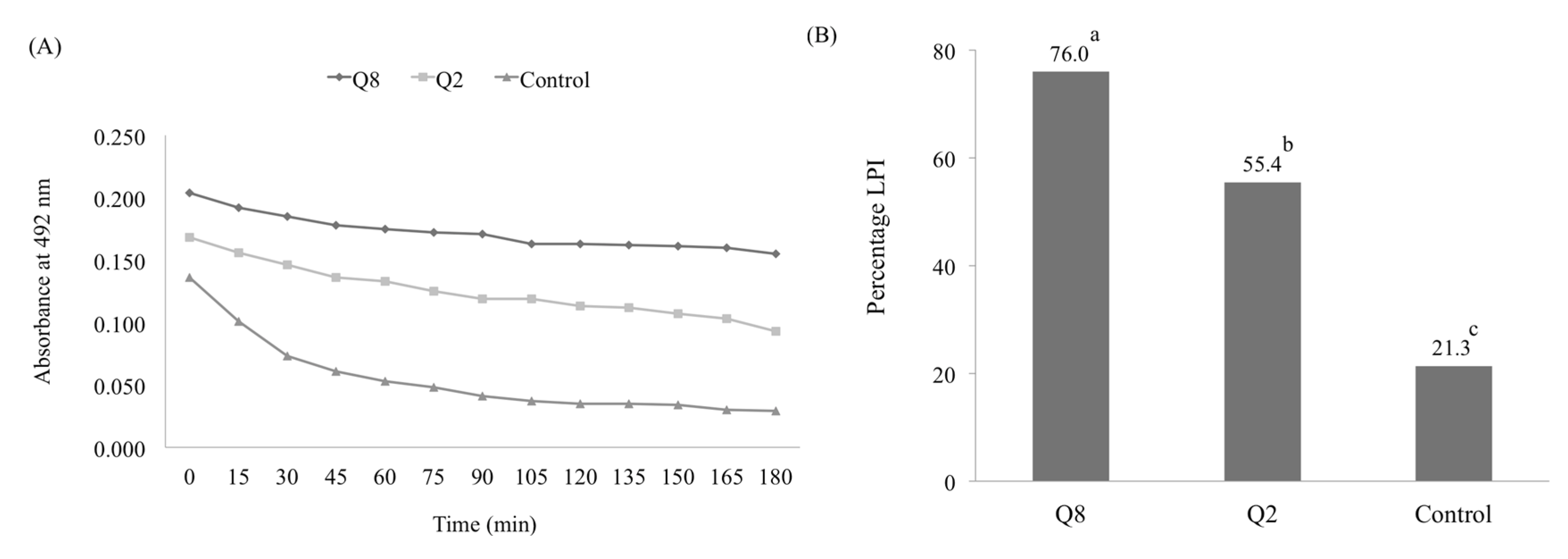
3.3. Effects of Water Deficient Stress on Antioxidant Capacity
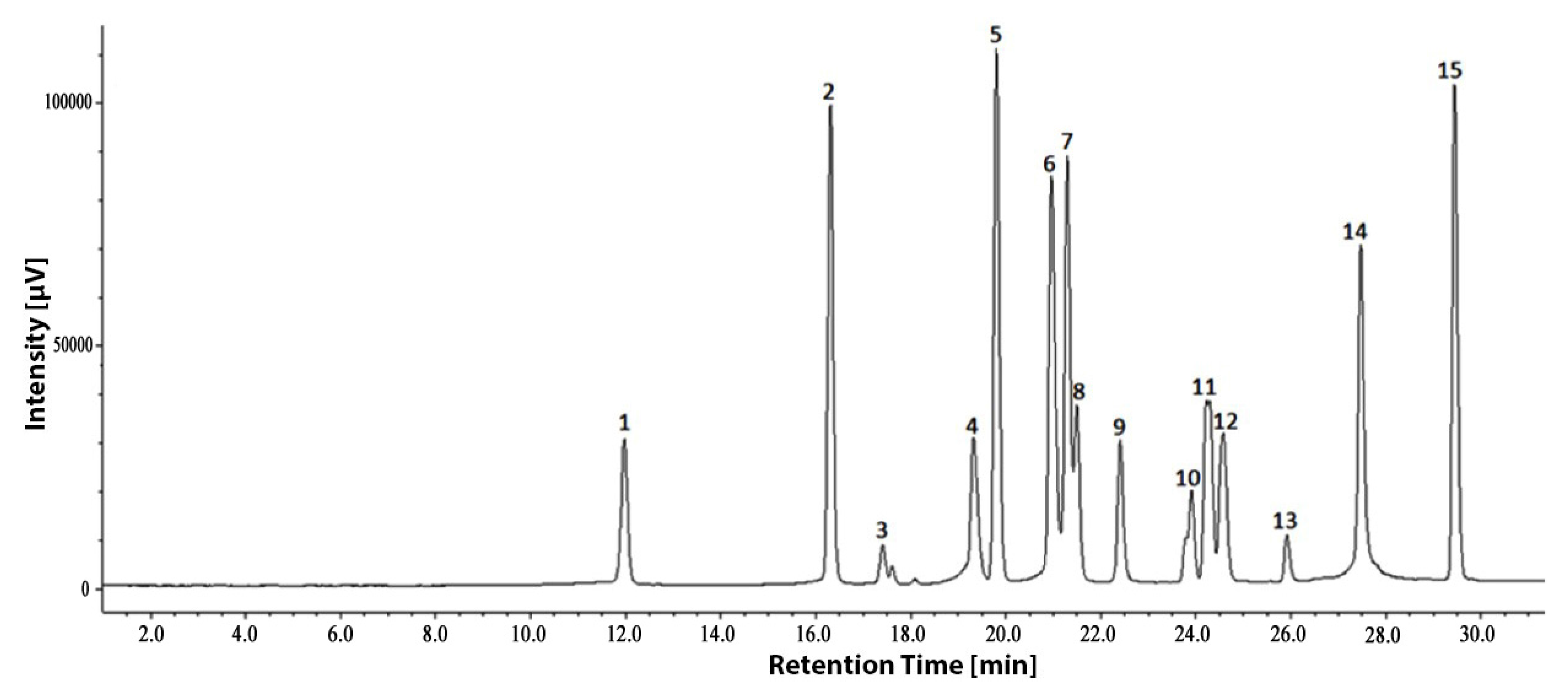
3.4. Changes of Phenolic Components under Drought Stress
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saikumar, S.; Gouda, P.K.; Saiharini, A.; Varma, C.M.K.; Vineesha, O.; Padmavathi, G.; Shenoy, V.V. Major QTL for enhancing rice grain yield under lowland reproductive drought stress identified using an O. sativa/O. glaberrima introgression line. Field Crops Res. 2014, 163, 119–131. [Google Scholar] [CrossRef]
- Sabar, M.; Arif, M. Phenotypic response of rice (Oryza sativa) genotypes to variable moisture stress regimes. Int. J. Agric. Biol. 2014, 16, 32–40. [Google Scholar]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.R.; Zaman-Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Jáuregui, O.; Codina, C.; Tiburcio, A.F.; Bastida, J.; Viladomat, F. Analysis of phenolic compounds by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry in senescent and water-stressed tobacco. Plant Sci. 2012, 182, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef]
- Fernández-García, N.; Olmos, E.; Bardisi, E.; García-De la Garma, J.; López-Berenguer, C.; Rubio-Asensio, J.S. Intrinsic water use efficiency controls the adaptation to high salinity in a semi-arid adapted plant, henna (Lawsonia inermis L.). J. Plant Physiol. 2014, 171, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Yildiz-Aktas, L.; Dagnon, S.; Gurel, A.; Gesheva, E.; Edreva, A. Drought tolerance in cotton: Involvement of non-enzymatic ROS-scavenging compounds. J. Agron. Crop Sci. 2009, 195, 247–253. [Google Scholar] [CrossRef]
- Wahid, A.; Ghazanfar, A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 2006, 163, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D. Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Landbauforsch. Volkenrode 2008, 58, 139–144. [Google Scholar]
- Markham, K.R.; Tanner, G.J.; Caasi-Lit, M.; Whitecross, M.I.; Nayudu, M.; Mitchell, K.A. Possible protective role for 3′, 4′-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry 1998, 49, 1913–1919. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Ahmad, N.; Saleem, B.A. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 2009, 195, 237–246. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Marchesan, E. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Biol. Technol. 2011, 54, 371–377. [Google Scholar] [CrossRef]
- Walter, M.; Marchesan, E.; Massoni, P.F.S.; da Silva, L.P.; Sartori, G.M.S.; Ferreira, R.B. Antioxidant properties of rice grains with light brown, red and black pericarp colors and the effect of processing. Food Res. Int. 2013, 50, 698–703. [Google Scholar] [CrossRef]
- IRRI (International Rice Research Institute). Standard Evaluation System for Rice, 2nd ed.; IRRI Los Banos: Laguna, Philippines, 1980; p. 44. [Google Scholar]
- Ti, H.; Zhang, R.; Zhang, M.; Li, Q.; Wei, Z.; Zhang, Y.; Ma, Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Elzaawely, A.A.; Tawata, S. Antioxidant capacity and phenolic content of Rumex dentatus L. grown in Egypt. J. Crop Sci. Biotechnol. 2012, 15, 59–64. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Tawata, S. Antioxidant activity of phenolic rich fraction obtained from Convolvulus arvensis L. leaves grown in Egypt. Asian J. Crop Sci. 2012, 4, 32–40. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Antioxidant and antibacterial activities of Rumex japonicus HOUTT. aerial parts. Biol. Pharm. Bull. 2005, 28, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.A.; de Souza, C.G.M.; Daniel, F.M.; Ferrari, G.P.; da Costa, S.M.G.; Peralta, R.M. Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chem. 2009, 112, 775–781. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E.; Terao, H.; Matsuo, M.; Khanh, T.D. Correlation between growth inhibitory exhibition and suspected allelochemicals (phenolic compounds) in the extract of alfalfa (Medicago sativa L.). Plant Prod. Sci. 2003, 6, 165–171. [Google Scholar] [CrossRef]
- Hu, H.; Dai, M.; Yao, J.; Xiao, B.; Li, X.; Zhang, Q.; Xiong, L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Dziurka, K.; Ostrowska, A.; Bączek-Kwinta, R.; Grzesiak, M. An increase in the content of cell wall-bound phenolics correlates with the productivity of triticale under soil drought. J. Plant Physiol. 2012, 169, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.N.; Hofmann, R.W.; Williams, W.M. Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ. Exp. Bot. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; del Mar Rubio-Wilhelmi, M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S.; Suda, L.; Sato, T. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J. Agric. Food Chem. 2002, 50, 7524–7529. [Google Scholar] [CrossRef] [PubMed]
- Subba Rao, M.V.; Muralikrishna, G. Evaluation of the antioxidant properties of free and bound phenolic acids from native and malted finger millet (Ragi, Eleusine coracana Indaf-15). J. Agric. Food Chem. 2002, 50, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Saha, P.K.; Gupta, P.D.; Ganguly, S.N.; Sircar, S.M. Chemical examination of viable and non-viable rice seeds. Physiol. Plant. 1976, 38, 307–308. [Google Scholar] [CrossRef]
- Karimi, E.; Mehrabanjoubani, P.; Keshavarzian, M.; Oskoueian, E.; Jaafar, H.Z.; Abdolzadeh, A. Identification and quantification of phenolic and flavonoid components in straw and seed husk of some rice varieties (Oryza sativa L.) and their antioxidant properties. J. Sci. Food Agric. 2014, 94, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Callaway, R.M.; Vivanco, J.M. Can plant biochemistry contribute to understanding of invasion ecology? Trends Plant Sci. 2006, 11, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, S.W.; Jung, K.H.; Hahn, T.R.; Cho, M.H. Transcriptomic analysis of UV-treated rice leaves reveals UV-induced phytoalexin biosynthetic pathways and their regulatory networks in rice. Phytochemistry 2013, 96, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthetic pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Beckmann, M.; Zubair, H.; Enot, D.P.; Caracuel-Rois, Z.; Overy, D.P.; Snowdon, S.; Talbot, N.J.; Draper, J. Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J. 2009, 59, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Lee, S.W. Phenolic phytoalexins in rice: Biological functions and biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Kim, B.G.; Kim, J.H.; Kim, H.; Lim, C.E.; Lim, J.; Lee, C.; Lim, Y.; Ahn, J.H. Four glucosyltransferases from rice: cDNA cloning, expression, and characterization. J. Plant Physiol. 2008, 165, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Terashima, N.; Mori, I.; Kanda, T. Biosynthesis of p-hydroxybenzoic acid in poplar lignin. Phytochemistry 1975, 14, 1991–1992. [Google Scholar] [CrossRef]
- El-Basyouni, S.Z.; Chen, D.; Ibrahim, R.K.; Neish, A.C.; Towers, G.H.N. The biosynthesis of hydroxybenzoic acids in higher plants. Phytochemistry 1964, 3, 485–492. [Google Scholar] [CrossRef]


| No. | Rice Cultivars | Codes | Origins |
|---|---|---|---|
| 1 | IRRI-C22 | C22 | PRC, Vietnam |
| 2 | Nep vang ong Hoa Binh | Q1 | PRC, Vietnam |
| 3 | Re nuoc | Q2 | PRC, Vietnam |
| 4 | Bau quai | Q3 | PRC, Vietnam |
| 5 | Nep chuoi Hoa Binh | Q4 | PRC, Vietnam |
| 6 | Nep re | Q5 | PRC, Vietnam |
| 7 | Lua rac | Q6 | PRC, Vietnam |
| 8 | Nep lai hoa vang | Q7 | PRC, Vietnam |
| 9 | Nep nanh ngua Hai Phong | Q8 | PRC, Vietnam |
| 10 | QTN-1 | T1 | AGI, Vietnam |
| 11 | QTN-2 | T2 | AGI, Vietnam |
| 12 | QTN-3 | T3 | AGI, Vietnam |
| 13 | QTN-4 | T4 | AGI, Vietnam |
| 14 | QTN-5 | T5 | AGI, Vietnam |
| 15 | QTN-6 | T6 | AGI, Vietnam |
| 16 | QTN-7 | T7 | AGI, Vietnam |
| 17 | QTN-BV5 | B5 | AGI, Vietnam |
| 18 | QTN-HTS1 | H1 | AGI, Vietnam |
| 19 | Khang dan 18 | K18 | AGI, Vietnam |
| 20 | Koshihikari | KO | Hiroshima, Japan |
| Scales | Description |
|---|---|
| Leaf rolling | |
| 0 | No symptoms (normal leaves) |
| 1 | Leaves starts folding (light V-shaped) |
| 3 | Leaves folding (deep V-shaped) |
| 5 | Leaves cupped fully (U-shaped) |
| 7 | Two leaf margins touching (O-shaped) |
| 9 | Leaves rolled tightly |
| Leaf drying | |
| 0 | No symptoms (normal leaves) |
| 1 | Slight leaf tip drying (extended to less than 1/4 length of leaves) |
| 3 | Tip drying extended to 1/4 length in 25% of all leaves |
| 5 | Tip drying extended from 1/4 to 1/2 length in at most 50% of all leaves |
| 7 | Tip drying extended to 2/3 length or more in at most 70% of all leaves |
| 9 | All plants dryly died |
| Leaf withering | |
| 1 | Leaves had a naturally green color (account for 95% all of the leaves) |
| 5 | The backside of all leaves transferred to yellow accounted for 70% |
| 9 | Leaves totally transferred to yellow color |
| Recovery | |
| 1 | 90%–100% of plants were recovered |
| 3 | 70%–89% of plants were recovered |
| 5 | 40%–69% of plants were recovered |
| 7 | 20%–39% of plants were recovered |
| 9 | 0%–19% of plants were recovered |
| No. | Rice Variety | Leaf Rolling | Leaf Drying | Leaf Withering | Recovering |
|---|---|---|---|---|---|
| 1 | C22 | 5.3 ± 2.3 | 4.0 ± 2.1 | 5.0 ± 2.3 | 3.5 ± 2.0 |
| 2 | Q1 | 4.7 ± 2.6 | 3.9 ± 2.7 | 5.0 ± 2.3 | 4.3 ± 2.4 |
| 3 | Q2 | 7.9 ± 1.1 | 7.1 ± 1.0 | 7.1 ± 1.9 | 5.5 ± 1.6 |
| 4 | Q3 | 5.9 ± 2.5 | 5.4 ± 2.3 | 5.3 ± 2.3 | 3.4 ± 2.0 |
| 5 | Q4 | 4.9 ± 2.6 | 4.0 ± 2.6 | 5.0 ± 2.3 | 4.3 ± 2.4 |
| 6 | Q5 | 5.3 ± 2.6 | 4.4 ± 2.6 | 5.0 ± 2.3 | 4.5 ± 2.2 |
| 7 | Q6 | 6.6 ± 2.2 | 6.1 ± 1.9 | 5.5 ± 2.4 | 5.0 ± 2.3 |
| 8 | Q7 | 6.7 ± 1.9 | 5.7 ± 2.0 | 5.5 ± 2.1 | 4.5 ± 2.0 |
| 9 | Q8 | 3.7 ± 2.7 | 3.1 ± 2.0 | 4.2 ± 2.4 | 3.0 ± 2.0 |
| 10 | T1 | 4.7 ± 2.4 | 4.2 ± 2.1 | 4.5 ± 2.4 | 3.3 ± 1.9 |
| 11 | T2 | 6.4 ± 1.3 | 5.9 ± 1.6 | 5.0 ± 2.3 | 4.9 ± 2.3 |
| 12 | T3 | 5.4 ± 2.2 | 4.9 ± 2.1 | 5.0 ± 2.3 | 4.3 ± 2.1 |
| 13 | T4 | 5.8 ± 2.2 | 5.0 ± 1.8 | 5.5 ± 2.1 | 4.3 ± 1.9 |
| 14 | T5 | 6.1 ± 2.2 | 5.7 ± 2.2 | 5.3 ± 2.3 | 4.9 ± 2.2 |
| 15 | T6 | 5.9 ± 2.5 | 5.4 ± 2.5 | 5.3 ± 2.3 | 5.0 ± 2.3 |
| 16 | T7 | 6.8 ± 1.6 | 6.2 ± 2.1 | 5.5 ± 2.4 | 5.7 ± 1.8 |
| 17 | B5 | 5.7 ± 2.6 | 5.5 ± 2.4 | 5.0 ± 2.3 | 3.7 ± 2.1 |
| 18 | H1 | 4.7 ± 2.3 | 4.1 ± 1.9 | 4.7 ± 2.3 | 3.7 ±1.9 |
| 19 | K18 | 6.1 ± 2.4 | 5.3 ± 2.3 | 5.0 ± 2.3 | 4.3 ± 2.1 |
| 20 | KO | 5.5 ± 2.0 | 5.1 ± 2.0 | 5.3 ± 2.1 | 4.1 ± 2.3 |
| No. | Phenolic Acids | Retention Times (min) | Q8 | Q2 | ||
|---|---|---|---|---|---|---|
| Controls (mg/g DW) | Drought Stress (mg/g DW) | Controls (mg/g DW) | Drought Stress (mg/g DW) | |||
| 1 | PHBA | 19.82 | - | 0.161 ± 0.08 | - | - |
| 2 | VA | 20.99 | - | 0.029 ± 0.02 ns | - | 0.009 ± 0.01 ns |
| 3 | SYA | 21.56 | 0.746 ± 0.37 a | 0.157 ± 0.08 b | - | - |
| 4 | V | 22.40 | 0.298 ± 0.03 ns | 0.447 ± 0.22 ns | - | 0.152 ± 0.08 ns |
| 5 | FA | 23.97 | 0.304 ± 0.15 ns | 0.638 ± 0.02 ns | 0.219 ± 0.11 ns | 0.299 ± 0.10 ns |
| 6 | PCA | 24.38 | 0.998 ± 0.12 ab | 1.295 ± 0.65 a | 0.598 ± 0.01b c | 0.576 ± 0.29 bc |
| 7 | BA | 26.02 | 0.332 ± 0.17 b | 1.016 ± 0.40 a | 0.274 ± 0.01 b | 0.407 ± 0.20 b |
| 8 | CA | 29.65 | 0.025 ± 0.01 ns | 0.053 ± 0.01 ns | - | 0.056 ± 0.004 ns |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Minh, L.T.; Bach, D.T.; Ha, P.T.T.; Elzaawely, A.A.; et al. Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.). Agriculture 2016, 6, 23. https://doi.org/10.3390/agriculture6020023
Quan NT, Anh LH, Khang DT, Tuyen PT, Toan NP, Minh TN, Minh LT, Bach DT, Ha PTT, Elzaawely AA, et al. Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.). Agriculture. 2016; 6(2):23. https://doi.org/10.3390/agriculture6020023
Chicago/Turabian StyleQuan, Nguyen Thanh, La Hoang Anh, Do Tan Khang, Phung Thi Tuyen, Nguyen Phu Toan, Truong Ngoc Minh, Luong The Minh, Do Tuan Bach, Pham Thi Thu Ha, Abdelnaser Abdelghany Elzaawely, and et al. 2016. "Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.)" Agriculture 6, no. 2: 23. https://doi.org/10.3390/agriculture6020023
APA StyleQuan, N. T., Anh, L. H., Khang, D. T., Tuyen, P. T., Toan, N. P., Minh, T. N., Minh, L. T., Bach, D. T., Ha, P. T. T., Elzaawely, A. A., Khanh, T. D., Trung, K. H., & Xuan, T. D. (2016). Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.). Agriculture, 6(2), 23. https://doi.org/10.3390/agriculture6020023











